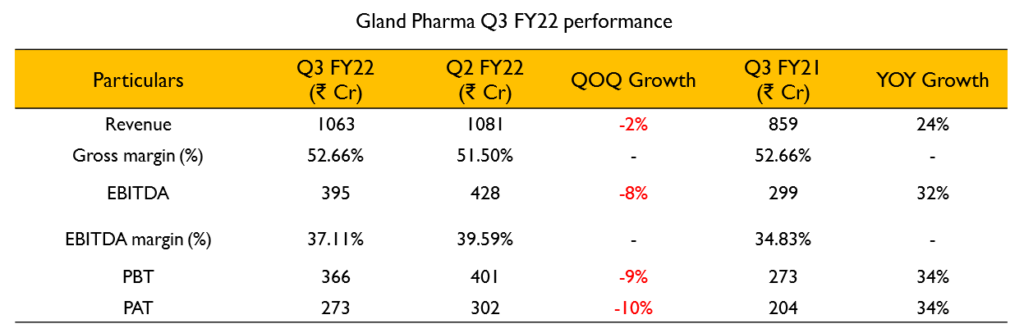
- Revenue – ₹1063 Cr (24% growth YoY).
- EBITDA – ₹394 Cr (32% growth YoY). EBITDA margin stood at 36%
- PAT – ₹273 Cr (34% growth YoY)
- Almost 17-18% growth has come from increase in volumes and new launches have given 6% growth. So major growth has come volumes and new launches so price changes do not affect them as much.
- Completed ANDA filings for 4 complex injectables that were targeted for this financial year – 3 are hormonal products and 1 is a complex peptide. The addressable market is a total of $1 Billion for these 4 products.
- Filed a total of 27 ANDAs for the 9 months this financial year. Also received 4 ANDA approvals during the quarter
- R&D expenditure for the quarter ~₹70 Cr. For 9 months R&D expenditure stands at 4.5% of sales.
- Strengthened presence in ROW markets by winning new tenders. ROW contributed to 19% of Q3 FY22 revenues. Key markets continue to remain MENA, LATAM and APAC. Enoxaparin Sodium was the biggest contributor to growth among the key products.
- Key markets(USA, Europe, Canada and Australia) accounted for 63% of revenues. New variant of COVID affected offtake of core portfolio in the regulated markets.
- Registered a 23% growth YoY in the US market. Key products driving the growth include Micafungin Sodium, Ketorolac Tromethamine and Heparin Sodium. Launched 6 new products during the quarter
- The India market accounted for 18% of revenues. Sales for the domestic market stood at 6% of revenue and sales for export markets (primarily the US market) stood at 12% of revenue. Ertapenem, which is a new launch in the US has shown strong demand from the end market.
- They have a total of about ₹3285 of cash which they intend to use for capex and inorganic growth strategies
- They are not interested in developing products in biosimilars themselves. They are looking to do development and manufacturing work for other biosimilar companies instead. Biosimilar revenue may kick in from Q1 FY23.
- Gross margin is irrelevant to them because of their model. They do contract manufacturing which has 100% gross margin and also have licensing income which has 100% gross margin. Various technology projects have varying gross margins. As the product mix changes, the gross margin varies. EBITDA and PAT are better measures to gauge their performance.
- Complex generics take a longer time to develop. The 4 filings were supposed to be done last year. They try not to delay filings and do it as soon as development work is done.
- For peptide products, they either use third party APIs or they buy intermediates and produce the API themselves. But they are dependent on external parties for critical APIs. They will be looking for an acquisition to de-risk from that in the long term.
- Biosimilars CDMO – They are looking at 2 types of businesses. First one is where the product is already generic and they do a tech transfer from the client and provide fill-finish service. Second is the new drugs CDMO where they will be looking to provide services like cell line development, scale-up batches or pilot scale batches. So they will be looking to work more with companies who are developing new drugs and less with generic biosimilar companies.
- They look at competition in the injectable space as a good thing for them because they get another partner who they can license their products to. Also a product portfolio in this space takes a long period of time to build, so the new companies will take time to get to that stage.
- Capex – Spent about ₹450Cr in 9 months and looking to spend another ₹100 Cr in the next quarter. So ₹550 Cr that was indicated for this financial year will be completely utilized. It has been spent mostly on the vaccine facility in Pashamylaram. Estimating about ₹300 Cr of capex in the next financial year. For further setting up the manufacturing lines in the facility. They are also adding capacity on the API side for more vertical integration.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: kamagra en ligne – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
achat kamagra: achat kamagra – achat kamagra
kamagra en ligne: Kamagra Commander maintenant – Kamagra Oral Jelly pas cher
pharmacies en ligne certifiГ©es: Medicaments en ligne livres en 24h – п»їpharmacie en ligne france pharmafst.com
pharmacie en ligne sans ordonnance: Pharmacie en ligne France – pharmacie en ligne france fiable pharmafst.com
The Real Person!
The Real Person!
achat kamagra: Kamagra Oral Jelly pas cher – kamagra 100mg prix
The Real Person!
The Real Person!
kamagra 100mg prix: achat kamagra – acheter kamagra site fiable
The Real Person!
The Real Person!
Cialis generique prix: Acheter Viagra Cialis sans ordonnance – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Tadalafil 20 mg prix en pharmacie: Tadalafil 20 mg prix en pharmacie – Tadalafil 20 mg prix en pharmacie tadalmed.shop
The Real Person!
The Real Person!
cialis prix: Pharmacie en ligne Cialis sans ordonnance – cialis prix tadalmed.shop
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: Kamagra Commander maintenant – kamagra 100mg prix
The Real Person!
The Real Person!
pharmacie en ligne fiable: pharmacie en ligne pas cher – pharmacie en ligne pas cher pharmafst.com
The Real Person!
The Real Person!
kamagra livraison 24h: acheter kamagra site fiable – Kamagra Oral Jelly pas cher
The Real Person!
The Real Person!
п»їpharmacie en ligne france: pharmacie en ligne livraison europe – pharmacie en ligne pharmafst.com
The Real Person!
The Real Person!
Kamagra pharmacie en ligne: kamagra 100mg prix – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
pharmacie en ligne sans ordonnance: Achat mГ©dicament en ligne fiable – pharmacie en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne avec ordonnance: Pharmacie en ligne livraison Europe – pharmacie en ligne france fiable pharmafst.com
The Real Person!
The Real Person!
Acheter Viagra Cialis sans ordonnance: Acheter Viagra Cialis sans ordonnance – Achat Cialis en ligne fiable tadalmed.shop
The Real Person!
The Real Person!
best canadian pharmacy to buy from: ExpressRxCanada – canadian drug pharmacy
The Real Person!
The Real Person!
reliable canadian pharmacy: best rated canadian pharmacy – canadian pharmacies comparison
The Real Person!
The Real Person!
online pharmacy canada: Canadian pharmacy shipping to USA – online canadian drugstore
The Real Person!
The Real Person!
indian pharmacy online shopping: medicine courier from India to USA – MedicineFromIndia
Medicine From India indian pharmacy online indian pharmacy
Medicine From India: Medicine From India – MedicineFromIndia
The Real Person!
The Real Person!
northwest pharmacy canada: ed drugs online from canada – online canadian pharmacy
mexico drug stores pharmacies mexican online pharmacy RxExpressMexico
The Real Person!
The Real Person!
mexican pharmaceuticals online: mexico pharmacies prescription drugs – mexico pharmacies prescription drugs
mexico pharmacy order online RxExpressMexico mexican drugstore online
The Real Person!
The Real Person!
canadian pharmacy prices: canadian pharmacy ltd – canadian pharmacy world reviews
MedicineFromIndia: indian pharmacy – indian pharmacy online shopping
The Real Person!
The Real Person!
RxExpressMexico: Rx Express Mexico – mexico pharmacy order online
best online pharmacy india indian pharmacy indian pharmacy
The Real Person!
The Real Person!
пин ап зеркало: пинап казино – пин ап казино официальный сайт
The Real Person!
The Real Person!
пинап казино: пин ап зеркало – пин ап вход
The Real Person!
The Real Person!
pin up azerbaycan: pinup az – pin up
The Real Person!
The Real Person!
vavada вход: vavada casino – вавада
The Real Person!
The Real Person!
пинап казино: пинап казино – пинап казино
The Real Person!
The Real Person!
pin up: pin up azerbaycan – pin up azerbaycan
The Real Person!
The Real Person!
вавада: вавада зеркало – вавада казино
The Real Person!
The Real Person!
vavada casino: вавада – вавада казино
The Real Person!
The Real Person!
pin up casino: pin up az – pin up az
The Real Person!
The Real Person!
pin up az: pin up azerbaycan – pin up casino
вавада зеркало: вавада официальный сайт – вавада официальный сайт
pinup az: pin-up – pin up azerbaycan
pin up вход: пин ап казино официальный сайт – pin up вход
pin up вход: pin up вход – пин ап казино
пин ап казино: пин ап казино официальный сайт – пин ап казино
pinup az: pinup az – pin up casino
пин ап вход: пинап казино – pin up вход
пин ап казино: пин ап казино официальный сайт – pin up вход
пин ап зеркало: пинап казино – пин ап вход
pin up вход: пин ап казино официальный сайт – пин ап зеркало
vavada: vavada – vavada вход
пинап казино: пин ап казино официальный сайт – pin up вход
The Real Person!
The Real Person!
cheap Viagra online: Viagra without prescription – trusted Viagra suppliers
The Real Person!
The Real Person!
generic tadalafil: order Cialis online no prescription – buy generic Cialis online
The Real Person!
The Real Person!
no doctor visit required: fast Viagra delivery – best price for Viagra
The Real Person!
The Real Person!
doctor-reviewed advice: Modafinil for sale – purchase Modafinil without prescription
The Real Person!
The Real Person!
secure checkout Viagra: best price for Viagra – generic sildenafil 100mg
The Real Person!
The Real Person!
modafinil pharmacy: buy modafinil online – doctor-reviewed advice
The Real Person!
The Real Person!
legit Viagra online: same-day Viagra shipping – fast Viagra delivery
The Real Person!
The Real Person!
discreet shipping ED pills: order Cialis online no prescription – secure checkout ED drugs
doctor-reviewed advice: doctor-reviewed advice – purchase Modafinil without prescription
http://modafinilmd.store/# safe modafinil purchase
The Real Person!
The Real Person!
doctor-reviewed advice: legal Modafinil purchase – purchase Modafinil without prescription
order Cialis online no prescription: online Cialis pharmacy – reliable online pharmacy Cialis
The Real Person!
The Real Person!
no doctor visit required: secure checkout Viagra – generic sildenafil 100mg
buy generic Viagra online: buy generic Viagra online – order Viagra discreetly
http://zipgenericmd.com/# best price Cialis tablets
The Real Person!
The Real Person!
safe online pharmacy: secure checkout Viagra – buy generic Viagra online
Viagra without prescription: buy generic Viagra online – cheap Viagra online
http://maxviagramd.com/# legit Viagra online
The Real Person!
The Real Person!
Modafinil for sale: modafinil 2025 – verified Modafinil vendors
The Real Person!
The Real Person!
buy generic Cialis online: Cialis without prescription – Cialis without prescription
The Real Person!
The Real Person!
can you buy prednisone over the counter in mexico: PredniHealth – PredniHealth
The Real Person!
The Real Person!
Amo Health Care: Amo Health Care – cost of amoxicillin prescription
The Real Person!
The Real Person!
PredniHealth: PredniHealth – PredniHealth
The Real Person!
The Real Person!
PredniHealth: PredniHealth – online order prednisone 10mg
cialis no perscrtion: TadalAccess – online cialis no prescription
tadalafil (tadalis-ajanta): Tadal Access – is generic tadalafil as good as cialis
liquid tadalafil research chemical: Tadal Access – is cialis covered by insurance
Discount pharmacy Australia Online medication store Australia Pharm Au24
cheap ed drugs: Ero Pharm Fast – Ero Pharm Fast
https://eropharmfast.com/# ed medications online
Over the counter antibiotics for infection: Biot Pharm – buy antibiotics online
over the counter antibiotics buy antibiotics online get antibiotics quickly
Pharm Au24: Discount pharmacy Australia – Discount pharmacy Australia
Pharm Au 24: online pharmacy australia – Buy medicine online Australia
PharmAu24: PharmAu24 – Licensed online pharmacy AU
http://pharmau24.com/# pharmacy online australia
Licensed online pharmacy AU: Online drugstore Australia – Buy medicine online Australia
buy antibiotics from canada buy antibiotics online over the counter antibiotics
Pharm Au24: PharmAu24 – Licensed online pharmacy AU
Ero Pharm Fast: online erectile dysfunction prescription – Ero Pharm Fast
http://biotpharm.com/# get antibiotics without seeing a doctor
buy antibiotics from india: buy antibiotics online – over the counter antibiotics
best online doctor for antibiotics: buy antibiotics online – Over the counter antibiotics pills
https://pharmau24.com/# Online drugstore Australia
Medications online Australia Licensed online pharmacy AU Licensed online pharmacy AU
Medications online Australia: pharmacy online australia – Online medication store Australia
online prescription for ed Ero Pharm Fast online erectile dysfunction
top canadian pharmacies
internet pharmacy no prior prescription
mail order pharmacies
online prescriptions canada without
non prescription canadian pharmacy
pharmacy price compare
prescription price checker
no prescription drugs canada
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
canada prescription drugs
top canadian pharmacies
online ed drugs no prescription
online pharmacy prescription
prescription drug pricing
Your article helped me a lot, is there any more related content? Thanks!
The Real Person!
The Real Person!
Привет всем!
Долго обмозговывал как поднять сайт и свои проекты и нарастить DR и узнал от крутых seo,
топовых ребят, именно они разработали недорогой и главное буст прогон Xrumer – https://www.bing.com/search?q=bullet+%D0%BF%D1%80%D0%BE%D0%B3%D0%BE%D0%BD
Прогон по базам форумов позволяет ускорить линкбилдинг. Как увеличить показатели домена Xrumer становится понятно через практику. Xrumer для продвижения сайта облегчает автоматизацию. SEO-прогон для новичков повышает видимость ресурса. Рассылки с помощью Xrumer экономят время специалистов.
вопросы на собеседовании seo, продвижение в яндексе молодых сайтов, линкбилдинг цена
линкбилдинг на форумах, анализ страницы на сео, продвижение сайта германия
!!Удачи и роста в топах!!
The Real Person!
The Real Person!
Привет всем!
Долго анализировал как поднять сайт и свои проекты и нарастить DR и узнал от гуру в seo,
профи ребят, именно они разработали недорогой и главное продуктивный прогон Xrumer – https://www.bing.com/search?q=bullet+%D0%BF%D1%80%D0%BE%D0%B3%D0%BE%D0%BD
Линкбилдинг что работа требует внимательного подхода. Линкбилдинг быстрый позволяет ускорить продвижение. Линкбилдинг линкбилдинг стратегии помогают системно создавать ссылки. Секреты работы с Xrumer открывают новые возможности. Как увеличить DR сайта Ахрефс зависит от качества ссылок.
продвижение сайта коммерческое предложение, продвижение сайта псков, линкбилдинг
Линкбилдинг через автоматические проги, сео что это за должность, seo статья что
!!Удачи и роста в топах!!
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?