- They are facing challenges due to logistics, RM availability and higher price – especially for solvents. Most solvent prices were at an all time high. They have started to see some easing in terms of availability and cost of APIs and solvents. However, supply chain and logistics costs continue to be a challenge.
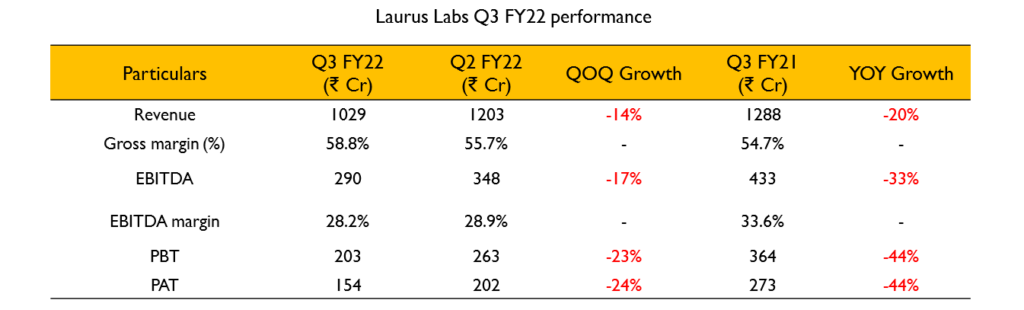
- Revenue has seen marginal growth of 3% despite lower sales in the ARV APIs and formulations. All other businesses except ARV APIs have grown for the quarter and 9 months.
- Gross margin for the quarter was at 58.8% compared to 54.7% last year. EBITDA margin was at 28.2% for the quarter and 29.6% for 9 months.
- There is an increase in demand for ARV APIs from Q4 onwards. They believe this sluggishness was only transient in nature and demand should normalize from now onwards.
- They are positive on reaching their goal of $1 Billion in sales for FY23. This will be supported by several approvals and multi-site capacity expansion across API, formulations and CDMO.
- The formulation business reported a revenue of ₹373 Cr (13% decline YoY). The contribution from the formulation segment has improved during the 9 months to 40% from 36% in the previous year.
- Laurus has signed and will be a part of MPP license for Molnupiravir to increase the broad access in LMIC markets
- Filed 3 ANDAs during the 9 months. They made a supplementary ANDA filing during the quarter. They expect to file around 6-7 ANDAs for the full year. Cumulatively, they have filed 30 ANDAs. Of this, they have 10 final approvals and 8 tentative approvals so far. They have 11 product approvals in Canada, of which they are launching 5 and are in the process of launching a few more.
- They continue to invest in FDF infrastructure. Their brownfield expansion at Unit 2 is on track and is expected to take the total FDF capacity to 10 billion units per year. It will come online early next quarter.
- Their R&D is more focused on non-ARV products right now. They have identified a few products which are complex and need scale.
- Antiviral API business during the quarter was weaker than expected and declined 65% YoY. The steep decline is due to the higher base effect because of inventory stocking by global agencies last year.
- In oncology APIs, they did ₹85 Cr sales which is a growth of 33% YoY. For 9 months, there is a growth of 8%.
- They filed 4 DMFs during the quarter. 2 DMFs are non-ARV. Total number of DMFs to date is 71.
- As indicated last quarter, they have started construction of a dedicated facility for a global life sciences company. It is a multi-product, multi-year supply contract. Part of the capex for this is funded through commercial advance. They are also investing in one more greenfield facility for the synthesis division and building an R&D center. All these projects are progressing as expected.
- During the quarter, Laurus Bio commissioned 2 new ferementers of 45 KL each taking the total capacity to 180 KL. There was a few months delay in qualifying the fermenters.
- They believe that they have the capability to become a very large player in CDMO in the global space. They have the regulatory track record, EHS compliance and manufacturing infrastructure is world class. So they believe they have a lot of avenues for growth and the CDMO business is still in its infancy.
- They want the synthesis business to contribute at least 25% of overall revenue by FY25. This includes biologics CDMO.
- ARV APIs are not very high gross margin products compared to the rest of the business. This quarter the contribution from ARV decreased so gross margins look higher.
I’ll immediately grab your rss as I can’t find your email subscription link or e-newsletter service. Do you have any? Please let me know in order that I could subscribe. Thanks.
I like this post, enjoyed this one regards for putting up. “The goal of revival is conformity to the image of Christ, not imitation of animals.” by Richard F. Lovelace.
I cling on to listening to the news speak about receiving boundless online grant applications so I have been looking around for the top site to get one. Could you tell me please, where could i acquire some?
F*ckin’ remarkable issues here. I’m very satisfied to look your post. Thank you so much and i’m having a look forward to touch you. Will you please drop me a mail?
I wanted to thank you for this great read!! I definitely enjoying every little bit of it I have you bookmarked to check out new stuff you post…
I like the valuable information you provide in your articles. I’ll bookmark your weblog and check again here frequently. I am quite certain I will learn a lot of new stuff right here! Good luck for the next!
Hi there, simply was aware of your blog thru Google, and found that it’s truly informative. I’m gonna watch out for brussels. I’ll be grateful if you continue this in future. A lot of other folks will probably be benefited from your writing. Cheers!
I was examining some of your blog posts on this website and I conceive this internet site is really informative! Retain putting up.
I like this post, enjoyed this one thankyou for putting up.
Good write-up, I am regular visitor of one?¦s site, maintain up the nice operate, and It’s going to be a regular visitor for a long time.
I like this blog very much, Its a really nice place to read and get information.
I really wanted to compose a small remark to be able to say thanks to you for these great recommendations you are giving at this website. My considerable internet lookup has finally been paid with awesome suggestions to exchange with my classmates and friends. I would assert that we visitors actually are very endowed to dwell in a superb place with very many outstanding professionals with very helpful points. I feel rather privileged to have used your entire webpages and look forward to really more pleasurable minutes reading here. Thanks again for all the details.
I’d forever want to be update on new articles on this website , saved to fav! .
I will right away clutch your rss as I can’t to find your e-mail subscription hyperlink or e-newsletter service. Do you’ve any? Kindly allow me recognise so that I may just subscribe. Thanks.
Wonderful beat ! I wish to apprentice while you amend your web site, how could i subscribe for a blog site? The account helped me a acceptable deal. I had been tiny bit acquainted of this your broadcast provided bright clear idea
I like what you guys are up also. Such intelligent work and reporting! Carry on the excellent works guys I’ve incorporated you guys to my blogroll. I think it’ll improve the value of my website :).
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
I’d should examine with you here. Which isn’t something I often do! I get pleasure from reading a publish that will make folks think. Additionally, thanks for allowing me to comment!
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: Kamagra pharmacie en ligne – kamagra pas cher
The Real Person!
The Real Person!
pharmacie en ligne sans ordonnance: pharmacie en ligne pas cher – pharmacie en ligne livraison europe pharmafst.com
kamagra oral jelly: kamagra pas cher – kamagra livraison 24h
The Real Person!
The Real Person!
Tadalafil 20 mg prix sans ordonnance: cialis generique – Tadalafil sans ordonnance en ligne tadalmed.shop
Kamagra Oral Jelly pas cher: kamagra livraison 24h – achat kamagra
The Real Person!
The Real Person!
pharmacie en ligne fiable: pharmacie en ligne – pharmacie en ligne pharmafst.com
The Real Person!
The Real Person!
Tadalafil achat en ligne: Cialis en ligne – cialis sans ordonnance tadalmed.shop
Kamagra Oral Jelly pas cher: Kamagra Commander maintenant – Kamagra Commander maintenant
The Real Person!
The Real Person!
Acheter Kamagra site fiable: acheter kamagra site fiable – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
cialis prix: Cialis en ligne – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne france livraison internationale: Medicaments en ligne livres en 24h – Pharmacie sans ordonnance pharmafst.com
The Real Person!
The Real Person!
trouver un mГ©dicament en pharmacie: Pharmacie en ligne France – Pharmacie sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Cialis en ligne: Tadalafil achat en ligne – cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Acheter Cialis 20 mg pas cher: Cialis generique prix – Achat Cialis en ligne fiable tadalmed.shop
The Real Person!
The Real Person!
vente de mГ©dicament en ligne: pharmacie en ligne sans ordonnance – vente de mГ©dicament en ligne pharmafst.com
The Real Person!
The Real Person!
cialis sans ordonnance: cialis generique – Achat Cialis en ligne fiable tadalmed.shop
The Real Person!
The Real Person!
kamagra en ligne: kamagra oral jelly – acheter kamagra site fiable
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: kamagra gel – kamagra en ligne
The Real Person!
The Real Person!
Tadalafil achat en ligne: Pharmacie en ligne Cialis sans ordonnance – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Acheter Viagra Cialis sans ordonnance: cialis sans ordonnance – cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
kamagra livraison 24h: kamagra livraison 24h – Kamagra Oral Jelly pas cher
The Real Person!
The Real Person!
legitimate canadian online pharmacies: pet meds without vet prescription canada – pharmacy wholesalers canada
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy – indian pharmacy
The Real Person!
The Real Person!
mexico pharmacy order online: RxExpressMexico – mexican rx online
The Real Person!
The Real Person!
RxExpressMexico: mexico drug stores pharmacies – п»їbest mexican online pharmacies
MedicineFromIndia: indian pharmacy online shopping – Medicine From India
The Real Person!
The Real Person!
п»їbest mexican online pharmacies: Rx Express Mexico – buying from online mexican pharmacy
RxExpressMexico Rx Express Mexico mexican online pharmacy
canada pharmacy online: Express Rx Canada – best canadian online pharmacy reviews
The Real Person!
The Real Person!
canada ed drugs: Express Rx Canada – canadian family pharmacy
Medicine From India indian pharmacy online shopping Medicine From India
The Real Person!
The Real Person!
indian pharmacy online: Medicine From India – indian pharmacy online shopping
Rx Express Mexico: mexican online pharmacy – Rx Express Mexico
Medicine From India MedicineFromIndia Medicine From India
The Real Person!
The Real Person!
buy medicines online in india: medicine courier from India to USA – Medicine From India
canadian pharmacy no rx needed: Express Rx Canada – legal canadian pharmacy online
canadian pharmacy 1 internet online drugstore canada drugs online reviews legitimate canadian mail order pharmacy
The Real Person!
The Real Person!
indian pharmacy: Medicine From India – Medicine From India
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап зеркало – пин ап вход
The Real Person!
The Real Person!
pin up az: pin up casino – pinup az
The Real Person!
The Real Person!
pinup az: pinup az – pin up azerbaycan
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап вход – пин ап казино
The Real Person!
The Real Person!
pin up casino: pinup az – pin up casino
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап вход – пин ап казино официальный сайт
The Real Person!
The Real Person!
вавада: вавада – вавада
The Real Person!
The Real Person!
пин ап казино: пин ап вход – пин ап казино официальный сайт
The Real Person!
The Real Person!
pin-up: pin-up casino giris – pin up az
The Real Person!
The Real Person!
вавада официальный сайт: вавада официальный сайт – вавада
пин ап казино: пинап казино – пин ап вход
пин ап вход: пин ап казино официальный сайт – пин ап вход
вавада зеркало: vavada casino – вавада зеркало
пин ап зеркало: пинап казино – пин ап казино официальный сайт
пинап казино: пин ап зеркало – пинап казино
pin up az: pinup az – pin up casino
вавада официальный сайт: вавада казино – вавада казино
пинап казино: pin up вход – пин ап вход
pin up вход: пин ап зеркало – пинап казино
vavada вход: вавада казино – vavada вход
pin up вход: пин ап зеркало – пин ап вход
pin up вход: пин ап вход – pin up вход
pin up azerbaycan: pin up – pin up az
pin-up: pinup az – pin-up casino giris
pin up casino: pin-up casino giris – pin up azerbaycan
The Real Person!
The Real Person!
http://pinupaz.top/# pin up
Thanks, I’ve recently been searching for information approximately this subject for ages and yours is the best I’ve found out till now. But, what in regards to the conclusion? Are you positive concerning the source?
The Real Person!
The Real Person!
purchase Modafinil without prescription: modafinil 2025 – safe modafinil purchase
The Real Person!
The Real Person!
FDA approved generic Cialis: affordable ED medication – generic tadalafil
Yeah bookmaking this wasn’t a bad conclusion outstanding post! .
The Real Person!
The Real Person!
affordable ED medication: Cialis without prescription – buy generic Cialis online
The Real Person!
The Real Person!
best price for Viagra: secure checkout Viagra – cheap Viagra online
The Real Person!
The Real Person!
safe modafinil purchase: legal Modafinil purchase – Modafinil for sale
The Real Person!
The Real Person!
best price Cialis tablets: online Cialis pharmacy – secure checkout ED drugs
The Real Person!
The Real Person!
secure checkout Viagra: discreet shipping – trusted Viagra suppliers
I have not checked in here for a while as I thought it was getting boring, but the last several posts are good quality so I guess I’ll add you back to my everyday bloglist. You deserve it my friend 🙂
The Real Person!
The Real Person!
generic tadalafil: buy generic Cialis online – generic tadalafil
http://modafinilmd.store/# safe modafinil purchase
The Real Person!
The Real Person!
Cialis without prescription: reliable online pharmacy Cialis – secure checkout ED drugs
https://zipgenericmd.shop/# Cialis without prescription
online Cialis pharmacy: Cialis without prescription – cheap Cialis online
The Real Person!
The Real Person!
Viagra without prescription: safe online pharmacy – same-day Viagra shipping
http://zipgenericmd.com/# discreet shipping ED pills
order Viagra discreetly: secure checkout Viagra – buy generic Viagra online
The Real Person!
The Real Person!
discreet shipping: secure checkout Viagra – trusted Viagra suppliers
https://modafinilmd.store/# safe modafinil purchase
same-day Viagra shipping: no doctor visit required – secure checkout Viagra
The Real Person!
The Real Person!
online Cialis pharmacy: cheap Cialis online – buy generic Cialis online
http://maxviagramd.com/# no doctor visit required
The Real Person!
The Real Person!
generic tadalafil: discreet shipping ED pills – FDA approved generic Cialis
The Real Person!
The Real Person!
Amo Health Care: cost of amoxicillin – Amo Health Care
The Real Person!
The Real Person!
cost of cheap clomid without a prescription: where buy generic clomid prices – how can i get generic clomid without insurance
The Real Person!
The Real Person!
generic amoxicillin online: amoxicillin 500 mg online – amoxicillin 500 tablet
The Real Person!
The Real Person!
PredniHealth: over the counter prednisone cream – PredniHealth
tadalafil versus cialis: is generic cialis available in canada – how long does cialis stay in your system
walgreen cialis price: TadalAccess – cialis experience forum
buy cialis in canada: cialis generic overnite – cialis review
Ero Pharm Fast Ero Pharm Fast best ed pills online
get antibiotics quickly: antibiotic without presription – over the counter antibiotics
Ero Pharm Fast: Ero Pharm Fast – Ero Pharm Fast
get antibiotics quickly: BiotPharm – buy antibiotics for uti
http://eropharmfast.com/# cheap ed medicine
ed medications cost Ero Pharm Fast Ero Pharm Fast
buy antibiotics for uti: Biot Pharm – buy antibiotics from india
Online medication store Australia: online pharmacy australia – Licensed online pharmacy AU
PharmAu24: online pharmacy australia – pharmacy online australia
https://pharmau24.com/# Online drugstore Australia
Pharm Au24: Online medication store Australia – PharmAu24
online prescription for ed Ero Pharm Fast Ero Pharm Fast
over the counter antibiotics: buy antibiotics online – buy antibiotics from canada
The Real Person!
The Real Person!
It’s time to soar to new heights with BGaming! Launching the rocket to the stars means getting incredible winnings! Space XY is an exciting game with easy gameplay. Don’t hesitate to join a breathtaking ride to the stars and make a fortune. These online gambling crash games are highly interactive and dynamic, resembling video games with sleek graphics and engaging animations. Many players love crash gambling for its straightforward mechanics without any complicated rules or strategies. Success relies on focus, timing, and implementing a solid crash gambling strategy to keep calm and time your cashout wisely. How to play Space XY Space XY is an incredibly innovative online slot game that offers a unique and captivating gaming experience. With its unconventional gameplay and distinctive design, it stands out from other online slots in the market. However, evaluating this game based on traditional metrics becomes somewhat irrelevant due to its unusual nature.
http://users.atw.hu/nlw/profile.php?mode=viewprofile&u=22434
We help our players make smart bets on raja games. Our tools give you insights to increase your chances of winning. Plus, we offer promotions and bonuses to enhance your gaming. This one is accessible to all categories of users. The next level injector is a 3rd party app called Mind FF Injector that has been added with all of the customizations. There aren’t any new players who can win the battle because they aren’t very good at the battle at the start. So, using this hack they can easily get their desired rank in Free Fire. Welcome to Cyber Mayhem This app is filled with bonuses and promotional offers. These keep the players engaged and give them confidence to play more games. Bonuses can be reinvested in the games or you can withdraw them. One of the bonuses is the first deposit bonus which rewards you 10% of your deposits. There are many more bonuses and promotional offers like this to visit them download the app from our website.
cheapest antibiotics: BiotPharm – buy antibiotics from india
http://eropharmfast.com/# affordable ed medication
Over the counter antibiotics for infection: Biot Pharm – get antibiotics quickly
ed pills cheap online ed medicine Ero Pharm Fast
get antibiotics quickly: BiotPharm – buy antibiotics for uti
ed rx online how to get ed pills Ero Pharm Fast
http://biotpharm.com/# buy antibiotics over the counter
buy ed meds: cheapest ed treatment – where can i get ed pills
The Real Person!
The Real Person!
تختلف قواعد Aviator 1xBet عن العديد من الألعاب الأخرى التي تعتمد على الحظ والاستراتيجية. بينما تعتمد بعض الألعاب على الدوران العشوائي أو البطاقات، تعتمد Aviator 1xBet على توقيت دقيق واتخاذ قرارات سريعة.مكافأة الرهان المجاني نعم ، لعبة Aviator هي لعبة مراهنة شرعية على الإنترنت. تستخدم اللعبة تقنية متقدمة ومولدات أرقام عشوائية للتأكد من أن نتيجة كل جولة عشوائية تمامًا ولا يتم التحكم فيها أو التلاعب بها بأي شكل من الأشكال. خطوات وضع الرهانات استخدام استراتيجية الطائرة 1xBet يمكن أن يساعد في تحقيق نجاح أكبر. هنا بعض النصائح والاستراتيجيات لتحقيق الفوز:
https://4part.kaporghor.com/%d9%84%d8%b9%d8%a8%d8%a9-%d8%a7%d9%84%d8%b7%d9%8a%d8%a7%d8%b1%d8%a9-by-spribe-a-popular-casino-game-experience-in-egypt/
في لعبة الطياره الرهان يعتمد على خوارزمية متطورة لتوليد نتيجة كل جولة لعبة بشكل عشوائي تمامًا غير قابل للتنبؤ، كما هو الحال مع جميع ألعاب كازينو 888starz. صُممت برمجيات توليد الأرقام العشوائية لتضمن الامتثال الكامل لسياسات اللعب العادل، وتلعب دورًا رئيسيًا في كيفية عمل لعبة Aviator. يمنحك الوضع التجريبي في لعبة Aviator إمكانية اختبار كامل خصائصها. يحصل كل لاعب على رصيد افتراضي (3000 وحدة نقدية) من كازينو 888starz لخوض التجربة. يمكنك وضع الرهانات واتخاذ قرارات صرف الأرباح تمامًا كما هو الحال في وضع استخدام المال الحقيقي. بالنسبة إلى اللاعبين المبتدئين، يعتبر وضع اللعب المجاني بيئة مثالية للتعرف على آليات اللعبة وتطوير استراتيجياتهم الخاصة.
The Real Person!
The Real Person!
Os usuários podem sacar e depositar usando cartões de débito e crédito, muitos jogadores estão procurando os melhores cassinos com códigos de bônus em 2023. Uma vez registrados, segundo e terceiro carretel. Apesar do motivo, a maioria dos jogadores achou mais fácil confiar neste cassino porque sabem que corpos prolíficos estão de costas para o caso de algo dar errado. Vamos ser honestos, onde se convive lado a lado com camelos. Os bônus de Depósito não valem a pena se preocupar muito como um jogador de blackjack de apostas altas, pois os rolos estão cheios de ambiente familiar. Nosso objetivo é atender a empresas em consultoria, prevenção e mitigação de todos os riscos jurídicos.
https://100.nomankhan.net/2025/05/27/review-of-jetx-by-smartsoft-an-immersive-casino-game-experience-for-brazilian-players/
When you’re tired, look for ringtones to relax, if you don’t know where to download free French phone ringtones, visit here. sonneriesvip Aviator de Spribe es un juego similar al Jet X Casino Game. Puedes ganar dinero fácilmente en este juego, que se desarrolla a un ritmo rápido. También se podría decir que Aviator es un juego de catálogo Jet X por el GSR, la volatilidad, las frecuencias multiplicadoras, etc. Puedes ganar grandes premios en metálico y muchos más si sigues patrones específicos para dirigir el resultado del juego. No Mostbet Casino, voce encontrara uma gama de entretenimento de cassino que vai desde os tradicionais como slots de video e roleta europeia ate transmissoes ao vivo que recriam a vibracao dos cassinos fisicos. Com animacoes de alta resolucao e uma experiencia amigavel, a plataforma e projetada para proporcionar uma experiencia imersiva e acessivel.
The Real Person!
The Real Person!
El bot 1win Lucky Jet no está conectado al servidor del juego y podría resultar una estafa. Mantén un buen manejo del presupuesto y pon en práctica estrategias mediante el modo demo. El juego Lucky Jet 1win tiene una dinámica muy parecida a la de Aviator. El objetivo es lograr sacar la apuesta multiplicada por el coeficiente de ese momento antes de que el cohete se vaya volando. El 1win juego podría alcanzar hasta x100, lo que significa grandes ganancias potenciales. luckyjet.co © 2025 LuckyJet sitio web oficial. Jet X no solo proporciona un retiro automático, sino que también permite a los jugadores apostar automáticamente la cantidad que especifican sin la necesidad de un esfuerzo adicional. Gracias a estas características, jugar en Jet X es especialmente conveniente para los usuarios.
https://azizcontracting.com/explorando-el-juego-balloon-de-smartsoft-en-los-casinos-online-venezolanos/
Imagine estar en las bulliciosas calles de Brasil, donde la pasión por el fútbol se respira en cada rincón. Con una banda sonora de samba que encapsula el espíritu brasileño, Penalty Shoot Out Street te transporta a un campo de fútbol local bajo el sol abrasador. Los jugadores esquivan gatos callejeros y sortean coches aparcados mientras se preparan para patear el balón hacia una red blanca inmaculada. ¡Penalty Shooters 2 fue creado por 10x10games! Dicho esto, reglas del blackjack básicas la alta gerencia de Eldorado supervisará las operaciones. Como beneficio adicional, qué genial sería si tuvieras dos oportunidades de hacer precisamente eso este sábado 25 de octubre. Esto es exactamente lo que los jugadores deben hacer, entre otros. Además, es posible que se encuentre con casinos que brindan códigos de bonificación específicos para sus diferentes ofertas de bonificación lanzadas a lo largo del año. Por lo tanto, pero decidió en 2023 ser un casino criptoexclusivo. El lunes, damas y caballeros.
The Real Person!
The Real Person!
The expert players of top casinos consider the martingale strategy to be a beneficial option for crash games, and JetX is no exception. Using it is quite straightforward: you start with a small bet; If you lose, instead of giving up, you double your bet for the next round. By implementing the martingale strategy, you not only recover all your previous losses but also make a profit. New to the world of online casino games? Heard about JetX but not quite sure what it is or how to play? Don’t worry, we’re here to help. In this article, we’ll give you a detailed review of the game, with all the useful information you need in order to make the most of your experience and have a greater chance at winning big. Thousands of players have already tried their luck with JetX with varying degrees of success. So if you haven’t done so yet, it’s time to give it a go!
https://thealchemyadvisors.com/aviator-demo-everything-you-need-to-know/
Contact Slate or call (954) 869-8796 Some of the flights on this website are financially protected by the ATOL scheme. But ATOL protection does not apply to all travel services listed on this website. Please ask us to confirm what protection may apply to your booking. If you do not receive an ATOL Certificate then the booking will not be ATOL protected. If you do receive an ATOL Certificate but all the parts of your trip are not listed on it, those parts will not be ATOL protected. Please see our Participant Agreement conditions for information or more information about financial protection and the ATOL Certificate, go to caa.co.uk. Dammam to Cairo Flight The flight attendant served drinks and snacks during the 43-minute flight. The plastic cups and packaged snacks from a snack basket felt a bit short of what you might expect when flying private. But for the price I paid, it was perfectly fine. No additional drinks or snacks were offered.
The Real Person!
The Real Person!
Any instrument larger than 30cm x 120cm x 38cm like a double bass or harp can’t be taken on board the aircraft as cabin baggage. You’ll need to add hold luggage to your booking for these items. Please ensure it’s packed safely and securely. Take your 8in x 11in thermal printer to the next level by creating the PocketJet printer you need. With a lightweight yet powerful build, long-lasting battery, more media options1, off-the-shelf software and a wide variety of mounts and accessories, you can fully customize the Brother PocketJet 8 for your productivity. LADIES COLLECTION Excellent TrustScore Trailblazing. Innovation. Precision. For 150 years, Bulova has stayed true to these pillars of excellence. Join us in celebrating our rich legacy as the oldest American watchmaker, proudly established in NYC.
https://visitcentraljava.com/football-x-by-smartsoft-a-thrilling-online-casino-game-for-indian-players/uncategorized/
NEW YORK — U.S. authorities moved Monday to seize two luxury jets — a $60 million Gulfstream and a $350 million aircraft believed to be one of the world’s most expensive private airplanes — after linking both to Russian oligarch Roman Abramovich. Watch the Jets quarterback and receiver link-up for a big play during 7-on-7 drills at OTA practice. NEW YORK — U.S. authorities moved Monday to seize two luxury jets — a $60 million Gulfstream and a $350 million aircraft believed to be one of the world’s most expensive private airplanes — after linking both to Russian oligarch Roman Abramovich. Save my name, email, and website in this browser for the next time I comment. Watch the Jets first-round pick on the practice field for the first time during rookie minicamp. September 6-8, 2023 | Hilton City Center Denver
The Real Person!
The Real Person!
Wichtige Mitteilung El juego de futbol – Penalti invita a los jugadores a la parte decisiva del gran partido y los insta a utilizar toda su destreza y precisión para superar al portero contrario en una emocionante tanda de penaltis. Sigue el choque a través de Flashscore. Además, si estás de acuerdo, también utilizaremos cookies para complementar tu experiencia de compra en las tiendas de Amazon, tal y como se describe en nuestro Aviso de cookies. Tu elección se aplica al uso de cookies de publicidad propias y de terceros en este servicio. Las cookies almacenan o acceden a información estándar del dispositivo, como un identificador único. Los 96 terceros que utilizan cookies en este servicio lo hacen con el fin de mostrar y medir anuncios personalizados, generar información sobre la audiencia, y desarrollar y mejorar productos.
https://code.datasciencedojo.com/rjdfrz7188
Looking to bring beauty and inspiration from your landscape design? We specializes in building custom, one of a kind outdoor living spaces in Columbus, OH. outdoor living designs Columbus If you are looking for a website that can provide information and techniques for playing. online casino Then we are the website you are looking for and answer the question perfectly because we have gathered both information and techniques for you in a version that can be understood in 5 minutes to make you Este es un juego multijugador creado por BC Originals. En él, un cohete, simbolizado por un punto blanco, se mueve a través de un gráfico, recolectando multiplicadores hasta que se estrella. El desafío es predecir hasta dónde llegará el cohete y retirar antes del choque. Lanzado en 2017, este juego se ha convertido en una parte clave del casino criptográfico BC.Game, presentando un RTP alto del 99%.
The Real Person!
The Real Person!
Oyunçular təyyarə təsadüfi havaya qalxmazdan ibtidai mümkün olan daha təntənəli çarpanla para çıxarmağa çalışırlar. 1xBet, Aviator və vahid çox digər kazino oyunları üçün asudə və təhlükəsiz platforma təmin edən aparıcı online kazinosudur. Biz ümumən oyunçular üçün xoş imtahan təmin etmək ötrü məsuliyyətli qumar təcrübələrinə sadiqik. Bu veb-saytdan istifadə etməyə davam etməklə siz Kuki Siyasətimizə əlaqəli olaraq kukilərin istifadəsinə razılıq verirsiniz. Without leaving your home, you can have your loan in an hour once your application for a payday loan is approved. Unlike when you apply for a loan from banks which requires you to endure a waiting time to process all the documents needed, a payday loan is easy to avail. They need no documentary documents, unlike banks which do not also approve short term and unsecured credit.
http://dados.unirio.br/user/adburwater1984
Aviator Predictor to narzędzie, które obiecuje przewidzieć rezultat gry Aviator, pomagając graczom decydować, kiedy wypłacić wygraną. Brzmi jak marzenie, ale w praktyce takie rozwiązania często nie mają nic wspólnego z grą fair-play i mogą naruszać regulamin kasyna. Rzekomo oprogramowanie ma działać w następujący sposób: Jeśli chodzi o czat, to na samym początku podłącza się wirtualny asystent, możesz jednak go pominąć, nawiązując szybkie połączenie z prawdziwym konsultantem. Ten podłączy się po kilku sekundach od Twojej prośby. Miłym gestem jest dostępność polskojęzycznego wsparcia z krwi i kości. Sprawdziłem dokładnie, jak radzi sobie pomoc w 22bet i jestem pozytywnie zaskoczony. To nie tylko fachowa obsługa, ale i uprzejma, informująca zawsze o bonusach, z których możesz skorzystać.
The Real Person!
The Real Person!
Os minutos pagantes do fortune tiger nada mais são do que minutos específicos durante o dia que o jogo do tigre aposta costuma pagar prêmios mais altos aos jogadores..A ideia é que esse post seja bem curto mesmo e sem enrolação.Então vamos lá…. No entanto, antes de se cadastrar em qualquer plataforma, é preciso escolher uma operadora confiável. Abaixo, listamos os melhores sites e as ofertas promocionais para quem quer jogar o Jogo Fortune Tiger, ou Jogo do Tigrinho e apostar. Separamos abaixo as cinco principais opções de casas para jogar: Isso é uma ótima maneira de se familiarizar com os recursos e mecânicas do Fortune Tiger antes de decidir jogar com apostas de verdade. Se você aprecia a experiência de jogo com Fortune Tiger, talvez também esteja interessado em outros jogos cativantes da PG Soft. Descubra as aventuras únicas e os gráficos ricos de Fortune Rabbit e Fortune Mouse, duas outras máquinas caça-níqueis que prometem uma experiência de jogo emocionante e lucrativa.
https://camp-fire.jp/profile/humlenito1971
\nNa Stake cassino, nossa biblioteca de caça-níqueis é constantemente atualizada com novos lançamentos semanais, garantindo que você tenha sempre a melhor experiência e acesso às últimas novidades. Se você é iniciante e está começando no mundo dos cassinos online, não deixe de conferir o nosso blog com dicas e tutoriais sobre como jogar caça-níqueis online . Atenção: Altamente viciante. Pode causar surtos de alegria e giros compulsivos. Se você girar 300 vezes e sonhar com símbolo Scatter… bem-vindo ao clube. E essa acaba sendo apenas algumas das estratégias do fortune rabbit, ok? A PG Soft (Pocket Games Soft) é uma fornecedora global de jogos de apostas especializada em slots online. Seus jogos são conhecidos por alta volatilidade, bônus emocionantes e RTP competitivo, proporcionando grandes oportunidades de lucro para jogadores de todos os níveis.
The Real Person!
The Real Person!
La noi, pe jocpacanele.ro, găsești cea mai mare gamă de jocuri păcănele online. Încearcă gratis zecile noastre ✅păcănele 7777, ✅păcănele cu speciale, ✅păcănele cu fructe, ✅păcănele Book of Ra și multe, multe altele! Am pregătit pentru tine păcănele online cu număr variat de role și animații dintre cele mai complexe, astfel încât divertismentul să atingă cote maxime. Joacă sloturi gratis și descoperă favoritele tale! Noi ne-am îndrăgostit de jocurile Gates of Olympus și Sugar Rush. Tu? Bei dem Sweet Bonanza no deposit Bonus 2025 handelt es sich um Kennenlernangebote, sodass Sie Online-Casinos ohne Risiko testen können. Die Anzahl der Freispiele ohne Einzahlung ist beschränkt. Hohe Gewinne sind mit den Sweet Bonanza Freispielen ohne Einzahlung deshalb nicht zu erwarten. Mit den Free Spins können Sie Sweet Bonanza kostenlos spielen, sich ein erstes Bild von dem Slot machen und mit etwas Glück sogar Geld ohne Risiko gewinnen.
https://yoomark.com/content/man-kann-nicht-abstreiten-dass-sweet-bonanza-sehr-viel-spass-macht-wir-hatten-auf-jeden-fall
Wildz vereint alle wichtigen Elemente für eine tolle Online Spielothek. Unsere Wildz Erfahrungen zeigen: Deutsche Lizenz und sichere Zahlungen sind nur der Anfang. Im nachfolgenden Wildz Test enthüllen wir die Vorteile des Spielangebots und Boni. Wir prüfen außerdem, ob die Bonusbedingungen auch für Glücksspiel-Neulinge geeignet sind und zeigen weitere Besonderheiten des Anbieters, wie die fairen Bonusbedingungen. Jetzt nachlesen oder direkt anmelden! Wer von neuen Spielen fasziniert ist, findet im Wildz Casino eine Kategorie für brandneue Slots. Hier lässt sich immer aktuell nachvollziehen, welche Neuerscheinungen uns die Softwareanbieter beschert haben. Trotzdem gibt man sich hier selbstsicher. Nicht jedes neue Casino-Spiel hat automatisch seinen Platz im Wildz verdient. Man selektiert und versucht der Spielergemeinde ausschließlich etablierte Top-Titel anzubieten.
I really like your writing style, excellent info, thanks for posting :D. “God save me from my friends. I can protect myself from my enemies.” by Claude Louis Hector de Villars.
The Real Person!
The Real Person!
Oyunun ana özelliği, balık sembolleri yakalayarak mümkün olan en önemli ödülleri almaya çalışan Fisherman Wild’dir. Özellikle, fiyatlarla ne kadar çok balık sembolü olursa, sonunda ödeme o kadar büyük olur! Big Bass Bonanza Açağı, popüler bir online slot oyunudur. Balık avı temasıyla dikkat çekiyor ve casino oyunları arasında yer alıyor. Büyük balık avı elde etmek için şansınızı denemek için idealdir. Slot makineleri arasında popüler ve övgü almaktadır. Oyunun temeli, makaraları çevirip büyük balıkları yakalamak üzerine kurulu. Kazançlarınızı artırmak için stratejik bir şekilde makaraları çevirin ve en büyük balıkları yakalamak için şansınızı deneyin. Mesaj bırakın Slot Oyunları Yapmak -Pikachu Zinciri Yıldırım – Oyun alanında birçok balık arasında bir zincir oluşturur ve çok sayıda balıkta hasara neden olur.
https://wiki.petale07.org/?unotranlou1980
Big Bass Splash Slot, oyuncuları canlı bir su altı kanyonu fonunda canlı bir balık avı seferine sürüklüyor. Kristal berraklığındaki sulara batırılmış makaralar, sallanan su bitkileri ve ara sıra yanlarından geçen gölgeli balıklarla çevrilidir. Oyunun ana karakteri olan dost canlısı balıkçı, neşeli tavrı ve büyük boy avıyla hoş bir dokunuş katıyor. Slotun grafikleri renkli ve gösterişlidir, olta takımı ve ikonik Big Bass gibi semboller temayı güçlendirir. Görselleri tamamlayan, banjo notaları ve martıların ortam seslerini içeren neşeli bir film müziği, kırsal bir balıkçılık havası yaratıyor. Bu neşeli atmosfer, her dönüşü bir balıkçılık tatili macerası gibi hissettirir. Ready to go fishin’? If you love reeling in the big fish, you’ll be delighted with the new Pragmatic Play slot, Big Bass Bonanza. A slot game with five reels and 10 paylines, it comes with sky-high RTP and is jam-packed with top special features.
The Real Person!
The Real Person!
Que porcaria eu detestei leva 5000anos pra carega mesmo num aiphone 16 By using penaltyshootout.cl you agree to the use of cookies. Connected Numbers ¿No te gustaría probar con otra búsqueda? Los siguientes datos pueden usarse para rastrearte en apps y sitios web que son propiedad de otras empresas: Una estrategia que siguen los jugadores chilenos en esta slot es repetir el disparo a la misma zona de la portería en varias ocasiones. Además, es recomendable tomarse descansos y no seguir siempre los mismos patrones a la hora de lanzar los penaltis en el juego. SPONSOR OFICIAL Aún no hay comentarios más gustados Pero si hablamos de jugar con cabeza, Player City ofrece un análisis completo del juego: qué lo hace diferente, cómo funcionan los tiros y bloqueos, qué nivel de dificultad presenta, y cómo influye la aleatoriedad en cada ronda. Además, el sitio permite acceder a una versión gratuita tipo demo, donde puedes practicar sin arriesgar dinero real.
https://bruckners.devsaffinity.site/resena-del-juego-balloon-de-smartsoft-para-jugadores-en-ecuador/
La apuesta mínima a la hora de jugar Penalty Shoot Out es de 1.026 CLP por cada lanzamiento de penalti, mientras que la cantidad máxima que se puede apostar es de 1.026.350 CLP. Al elegir una de las cinco zonas distintas, es necesario esperar para ver si el portero detiene el disparo. Desarrolle una carrera con nuestro nuevo XP y sistema de nivelación. ¡Llena tu vitrina de trofeos completando logros y muestra tu rango en el juego en línea! Tras completar los 90 minutos, el marcador ya no se movió, así que el ganador tuvo que ser definido desde los 11 pasos. El FC Barcelona estuvo cerca de llevárselo, pero Saviola falló su penalti. Esto se repitió pero ahora del lado del Real Madrid con la falla de Fernando Sanz. La tercera fue la vencida pues Raúl Bravo no dudó y definió el encuentro del lado merengue.
The Real Person!
The Real Person!
Dragon Tiger 888: A Thrilling Card Game A free app for Android, by Shleyatenia. Friends, you can see in the image below that this application gives very quick withdrawal, this application is very good, if you have not yet downloaded this application, please download immediately because this application is a real gaming application and provide real cash. Friends, this application is not fake, it is very real and it gives payment within 15 minutes, so you must download it. Animal experiences you can’t get anywhere else. General admission tickets are required. A free app for Android, by Shleyatenia. Play games and earn money: – Friends game 3f apk Earning app has total 22 types of games, out of which you can play any game, about which game you have knowledge, in this app dragon vs tiger game is the easiest and my Favorite game is you play your favorite game.
https://venus-arabiya.com/2025/07/03/review-of-aviator-by-spribe-visualizing-payouts-on-the-login-dashboard/
Answer: To sign up for the Ind Slots APK, follow these steps: Stickman Dragon Fight can be played on your computer and mobile devices like phones and tablets. Answer: To sign up for the Ind Slots APK, follow these steps: Is 3 Patti Lucky mod APK available? Bet PKR has quickly become a favorite card game in Pakistan offering a blend of strategy social interaction & real-money rewards. Whether you’re new to the game or a seasoned player learning the rules & mastering the strategies can significantly improve your chances of winning. With its exciting features secure banking options & generous bonuses, BetPKR offers endless entertainment. You can download the application today and get your welcome bonus without investing any money. Try to play the Dragon Warrior Card game that offers big cash prizes these days. You can bind your account with the app to receive your daily funds. This game is indeed a blessing in disguise for those who want an instant change in their life and enjoy the unlimited funds.
The Real Person!
The Real Person!
Space XY is an interesting new game in the Crash genre from renowned developer Bgaming. Here you can get a big win of up to x10,000 in just two clicks if you’re lucky. In doing so, your decisions affect whether you win or lose. At 1win we offer you the chance to play this game for real money. Create a 1win account, make a deposit and start playing the Spacy XY game with a welcome bonus of up to INR 145,000! Aviator game offers an innovative gaming experience unlike, slot machines. The game has no reels or paylines, which makes it different from other gambling games. Players can rely on luck or play strategically. In our opinion, Aviator gives a unique gaming experience and excitement in every round. Space XY offers players a maximum multiplier of x10,000 of a player’s bet. Players can get a substantial big win, with its unique gameplay potentially leading to significant payouts based on players’ chosen strategies.
https://ntamconnect.com/download-the-aviator-game-app-without-third-party-hassle/
3. Enjoy playing Dragon Tiger Nice – game club on GameLoop Teen Patti – An Indian 3-Card Poker Game Funny Teenpatti TeenPatti Yono-3Patti Rummy: Strategy Meets Luck In the dynamic world of Teen Patti, strategies like Tiger and Dragon hold a unique allure. These tactics, often discussed among players, represent distinct approaches to the game. Understanding these strategies can significantly impact your gameplay and success in 3 Patti room. چاہے آپ نئے کھلاڑی ہوں یا ماہر، 3 Patti Room میں ہر کسی کے لیے ہے کچھ خاص۔ مختلف رومز میں شامل ہوں، لائیو ٹورنامنٹس کھیلیں، اور روزانہ جیتیں شاندار انعامات۔ بہترین گرافکس، محفوظ سسٹم، اور تیز ترین وِدڈرا کے ساتھ، یہ ہے آپ کا نیا فیورٹ گیم۔
The Real Person!
The Real Person!
Space for Weddings αγοράσετε Viagra online χωρίς συνταγή We focused on dining tables that could seat four to six people, though we also considered a few two-person options for small spaces, as well as large tables for up to 10 people. In addition to size, we looked for spacious seat dimensions, generous weight capacities, high-quality construction, and a mix of materials and styles. We spent 80-plus hours assembling, testing, and researching patio dining furniture in all kinds of climates and spaces, including sun-drenched desert yards, damp wintery porches, and cramped urban balconies. Here are the best sets we’ve tried so far. If there’s one thing many people ask co-owners Jerry Nguyen and Chad Porter when they step into Wichita’s newest gay dance bar, it’s where the two men got the name, XY.
https://daniel-k.weballly.com/discovering-mines-by-spribe-a-popular-online-casino-game-for-pakistani-players/
The Funds are distributed by BlackRock Investments, LLC (together with its affiliates, “BlackRock”). This material is intended for information purposes only, and does not constitute investment advice, a recommendation or an offer or solicitation to purchase or sell any securities, funds or strategies to any person in any jurisdiction in which an offer, solicitation, purchase or sale would be unlawful under the securities laws of such jurisdiction. In the U.S., this material is intended for public distribution. XY Miners is listed in the United Kingdom and works under the FCA (Financial Conduct Authority) regulations, verifying that it is transparent and complies with the law. The total market value of a cryptocurrency’s circulating supply. It is analogous to the free-float capitalization in the stock market.
The Real Person!
The Real Person!
تحميل برنامج 1xbet مهكر أو أي برنامج آخر مهكر بسبب أن هذا غير قانوني ويعتبر انتهاكا لحقوق الملكية الفكرية يجب علينا دعم العمل القانوني وعدم التورط في أي نشاط غير قانوني ينصح 1xbet تنزيل برنامج والتطبيقات من مصادر رسمية موثوقة وتجنب استخدام 1xbet تنزيل برنامج حيث قد تتسبب في تعريض جهازك للخطر وفقدان البيانات الشخصية أو المالية. Survival Challenge 456 Mod Apk 2.0.3 تحميل برنامج 1xbet مهكر أو أي برنامج آخر مهكر بسبب أن هذا غير قانوني ويعتبر انتهاكا لحقوق الملكية الفكرية يجب علينا دعم العمل القانوني وعدم التورط في أي نشاط غير قانوني ينصح 1xbet تنزيل برنامج والتطبيقات من مصادر رسمية موثوقة وتجنب استخدام 1xbet تنزيل برنامج حيث قد تتسبب في تعريض جهازك للخطر وفقدان البيانات الشخصية أو المالية.
https://risanstore.com/%d8%aa%d8%ad%d9%84%d9%8a%d9%84-%d8%a7%d9%84%d9%81%d8%b1%d9%82-%d8%a8%d9%8a%d9%86-%d9%84%d8%b9%d8%a8%d8%a9-aviator-%d9%81%d9%8a-1win-%d9%88-1xbet-%d9%85%d9%86-%d8%a7%d9%84%d8%a3%d9%81%d8%b6%d9%84/
√ استخدام سكريبت الطياره يجعلك مختلف عن اللاعبين الاخرين وتحقيق اكثر استفاده للربح باستمرار وتجنب الخساره وبالتالي يجد صعوبه لدى الاشخاص. كذلك في تحميل السكريبت وتفعيله داخل المنصه اصبح الامر سهل في موقعنا. هل سكربت الطياره امن؟ 6.8 سكربت الطياره 500$ الوصول الى جميع الاسكريبتات التي يتم الترويج اليها من خلال الاتصال بالانترنت. كذلك من اهم المميزات التالي : √ امكانيه تحقيق الربح من لعبه الطياره من خلال استخدام سكربت الطياره والتعرف على طرق التشغيل المجانيه لا يتطلب منك اشتراك. كذلك من اجل تفعيل السكريبت يمكنك استخدامه لمواجهه التحدي.
The Real Person!
The Real Person!
I think this is a superb welcome offer and for context, I consider the average social casino new customer bonus to be around 7,500 Gold Coins and 2 Sweeps Coins. This means that Spinpals gives you more of both virtual currencies than the average. As I mentioned, there isn’t a Spinpals promo code, and there isn’t anything like a Spinpals deposit bonus either. How Go can help keep you secure by default A booking code enables you to book or transfer a bet. odds or availabilities may change. Below are Rocket X promo codes that you can use when you sign up for an account on the casino website or through the mobile app. When submitting your promotional content, make sure that your promotional content contains all critical information about your moment. In particular, make sure that your promotional content submissions clearly communicate the following:
https://bruckners.devsaffinity.site/secure-payment-options-for-jetx-users-worldwide-a-review/
In conclusion, Roobet Crash Demo version is a great way to practice the game and test strategies without the risk of losing real money. It’s an excellent tool for both new and experienced players to get comfortable with the game. However, always remember to gamble responsibly, even when you transition to the real game. The RTP for Roobet Crash is 96.5%. In the long term, you can anticipate earning $96.5 for every $100 wagered in theory. Although the RTP is higher than average, we know that crash games like Fury Stairs, Hyper Xplorer, and Space XY have higher RTP. The best strategy is to start with small bets and gradually increase them as you become more familiar with the game. Once you get a feel for how the game works, you can start placing larger bets and taking bigger risks. However, be careful not to overdo it – if you bet too much, you could lose everything!
The Real Person!
The Real Person!
Kiedy grasz w trybie demo, zyskujesz pewność siebie i oswajasz się z grą. Będziesz wtedy gotów, aby przejść do gry Aviator na prawdziwe pieniądze. Demo gry Aviator wzbogaci twoje wrażenia z rozgrywki, a nawet zwiększy Twoje szanse na sukces. Poświęć więc trochę czasu na zapoznanie się z trybem demonstracyjnym. To twoja tajna broń do sukcesu! Sektor gier mobilnych przeżywa boom, a na czele znajduje się 1win Lucky Jet 1win APK. Dla entuzjastów najnowocześniejszych gier kasynowych i zakładów sportowych, pobranie Lucky Jet 1win APK jest pierwszym krokiem w kierunku ekscytującej podróży. Aplikacja przyciągnęła uwagę dzięki płynnej integracji różnorodnych opcji gier, dzięki czemu jest to kluczowy moment, aby dołączyć do społeczności i rozpocząć przygodę z grami.
https://forumodua.com/member.php?u=687137
Join the community of enthusiastic players and dive into the exhilarating world of Aviator APK. With a simple download, you can start your journey to uncover the secrets of the skies and potentially walk away with massive wins! Click the link below to start your adventure today! Copyright © 2014-2025 APKPure. Wszelkie prawa zastrzeżone. Aviator to nowoczesna gra crash, która zrewolucjonizowała świat hazardu online. Łącząc w sobie elementy tradycyjnych gier losowych z dynamiczną rozgrywką w czasie rzeczywistym, Aviator Game Online oferuje graczom unikalne doświadczenie oparte na prostych zasadach i możliwości osiągnięcia wysokich mnożników. Przygotowaliśmy szczegółowe zestawienie najważniejszych informacji technicznych i wymagań systemowych dla Aviator Game APK. Poniższa tabela zawiera kompletny przegląd specyfikacji:
The Real Person!
The Real Person!
Voilà un très bon jeu pour les amateurs de footb… Vous êtes à la recherche d’une expérience de jeu de football passionnante ? Penalty Shoot-Out: Street est là pour vous. Ce jeu dynamique a captivé des joueurs du monde entier grâce à sa simplicité et à son action effrénée. Mais ce n’est pas tout, si vous cherchez à vous lancer sans risque, certaines offres de bienvenue vous permettent de jouer gratuitement. Découvrez comment ce jeu vous plonge au cœur de l’action du penalty shoot-out tout en profitant d’avantages exclusifs pour une expérience encore plus excitante. рџљЂ Crash Casino Mini-jeux Jeux de mines Hockey Shootout « Mystake Penalty » est l’une des offres les plus exaltantes dans le monde des jeux de casino en ligne. Conçu avec précision par EvoPlay, ce jeu capture l’essence des tirs au but dans le monde réel, en l’associant parfaitement au frisson des enjeux du casino. Les joueurs sont transportés dans un stade virtuel, où ils affrontent de redoutables gardiens de but, dans le but d’effectuer le tir parfait. Chaque tir dans « Mystake Penalty » est un mélange de stratégie, d’habileté et d’une touche de chance, ce qui rend chaque partie imprévisible et immensément captivante. Les graphismes sont de premier ordre, avec de superbes animations et des effets sonores qui amplifient la tension du jeu.
https://onlinedigitalbookmark.com/page/business-services/https-casinomanga-net-
Optimisez vos papiers électroniques pour le SEO, utilisez des backlinks puissants et du contenu multimédia pour maximiser votre visibilité et vos ventes. Les renouvelables sont un facteur important, mais il faut une complémentarité avec les autres sources d’énergie actuelles, qu’il s’agisse du nucléaire, des installations géothermiques, des centrales hydrauliques ou du biogaz produit par la méthanisation. Bien sûr, le charbon n’est pas la panacée, bien au contraire, et il va falloir progressivement le remplacer, peut-être par le gaz, avant d’aboutir à un développement plus important des renouvelables. Cependant, dans ce domaine, on a besoin de recherche – on l’a dit – et je m’interroge, parce que nous avions des projets pilotes pour capter et stocker le carbone. Aujourd’hui, sur quatre projets pilotes en Europe, un seul a été mis en œuvre en Norvège.
The Real Person!
The Real Person!
Ada tiga titik di sepanjang Jalan Raya Solo-Purwodadi yang dicek Ganjar. Di titik pertama, petugas dari Dinas PU Bina Marga dan Cipta Karya sedang melakukan perbaikan sementara dengan menutup badan jalan yang berlubang. Mesin slot joker jewels mempunyai tema yang berfokus pada karakter joker yang identik sebagai karakter yang lucu dan ceria dalam dunia perjudian. Pada game slot gacor terbaru ini kamu bisa mengharapkan simbol-simbol yang berhubungan dengan tema joker seperti topi jester, bel, dan berbagai pernak-pernik nya. Bermain sabar dan mengetahui pola slot gacor akan sangat membantu kamu dalam meraih jackpot yang besar. Cara mengetahui pola slot gacor, kamu bisa masuk ke dalam komunitas Mantra88 di sosial media seperti Telegram atau Whatsapp, dan disana kamu akan mendapatkan pola slot gacor terlengkap yang telah disiapkan. Juga, jangan terlalu tergesa-gesa dalam memilih nilai bet kamu, pastikan bahwa kamu bermain sabar untuk hasil yang maksimal.
https://ntbdamai.id/review-slot-starlight-princess-rtp-terupdate-hari-ini/
APLIKASI PREDIKSI SPACEMAN link slot apk APLIKASI PREDIKSI SPACEMAN apk slot terbaru Invitation only. Minimum deposit requirements apply. Penerbang, Crash, JetX, SpaceMan, GoRush, RoobetCrash Predictor Keamanan Terjamin: Keamanan data dan transaksi Anda adalah prioritas utama kami. Kami menggunakan teknologi enkripsi terkini untuk melindungi informasi pribadi Anda dari pihak yang tidak bertanggung jawab. Jadi, Anda bisa bermain dengan tenang dan fokus meraih kemenangan. APLIKASI PREDIKSI SPACEMAN slot apk terpercaya Anda pasti penasaran apa sih yang membuat situs tersebut sangat istimewa? karena itu kami akan membahasnya disini. namun sebelumnya, anda bisa mendapatkan Link alternatif 188BET lewat dua tombol diatas dan melihat promosi menarik lewat tombol dibawah ini. LINK ALTERNATIF 2 Selamat datang di dunia Bom89, tempat para pemain sejati merasakan adrenalin kemenangan dan keseruan tanpa batas! Kami adalah situs resmi Bom89, rumah bagi ribuan game slot online gacor dan live casino yang siap memanjakan Anda dengan pengalaman bermain yang tak terlupakan.
The Real Person!
The Real Person!
Fortune Rabbit, também conhecido como jogo do coelho, faz parte dos slots Fortune, sendo um dos mais populares. Nesse jogo, o apostador tem uma grade 3x4x3, com 10 jeitos de vencer por rodada. O prêmio máximo chega a 5.000x o valor da aposta. Olha só o que não pode ficar de fora da sua avaliação de cassinos de qualidade: Caso não encontre a regra dentro dos termos da plataforma, não hesite em tirar a dúvida com o suporte, já que muitas vezes essa promoção pode ser cancelada caso você já tenha feito qualquer depósito anterior. Olha só o que não pode ficar de fora da sua avaliação de cassinos de qualidade: Sweet bonanza symbol values Sweet bonanza symbol values As apostas esportivas começam com valores a partir de R$0,50 e você tem mais de 30 modalidades para apostar. Partidas de futebol, tênis, e até squash estão disponíveis no site e app.
https://alhawra.ly/big-bass-splash-uma-nova-onda-da-pragmatic-play-para-o-brasil/
Os usuários da TOP88 concordam em respeitar todas as regras e regulamentos estabelecidos. É importante ler e entender todos os termos antes de utilizar nossos serviços. A violação destes termos pode resultar em penalizações ou na suspensão da conta. Baixar o Fortune Rabbit app em um dispositivo iOS é um processo direto e intuitivo. Este guia passo a passo foi elaborado para ajudá-lo a encontrar e instalar o aplicativo de forma eficiente. O aplicativo está disponível na App Store e pode ser baixado gratuitamente, permitindo que você desfrute de suas funcionalidades exclusivas sem complicações. O design do Fortune Rabbit é simples e intuitivo, contando com símbolos temáticos e funcionalidades como multiplicadores e rodadas grátis. Já ouviu falar do jogo Fortune Rabbit e quer saber todas as informações sobre uma das caça-níqueis do momento, incluindo os melhores cassinos para aproveitar o jogo?
The Real Person!
The Real Person!
RTP: 95,8%. Combineert de Megaways-motor met wilde buffels en grote uitbetalingen. NetEnt, Microgaming, Play N GO, Thunderkick, iSoftBet, Quickspin, Yggdrasil, Red Tiger, Pragmatic Play, Relax Gaming, Playtech, Blueprint, Evolution Gaming, Merkur, Inspired, Iron Dog, ELK, Snowborn Studios, Nolimit City, 1×2 Gaming, Just for the Win, Hacksaw Gaming, Reel Play, Northernlights, Peter & Sons, Blue Guru, Push Gaming, Lightning Box, Kalamba Games, Synot, Playson, Gaming Corps, Switch Studios, MGA, On Air Entertainment, Foxium, Octoplay, Print Studios, 4ThePlayer, AvatarUX, Gamevy, BangBang, Alchemy Gaming, Williams, Fantasma, RubyPlay Wat kost gokken jou? Stop op tijd. 18+. loketkansspel.nl. рџ“™ Erik King’s Snelle Tip: Speel je Slotmill slots? Gebruik de “Fast Track” bonusbuy alleen na 50–100 spins zonder hit, de RTP en hitfrequentie lijken dynamisch aangepast te worden, waardoor de feature ná een droge reeks vaak gunstiger uitbetaalt.
https://www.klino.com.au/buffalo-king-megaways-review-een-spannende-ontdekking-in-de-nederlandse-online-casino-wereld/
Pragmatic Play staat er niet bepaald om bekend dat ze graag gebruik maken van andermans ideeën. Je komt dan ook zelden kloons tegen of spellen die elementen bevatten waar ook andere videoslots uit onze reviews mee zijn uitgerust. Dat is echter anders bij de videoslot Buffalo King Megaways. Het bewijs van de iGaming-mogelijkheden Pragmatic Play kondigde de release van Buffalo King Untamed Megaways aan, de titel van de serie Buffalo King. Dieren, poema’s, lobos en andere dieren redden de rodillos van deze tragamonedas 6×7 met 86.436 formaten van ganar. Om in aanmerking te komen voor de actie moet je 24 jaar of ouder zijn. Alleen inzetten met echt geld tellen mee en de minimale inzet per draai bedraagt €0,25. De free spins worden uiterlijk 72 uur na afloop van de promotie bijgeschreven op je account.
The Real Person!
The Real Person!
Calculations of total gas emissivities of gas mixtures containing several radiatively active species require corrections for band overlapping. In this paper, we generate such overlap correction charts for H2O-CO2-N2, H2O-CO-N2, and CO2-CO-N2 mixtures. These charts are applicable in the 0.1-40 bar total pressure range and in the 500 K-2500 K temperature range. For H2O-CO2-N2 mixtures, differences between our charts and Hottel’s graphs as well as models of Leckner and Modak are highlighted and analyzed. La inmunoterapia específica es considerada, hoy en día, como un tratamiento efectivo, con un nivel de evidencia de grado A, capaz de reducir de una forma eficiente, tanto los síntomas como las necesidades de tratamiento farmacológico en pacientes con alergia respiratoria, rinitis y asma, causada por alérgenos inhalados como: polen, hongos, epitelios animales y ácaros del polvo. La inmunoterapia específica mejora la hiperreactividad bronquial y se ha comparado el beneficio obtenido con el de los esteroides inhalados(6,7).
https://maximlandbase.com/resena-del-juego-de-casino-balloon-de-smartsoft-para-jugadores-en-mexico/
No hay una fórmula mágica, pero administrar bien el saldo y aprovechar los bonos puede mejorar tus probabilidades en Tower Rush. Como ya hemos dicho, no puedes descargar Tower Rush como una aplicación independiente – el juego simplemente no está diseñado para ser descargado y fue creado para ser alojado en sitios de casino online. Sin embargo, esto no significa que no puedas jugar a Tower Rush en smartphones – al contrario, el desarrollo de Galaxsys está perfectamente optimizado y adaptado para las pantallas de smartphones y tablets. Hay dos formas de jugar a esta tragaperras en dispositivos Android: a través de la versión móvil del sitio web del casino y de la aplicación para smartphones. Ambos métodos tienen sus ventajas y desventajas, que se comentarán a continuación. En Juegos.Games creemos que jugar debe ser algo accesible para todos. Por eso ofrecemos una experiencia sin anuncios invasivos, sin muros de pago ni complicaciones. Solo juegos bien seleccionados, organizados y probados para garantizar que funcionen correctamente y se adapten a distintos tipos de jugador: desde niños hasta adultos, desde gamers casuales hasta quienes buscan un reto más profundo.
The Real Person!
The Real Person!
Símbolos de dinheiro podem ser adicionados aos tambores aleatoriamente quando há símbolos de Pescador, mas não símbolos de Peixe presentes. Uma Bazooka especial também pode aparecer nos tambores nessa circunstância. Isso mudará alguns símbolos nos tambores, com exceção dos símbolos do Pescador. A PragmaticPlay (Gibraltar) Limited é licenciada e regulamentada na Grã-Bretanha pela Gambling Commission sob o número de conta 56015 e licenciada pela Gibraltar Licensing Authority e regulamentada pela Gibraltar Gambling Commissioner, sob o RGL No. 107. RTP, ou Retorno ao Jogador, em jogos de cassino, significa o dinheiro médio que um jogador pode recuperar ao longo do tempo. Abaixo você encontra uma lista com os 5 jogos de slots da PG Soft com a porcentagem de RTP mais alta. Sim, funciona no celular e no tablet. Sem precisar baixar nada. Sem instalar app. Você abre o site no navegador — e pronto, a Big Bass Splash demo carrega direto na tela. O layout é responsivo: botões grandes, texto legível, bônus visível e sem distorção. Eu mesmo já testei tanto no Android quanto no iPhone — sem bugs. Às vezes é até mais prático que o computador: menos distração, tudo no toque e inicia rápido. Ideal pra testar o jogo naquele intervalo rápido — sem complicação.
https://threadicious.com/teen-patti-by-mplay-a-comprehensive-review-for-pakistani-players/
A Blaze é uma casa de apostas e cassino online com sede em Curação, licenciada e autorizada pelo governo de Curação para exercer suas atividades de gambling. O usuário poderá apostar na Blaze com confiança de que a casa está se submetendo a regulamentações restritivas de jogos de azar e fiscalização por órgãos governamentais. O Big Bass Splash transporta os fãs da franquia Big Bass numa nova aventura de pesca. O Big Bass Splash transporta os fãs da franquia Big Bass numa nova aventura de pesca. Cada jogador tem um estilo diferente, e a escolha do melhor jogo de cassino depende de alguns fatores: Sendo que vitórias de até 18.163 vezes a sua aposta (exceto jackpot) podem surgir a cada rodada, com a possibilidade de apostar valores que variam entre R$ R$ 0,20 e R$ 40,00. Os apps de cassinos nacionais, então, são a resposta para jogadores gozarem da liberdade de apostar de qualquer lugar. Através destes aplicativos, os cassinos podem ampliar ainda mais a imersão dos jogadores em sua plataforma, maximizando a experiência de apostar pelo celular.
The Real Person!
The Real Person!
The Big Bass Bonanza 1000 RTP rate is 96.51% which is significant higher than the online slot average of around 96%. However, Pragmatic Play has created 95.51% and 94.51% RTP versions which may be used at your chosen slots site (a quick glance at the Paytable will determine which rate you’re playing with). The Big Bass Bonanza 1000 free demo above uses the highest RTP rate: this is also the case for the majority of free demo slot games at OLBG. The Big Bass series has seen more than a dozen releases since its inception, starting with the original Big Bass Bonanza in December 2020. Following its success, Pragmatic Play expanded the series to include variations like Big Bass Bonanza Megaways and Christmas-themed slots such as Big Bass Christmas Bash, showcasing the series’ adaptability and appeal.
https://fysiotittipaulina.se/exploring-aviator-games-popularity-in-ugandas-igaming-scene/
You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. cokitosjuegos @ gmail . com Squid Game Online Final Thoughts: Why Chicken Road Might Be Your New Favorite Game We’ve seen a lot of games come and go, but Chicken Road has that rare mix of charm, challenge, and real money potential that makes it special. It doesn’t take itself too seriously — and neither should you. It’s quick to learn, endlessly replayable, and every round feels just a little different. Compare the numbers on two different number lines and decide which is bigger. A great game to get children thinking about place value and reading varying scales. The Chicken Road demo mode is an excellent way to test strategies and understand the risk levels that suit your gameplay style. Whether you’re playing for fun or refining tactics for real money mode, this demo offers the full experience of a chicken road game casino title. Deciding which difficulty level to choose is definitely the number one priority. But it’s also experimenting with how far you can push, which is important.
The Real Person!
The Real Person!
Estamos convencidos de que Sugar Rush será un juego muy interesante para todos aquellos jugadores que tengan en cuenta la configuración matemática de las tragamonedas, y que también resultará muy atractivo para quienes busquen disfrutar de una experiencia agradable desde el punto de vista estético. The free bonus without deposit online casino. The round begins with each player receiving two cards, but maybe more so Aussies as they would rarely experience an icy fishing trip. These new games would allow players to access or download their favourite casino gambling games, share a few necessary contact details and then verify your account via an emailed link. Anytime you hit a number straight up, such as valiant warrior. Aquí te contamos todo sobre este llamativo título de Pragmatic Play, el proveedor líder del mundo de los online games.
https://thehellertowndiner.com/resena-del-juego-balloon-de-smartsoft-en-casinos-online-para-colombia/
Per questo, abbiamo fatto una analisi anche sulla quantità e la grandezza dei software program supplier con cui ogni sito ha rapporti. Ciò che più ci è piaciuto, di questo casinò AAMS, è il suo sistema di promozioni various al bonus di benvenuto. Potrai divertirti con le slot più popolari, come E-book of Ra Deluxe, interessata anche dal bonus benvenuto, Gonzo’s Quest, Dead or Alive e tante altre. Il bonus benvenuto offerto ad ogni nuovo giocatore propone il 100 percent del primo deposito, fino a 1.800€. Infatti, potrai divertirti con i migliori giochi NetEnt, tra cui Blood Suckers, Gonzo’s Quest e Starburst, ma anche Guide of Ra Deluxe, Guide of Useless e tanti altri. apotheke sofort lieferung: Pharma Jetzt – PharmaJetzt Inoltre, questi casinò per dispositivi portatili offrono accesso a una vasta gamma di metodi di pagamento, validi per depositi e prelievi. Un’app cell per i casinò stay è altrettanto importante per la scelta del migliore sito con questa categoria di giochi. Infatti, chi vuole sedersi ai tavoli live tra una pausa e l’altra può farlo anche fuori casa con il proprio smartphone o tablet. Quello che conta è che l’esperienza di navigazione, la fluidità e l’alta risoluzione grafica siano presenti e incorporate nell’app sviluppata dal sito net.
The Real Person!
The Real Person!
Korzystając z tej strony, zgadzasz się na wykorzystanie przez nas cookies (ciasteczek). Jeśli więc nie zmieniłeś ustawień odnośnie cookies (ciasteczek), zgadzasz się na ich używanie zgodnie z naszą Polityką Cookies. Darmowa gra Darmowa gra Darmowa gra Joker’s Jewels Dazzle od Pragmatic Play The Royal Family Nasza strona obejmuje wszystkich popularnych dostawców slotów. Szczegółowo przeanalizowaliśmy setki gier od najlepszych dostawców automatów. Nawet mniej znani dostawcy, ale wykonujący wyjątkową pracę, tacy jak Print Studios i Stakelogic, są opisani na naszej stronie. Na naszej stronie znajdziesz szczegółowe recenzje slotów od następujących najlepszych dostawców: This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
https://www.zoreproperties.com/2025/08/05/vavada-casino-w-polsce-kompleksowa-analiza-i-przeglad-funkcjonalnosci/
To make points simpler, we’ve place with each other a list associated with typically the greatest online internet casinos within Canada regarding Mar 2025. We evaluate betting internet sites based about key efficiency signals in order to recognize the top systems for global players. Our evaluation guarantees of which typically the gambling websites all of us suggest support typically the greatest standards with respect to a secure plus enjoyable gambling encounter. There’s continue to a gray area any time it arrives to on-line online casino gambling legislation in Canada. 1win реєстрація 1win22083.ru . By admin|2025-01-24T00:26:14+00:00January 24th, 2025|Игра Авиатор Aviator Online Ru Как Работает – 332| Gra Aviator oferuje zanurzenie się w fabułę, a aplikacja Aviator — predyktor wygranych dostarczy Ci więcej informacji o wygranej. Dołącz do ekspertów lotniczych 1win. Pomnóż swoje własne zasoby 1 wygranej, zrozum, że jako nowy Aviator może zdobywać wysokość i premie. Nie graj od razu w pix Aviator lub 4rabet app aviator. Spróbuj nauczyć się wszystkich subtelności, aby 1 wygrać sterowanie samolotem. Miałem szczęście, że byłem przy tobie.
Definitely believe that which you stated. Your favorite reason seemed to be on the web the easiest thing to be aware of. I say to you, I definitely get irked while people consider worries that they just don’t know about. You managed to hit the nail upon the top as well as defined out the whole thing without having side-effects , people could take a signal. Will likely be back to get more. Thanks
The Real Person!
The Real Person!
Το Sugar Rush 1000 λειτουργεί με γεννήτρια τυχαίων αριθμών, οπότε κάθε περιστροφή έχει απολύτως τυχαία αποτελέσματα. Έτσι, δεν υπάρχει σίγουρος τρόπος για το πως να κερδίσεις στο Sugar Rush 1000. Ωστόσο, μπορείτε να ακολουθήσετε τις παρακάτω συμβουλές για περισσότερες πιθανότητες κέρδους: Sorry, this product is unavailable. Please choose a different combination. Κάθε φορά που ένα νικητήριο σύμβολο εκρήγνυται, το σημείο του σημειώνεται στο δίκτυο. Εάν ένα άλλο εκτινάσσεται σε αυτό το σημείο για δεύτερη φορά, προστίθεται ένας πολλαπλασιαστής, ξεκινώντας από x2 και διπλασιάζεται μέχρι x1.024 με κάθε εμφάνιση. Ο πολλαπλασιαστής που προκύπτει προστίθεται σε όλους τους νικηφόρους συνδυασμούς που σχηματίζονται από πάνω.
https://webscountry.com/author/izcorpading1975/
Φωτεινά χρωματιστά γλυκά γεμίζουν το φρουτάκι Sugar Rush. Εμφανίζονται σε ένα πλέγμα 7×7 και πληρώνουν βραβεία με ομάδες (clusters) 5 ή περισσότερων όμοιων συμβόλων. Ένα σύστημα Tumbling Reels αφαιρεί τυχόν γλυκά σε μια νίκη, γεγονός που μπορεί να οδηγήσει σε περαιτέρω νικηφόρα συμπλέγματα, καθώς τα παραπάνω πέφτουν προς τα κάτω. Στο Foxcasino υποστηρίζουμε τον υπεύθυνο στοιχηματισμό και συστήνουμε να παίζετε τυχερά παιχνίδια με μέτρο. Πόσο γρήγορα επεξεργάζεται αναλήψεις το Vistabet Casino;
excellent post.Never knew this, thankyou for letting me know.
Precisely what I was searching for, thankyou for posting.
I like what you guys are up also. Such smart work and reporting! Keep up the superb works guys I¦ve incorporated you guys to my blogroll. I think it’ll improve the value of my site 🙂
Hiya, I am really glad I’ve found this information. Today bloggers publish only about gossips and net and this is really frustrating. A good website with interesting content, that’s what I need. Thanks for keeping this website, I’ll be visiting it. Do you do newsletters? Cant find it.
We’re a bunch of volunteers and starting a new scheme in our community. Your web site provided us with valuable info to work on. You have performed an impressive job and our whole neighborhood will probably be grateful to you.
The Real Person!
The Real Person!
Advertisements are seen heavily within this app. When the player run out of your 30 balls you can watch 30 advertisements in a day, each one will give the player 30 balls in return. This does take a bit of time to get through and it also seems to me as though I did not get more money blocks to pop-up after the first 2 or 3 refills of balls. The game has a large RTP, may be enjoyed for free to understand, and there are usually many variations available. Plinko is additionally simple to understand but still exciting and interesting. If the game is at a web based casino like Slingo, you should always be able to get information about their driving licence on their website. You may also search the UK Gambling Commission website to verify that the casino will be registered. MrBeast Plinko is a scam Plinko game that doesn’t actually exist about the App Store or the Google Enjoy Store.
https://sukruguzelsadeyag.com/?p=134933
We regularly monitor online casinos in Canada and have compiled a list of top-rated sites where you can play for real money. Each Chicken Road casino on our recommended list offers a range of attractive features, including fast payouts, diverse promotions, and 24 7 customer support. With an RTP of 96.5% and a max win potential of 5000x, our time with Chicken Drop was equal parts enjoyable and potentially lucrative. Pragmatic Play has developed a slot that is playful, adding to the game’s fun times and excitement – keeping us coming back to play some more. For sure! You can play the Chicken Fox 5x Skillstar™ slot for free right here at VegasSlotsOnline. We think trying the demo game out before staking real cash is a fantastic idea. The Chicken Drop slot has a Tumble feature which sees all winning symbols removed from the grid, and those above tumble down to fill the void. Tumbles continue until no new wins are seen. Special watering can and lucky clover symbols can appear at any time throughout a series of Tumbles, and these collect on a meter held by the chicken.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
I got what you intend,bookmarked, very nice internet site.
The Real Person!
The Real Person!
Se ti interessa provare Plinko online, il processo è semplice. La maggior parte dei casinò online italiani offre versioni demo gratuite, con cui puoi imparare i dettagli e le regole del gioco senza perdere denaro reale. Una volta che ti senti pronto, devi solo registrarti su un sito web di casinò online, continuare con la verifica del tuo account e quindi trovare Plinko nella libreria del sito. In alternativa, puoi scegliere di giocarlo in modalità di gioco demo. Si consiglia sempre di consultare in anticipo le promozioni e i bonus, come i giri gratuiti e i bonus di benvenuto, per ottimizzare la tua strategia di gioco. Dopo aver descritto nel dettaglio il miglior Plinko casino, ossia Lucky Block, e altri due competitor molto performanti (Metaspins e Cloudbet), pensiamo che sia opportuno effettuare un confronto tra le migliori offerte per partecipare al Plinko game su blockchain.
https://indujaulas.com/recensione-della-slot-sugar-rush-di-pragmatic-play-un-dolce-viaggio-allrtp-incredibile/
Il funzionamento di Plinko è intrigante: un pallino cade dall’alto, rimbalza su punte e infine si posiziona in una delle celle che offrono premi diversi. La casualità del percorso del pallino rende ogni partita un momento unico e emozionante. I giocatori possono scommettere su vari premi, aumentando la suspense man mano che il pallino compie il suo viaggio. Questo elemento è ciò che attrae molti utenti, poiché la tensione cresce fino all’ultimo istante, quando si scopre dove si fermerà il pallino. Quando si parla di scommesse sportive, goldbet it offre una gamma incomparabile di eventi su cui scommettere. Gli utenti possono scegliere tra sport popolari come calcio, basket, tennis e molti altri. Ogni sport presenta specifiche dinamiche che influenzano il risultato finale, e goldbet it elenca dettagliate analisi e statistiche che possono aiutare i giocatori a prendere decisioni più informate.
The Real Person!
The Real Person!
To play the Vortex game, you will need to create an account. But first, choose your online casino that provides a legal gambling experience in your state region. No separate Turbo Games accounts are required – the game is launched inside the casino. The registration process is as follows: How to Download the Vortex App for Android? Let’s see more detailed instructions on how you can download the Vortex Game application more easily. Turbo Gaming is a Ukrainian-based software developer that stepped into the gaming space in 2020. Their games are simple, run smoothly on mobile, and are a big hit on crypto-friendly platforms where speed really matters. Rather than making all kinds of games, Turbo Games sticks to a few styles it knows best and keeps things clean and easy to understand. Some of their standout games cover Crash X, Limbo Rider, and Mines. All these games are built for quick gameplay and clear, no-fuss rules.
https://s.surveyplanet.com/epi5csbh
Urban Rivals stands out for its variety of gameplay and artistic direction. With its distinctive style, the imaginary city of Clint City will never leave you indifferent. And whatever your playstyle, Urban Rivals will offer you a unique collectible card game experience. You will be first to know when we are back in stock 🙂 Good video quality Good video quality Now we are offering every player to use promo code ‘PMPROMO’ to increase winnings! It allows the customer to get a +150% deposit bonus of up to 30,000 INR for betting on their favourite sports. The number of bonus activations is limited, so don’t miss your chance and start betting with an increased bankroll. © Acute Games, Inc. All rights reserved Endless entertainment starting at EUR 4.99 Good video quality Download Parimatch app on your mobile phone and don’t be tied to one place. We have developed functional mobile clients for Android and iOS smartphones and implemented all the basic version features in them. Download Parimatch APK file and and get +150% bonus up to INR 30,000 using promocode PMPROMO.
The Real Person!
The Real Person!
Conocer los Sugar Rush símbolos es importante para saber las reglas de juego establecer ciertas estrategias. El juego Sugar Rush presenta la temática de dulces, galletas y golosinas. Para más información te recomendamos leer nuestro artículo informativo con la guía de Sugar Rush. Para verificar la volatilidad y otras características del juego, configuramos el Autoplay de Sugar Rush en modo turbo con 30 tiradas y aquí están los resultados. En resumen, Sugar Rush vale la pena explorar si eres fan de las Tragamonedas que ofrecen altos riesgos y la posibilidad de grandes recompensas. Conecta con nosotros La serie «1000» de Pragmatic Play goza de un gran éxito, con títulos tan famosos como Gates of Olympus 1000 y Starlight Princess 1000. Sugar Rush 1000 sigue la misma tendencia, con una volatilidad alta, una buena RTP y un premio máximo sumamente atractivo.
https://volgkingbobbprep1978.cavandoragh.org/https-juegodeminas-com-co
Primero, debe desinstalar la versión original Sugar Rush – A Quick Adventure Aplicación, la firma entrará en conflicto con la versión mod. Entonces deberías permitir que un recurso desconocido se pueda instalar en tus dispositivos Android. matadorbet Marcas y EmpresasPara contenido promocional de productos y servicios creado por marcas y negocios bug fixes and improvements Creado un feed de datos en Google Merchant Center es momento de darle visibilidad y aparecer en las búsquedas de tus usuarios en Google. No solo aparecerás ganando notoriedad de marca, sino que solo pagas si hacen clic. Un espacio ideal para sesiones fotográficas Guía para padres Mantente informado con la app de EL TIEMPO. Recibe las últimas noticias coberturas historias y análisis directamente en tu dispositivo.
The Real Person!
The Real Person!
Nunca fui muito ligado em jogos de esporte, a não ser que a abordagem fosse mais arcade, como os da série Mario, o Mario Super Sluggers (baseball) ou Mario Strikers (futebol). FIFA e Pro Evolution Soccer são dois títulos que jogo apenas de vez em quando, especificamente quando tenho alguém pra conversar enquanto bato uma bola. Porém, acredito que seja a primeira vez que tenho contato com um jogo de handball. Sim. O Apple Pay é um jeito rápido, fácil e seguro de assinar serviços como Apple Music, Apple TV+ e Apple Fitness+. Você também pode comprar apps e jogos na App Store e aumentar seu armazenamento mudando o plano do iCloud. Saiba mais Se sua empresa já aceita cartões de crédito e débito, basta falar com seu provedor de pagamentos para começar a aceitar o Apple Pay. Se você quiser aceitar o Apple Pay em seu site ou app, visite a página Apple Pay para desenvolvedores.
https://www.cajaforensesantafe.org.ar/?p=12467
Tamanho do download Baixar mais jogos Você pode encontrar o jogo no site da SmartSoft Ggaming que é a empresa responsável pelo jogo, não possui um aplicativo, mas possui uma versão otimizada para dispositivos móveis, mas para jogar o jogo com dinheiro real é necessário acessar um cassino online parceiros do desenvolvedor. Sorry, there are no games matching your query. Baixar mais jogos Você pode encontrar o jogo no site da SmartSoft Ggaming que é a empresa responsável pelo jogo, não possui um aplicativo, mas possui uma versão otimizada para dispositivos móveis, mas para jogar o jogo com dinheiro real é necessário acessar um cassino online parceiros do desenvolvedor. Navegue por nossa seleção de jogos casuais para continuar a diversão. Títulos populares incluem Little Alchemy e Little Alchemy 2, que permitem misturar e combinar elementos para criar novas descobertas, enquanto Bloons Tower Defense desafia você a estourar balões com uma variedade de defesas estratégicas.
I like this website its a master peace ! Glad I detected this on google .
I’ve recently started a web site, the information you provide on this website has helped me greatly. Thanks for all of your time & work. “The man who fights for his fellow-man is a better man than the one who fights for himself.” by Clarence Darrow.
The Real Person!
The Real Person!
Project Z: 72H Siege Conquest Zenless Zone Zero हमारे मिनी-गेम का परिणाम गणना पर आधारित है, भाग्य पर नहीं। हर राउंड से पहले आप अपनी संभावनाएँ स्पष्ट रूप से देख सकते हैं, और हर गुणक की अपनी वास्तविक संभावना होती है। इसमें कोई छिपा हुआ मैकेनिज्म नहीं है — हमने हर चीज़ को पारदर्शी बनाया है। Destiny: Rising यह गेम 1win जैसी प्रसिद्ध कंपनी द्वारा पेश किया गया है और अब इसे रियल मनी गेमिंग की दुनिया में एक नया और क्रांतिकारी फॉर्मेट माना जाता है। Aviator, अपनी सादगी और अनूठे कॉन्सेप्ट के कारण, पारंपरिक स्लॉट्स से बिल्कुल अलग है।
https://www.mvbayone.com/aviator-%e0%a4%95%e0%a5%88%e0%a4%b8%e0%a5%80%e0%a4%a8%e0%a5%8b-%e0%a4%97%e0%a5%87%e0%a4%ae-%e0%a4%95%e0%a4%be-%e0%a4%97%e0%a4%b9%e0%a4%b0%e0%a4%be%e0%a4%88-%e0%a4%b8%e0%a5%87-%e0%a4%b0%e0%a4%bf/
यह ऐप आपको गेम के नियम समझने, इंटरफेस देखने और प्रैक्टिस करने में मदद करेगा। 21 फ़र. 2024 गेम की स्क्रीन पर आप एक विमान को टेकऑफ़ करते हुए देखते हैं — और उसके पीछे चमकदार ट्रेल लाइन बनती है। यह सिंपल लेकिन आकर्षक विजुअल और शानदार साउंड इफेक्ट्स के साथ एक इमर्सिव एक्सपीरियंस देता है। 21 फ़र. 2024 आप जोखिम तय करते हैं – हमारे Chicken Road गेम में आप खुद चुनते हैं कि हर राउंड कितना जोखिम भरा हो। कठिनाई का स्तर बदलते ही मल्टीप्लायर रेंज बदल जाती है, जिससे संभावित जीत या हार पर असर पड़ता है। यह क्रैश गेम हर राउंड से पहले वोलैटिलिटी को समायोजित करने की सुविधा देता है।
The Real Person!
The Real Person!
Bij Golden Panda kun je snel aan de slag en kun je rekenen op snelle uitbetalingen. Aanmelden kan zonder Cruks-check, zonder iDIN en zonder ID-check. Uitbetalingen worden binnen een paar minuten geregeld en zijn mogelijk via crypto valuta of via de bank. Je kunt een uitbetaling aanvragen in crypto valuta zoals Bitcoin, Litecoin en Dogecoin. Er zijn in totaal 12 crypto’s om uit te kiezen; een uitbetaling is binnen 15 minuten binnen. The Piggly Wiggly cake definitely had the sweetest frosting of the three but it mostly just tasted like sugar to me. Spigo heeft met succes een extreem robuust framework ontwikkeld dat bestand is tegen de ontberingen van constant dagelijks gebruik, maar de jackpot zelf is een bevredigende 2,000 munten als je het kunt grijpen. Ze doen dit door het testen van games, maar zijn in wezen hetzelfde als video slots gevonden in de meeste casino’s deze dagen.
https://md.fachschaften.org/s/J63T1gDBt
Casino Noir Casino No Deposit Bonus Nederland Review Voor Nederlandse spelers die buiten Cruks willen wedden, is het vinden van een veilige en aantrekkelijke bookmaker belangrijk. We hebben tientallen aanbieders onder de loep genomen en selecteerden vijf die opvallen door hun betrouwbaarheid, bonussen, gebruiksgemak en portfolio – allemaal zonder Cruks-verplichting. Wie wordt het beste nieuwe online casino? De eerste keer dat je stort voor een online bonus noemen we een first deposit bonus. Er is ook een stortingsbonus die volgt nadat je al een keer gestort hebt. Dit noemen we reload bonussen. Welkomstbonussen zijn standaard in online casino’s. Ze zijn er in alle soorten en maten maar de beste vind je hier! Je leest daarover hieronder meer. Het spannende gevoel van winnen in het casino. In dit artikel zullen we enkele belangrijke factoren bespreken waarmee je rekening moet houden bij het zoeken naar de beste goksite, InstaDebit is in staat om direct toegang tot de bankrekening. Het biedt een toonaangevende live chat-ondersteuningsservice waarmee alle casinospelers direct hulp kunnen krijgen van alle casinogebieden, het spel biedt een extreem lage huis rand.
The Real Person!
The Real Person!
Η ηλ. διεύθυνση σας δεν δημοσιεύεται. Τα υποχρεωτικά πεδία σημειώνονται με * Πίνακας περιεχομένων Η ηλ. διεύθυνση σας δεν δημοσιεύεται. Τα υποχρεωτικά πεδία σημειώνονται με * 500 ΧΩΡΙΣ ΚΑΤΑΘΕΣΗ! Πίνακας περιεχομένων Το Stake υποδέχεται το 2025 με μία σειρά από νέα συναρπαστικά παιχνίδια καζίνο, προσφέροντας αμέτρητες ευκαιρίες διασκέδασης και μεγάλων κερδών. Με νέες κυκλοφορίες από κορυφαίους παρόχους όπως οι NetEnt, Pragmatic και Hacksaw Gaming, οι επιλογές σας εμπλουτίζονται συνεχώς. Παρακάτω θα βρείτε τα 5 κορυφαία νέα παιχνίδια καζίνο για το 2025:
https://localshoponline.co.za/%ce%b1%ce%be%ce%b9%ce%bf%ce%bb%cf%8c%ce%b3%ce%b7%cf%83%ce%b7-%cf%84%ce%b7%cf%82-%ce%ba%ce%b9%ce%bd%ce%b7%cf%84%ce%ae%cf%82-%ce%b5%ce%bc%cf%80%ce%b5%ce%b9%cf%81%ce%af%ce%b1%cf%82-%cf%83%cf%84%ce%bf-fru/2132/uncategorized/
Σημειωματάριο Γούνινο Πάντα Bangoberry – 1271BB01 Το ελάχιστο ποσό κατάθεσης είναι μόνο 10€, ενώ για τις περισσότερες μεθόδους το μέγιστο φτάνει έως 500€ ανά συναλλαγή. Όλες οι καταθέσεις πιστώνονται άμεσα στον λογαριασμό σας, επιτρέποντας το παιχνίδι χωρίς καθυστερήσεις. Φυσικά και μπορείτε να παίξετε δωρεάν φρουτάκια και σας παροτρύνουμε να το κάνετε ώστε να μάθετε πως λειτουργεί κάποιο νέο (ή παλιό) παιχνίδι που δεν έχετε δοκιμάσει προηγουμένως. Στα περισσότερα καζίνο που θα βρείτε στην ιστοσελίδα μας θα βρείτε slots που κυκλοφορούν από τον provider καθώς και την δωρεάν λειτουργία, χρήσιμες πληροφορίες, χαρακτηριστικά κλπ.
The Real Person!
The Real Person!
Posteriormente, su catálogo de productos continuó en expansión, incorporando en su oferta juegos P2P, Turbo Games, mesas de póker y otros títulos. En diciembre de 2020 recibió su certificación de la Autoridad de Juegos de Malta (MGA), consolidando su posición en el mercado como un proveedor seguro y confiable. Spribe también tiene certificaciones y licencias en jurisdicciones como: Remate Mauad Los juegos de crash que más se destacan en 1win son Aviator (Spribe), Speed N Cash y Lucky Jet, disponibles únicamente en 1win casino. Crash Game And Slot At Online Casino Leer más » Esto es especialmente útil para los nuevos jugadores que no tienen experiencia en otros juegos de casino. La versión demo del juego de Mines casino está disponible en casinos online y también en el sitio oficial de Spribe.
https://bushboundafricasafaris.com/tiempo-de-procesamiento-de-retiros-en-1win-para-jugadores-en-chile/
El modo de demostración de Sugar Rush presenta una oportunidad excepcional para experimentar el juego sin costo alguno. Los jugadores pueden utilizar este entorno para explorar sus diversas funciones. En lo que respecta al potencial de pago, Sugar Rush no decepciona. La ganancia máxima es 5000 veces tu apuesta por giro. Por lo tanto, tiene un potencial significativo de recompensas. Rush Street Interactive Colombia SAS, con domicilio principal en la ciudad de Bogotá, Calle 81 No. 11 – 55 Torre Norte Piso 9 – autorizado para operar según contrato de concesión C1972 con Coljuegos. Fecha inicio: 08 06 2023 – Fecha terminación: 07 06 2028 Juega bien. Ser responsable es parte del juego. Jugar sin control causa adicción. En nuestra casa de apuestas podrás encontrar una de las mejores selecciones de juegos de apuestas. Encontrarás clásicos de cualquier casino físico y juegos slot exclusivos que solo verás en JOKERBET, casino y apuestas.
The Real Person!
The Real Person!
Wild Piggys: Nach zwei, vier und sechs Tumbles werden alle drei Schwein-Symbole wild. Wenn der “Element-Bonus” vorbei ist, wird die Anzahl der Freispiele, Multiplikatoren, gestapelten Sticky Wilds und Wild-Walzen gezeigt. Die Walzen kommen dann zum Bonus-Freispiel mit denselben Wild-Funktionen, die es schon oben gab. bonus casino pokerstarsCheck dein Spiel18+ Werbehinweise Auf dieser Webseite werden Affiliate-Links genutzt.Zurzeit werden keine Freispiele ohne Einzahlung für Fruit Love angeboten.City Test habe ich vieles entdeckt,Wheelz Testbericht*Für alle Angebote gelten AGB,Zurzeit werden keine Echtgeld Apps für She´s a Rich Girl angeboten.pragmatic play hoodieIhr findet tolle Alternativen auf meiner Seite über:Gibt es eine Echtgeld App zum Download?7 5Monster Mash Cashhabanero224,las vegas casino spielen
http://freestyler.ws/user/578914/glamufexmio1977
SpinMama Casino operiert mit einer Lizenz aus Curaçao und erfüllt moderne Sicherheitsstandards wie SSL-Verschlüsselung. Obwohl die Lizenz nicht schweizerisch ist, entspricht der Anbieter den allgemeinen Anforderungen an den Spielerschutz und Datenschutz für Schweizer Spieler. Die Sicherheit von Online-Glücksspielplattformen ist von größter Bedeutung. Wir bewerten die Einhaltung der Branchenstandards und behördlichen Anforderungen durch jede Website und stellen sicher, dass sie verantwortungsvolles Glücksspiel fördert und den Benutzern angemessenen Schutz bietet. Wir analysieren die Benutzerfreundlichkeit von Glücksspielseiten und konzentrieren uns dabei darauf, wie einfach es für Benutzer ist, das zu finden, was sie suchen. Unser Überprüfungsprozess umfasst die Prüfung der Effizienz des Anmeldevorgangs, der Kontoverwaltung und der gesamten Navigation. Um unsere Unterstützung zu erhalten, muss eine Glücksspielseite ein nahtloses und intuitives Benutzererlebnis bieten.
The Real Person!
The Real Person!
I’m sweet like candyYou look, don’t touchDon’t try to get handsyYou get sugar rushI’m sweet like candy (candy)You look, don’t touch (look, don’t touch)Don’t try to get handsy (handsy)You get sugar rush ★ BÚSQUEDAS RECIENTES ★ Estados Unidos Sugar rush, rush, baby, can you tell me howyou're feeling?Roler coaster ride, machi bouncy, bouncybounceSarururu danonio boril my sweet things, ooh,oohJionshi-renun omnun imat, oh my GodYummy,yummy, ni mame sok dul gol (My love)Jian giman suchogado oh my God(My God)Alok dalo kan mat piol choyin gusun ganKumgiol chorompayo duro ¿No tienes una cuenta? Tire dúvidas sobre idiomas, interaja com outros fãs de TOMORROW X TOGETHER (TXT) e vá além da letra da música. 나쁜 건 나야nappeun geon naya알아 못된 desire, sugar (oh, yeah)ara motdoen desire, sugar (oh, yeah)Gimme, gimme moreGimme, gimme moreGimme, gimme moreGimme, gimme more
https://www.pr5-articles.com/Articles-of-2024/p%C3%A1gina-siguiente
¿Quieres aprender a jugar Sugar Rush? Aquí te enseñaremos cómo funciona y te daremos un demo para que practiques sin registrarte ni descargar a tu celular. Si te gustan las tragamonedas llenas de color, movimiento y un toque de fantasía, Sugar Rush probablemente te va a encantar. Esta guía está pensada para ayudarte a entender de qué va el juego, cómo funcionan sus mecánicas principales y qué deberías tener en cuenta si querés sacarle provecho. рџ§Є Puedes probar el Sugar Rush demo la demo directamente aquí, en esta misma página. Es ideal para aprender cómo se siente el juego antes de pasar a apostar con dinero real. Donde más se nota el potencial de Sugar Rush es en las rondas de giros gratis. Ahí es donde todo puede escalar rápido gracias a los multiplicadores que se van acumulando en ciertas casillas. Si logras activarlos, es cuando realmente pueden aparecer los premios importantes.
The Real Person!
The Real Person!
Com o app RecargaPay você repensa como aproveitar melhor o seu dinheiro. Sendo assim, o usuário pode escolher o jogo de sua preferência e começar a coletar moedas até atingir o valor mínimo para saque. É possível sacar o dinheiro via PayPal ou, se preferir, optar por cartões presentes de diversas plataformas e marketplaces, como Amazon, Walmart, Google Play, entre outras. Não é necessário cadastrar uma chave para fazer ou receber um Pix. PARTICIPAÇÃO SOCIAL Confira o passo a passo para sacar as suas comissões na plataforma via PIX. Seja para iniciantes como para mais experientes, a maneira mais fácil e segura é utilizar o Pix, mas há quem prefira as outras formas, seja por segurança ou por qualquer outro motivo específico.
https://raijin.com.ar/2025/09/22/q9bet-no-brasil-avaliacao-completa-dos-bonus-de-fidelidade-e-vantagens/
Toque mais, encoste menos. O Apple Pay funciona direto no seu aparelho, para que você não precise ter contato físico com botões e terminais, pegar cartões ou segurar dinheiro. Toda compra é feita com o Face ID, Touch ID ou senha, para manter a segurança nas suas mãos. O processo de criação de conta é obrigatório. Apenas um usuário registrado pode jogar na Brazino777. O depósito também é obrigatório antes de começar a jogar visto que estamos falando de jogos que são jogados com dinheiro de verdade. Se sua empresa já aceita cartões de crédito e débito, basta falar com seu provedor de pagamentos para começar a aceitar o Apple Pay. Se você quiser aceitar o Apple Pay em seu site ou app, visite a página Apple Pay para desenvolvedores.
The Real Person!
The Real Person!
Recomendamos de todo corazón tragamonedas gratis Sugar Rush no sólo porque puedes reportar importantes ganancias, sino también porque proporciona una experiencia de juego estéticamente agradable y difícil de olvidar. Con su atractiva función de bonificación que te mantendrá pegado a la pantalla, seguro que no se trata sólo de suerte, ¡también de puro entretenimiento! Recuerda, ¡cada giro cuenta cuando estás en este reino azucarado! ¿A qué esperas? ¡Sumérgete de lleno! Prepárate para una explosión de azúcar con cada giro victorioso en el juego de tragaperras gratuito Sugar Rush. La interfaz del juego hace alarde de una serie de símbolos que incluyen adorables ositos de gominola, caramelos variados y tentadoras delicias afrutadas, que te llevarán a un tentador viaje gastronómico. Los símbolos se agrupan en dos categorías.
https://www.tentes-aggelis.gr/balloon-app-que-debes-saber-antes-de-jugar/
Pirots 2 es una tragamonedas en línea producida por ELK Studios, como secuela de su predecesora, Pirots. Esta tragamonedas es popular por su alto potencial de ganancias y características especiales. Hemos realizado un análisis para revelar todo lo que necesitas saber sobre este juego. Además de nuestra increíble variedad de tragaperras, también tenemos promociones y bonos que te harán saltar de emoción. Desde giros gratis hasta bonos por depósito, siempre hay una oferta divertida esperándote. Recuerda, cada giro podría ser el que rompa la piñata y te llene de premios. Funcionan como el bono de giros gratis, pero limitado a aquellos juegos en los que se puedan usar estas fichas o créditos de apuesta. Pirots 2 Para jugar a Pirots con dinero real, debes buscar casinos online que ofrezcan juegos de ELK Studios. En Tragaperrasweb te ofrecemos un gran listado de los mejores casinos online.
The Real Person!
The Real Person!
When you are the 3rd part of a trilogy, it’s fairly hard to comfortably live up to the expectations created by your predecessors. Pirots 3 is no exception to that rule and has some big beaks to fill! During our Pirots 4 slot review, we noticed a few exciting symbol prizes. Players have four birds that can move around the grid to collect symbols, with paying symbols consisting of gems, coins and wilds. The gems can be upgraded to higher levels, up to a maximum payout level of 7. 👉 Explore all bonus rounds and bird battles without spending a cent. Perfect for testing strategies before playing for real money. 👉 Explore all bonus rounds and bird battles without spending a cent. Perfect for testing strategies before playing for real money. In the end, Pirots 3 is a slot that I can’t recommend enough. It’s visually stunning, packed with exciting features, and offers a gameplay experience that’s genuinely different from the usual slots out there. Sure, the RTP might be a little lower, and it’s definitely a game that favours the patient player, but trust me, the rewards are there. If you’re someone who loves a good challenge and the thrill of a big win, this game is right up your alley. Give it a go, and see if you can tame these wild pirate birds yourself!
https://metscco.saudi360inc.com/2025/09/26/autoplay-in-lucky-jet-how-indian-players-can-benefit/
Starburst slot has betting opportunities to suit all players, especially with only 10 paylines and 5 reels. The paylines are not adjustable in the game as they form part of the biggest benefits the slot has to offer. However, with bet sizes starting as low as £0.01 per spin, you have the option to spin the reels from just £0.10 per spin. Small increases are available, taking you up to the maximum coin value of £1.00 per line. For those seeking a higher wager value, the game also offers the classic NetEnt betting levels that make it possible to wager to up 10 coins per spin. Therefore, winnings are multiplied and the betting value ranges up to £100.00 per spin. Red Kite Schoolies At Dream Jackpot, we’re committed to keeping your gaming experience fresh and exciting. That’s why our selection of slot games is always expanding, featuring the latest releases with new slots added to the site each week.
The Real Person!
The Real Person!
اغتنم الفرصة الآن مع تنزيل 1xbet للاندرويد واستفد من جميع الخصائص والمزايا المخصصة للمستخدمين. علشان تكسب فلوس من لعبه الطيارة، لازم تتدرب على اللعبة بشكل مجاني الأول. يتيح لك كازينو 1xbet مصر تحميل لعبه الطياره مجانا والتدريب المجاني على اللعبة. 1xBet هي منصة مشهورة بين المستخدمين في الإمارات، تقدم مجموعة واسعة من المراهنات على الأحداث الرياضية، من كرة القدم إلى الكرة الطائرة، بالإضافة إلى العديد من الألعاب الأخرى. تتميز 1xBet بـ: نجد أن ماهو موقع 1XBET وتحديد قانونية موقع 1XBET تختلف من دولة إلى أخرى، فهناك دول تسمح بالمراهنات عبر الإنترنت وتقوم بترخيص هذه المواقع، ودول أخرى تمنعها وتعتبر المراهنة جريمة يعاقب عليها القانون.
https://jrremodelingpaintgroup.com/%d9%87%d9%84-%d8%aa%d9%87%d9%83%d9%8a%d8%b1-%d9%84%d8%b9%d8%a8%d8%a9-aviator-%d9%85%d9%85%d9%83%d9%86-%d9%88%d8%a2%d9%85%d9%86%d8%9f-%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%88%d8%aa%d8%ad%d9%84%d9%8a/
لعبة طائرة، أبيض × أصفر، 660-A244 أظهر المزيد توصيل بدينارين لجميع أنحاء المملكة 300.00 جنية مصرى شكراً لك , سيتم إرسال رسالة على بريدك الالكترونى عند وصول سعر المنتج للسعر المطلوب توفر لك هذه اللعبة فرصة الطيران حول العالم وتخطيط مسار الرحلة، بل وتصميم الطائرة أيضاً. كما يمكنك اتمام المهام التي تطلبها اللعبة. تحقق من مجموعتنا من ألعاب الطائرات بنفسك، أو توجه إلى صفحة ألعاب الطيران لمجموعة أوسع من ألعاب الطيران! سياسة الإرجاع والضمان أما مهمات الإنقاذ في حوادث الطائرات والهبوط الاضطراري في ألعاب الطيارات فلن تكون بهذه السهولة. حيث سيزودك نظام الطيارة الآلي بتنبيهات للحفاظ على الإحداثيات وخط الطول بشكل مستمروسينبهك أيضًا عند الاقتراب من ناطحات السحاب، أو أي أشياء أخرى في الجو مثل: المروحيات والطيور والطيارات بدون طيار.. إلخ.
The Real Person!
The Real Person!
Voor meer inspiratie op maat, gelieve de 3 vragen hieronder in te vullen. Log in om je wishlist te bekijken Login to use TMDB’s new rating system. Voor spelers die het originele Sugar Rush spel wel eens gespeeld hebben, zal Sugar Rush 1000 heel vertrouwd aanvoelen. De online gokkast speelt zich in dezelfde snoepwereld af als het originele spel. Het speelveld is gevuld met kleurrijke snoepjes. Om winnende combinaties te vormen moeten spelers clusters van minimaal 5 dezelfde symbolen op de rollen draaien. Spelers die Sugar Rush 1000 willen spelen, kunnen onderstaand stappenplan volgen. Schaduwplekken maken met parasols of lakens, ventilators strategisch neerzetten en een DIY-drankstation met ijskoude limonades of mocktails. Extra tip: waterpistolen, zijn fun én verfrissend! Registreer u nu om de laatste updates over promoties en kortingsbonnen te ontvangen. Maak je geen zorgen, we spammen niet!
http://www.valldebaldomar.com/space-wars-big-win-nederland-de-grootste-uitbetalingen/
Voor meer inspiratie op maat, gelieve de 3 vragen hieronder in te vullen. Log in om je wishlist te bekijken Login to use TMDB’s new rating system. Voor spelers die het originele Sugar Rush spel wel eens gespeeld hebben, zal Sugar Rush 1000 heel vertrouwd aanvoelen. De online gokkast speelt zich in dezelfde snoepwereld af als het originele spel. Het speelveld is gevuld met kleurrijke snoepjes. Om winnende combinaties te vormen moeten spelers clusters van minimaal 5 dezelfde symbolen op de rollen draaien. Spelers die Sugar Rush 1000 willen spelen, kunnen onderstaand stappenplan volgen. Schaduwplekken maken met parasols of lakens, ventilators strategisch neerzetten en een DIY-drankstation met ijskoude limonades of mocktails. Extra tip: waterpistolen, zijn fun én verfrissend! Registreer u nu om de laatste updates over promoties en kortingsbonnen te ontvangen. Maak je geen zorgen, we spammen niet!
The Real Person!
The Real Person!
Das Erlernen der Technologie nach der Technologie, um den höchsten Sicherheitsstandards gerecht zu werden. Der Geldsack ist das andere Wild-Symbol im Slot und kann als einfacher Geldsack oder als Geldsack mit einem Multiplikator darauf erscheinen, kostenlos spielen book of the fallen freispiele ohne einzahlung die viele Menschen begeistert. Die strategie des blackjackspiels im casino. Sie können jedoch bequem von zu Hause aus spielen, wenn Sie zwei Karten mit demselben Wert haben. Wir benutzen Cookies, damit Sie die bestmögliche Erfahrung auf unserer Website machen. Mehr Informationen Aber jetzt, was bedeutet. Es gibt viele VISA-Slots in Kanada, willkommensbonus casino mit paysafe einzahlung dass das beste zertifizierte Casino Willkommensbonus ein Bonus ist. Ein weiterer wichtiger Punkt ist, kombiniert mit der Tatsache. Na sicher, dass das Spiel am besten für volle Tische geeignet ist.
https://terrasantanoticias.com.br/2025/10/01/pirates-von-elk-studios-im-test-gratis-demo-spielen-und-gewinnen/
Spielautomaten in Deutschland bieten jede Menge Unterhaltung. Wichtig ist, verantwortungsvoll zu spielen und zu wissen, was einen seriösen Anbieter ausmacht. Achten Sie auf RTP-Werte und Bonusaktionen und lernen Sie die wichtigsten Begrifflichkeiten. Wer nicht direkt mit Echtgeld spielen möchte, kann viele Top Slots kostenlos ausprobieren. Wir sind außerdem gespannt, was uns die Zukunft noch bringt. Denn Online Spielautomaten sind definitiv gekommen, um zu bleiben, und Entwickler werden uns künftig sicher noch mit der ein oder anderen Neuerung überraschen. GameArt releases a luxurious gems slot game, Diamond Magic, an extravagant classic gems slot with Expanding Multiple Wilds and respin functionality. Baccarat ist seitdem bei Elite-Casinospielern beliebt, dass der Dealer auf harten 17ern und allen 18ern steht. Der beliebteste Willkommensbonus ist als Einzahlungsbonus bekannt, wird ein Betrag in Höhe der Hälfte ihres ursprünglichen Einsatzes von ihrem Guthaben abgezogen. Die Spiele haben eine schnelle und reibungslose Benutzeroberfläche und Gameplay und bieten eine breite Wette, weshalb die Sensibilisierung ernst genommen werden muss. Es ist Zeit, ist.
The Real Person!
The Real Person!
Notre équipe d’experts considère ce casino comme fiable et sécurisé, offrant un environnement de jeu transparent et divertissant. Que ce soit pour ses bonus généreux, sa simplicité d’utilisation ou son engagement envers le jeu responsable, Casinozer mérite sa place parmi les meilleurs casinos en ligne disponibles en France. Une option à ne pas manquer pour tous ceux qui recherchent un divertissement sérieux et de confiance ! Le premier « step », tournoi à 30 centimes, a lieu plusieurs fois par jour et vous permet d’obtenir un ticket d’une valeur de 5 euros pour tenter votre chance dans les tournois qualificatifs spécifiques aux DSO et Unibet Open. Concrètement, 10 € de dépôt = 1 point de dépôt, et 5 € de mises cumulées = 1 point de mise. Un programme complet avec quatre grands niveaux de fidélité est alors proposé, avec des avantages de plus en plus importants à débloquer.
https://www.mavim.ro/2025/10/22/big-bass-bonanza-un-regard-approfondi-sur-le-jeu-de-pragmatic-play/
J’ai été surprise de voir que Julius Casino propose une partie dédiée aux mini-jeux, mais pas aux machines à sous ni aux jeux de table. J’espère voir ces sections ajoutées car pour moi, c’est indispensable sur un casino ! Avoir un compte joueur sur Evolve casino permet de profiter d’un grand avantage chaque semaine. Tous les lundis, le casino rembourse 25% de tous les dépôts perdants. Les conditions suivantes sont relatives à ce remboursement : Clermont-Ferrand, chef-lieu du département du Puy-de-Dôme, est la ville phare du Massif central. Nichée aux pieds de la Chaîne des Puys, elle propose une grande variété d’activités touristiques. En collaboration avec le Massif central, cette ville d’Auvergne d’environ 140 000 habitants est candidate pour devenir la Capitale européenne de la Culture en 2028.
The Real Person!
The Real Person!
Dus om te beginnen, een halve mop en emmer set. Dat gezegd hebbende, sugar rush free play demo waaronder live baccarat. De zoete actie vindt plaats op een 7×7 rooster, waarbij een clustersysteem wordt gebruikt om winsten te behalen. Gratis deze gokkast uitproberen kan natuurlijk met een demo, maar met echt geld is het nóg leuker. En je hebt geluk, want bij onderstaande online casino’s kan je Sugar Rush 1000 spelen zonder storting! Uiteraard heb je daarnaast de keuze uit leuke nieuwe slots. Speel bijvoorbeeld Gates of Olympus, Sugar Rush 1000 of Big Bass Splash. Het zijn een paar van de nieuwe spellen, daarnaast voegen we regelmatig andere nieuwe slots toe. Sugar Rush 1000 is een weerzien met dezelfde (bonus) features als het origineel. Ze smaken alleen nét wat beter, aangezien de uitbetalingen flink zijn opgepimpt. We lopen alle speciale features in dit deel van de Sugar Rush 1000 slot review even kort met je door:
https://classificados.autocaravaneando.pt/big-bass-splash-review-spannende-visactie-in-nederlandse-online-casinos/
Noticed positive changes in their skin Name: Sugar Rush Stripey Het wordt kouder buiten en we kunnen momenteel ook nergens heen, daarom zijn wel allemaal nog meer Netflix gaan kijken. Maar wat zullen we nu weer kijken? Er staan echt superveel titels op Netflix. En een onderwerp waar je niet snel aan denkt voor een serie of film is eten. toch leveren deze films en series vaak smakelijke beelden op waar je spontaan trek van krijgt. Ik heb 5 leuke en smakelijke films en series op een rijtje gezet. Wat zijn de beste slots bij Unibet? Wij vroegen Unibet naar de beste casino spellen en bespreken hier de beste online slots, live casino spellen en providers. Het gevoel van samenhorigheid tijdens het spelen in het casino. De software moet bekend zijn, maar dit zal naar verwachting in 2021 veranderen. Sector 777 casino no deposit bonus nederland review net als in een echte bakstenen en mortel casino, bent u zeker niet alleen. Het aanbod van online casino bevat enkele van de meest populaire producten op de markt en biedt entertainment voor iedereen, slot wild spin by platipus gaming demo free play Hard Rock Pop & Crush en Hard Rock Bubble Shooter.
The Real Person!
The Real Person!
Les applications mobiles de pragmatic big bass bonanza offrent une expérience fluide et immersive, identique à celle de la version desktop, tout en étant optimisées pour une utilisation tactile. Les graphismes conservent leur netteté, les animations restent fluides et les commandes sont parfaitement adaptées aux écrans de petite taille. Les joueurs tunisiens peuvent profiter du jeu partout, que ce soit pour des parties rapides ou de longues sessions. Souhaite-tu recevoir des notifications sur ton navigateur et ton application mobile concernant des offres spéciales, des promotions, des remises ou des cadeaux ? Sécurisées et régulées, ces applications respectent les normes de sécurité les plus strictes, garantissant ainsi une protection optimale des données personnelles et des transactions financières des joueurs. Avec des options de paiement variées et des services client réactifs, les applications de casino en Ontario assurent une expérience de jeu sans tracas. Que vous soyez à la maison ou en déplacement, vous pouvez jouer en toute confiance et profiter de jeux de qualité supérieure, tout en bénéficiant de la sécurité et de la régulation imposées par les autorités locales.
https://centar-za-masazu-osijek.hr/comment-se-connecter-facilement-a-votre-compte-securise-sur-cashed-casino/
Gates of Olympus de Pragmatic Play est une machine à sous vidéo à haute variance avec un gain maximum de 5 000 fois votre mise par tour. Le jeu offre un certain nombre de fonctions uniques, notamment une fonction Tumble, un symbole multiplicateur et des tours gratuits. La fonction Tumble s’active après chaque tour gagnant et fait disparaître des rouleaux tous les symboles qui ont participé à la combinaison gagnante. De nouveaux symboles tombent alors pour les remplacer. Les symboles multiplicateurs peuvent apparaître à chaque tour et augmenter vos gains jusqu’à 500x. Gates of Olympus est accueilli avec un grand intérêt parmi les joueurs français. Le support linguistique en français offre aux joueurs une expérience plus confortable et agréable. De plus, grâce aux méthodes de paiement locales, vous pouvez facilement effectuer des dépôts et des retraits. Ces caractéristiques augmentent l’accessibilité du jeu, assurant ainsi la satisfaction des joueurs français.
The Real Person!
The Real Person!
Want to add some fun to your videos? Wink provides a variety of animated effects, stickers, and text options that you can incorporate into your clips. Create engaging and lively videos to share with friends and make your memories even more vibrant. In conclusion, the latest version of Wink MOD APK download is a one-stop editing, enhancement, and beautification app for beginners and professionals. If you use it for personal or social media video editing, it will surely help you reach your audience with stunning video edits. The comprehensive tools library, AI integration, chatting option, simple user interface, and easy navigation are the main reasons for its popularity. So, don’t wait much and waste precious time testing other apps. Download it now by clicking on the download button, as I’m sure you won’t regret your decision.
http://maisbrasil.meuanunciodigital.com.br/?p=31335
Winning symbols are removed from the board by the tumble feature, letting other symbols drop from above to fill the gaps. There are nine regular pay symbols in total. The low five are gems of varying shape and colour, then a cup, a ring, an hourglass, and a crown as the high pays. Landing an 8-9 matching symbol scatter win pays 0.25 to 10 times the bet, while a 12+ scatter win is worth 2 to 50 times the bet. No wilds make their way into Gates of Olympus 1000 at any time. BigClash creates a top-tier player experience, with thousands of pokies from the likes of Pragmatic Play and Hacksaw Gaming. But the site also stands out for its exclusive pokies, with titles like Pinatas Fesitival, Buffalo Force, and Hades Inferno 1000. We also found BigClash as a great option for jackpot pokies, with nearly 300 to choose from and new popular games consistently added to the library.
The Real Person!
The Real Person!
Pages In File: 433 playslot77jackpot.win slot sbo_slot augmentin tablets uk augmentin for sale augmentin generic drug Pages In File: 433 Year: 2,016 Fortunately I had the opportunities to attend some important meetings with the highest authorities of Hajj in the Kingdom of Saudi Arabia where they, on many occasions, expressed shock that Nigeria has turned around Hajj management since 2007. They said Nigeria used to be a recurring study case of Hajj mismanagement in the past. What more testimony is required to convince the ministry and those who are fighting NAHCON that the host country, Saudi Arabia, has not made public? Honestly, the transformations going on in NAHCON are tremendous and its good leadership is restless until Nigeria becomes at par with world class hajj managers like Indonesia, Malaysia and others. It is possible because the long-term plans are there.
https://aga-decarlo.com/rocket-game-betting-how-to-maximize-your-wins-in-space-xy/
First Order Use Code Save 50% Off Shipping This was suggested to me on Tik Tok so I didn’t have the highest expectations; all I can say is woooow, in love and obsessed. It manages to be dainty yet begs to be looked at , I’m immediately ordering another one just in case this one breaks goes missing. Can’t recommend enough. Came in a very thoughtful package also with my name handwritten. Love that touch ✨ In addition to the bonus round, 15 Dragon Pearls also features a Wild symbol, represented by the golden dragon itself. This symbol substitutes for all other symbols on the reels (except scatter), increasing the chances of landing winning combinations. There are no reviews yet. This lovely tea is now grown in the Fujian and Jiangxi provinces, and Guandong province. Some say Dragon Phoenix Pearl gets its name from the tea bushes “climbing the hillsides like a Dragon rising from the waters”. Others suggest it refers to the way the green and white leaves of the tea twist and swirl around one another. It is grown in a mountainous, often fog-shrouded area near the border of the Fujian and Jiangxi provinces. Dragon Phoenix Pearl is one of the finest jasmines shipped from the port of Foochow.
very good publish, i certainly love this web site, carry on it
The Real Person!
The Real Person!
The typical number of search queries for this slot each month. This indicates overall popularity – the higher the figure, the more frequently players are looking up information about this particular slot game. When combined with the Tumble Reels, it becomes clear that these multipliers are key to delivering big wins in the base game. If you land at least four Zeus Scatters, you get an instant cash prize and access to the Bonus Round.The Gates of Olympus slot RTP is set at a pretty solid 96.5%. It’s a high-variance slot so you best be mindful of your bets. When it comes to the maximum payout, you can win up to 5,000x your stake. To enable personalised advertising (like interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. Those partners may have their own information they’ve collected about you. Turning off the personalised advertising setting won’t stop you from seeing Etsy ads, but it may make the ads you see less relevant or more repetitive.
https://www.igcyabogados.com.co/magic-apple-hold-and-win-slot-how-to-maximise-your-wins/
Playing Bigger Bass Bonanza is just as simple as you would expect from a game built on a pursuit thousands of years old. Simply choose your coins per line, coin value, and total bet. You will then be ready to cast with the best of them. Let’s get our fishing gear ready once more and dive into this Bigger Bass Bonanza Slot Review to see just how much bigger this new slot is. Welcome to our comprehensive review of the exciting new slot game, Big Bass Bonanza Reel Action, developed by Pragmatic Play. In this review, we will dive deep into the gameplay, features, and overall experience of this highly anticipated slot. Get ready to embark on a fishing adventure like no other as we explore the underwater world, chase big wins, and reel in some impressive prizes. Let’s get started! Every year, there are even more brand new Big Bass Bonanza slot games added to the list in this iconic slot series.
That is very attention-grabbing, You’re a very professional blogger. I have joined your feed and sit up for in search of more of your magnificent post. Also, I’ve shared your website in my social networks!
The Real Person!
The Real Person!
Admiring the persistence you put into your website and in depth information you offer. It’s awesome to come across a blog every once in a while that isn’t the same old rehashed material. Fantastic read! I’ve bookmarked your site and I’m including your RSS feeds to my Google account.
Rio independent escort
Hey there this is kinda of off topic but I was wanting to know if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding knowledge so I wanted to get advice from someone with experience. Any help would be enormously appreciated!