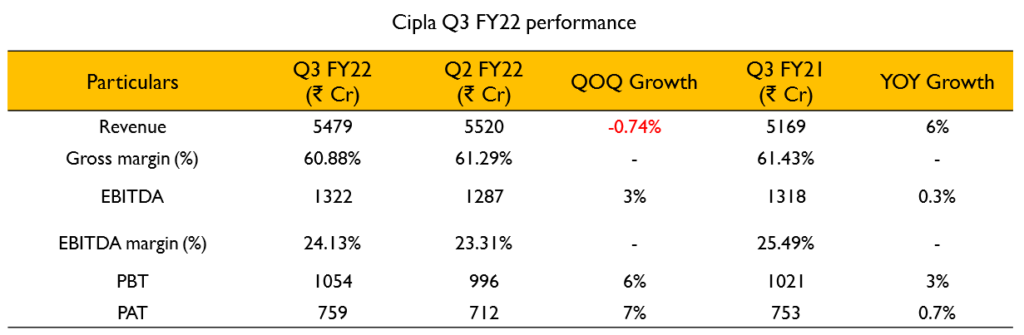
- Revenue growth was at 6%. EBITDA margins were at 22.7% which tracks with their full year guidance despite increased RM and freight costs. The cost increases were offset by increased share of the complex and chronic launches, continuous rigor on cost control and operating efficiencies.
- One India reported 13% growth YoY. The core prescription business in India excluding COVID grew strongly by 16% YoY. The branded prescription business is on track to achieve the $1 Billion mark
- The US revenue for the quarter was $150 million, one of the highest in recent quarters led by strong fraction in the respiratory and other portfolio. South Africa private business maintains a market meeting trajectory driven by steady launch momentum.
- Q4 is a seasonally reverse quarter for India and EBITDA will respond to the change in mix.
- The COVID portfolio declined by almost 10% YoY and 17% sequentially. They do expect to see some traction in the coming quarter in line with the caseloads amid the ongoing third wave in India.
- Total R&D investment for the quarter is at ₹262 crores. All the priority projects continue to be on track. Expenditure is expected to increase as the respiratory assets progress in the clinical trials.
- During the quarter the company unlocked a major peptide asset in the US with the approval of Lanreotide. They are expecting a sustainable ramp up over the medium term. They will continue to expand the peptide portfolio through internal development and partnerships
- On Advair they remain closely engaged with the US FDA on their file. They are waiting to hear from the FDA on the inspection schedule for the Goa plant.
- Lanreotide will be manufactured at their partner’s site. Although they are not ready to disclose where that is yet
- Next year R&D is expected to be higher around 7-7.5%.
- They are aware that large epharmacy players can cause disruption in the business and they are working on a strategy around it.
- Europe is more sensitive to market entry than the US is on this product. So in the US if you are even third or fourth player because of the volumes in the market, people can carve out their own shares and stay sustainable. Whereas in Europe the equation changes very rapidly after the second or third entrant, the market begins to be more responsive on price than you have seen in the US.
- If you really want to bring more products and more innovative products into the market which are patent protected and innovation driven, you have to partner with a multinational corporation. There is no other way to get innovation into India. For example yesterday there was news that Novo Nordisk is launching the oral Semaglutide in India, now that is a great product. If it was not Novo themselves and supposing Novo would be looking for a partner, many Indian companies would have offered to partner for this. The therapy is a game changer, right? So why would anyone sit out when there is innovation that we can bring to this market which can spurt the market growth and create the unmet need demand.
- The infrastructure requirement of launching products from innovators isn’t anything new for the top 10 or 15 Indian companies, because everyone will have a cardiac field force, everyone will have a diabetic field force and every time there is a new product that comes in, they kind of eliminate the tail products so that productivity is maintained.
Wow! This could be one particular of the most helpful blogs We’ve ever arrive across on this subject. Actually Excellent. I’m also a specialist in this topic therefore I can understand your hard work.
Hello there, I found your website via Google while looking for a related topic, your website came up, it looks great. I’ve bookmarked it in my google bookmarks.
It?¦s actually a nice and helpful piece of info. I am glad that you shared this helpful information with us. Please keep us informed like this. Thank you for sharing.
I have been browsing online more than three hours today, yet I never found any interesting article like yours. It is pretty worth enough for me. In my view, if all webmasters and bloggers made good content as you did, the internet will be a lot more useful than ever before.
Whats up very nice website!! Guy .. Beautiful .. Amazing .. I will bookmark your web site and take the feeds also?KI’m happy to find a lot of useful info right here in the put up, we’d like work out more strategies in this regard, thank you for sharing. . . . . .
Thanx for the effort, keep up the good work Great work, I am going to start a small Blog Engine course work using your site I hope you enjoy blogging with the popular BlogEngine.net.Thethoughts you express are really awesome. Hope you will right some more posts.
Fantastic beat ! I would like to apprentice while you amend your web site, how can i subscribe for a blog web site? The account aided me a acceptable deal. I had been tiny bit acquainted of this your broadcast offered bright clear idea
Hello there! This post could not be written any better! Reading this post reminds me of my good old room mate! He always kept chatting about this. I will forward this page to him. Pretty sure he will have a good read. Many thanks for sharing!
I like your writing style truly enjoying this site.
Thanks for the sensible critique. Me and my neighbor were just preparing to do some research on this. We got a grab a book from our local library but I think I learned more from this post. I am very glad to see such great info being shared freely out there.
you’re really a good webmaster. The web site loading speed is incredible. It seems that you’re doing any unique trick. Also, The contents are masterpiece. you’ve done a excellent job on this topic!
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Hey superb blog! Does running a blog similar tto this take a great deal
of work? I’ve no understanding of coding but I was hoping to start my
own blog in the near future. Anyway, should you have any ideas
or techniques for new blog owners please share. I know this is off subject
but I just needed to ask. Thank you! https://menbehealth.wordpress.com/
Pretty grteat post. I simply stumbled upon your blog and wanted to
menttion that I have truly enjoyed surfing
around your weblog posts. After all I will be subscribing on your rsss
feed and I am hoping you write once more very soon! http://www.leefairshare.org/casino-reviews-for-bdmbet-play-new-games-on-your-account-and-get-up-to-hours-of-enjoyment-terms-and-additional-content/
Thanks for a marvelous posting! I certainly enjoyed reading
it, you’re a great author. I will ensure that I bookmark your
blog and will often come back dopwn the road.
I want to encourage you to continue your great writing, have a nice weekend! https://Gtcs.co.in/employer/quality-dissertation-writing/
I think the admiin oof this site is really working hard
in support of his website, because here every data is
quality based stuff. https://WWW.Thempower.co.in/employer/thesis-proposal-specialist/
If you wwish for to increase your experience simply keep
visiiting this site and be updated with the hottest information posted here. https://technosfer.co/employer/dingle/
MetaMask Extension keeps improving! Regular updates enhance security and performance, making it the best crypto wallet.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
MetaMask Chrome never disappoints. Transactions are smooth, and the user interface is easy to navigate. A must-have for crypto users!
MetaMask Extension is my favorite crypto wallet. It ensures safe and easy access to Web3 and decentralized applications.
The Real Person!
The Real Person!
kamagra 100mg prix: Kamagra pharmacie en ligne – kamagra gel
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: cialis generique – Cialis en ligne tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne fiable: Medicaments en ligne livres en 24h – pharmacie en ligne avec ordonnance pharmafst.com
Tadalafil achat en ligne: Cialis sans ordonnance 24h – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Kamagra Commander maintenant: Kamagra Commander maintenant – kamagra oral jelly
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne pas cher – acheter mГ©dicament en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Tadalafil 20 mg prix en pharmacie: Tadalafil achat en ligne – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
kamagra pas cher: acheter kamagra site fiable – kamagra gel
kamagra gel: kamagra pas cher – achat kamagra
The Real Person!
The Real Person!
trouver un mГ©dicament en pharmacie: pharmacie en ligne sans ordonnance – pharmacie en ligne livraison europe pharmafst.com
MetaMask Chrome is perfect for managing multiple assets. I can easily switch between different networks and trade with confidence.
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: achat kamagra – Kamagra pharmacie en ligne
The Real Person!
The Real Person!
pharmacies en ligne certifiГ©es: pharmacie en ligne – acheter mГ©dicament en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Tadalafil 20 mg prix sans ordonnance: cialis sans ordonnance – Tadalafil 20 mg prix sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne france livraison belgique: Pharmacies en ligne certifiees – pharmacie en ligne pas cher pharmafst.com
The Real Person!
The Real Person!
cialis sans ordonnance: Cialis sans ordonnance pas cher – cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: kamagra oral jelly – acheter kamagra site fiable
The Real Person!
The Real Person!
pharmacie en ligne fiable: pharmacie en ligne sans ordonnance – Pharmacie Internationale en ligne pharmafst.com
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: cialis sans ordonnance – cialis prix tadalmed.shop
The Real Person!
The Real Person!
cialis generique: Cialis sans ordonnance pas cher – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
kamagra oral jelly: Kamagra Commander maintenant – kamagra en ligne
The Real Person!
The Real Person!
pharmacie en ligne livraison europe: Livraison rapide – pharmacie en ligne avec ordonnance pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne sans ordonnance: Achat mГ©dicament en ligne fiable – п»їpharmacie en ligne france pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne livraison europe: Pharmacies en ligne certifiees – pharmacie en ligne pas cher pharmafst.com
The Real Person!
The Real Person!
Acheter Viagra Cialis sans ordonnance: Tadalafil 20 mg prix en pharmacie – Tadalafil 20 mg prix sans ordonnance tadalmed.shop
Hi Dear, are you actually visiting this website daily, if soo after that you will absolutely take fastidious knowledge. https://www.linkedin.com/pulse/stakeholders-education-responsibilities-concerns-edu-reviewscom-xsmve/?trackingId=oks7a8BhlYe91KK5n%2F%2BP%2Fg%3D%3D
The Real Person!
The Real Person!
mexican online pharmacy: mexico pharmacy order online – mexico drug stores pharmacies
The Real Person!
The Real Person!
indian pharmacy online: medicine courier from India to USA – indian pharmacy online
The Real Person!
The Real Person!
Rx Express Mexico: mexican rx online – mexico pharmacy order online
The Real Person!
The Real Person!
mexican online pharmacy: mexico drug stores pharmacies – mexican rx online
mexican online pharmacy mexico drug stores pharmacies mexico pharmacies prescription drugs
canadian compounding pharmacy: real canadian pharmacy – canadian pharmacy com
The Real Person!
The Real Person!
canadian pharmacies compare: Express Rx Canada – canadian pharmacy in canada
mexican online pharmacy mexican online pharmacy mexican rx online
The Real Person!
The Real Person!
Rx Express Mexico: Rx Express Mexico – RxExpressMexico
canadian drugs online: Express Rx Canada – canadian discount pharmacy
mexican online pharmacy mexico pharmacy order online mexico drug stores pharmacies
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy online shopping – MedicineFromIndia
Medicine From India: Medicine From India – medicine courier from India to USA
online canadian pharmacy Generic drugs from Canada canadian neighbor pharmacy
The Real Person!
The Real Person!
mexico pharmacies prescription drugs: mexico pharmacies prescription drugs – RxExpressMexico
The Real Person!
The Real Person!
pin up вход: pin up вход – пин ап зеркало
The Real Person!
The Real Person!
pin-up: pin up azerbaycan – pinup az
The Real Person!
The Real Person!
vavada: vavada – вавада официальный сайт
The Real Person!
The Real Person!
pin up вход: пин ап вход – пин ап казино официальный сайт
The Real Person!
The Real Person!
vavada вход: вавада официальный сайт – vavada
The Real Person!
The Real Person!
пинап казино: пин ап зеркало – пинап казино
The Real Person!
The Real Person!
pinup az: pin up az – pin-up casino giris
The Real Person!
The Real Person!
pinup az: pin-up – pinup az
The Real Person!
The Real Person!
вавада зеркало: vavada casino – вавада официальный сайт
The Real Person!
The Real Person!
vavada: вавада – вавада казино
vavada casino: вавада официальный сайт – вавада казино
pin-up casino giris: pin up az – pin-up casino giris
pin up casino: pin up – pin up azerbaycan
pin up: pin up azerbaycan – pin up az
pin up: pin up az – pin up
pinup az: pin up azerbaycan – pin up casino
пинап казино: пин ап казино официальный сайт – пин ап вход
pin up вход: пин ап казино – pin up вход
pin up casino: pin-up – pin-up
vavada: vavada вход – vavada
vavada: вавада зеркало – vavada
pin up casino: pin up az – pin up azerbaycan
pinup az: pin up azerbaycan – pin-up
пин ап зеркало: пин ап казино официальный сайт – пин ап зеркало
The Real Person!
The Real Person!
http://pinupaz.top/# pin up azerbaycan
Super-Duper site! I am loving it!! Will come back again. I am bookmarking your feeds also.
The Real Person!
The Real Person!
discreet shipping: same-day Viagra shipping – Viagra without prescription
The Real Person!
The Real Person!
discreet shipping ED pills: FDA approved generic Cialis – generic tadalafil
The Real Person!
The Real Person!
buy modafinil online: verified Modafinil vendors – legal Modafinil purchase
I?¦m no longer sure the place you are getting your info, but great topic. I must spend a while learning much more or working out more. Thanks for excellent info I was on the lookout for this info for my mission.
A person essentially assist to make seriously articles I might state. This is the first time I frequented your website page and to this point? I amazed with the analysis you made to create this actual post incredible. Great task!
The Real Person!
The Real Person!
Modafinil for sale: buy modafinil online – purchase Modafinil without prescription
The Real Person!
The Real Person!
Modafinil for sale: buy modafinil online – modafinil 2025
The Real Person!
The Real Person!
fast Viagra delivery: same-day Viagra shipping – discreet shipping
The Real Person!
The Real Person!
safe online pharmacy: order Viagra discreetly – generic sildenafil 100mg
I like what you guys are up also. Such clever work and reporting! Carry on the superb works guys I have incorporated you guys to my blogroll. I think it’ll improve the value of my website 🙂
The Real Person!
The Real Person!
legal Modafinil purchase: safe modafinil purchase – purchase Modafinil without prescription
The Real Person!
The Real Person!
cheap Viagra online: trusted Viagra suppliers – same-day Viagra shipping
secure checkout ED drugs: secure checkout ED drugs – online Cialis pharmacy
The Real Person!
The Real Person!
cheap Viagra online: discreet shipping – generic sildenafil 100mg
no doctor visit required: fast Viagra delivery – discreet shipping
http://zipgenericmd.com/# secure checkout ED drugs
The Real Person!
The Real Person!
buy generic Cialis online: generic tadalafil – FDA approved generic Cialis
cheap Viagra online: no doctor visit required – Viagra without prescription
The Real Person!
The Real Person!
best price for Viagra: fast Viagra delivery – safe online pharmacy
The Real Person!
The Real Person!
generic amoxicillin online: Amo Health Care – amoxicillin 500mg buy online uk
The Real Person!
The Real Person!
PredniHealth: prednisone without prescription medication – PredniHealth
The Real Person!
The Real Person!
apo prednisone: PredniHealth – PredniHealth
The Real Person!
The Real Person!
prednisone online pharmacy: PredniHealth – PredniHealth
The Real Person!
The Real Person!
amoxicillin 500 coupon: canadian pharmacy amoxicillin – purchase amoxicillin online
does cialis lowers blood pressure: Tadal Access – vidalista 20 tadalafil tablets
cialis black in australia: how well does cialis work – prescription free cialis
best price for tadalafil: TadalAccess – best price cialis supper active
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
online pharmacy australia: PharmAu24 – Medications online Australia
http://eropharmfast.com/# cheap ed medicine
Buy medicine online Australia PharmAu24 Licensed online pharmacy AU
best online doctor for antibiotics: cheapest antibiotics – buy antibiotics from india
get antibiotics without seeing a doctor: buy antibiotics online – buy antibiotics over the counter
Medications online Australia: pharmacy online australia – Online medication store Australia
online ed medications generic ed meds online online ed medication
https://pharmau24.com/# Pharm Au24
Buy medicine online Australia: Buy medicine online Australia – Pharm Au 24
get antibiotics without seeing a doctor: buy antibiotics from canada – buy antibiotics over the counter
buy antibiotics online: buy antibiotics online – cheapest antibiotics
https://biotpharm.shop/# get antibiotics quickly
online ed meds Ero Pharm Fast Ero Pharm Fast
antibiotic without presription: buy antibiotics online uk – over the counter antibiotics
buy antibiotics over the counter: Biot Pharm – Over the counter antibiotics pills
buy antibiotics online antibiotic without presription buy antibiotics from canada
https://pharmau24.com/# Online drugstore Australia
Ero Pharm Fast: cheap ed pills online – Ero Pharm Fast
Online medication store Australia Pharm Au24 Online medication store Australia
There are definitely loads of particulars like that to take into consideration. That could be a great level to convey up. I provide the ideas above as common inspiration but clearly there are questions just like the one you convey up the place crucial factor will probably be working in trustworthy good faith. I don?t know if greatest practices have emerged around things like that, but I am sure that your job is clearly recognized as a fair game. Both boys and girls feel the affect of just a second’s pleasure, for the remainder of their lives.
Thanks a bunch for sharing this with all of us you really know what you’re talking about! Bookmarked. Kindly also visit my web site =). We could have a link exchange agreement between us!
whoah this blog is fantastic i love reading your articles. Keep up the good work! You know, lots of people are hunting around for this information, you could help them greatly.
certainly like your web-site but you have to check the spelling on several of your posts. Several of them are rife with spelling problems and I find it very troublesome to tell the truth nevertheless I’ll certainly come back again.
I just like the helpful info you provide to your articles. I will bookmark your weblog and test again right here regularly. I’m moderately certain I will be informed many new stuff right here! Best of luck for the following!
Very quickly this website will be famous amid all blog people, due to it’s good articles https://marylands.mystrikingly.com/
Hi everyone, it’s my first pay a quick visit at this web page,
and piece of writing is genuinely fruitful in favor of me, keep up
posting suh posts. https://checkerss.mystrikingly.com/
Hello, all the time i used too check web site posts here
early inn the break of day, as i love to find out more and more. https://Online-Aassignments.Blogspot.com/2025/08/the-art-of-online-assignments-students.html
Hello i aam kavin, its myy first occasion to
commenbting anywhere, when i rsad this post i thought i could also make comment due to this good
paragraph. https://top8studentjobs.Wordpress.com/
When I originally commented I clicked the -Notify me when new comments are added- checkbox and now each time a comment is added I get four emails with the same comment. Is there any way you can remove me from that service? Thanks!
Your means of describing all in this piece of writing is truly nice,
every one be able to simply know it, Thanks a lot. https://Z42Mi.Mssg.me/
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Great V I should certainly pronounce, impressed with your website. I had no trouble navigating through all tabs and related information ended up being truly simple to do to access. I recently found what I hoped for before you know it at all. Quite unusual. Is likely to appreciate it for those who add forums or something, site theme . a tones way for your customer to communicate. Nice task..
I have recently started a website, the info you offer on this website has helped me tremendously. Thanks for all of your time & work.
Perfectly composed articles, thank you for entropy. “No human thing is of serious importance.” by Plato.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. Binance推荐码
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
I truly enjoy examining on this site, it contains wonderful posts. “Heavier-than-air flying machines are impossible.” by Lord Kelvin.