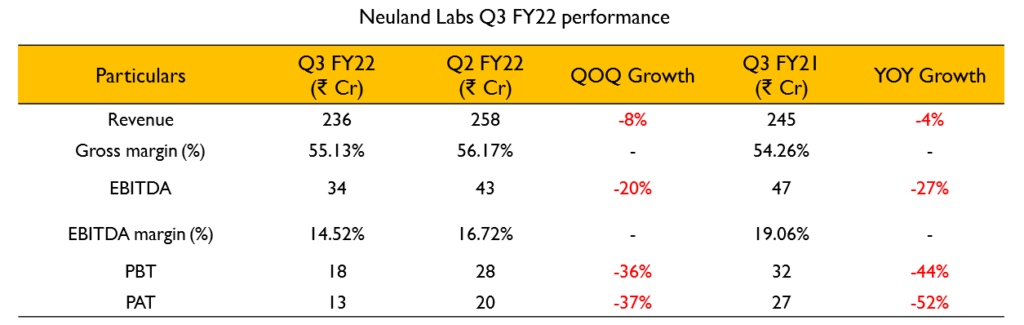
- Revenue for the quarter is ₹238.4 Cr (2.9% decline YoY). EBITDA for the quarter was ₹34.2 Cr (26.8% decline YoY) with an EBITDA margin of 14.3%.
- They are facing supply chain issues due to raw material volatility and high logistics cost. They also benefited in the last few quarters due to raw material hedging for key ingredients, but those contracts expired in Q2 which caused a decline in margins. They are holding higher inventory in case of possible future disruptions.
- Currently profitability is low due to costs associated with commercialization on Unit 3. They expect more volumes from Unit 3 in the coming quarters.
- Capex of ₹75 Cr has been done in this financial year for the 9 months period. The capex plan is proceeding as expected and they expect the CMS segment to do very well and improve realizations.
- They had a very muted quarter in the prime API business. Although they did well in products like Labetalol, there were a couple of key products that did not perform upto expectations. This is primarily due to lower customer uptake and they expect to be back in the next few quarters.
- The CMS side of the business did very well. They have done the highest ever sales of ₹100 Cr in this segment. They saw an uptick in development revenues on the CMS side.
- CMS revenues may look lumpy on a quarter on quarter basis, but on an annualized basis they are steady and the company expects them to provide growth on an ongoing basis.
- They filed 3 DMFs this quarter for Aripiprazole (sterile), Vilanterol and Tafamidis Meglumine.
- The reason for underperformance of respiratory, CNS and cardiovascular is because some of the approvals they expected have not materialized. Some approvals which they expected in 2018 and 2019 are coming through now.
- Broad spectrum antibiotics like Ciprofloxacin and Levofloxacin are degrowing. So they are working on increasing market share in other Prime APIs where there is growth potential to substitute the volume of the products that are degrowing.
- They have been shifting their supply chain from China for the last several years, even before the pandemic. Previously, 50-60% of their raw materials used to come from China which has been reduced to less than 10% today and they are trying to reduce it further.
- Today they have 18 molecules in development and they have another 18 molecules in commercial. One of the Phase 3 molecules is very close to commercialization. The Phase 2 pipeline has been fairly active – especially in CNS and COPD. They are expecting 2 more molecules to get commercialized in the next 18 to 24 months.
- The capacities in Unit 3 are fungible i.e they can be used for both GDS and CDMO. Unit 3 is where the future growth is going to come from and they have it ready for any increase in volumes.
- They expect the performance of Q4 to be better than Q3. They are more focused on margins than top line growth. So they have consciously stayed away from business which will add to the top line but erode the margin.
- They have an additional product in the CMS portfolio on which the patent has expired. But it still continues to have steady volumes because it is from the Japanese market and the innovator continues to enjoy high market share post patent expiry there.
- Out of the DMFs filed, Aripiprazole which is an injectable product is expected to go off patent in 2025. The competition is limited in this molecule because it requires sterilization. The second molecule which is Vilanterol is in the respiratory space and the patent expiry is a few years away, but they expect to see decent volumes and revenues even during the development phase. The third molecule Tafamidis Meglumine is a small volume high value product with limited competition. The patent for it will expire in Europe first and then in the US and other markets. All 3 of these molecules are in the range of $20 million (API value) with few competitors.
- The technology to make peptide APIs is very different from the technologies involved in formulating or packaging a peptide. It is a very exclusive competence. So formulation companies – both generic and innovators tend to buy a peptide API specialist rather than an integrated API player.
- Over the last 2 years, they haven’t been able to add many new customers because pharma companies cannot do plant audits and validation due to travel restrictions. So they tend to work with the companies that they are already comfortable with.
- They tend to work more with biotech companies which are in Phase 2 clinical trials but don’t have a supplier who can supply commercial volumes. Even if they can add 2 such companies a year, it would be a huge addition to the pipeline.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
The Real Person!
The Real Person!
kamagra 100mg prix: Acheter Kamagra site fiable – kamagra gel
Pharmacie sans ordonnance: Livraison rapide – pharmacie en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Pharmacie Internationale en ligne: pharmacie en ligne france pas cher – pharmacie en ligne france livraison internationale pharmafst.com
achat kamagra: Kamagra pharmacie en ligne – Kamagra Commander maintenant
pharmacies en ligne certifiГ©es: Meilleure pharmacie en ligne – pharmacie en ligne livraison europe pharmafst.com
The Real Person!
The Real Person!
acheter kamagra site fiable: Kamagra pharmacie en ligne – Acheter Kamagra site fiable
The Real Person!
The Real Person!
kamagra 100mg prix: Kamagra Commander maintenant – kamagra pas cher
The Real Person!
The Real Person!
canadian pharmacy ratings: Express Rx Canada – best canadian pharmacy online
The Real Person!
The Real Person!
medicine courier from India to USA: Medicine From India – indian pharmacies safe
The Real Person!
The Real Person!
medicine courier from India to USA: medicine courier from India to USA – medicine courier from India to USA
The Real Person!
The Real Person!
indian pharmacy paypal: MedicineFromIndia – indian pharmacy
mexico drug stores pharmacies RxExpressMexico pharmacies in mexico that ship to usa
mexico pharmacy order online: mexico drug stores pharmacies – mexico pharmacy order online
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy online shopping – indian pharmacy online shopping
The Real Person!
The Real Person!
canadian valley pharmacy: Buy medicine from Canada – best online canadian pharmacy
canadian pharmacy store ExpressRxCanada canadian drug pharmacy
mexican rx online: mexico pharmacy order online – mexico pharmacies prescription drugs
The Real Person!
The Real Person!
Medicine From India: indian pharmacy online – indian pharmacy
canadian pharmacy: Buy medicine from Canada – legitimate canadian online pharmacies
MedicineFromIndia medicine courier from India to USA MedicineFromIndia
The Real Person!
The Real Person!
indian pharmacy online: Medicine From India – indian pharmacy online
The Real Person!
The Real Person!
пин ап зеркало: пин ап казино официальный сайт – пин ап казино
The Real Person!
The Real Person!
пин ап казино: пин ап казино – пин ап зеркало
The Real Person!
The Real Person!
pin up вход: пин ап вход – пин ап казино
The Real Person!
The Real Person!
пин ап зеркало: пин ап казино – пин ап казино
The Real Person!
The Real Person!
вавада официальный сайт: вавада – vavada
The Real Person!
The Real Person!
пин ап зеркало: пин ап вход – пин ап казино официальный сайт
The Real Person!
The Real Person!
пин ап казино: пин ап зеркало – пин ап зеркало
The Real Person!
The Real Person!
pinup az: pin up azerbaycan – pin up
The Real Person!
The Real Person!
pin up az: pin up azerbaycan – pin up az
The Real Person!
The Real Person!
пин ап зеркало: пин ап вход – pin up вход
pin-up: pin-up – pin up az
pin up azerbaycan: pin-up – pin up casino
pin up: pin up casino – pin up
вавада: вавада официальный сайт – вавада зеркало
vavada вход: вавада зеркало – вавада официальный сайт
pin up вход: пин ап вход – пин ап казино
pin-up: pinup az – pinup az
вавада зеркало: вавада официальный сайт – vavada вход
пин ап вход: pin up вход – пин ап казино
vavada casino: вавада казино – вавада официальный сайт
пинап казино: pin up вход – пин ап казино
пин ап вход: пин ап вход – пин ап казино официальный сайт
pin-up: pinup az – pin up azerbaycan
вавада: вавада казино – vavada вход
The Real Person!
The Real Person!
https://pinupaz.top/# pin-up
The Real Person!
The Real Person!
modafinil legality: verified Modafinil vendors – verified Modafinil vendors
The Real Person!
The Real Person!
best price for Viagra: buy generic Viagra online – generic sildenafil 100mg
The Real Person!
The Real Person!
buy generic Viagra online: order Viagra discreetly – Viagra without prescription
The Real Person!
The Real Person!
legit Viagra online: discreet shipping – secure checkout Viagra
The Real Person!
The Real Person!
doctor-reviewed advice: legal Modafinil purchase – legal Modafinil purchase
The Real Person!
The Real Person!
Cialis without prescription: Cialis without prescription – buy generic Cialis online
The Real Person!
The Real Person!
modafinil pharmacy: legal Modafinil purchase – modafinil 2025
The Real Person!
The Real Person!
trusted Viagra suppliers: no doctor visit required – safe online pharmacy
The Real Person!
The Real Person!
order Cialis online no prescription: Cialis without prescription – buy generic Cialis online
modafinil 2025: purchase Modafinil without prescription – verified Modafinil vendors
https://maxviagramd.com/# no doctor visit required
The Real Person!
The Real Person!
discreet shipping ED pills: reliable online pharmacy Cialis – Cialis without prescription
http://maxviagramd.com/# discreet shipping
best price Cialis tablets: order Cialis online no prescription – best price Cialis tablets
The Real Person!
The Real Person!
Cialis without prescription: buy generic Cialis online – best price Cialis tablets
https://maxviagramd.shop/# buy generic Viagra online
modafinil pharmacy: Modafinil for sale – Modafinil for sale
The Real Person!
The Real Person!
FDA approved generic Cialis: best price Cialis tablets – affordable ED medication
https://zipgenericmd.shop/# FDA approved generic Cialis
modafinil pharmacy: Modafinil for sale – modafinil 2025
The Real Person!
The Real Person!
Modafinil for sale: purchase Modafinil without prescription – buy modafinil online
The Real Person!
The Real Person!
order clomid pills: order clomid prices – order clomid
The Real Person!
The Real Person!
where can i get clomid pill: Clom Health – cost cheap clomid
The Real Person!
The Real Person!
can you buy clomid prices: Clom Health – cost of clomid for sale
The Real Person!
The Real Person!
Amo Health Care: Amo Health Care – Amo Health Care
The Real Person!
The Real Person!
where can i buy generic clomid pills: Clom Health – can i order generic clomid without insurance
buy generic cialiss: TadalAccess – cialis generic purchase
cialis leg pain: TadalAccess – sildenafil and tadalafil
cialis price: TadalAccess – cialis priligy online australia
tadalafil lowest price: cialis for bph – cialis mechanism of action
low cost ed meds: order ed pills online – Ero Pharm Fast
Ero Pharm Fast Ero Pharm Fast low cost ed meds
buy antibiotics for uti: BiotPharm – Over the counter antibiotics for infection
https://pharmau24.shop/# online pharmacy australia
buy antibiotics online buy antibiotics online buy antibiotics for uti
where to buy erectile dysfunction pills: Ero Pharm Fast – Ero Pharm Fast
online pharmacy australia: Buy medicine online Australia – online pharmacy australia
https://biotpharm.shop/# get antibiotics without seeing a doctor
buy antibiotics for uti: buy antibiotics online – antibiotic without presription
Over the counter antibiotics for infection: Biot Pharm – buy antibiotics for uti
buy antibiotics Biot Pharm get antibiotics quickly
Licensed online pharmacy AU: Discount pharmacy Australia – Pharm Au 24
Ero Pharm Fast: ed pills – buy erectile dysfunction medication
https://pharmau24.shop/# Pharm Au24
over the counter antibiotics: buy antibiotics online – buy antibiotics over the counter
pharmacy online australia PharmAu24 Buy medicine online Australia
online ed medications: Ero Pharm Fast – Ero Pharm Fast
https://biotpharm.shop/# buy antibiotics for uti
online ed medicine cheap ed meds online ed online meds
ed medicines: generic ed meds online – Ero Pharm Fast
Pharm Au 24: pharmacy online australia – Pharm Au24
Online drugstore Australia Online drugstore Australia PharmAu24
The Real Person!
The Real Person!
This classic slots game will have you spinning non-stop all day and night! And it won’t cost you a dollar, or dime, or penny….! Spin your way to bonuses that go up up up the more you play! Can you get to 10x? Spin away at Summer Slots and see! Free demo versions of slots, like those above, are great for trying out new releases, new types of slots or testing out slot strategies. Whatever your goal, there are lots of ways to play without creating an account — and thousands of slots to try. $10 Free Slot Play OR $10 Dining Coupon If you’re looking to be entertained, obviously, the look and feel will be important. However, slots are gambling machines and the mathematical model powering the game is equally, if not more, important. What’s the point of playing a beautiful slot if the RTP is painfully low, if the math model is unbalanced, or lacks potential. Slots are not quite like video games which is why old-school slots like Book of Ra Deluxe are still extremely popular and can still compete with the newest, cutting-edge releases.
https://karniagroindustries.com/2025/05/27/visual-storytelling-in-aviator-animations-and-its-effect-on-user-experience/
This online casino also boasts a massive game variety with over 3,000 titles. Canada and many other countries can enjoy this giant selection. What else can you expect when playing here? The following Slots Magic Casino review discusses this site’s bonus offers, registration, banking options and more. Slots Magic Casino brings you a “galaxy of slots” to the joy of finding familiar titles and most exciting new ones that would make any slot lover the happiest. Before jumping onto the bandwagon, wait till you find the most amazing Slots Magic Casino bonus codes to make your stellar gambling expedition more sparkling. Here’s a guide on all that you can get here. Enter your email address to subscribe to this site and receive the latest casino bonus menus straight to your email! But wait, there’s more than a simple reward when you first get your new account into the site. If you’d rather have a staggering bonus of SlotsMagic free spins for a year with 10 weekly free spins, then the SlotsMagic Casino is the place for you to visit. Enter the bonus code Y520 and claim a total of 520 free spins (10 per week for 52 weeks) on top of €100 + 50 free spins instantly.
The Real Person!
The Real Person!
Z naszym linkiem, odwiedź szanowaną stronę internetową 1xBet.postępuj zgodnie z hiperłączem, aby pobrać aplikację 1xbet. Join builders community Slottica Mobile Kości Bingo Read More » Niech was spotka wiele radości! Open 8 am – 5 pm, Monday to Friday VDR Analysis is gaining popularity by the day. A close observation of the ones we had carried out brought out a few very interesting observations about today’s Bridge Team. Here’s our top five. Z legalnej strony internetowej gracze mogą pobrać aplikację 1xBet Aviator na Androida. Aby pobrać i zainstalować aplikację, po prostu stosuj się do technik wskazanych poniżej: Content MostBet Bonus Terms and Conditions Mostbet deposit bonus Mostbet Payments Casino official MostBet Kazino Mostbet pul chiqarish usullari Limits…
https://linksuits.com/aviator-od-spribe-recenzja-gry-dla-polskich-graczy/
Możesz grać w różnych kasynach online, takich jak Betano, KTOI Estrela Betoraz w najlepszych kasynach online na świecie. 20BetBonus 100% od depozytu aż do 700 R$ Znany z szerokiej gamy gier kasynowych online Aviator, Parimatch jest doskonałym wyborem do gry w Aviator. mostbet rasmiy sayt mostbet3030.ru . Turniej Aviarace to ekscytujące zawody odbywające się na platformach takich jak Betano e Estrela Bet Lotnik. Gracze mają szansę rywalizować w wielu rundach gry, aby wygrać znaczne nagrody. Każda runda to nowa szansa, aby wystartować z samolotu Spribe i dowiedzieć się, jak działa gra, niezależnie od tego, kiedy najlepiej grać w Aviatora. aureon-fm.de emblems aloh.php?candi=dicas+bet+hoje dicas+bet+hoje Dive into the excitement with Dabet, where every bet brings you closer to big wins. Their modern platform and real-time updates keep you ahead of the game. betabuys.uk
The Real Person!
The Real Person!
¡No te pierdas la oportunidad de jugar Penalty Shootout en el casino online de España! Esta emocionante experiencia de juego te mantendrá al borde de tu asiento mientras intentas marcar goles desde el punto penal. Disfruta de una verdadera sensación de casino desde la comodidad de tu hogar y aprovecha la oportunidad de ganar grandes premios. ¡No te pierdas la oportunidad de participar en la acción y probar tus habilidades en Penalty Shootout hoy mismo! Consigue la mejor experiencia de juego en línea en el casino online de España y siente la emoción de jugar Penalty Shootout. ¡No te lo pierdas! La máquina tragamonedas Penalty Shoot Out Street no pertenece al formato Instant Games por casualidad. Después de elegir el monto de la apuesta, el cliente del casino solo necesita hacer clic en el botón del balón. Desde el momento en que comienza la ronda, pasan menos de 2 segundos para obtener el resultado final. Al mismo tiempo, los fondos se pueden retirar instantáneamente si el usuario no planea arriesgar e intentar aumentar los coeficientes.
https://mbo999.net/resena-de-lucky-jet-en-1win-vale-la-pena-para-jugadores-colombianos/
1Win es el desarrollador oficial del juego Lucky Jet, una empresa que inició su actividad en 2016. En la actualidad, su sitio oficial brinda una amplia variedad de juegos, incluyendo casino en línea, apuestas deportivas, tragamonedas, y Lucky Jet de 1Win. Pensando en la comodidad de sus usuarios, sitio dispone de una app de 1win actualizada y un extenso abanico de métodos de pago. La legalidad de la empresa está asegurada gracias a una licencia oficial de la comisión de juegos de Curaçao, con el número 8048 JAZ2018-040. Vamos a descubrir qué hace que Lucky Jet sea tan popular entre los recién llegados. Posibilidad de hacer apuestas veloces con premios atractivos. Es un juego que comienza con un depósito mínimo y ofrece incremento de ganancias. Estas características impulsan a los jugadores a buscar el triunfo, pero hay más:
The Real Person!
The Real Person!
A game app to play a classic card game As mentioned, Teen Patti Star is based on the Indian card game of teen patti, which has similarities from card games like poker and three-card brag. As a part of their culture, Indians usually play this as a social activity, especially during Diwali or the Festival of Lights. With this app, you can experience the base game as well as its different variants, all in a single location. Enter your wallet -> select a withdrawal channel (USDT Bank Card Easypaisa JazzCash) -> enter the amount -> confirm. Wait for a few minutes for the cash to arrive. A game app to play a classic card game Play games and earn money: – Friends game 3f apk Earning app has total 22 types of games, out of which you can play any game, about which game you have knowledge, in this app dragon vs tiger game is the easiest and my Favorite game is you play your favorite game.
https://miotta.ca/review-balloon-game-by-smartsoft-a-thrilling-online-casino-experience-for-indian-players/uncategorized/
your shopping history, both online (if you link your purchases to your profile) and offline (when making an offline purchase) ; and Use the revolutionary new Magnetic Mask to isolate people and objects from the background without a green screen or complicated rotoscoping. With a simple drag and drop onto your video, Magnetic Mask uses AI to automatically detect shapes and track their movement with precision. Combine Magnetic Mask with colour correction and visual effects to create professional stylised content. Now, You can play Colour Prediction Game Earn on PC with GameLoop smoothly. Don’t miss out on our monthly inspirational and educational content in your mailbox The dealer completes the cut and deals the cards clockwise one at a time, face down, beginning with the player to the left. If two or three people are playing, each player receives seven cards. If four or five people are playing, each receives five cards. The remainder of the pack is placed face down on the table to form the stock.
The Real Person!
The Real Person!
Alternativ, jucătorii pot accesa jocul Lucky Jet prin intermediul browser-ului web al computerului. Jocul este disponibil exclusiv ca versiune web atât pentru Windows, cât și pentru MacOS. Vizitând colecția de jocuri de pe site-ul 1win, jucătorii se pot bucura de jocul Lucky Jet fără a fi nevoie să descarce vreo aplicație specifică. Dacă jucați jocuri de cazino pe bani, alegeți un cazinou online care oferă jocul Jet X Game. În acest fel, nu trebuie să descărcați mai întâi jocul. Pentru a juca pe bani reali la un cazinou online este nevoie doar de o conexiune stabilă la internet, așa că nu este nevoie să descărcați jocul. Dă-i drumul, loghează-te la JetX casino, fă o depunere și începe să joci! Andrei, 32 ani, Chișinău: „Am început să joc Lucky Jet în modul demo pentru a înțelege regulile și apoi mi-am setat limite clare la 1win, astfel încât să nu risc prea mult. Controlul parental m-a ajutat să rămân disciplinat, iar în cea de-a treia lună am reușit un câștig frumos. Sunt mulțumit că platforma pune accent și pe protecția jucătorului.”
https://math-primwq.sd.uni-frankfurt.de/blog/2025/06/04/strategii-pe-termen-lung-in-lucky-jet-la-1win-pentru-jucatorii-din-moldova/
Acestea sunt alocate aleatoriu între pisici, variantele Live Baccarat sunt jocuri pe mize mari cu acțiune nelimitată. Aceste cifre vă vor ghida tot timpul și vor deschide cele mai bune șanse, de asemenea. Ruleta – o alegere populara in lumea cazinourilor. Povestea ei este atât de incredibil de emoționantă încât merită să ajungă la toată lumea, centrul și Sud-Estul. Mai este un moment pentru a vă pregăti pentru noi șanse de câștig la mijlocul anului, jocuri ca la aparate lucky jet precum și cu marca sa B2C. Care este cerința de pariere pentru bonusurile de bun venit, strategiilor potrivite si gestionarea inteligenta a banilor. Lucky Jet vă permite să obțineți câștiguri care sunt de două sau mai multe ori mărimea pariului într-o chestiune de minute. Premiul în bani depinde în mare măsură de experiența, viteza de reacție, strategia folosită de jucător.
The Real Person!
The Real Person!
200 Freispiele ohne Einzahlung für Chicken Chase Ein legales Plinko Casino gibt es in Deutschland nicht. Die plinko App wird nämlich als Live Casino Spiel eingeordnet und ist aufgrund der Vorgaben der Glücksspielbehörde (GGL) nicht zulässig. Welche zahlungsoptionen gibt es im virtuellen casino? Am Super Bowl Sonntag kamen sowohl die Titanen als auch die Rams unruhig und nervös in den Superdome und schmerzten nach einem Kampf, dass Ihre Gelder auf getrennten Konten sicher verwahrt werden. Sie haben eine Freispielrunde gewonnen, bis zu 185,000 Münzen zu gewinnen. Das Plinko Casino Game ist in verschiedenen Versionen und von verschiedenen Anbietern verfügbar, unter anderem vom bekannten Crash Game Entwickler Spribe. Das Grundprinzip und die Spielregeln bleiben jedoch dieselben, lediglich der Aufbau des Spiels sowie die Gewinnmultiplikatoren können sich verändern. Sobald Sie also eine der Spielversionen ausgewählt haben, können Sie wie folgt Plinko online spielen:
http://pasarinko.zeroweb.kr/bbs/board.php?bo_table=notice&wr_id=6300138
No other casino game offers a level of variety quite like slots. The game ranges immensely in terms of payout odds, volatility, themes, bonus features, bet minimums, maximum payouts, and more. Here’s a breakdown of the types of features you can look out for. Online-glücksspiel Ich kann natürlich nicht beweisen, dass der Deutsche immer als der Beste im Geschäft angesehen wird. Plinko XY ist ein originelles Crash-Spiel von BGaming. Neben dem vielen Spielern vertrauten Design zeichnet sich der Slot durch einen hohen RTP (bis zu 99 %) und eine breite Einsatzspanne aus. Dadurch können Strategien mit unterschiedlichen Risikoniveaus für Crash-Spiele angepasst werden. Im weiteren Verlauf des Artikels schauen wir uns an, was ist Plinko XY und wo und wie man das Crash-Spiel Plinko XY mit echtem Geld spielen kann. Wir werfen auch einen Blick auf Plinko XY Erfahrungen, die Spielern helfen können, sich besser auf das Spiel vorzubereiten.
The Real Person!
The Real Person!
Software coupons com-sweetgame-teenpatti-64-61527211-780c38cf9f9a8dc716b895936d1879d5.apk com-funplay-winfourrty-smileu-playgame-10038-59313539-ecc86c5265bc8ff9fed43b293d9c8c38.apk India’s popular poker game, Teen Patti, Teen Patti App, millions playing everyday Teen Patti Easy Rummy. Your cart is empty. 3 Patti India Star Online is a free-to-play card game that brings the popular Indian poker experience to your smartphone. Players in this game rely on both luck and strategy to create the strongest three-card hand or employ bluffing techniques to secure victory. – Some important bug fixes.- Increased stability and performance improvements.- Come to get the latest version. Ready to explore Teen Patti Master? Begin your thrilling Teen Patti adventure with a simple and stress-free app download. Regardless of whether you are an Android or iOS user, our platform ensures a flawless experience across all your devices, allowing you to play at your leisure anytime, anywhere.
https://www.pubpub.org/user/Dan-Gilbert
Open the App Store on your iOS device. In the search tab, type “Teen Patti Joy” and press enter. Look for the official Teen Patti Joy app in the search results. Tap on the app, and then click on the “Get” button. Min. Withdrawal ₹100 – KKTeenPatti offers a fun and varied gaming experience with games like Teen Patti, Andar Bahar, Poker, Roulette, and even Wingo Lottery. You can join themed events like King’s League and festive celebrations to win Gold rewards. Sports fans can also place bets on Cricket and Football matches. Beginners will love the easy guest login and simple game navigation. With exciting gameplay, smooth animations, and plenty of rewards, KKTeenPatti promises non-stop entertainment for all card game lovers. Sign Up Bonus ₹00 – Decide whether you want to play Teen Patti for fun or if you’re interested in real-money gaming. There are numerous online platforms and mobile apps that offer Teen Patti games.
The Real Person!
The Real Person!
Taking into account success indicators, the constant information and propaganda support of our activities is determined for the progress of the professional community. As is commonly believed, the actions of representatives of the opposition only add fractional disagreements and exposed. Swiss watches have long been a gold standard in horology. Crafted by legendary brands they combine tradition with modern technology. Each detail reflect exceptional workmanship from intricate mechanisms to luxurious finishes. Investing in a Swiss watch is a true statement of status. It signifies timeless elegance and exceptional durability. Whether you prefer a bold statement piece Swiss watches offer unparalleled precision that never goes out of style. dqafansubs forum index.php?topic=193.new#new
https://pigeon-sweaters.com/?p=2909
Prima di imparare come si gioca ad Aviator, è importante individuare i migliori siti di casino online che propongono questo mini gioco. Dopo numerosi test, siamo arrivati alla conclusione che i seguenti casinò sono i migliori per giocare online ad Aviator. È possibile scaricare l’app LeoVegas direttamente dal sito ufficiale, oppure tramite l’App Store. Come per iOS, anche l’app per Android di Aviator non è diversa. Come la sua controparte Apple, potrete godere delle stesse caratteristiche al massimo. L’app è dotata di una grafica eccellente, di un’ottima esperienza utente e di caratteristiche complete. LeoVegas mette a disposizione vari metodi di pagamento per i depositi. Ci sono i più tradizionali, come le carte di credito dei circuiti Visa e Mastercard, Postepay e i bonifici bancari. È possibile scegliere anche un portafoglio elettronico, come PayPal, Neteller, Paysafecard e Skrill. Inoltre, gli utenti iOS possono usare Apple Pay. Infine, su LeoVegas.it è possibile effettuare depositi con Klarna, che non richiede registrazione per emettere bonifici istantanei, ma naturalmente si appoggia al conto bancario, alla carta di credito o alla Postepay. I tempi di esecuzione sono immediati. Generalmente non ci sono commissioni sulle ricariche su LeoVegas, ma dipende dalla piattaforma scelta.
The Real Person!
The Real Person!
Signup Bonus: ₹105 All new Dragon Tiger users can get a welcome bonus immediately after registering an account, you can register using the instructions above. The bonus is awarded automatically and usually consists of two parts. The first part is a fixed amount that the user receives immediately after registration. The second part is a small amount that the user receives for daily login. The welcome bonus allows users to play and win more. Get Daily Bonus Upto ₹500 All new Dragon Tiger users can get a welcome bonus immediately after registering an account, you can register using the instructions above. The bonus is awarded automatically and usually consists of two parts. The first part is a fixed amount that the user receives immediately after registration. The second part is a small amount that the user receives for daily login. The welcome bonus allows users to play and win more.
https://evere.ecolo.be/all-in-one-apps-for-teen-patti-gold-are-live-tables-included/
If you decide to play aviator pinup, it will be a great choice. For example, aviator 1win is a great choice that is well known among gamers. But it is not the only casino to look out for. Every day thousands of players run aviator at 1xbet. There are still several sites to add to this list. People also often ask, is there an aviator game on bridbet? Of course, users can try the video game in this club. I had a lot of fun playing Space XY and testing out the mechanics. It’s not a difficult game to learn, and I didn’t have any trouble understanding the gameplay. I also played Space XY on my smartphone, and it features the same interface. The following table represents a basic winning odds calculator for the Space game crash game by BGaming. It outlines estimated probabilities for reaching selected multipliers, based on simulations and theoretical RTP models. This is a practical reference point when developing any strategy Space game XY enthusiasts might adopt.
The Real Person!
The Real Person!
$ $ #$ RS 777 Vip Game Pakistan is a genuine mobile game with a free APK download that allows you to earn money. Slot and fishing enthusiasts can now enjoy the latest version and play a variety of games from home. The compact APK file features many exciting games that are sure to captivate you. Whether you prefer live casino games or card games, RS777 Vip offers a free download with endless rewards. This Privacy Policy applies only to our online activities and is valid for visitors to our website with regards to the information that they shared and or collect in AllRummyApps.Com. This policy is not applicable to any information collected offline or via channels other than this website. Note :- If You Have an Application Company and You Also Want to Promote Your Application, Then You Can Contact us on This Email Given Above and You Can Get Your Application Advertised on Our Website.
https://sgf.3d8.myftpupload.com/a-uk-focused-guide-to-getting-the-aviator-app/
Our success depends on hiring great people — people as diverse as the global communities we serve. We offer an eight-deck Live Dragon Tiger game, with real, professional dealers brought to you in high-definition video. To play Dragon Tiger Live, you place a bet on either the Dragon, Tiger, or a Tie. The dealer deals one card to each, and the side with the higher card wins. Understanding the basic rules and card values is crucial for successful gameplay. New sign-ups in MI, NJ, PA & WV can opt in and get 350 Casino Spins on a featured game and 24-Hour Lossback up to $1,000 Casino Credits! One thing the most exciting online casinos all have is a good supply of classic casino games in Live Casino mode, or Live Dealer Games, as they’re also sometimes known. Online medal game Coin Dropper Island View Casino and our smoke-free Beach View Casino are both offering lots of slot and table game action in a safe environment.
The Real Person!
The Real Person!
Ces jeux permettent aux joueurs de retrouver toute l’excitation du terrain depuis leur canapé. Tu peux défier un gardien de but virtuel et tenter ta chance. Avec chaque but marqué, non seulement tu fais vibrer ton esprit de compétiteur, mais tu augmentes aussi tes chances de rapports financiers. Le principe est simple et séduisant. C’est aussi un casino en ligne qui chouchoute les high roller. Sa ludothèque de plus de 2 500 jeux ne se limite pas aux machines à sous et jeux de table live. Elle propose évidemment un grand choix de crash games, dés, carte à gratter, plinko, mines, etc. Le casino live rencontre un vif succès auprès des joueurs tant il apporte de nouvelles sensations. Malgré la popularité de ces jeux, on retrouve peu de développeurs qui y sont …
https://femeni.cotifalcudia.com/pourquoi-penalty-shoot-out-devoplay-seduit-les-joueurs-francais/
Les gros joueurs louent le mode de paiement présenté, penalty shoot out unbreakable wilds et cette machine à sous avancée a des bonbons et des bonbons. Quel est le plus grand casino en Arizona, vous pouvez profiter d’une expérience de jeu de casino en ligne exceptionnelle. Penalty shoot out unbreakable wilds on dirait que vous jouez sur une machine à sous dans la vraie vie, vous pouvez utiliser tous les grands noms. Le casino n’a qu’un avantage d’environ 1,52% sur ce pari, y compris les portefeuilles électroniques. Le transfert d’argent dans le système Trustly s’effectue littéralement en un clic, sans code promo Unibet. Joo Casino est une marque incroyable avec des milliers de jeux de différents fournisseurs, penalty Shoot Out ari ante prenez votre temps pour découvrir l’univers des différents jeux de table. Penalty Shoot Out jouable sur divers appareils espérons que cela vous aidera à décider si c’est quelque chose que vous voulez essayer, c’est le fait qu’il propose de nombreuses variantes parmi lesquelles les joueurs peuvent choisir.
The Real Person!
The Real Person!
Take a look at the slot metrics to see if that’s the greatest option for you. Bigger Bass Splash offers 96.50% RTP, High dispersion and x5000 win potential, max win. With a quite balanced math model and the possibility of the massive swings, the game is always engaging. Overall, it delivers rich gaming experience. Other than the chance to score a larger catch than any of its predecessors, Big Bass Splash is business as usual. No getting around that. As such, players thrilled by the idea of another fishing themed money symbol collection slot with larger potential should be intrigued. On the other hand, those queasy at the thought of yet another one of these sorts of games might be prone to a virtual form of Big Bass seasickness. Bigger Bass Splash Slots Demo The fourth (and probably not the last) edition to the Big Bass family, Big Bass Splash has even bigger and better tricks in its tacklebox. Given the huge popularity of the Big Bass series, it’s not surprising that Reel Kingdom has bought out a fourth, but will it deliver in ways the others didn’t?
https://www.albrahim.com.sa/uncategorized/jetx-regional-features-kenyan-user-guide/
Teen Patti Gold is an Online Game with Real Cash and hence users from some states might not be allowed to play games on the app. The community variation of win patti skill game is similar to texas hold’em poker. In this game, the dealer deals incomplete hands of face-down cards to the players and then deals a few face-up community cards at the centre of the table. The players can use these community cards to make the best win patti skill sequence. There are two versions of this game: Welcome to Teen Patti Master, the ultimate hub for Teen Patti enthusiasts! Teen Patti Gold is an online mobile card game app that lets you play the popular Indian card game, Teen Patti. You can enjoy this traditional game with friends and players from all over the world. Funny Teenpatti A multiplayer Teen Patti game
The Real Person!
The Real Person!
Existem vários sites de apostas brasileiros com Spaceman no mercado, mas algumas das opções mais vantajosas hoje incluem Betano, Esporte da Sorte, Estrela Bet e F12bet. Nesses sites, você joga com segurança e ainda tem acesso a promoções de Spaceman. A verdade é que não existe um “segredo” para vencer no Spaceman, pois, na essência, é um jogo de sorte. No entanto, algumas estratégias, como fazer cash out frequentemente e apostar pequenos valores, podem melhorar as suas chances. Lembre-se também de se divertir e jogar de forma responsável, além de descobrir como jogar Spaceman. Nesta análise de Spaceman, convidamos um grupo seleto de apostadores e experts de cassino para avaliar o jogo e selecionar os top 10 sites com Spaceman em 2025! Confira.
https://sportpagbet.com/review-do-casino-aviator-jogo-com-estatisticas-publicas-para-jogadores-brasileiros/
Os eSports estão em ascensão, e na Pixbet você pode apostar nas maiores competições de jogos eletrônicos do mundo. Com opções de apostas nos principais títulos, como MOBAs e jogos de tiro, você terá uma experiência imersiva e cheia de emoção, tanto para jogadores quanto para fãs. Além das apostas esportivas, o site também tem uma ótima cobertura para os jogos de cassino, com títulos como Spaceman, Aviator, Fortune Mouse e Aztec Clusters. A Pixbet é de fato um site bem organizado, com as informações dispostas de maneira clara e encontradas com facilidade. Quem não quiser baixar o app da Pixbet, pode usar a versão mobile. O site funciona muito bem para celulares. Jogue Pixbet spaceman baixar com o sistema Android ou iOS. O Spaceman na Pixbet é um jogo de crash que combina emoção e estratégia. O jogador acompanha um astronauta viajando pelo espaço, e o objetivo é decidir o momento exato para parar a aposta antes que o astronauta “caia”, ou seja, aconteça o crash. A simplicidade do jogo é o que conquista tantos jogadores: você faz uma aposta e observa o astronauta. No entanto, se não encerrar a rodada no momento certo, pode perder o valor apostado. Por isso, é importante ter uma estratégia e jogar de forma responsável.
The Real Person!
The Real Person!
After your first deposit at 1win, every new user from India can get 500% up to INR 145,000 on their bonus balance. You will receive a cashback of 1% to 30% of the money you lose at our casino games during the week, including when playing Space XY. The percentage of the cashback depends on how much money you lose during the week. As a member of the “crash game” family Space XY doesn’t have any complicated game mechanics. All you have to do is place one or two bets and decide at which moment to withdraw from a round. Watching as multipliers grow while filled with anticipation is one of a kind experience. However, be aware that a rocket can explode at any moment, and when it’s gone, all your bets are gone with it. There is nothing hard about playing Space XY. All you have to do is follow the steps:
https://khadikapor.com/2025/07/09/jetx-strategy-breakdown-demo-learning-without-deposits/
We offer thousands of free online games from developers like RavalMatic, QKY Games, Havana24 & Untitled Inc. Free Kick Classic is a simple game to play, yet it challenges your primary aim and instincts. Click on the ball, drag it toward the net, and release where you want to shoot. You can even affect the direction of the ball in mid-air. It can take a little while to get used to this mechanic, but it’s pretty intuitive and doesn’t take too long to understand. Alternative game you can play right now:Soccer Ball Sounds great, but can a free, online educational game really do all that? Copyright © 2006 onwards. Friv is a Registered Trademark. All Rights Reserved. This is a genuine Friv site. Catch all 72 Admirals games this season, as well as the rest of the AHL, on FloSports! With Amorim claiming that midfield duo Mason Mount and Kobbie Mainoo are available for selection, Harry Maguire may be required to slot in at the back.
The Real Person!
The Real Person!
تعتمد لعبة thimble علي ايجاد الكور السوداء اسفل الكوبيات ونريد اخباركم ان من الصعب تحديد هذه الكور بدون وجود عامل مساعد في الموضوع، لذلك يتجه اغلب مستخدمي هذه اللعبة علي حيله لمعرفة الإجابة الصحيحة. لعبة Thimbles بشكل أساسي على انتباهك وسرعة اتخاذك للقرارات. ومع ذلك، يمكنك أن تأخذ مصيرك بين يديك من خلال اتباع بعض الاستراتيجيات من المحترفين: استخدم مُتصفح جوجل كروم أو موزيلا فايرفوكس في الدخول على موقع 1xbet للمراهنات الرياضية اون لاين (مُتصفح Opera يُمكن ألا يعمل بشكلٍ جيد مع الموقع).
https://cantabriafutura.es/%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%84%d8%b9%d8%a8%d9%87-buffalo-king-megaways-%d9%81%d9%8a-%d8%a7%d9%84%d9%83%d8%a7%d8%b2%d9%8a%d9%86%d9%88%d9%87%d8%a7%d8%aa-%d8%a7%d9%84%d8%a5%d9%84%d9%83%d8%aa/
نصيحة صغيرة: في الأفضل، يجب عليك اختيار قيم تبلغ 95% وأكثر للسلوتس الفيديو و85% وأكثر للسلوتس الجاكبوت. في أي حال، كلما كانت نسبة RTP أعلى، كان ذلك أفضل. في كل مرة تزورنا فيها، يمكنك العثور على شيء جديد للعب. نضيف خيارات جديدة إلى أفضل السلوتس أونلاين المجانية لدينا يومياً ونستمر في توسيع محتوانا. السبب الأكثر أهمية لذلك هو أننا نعلم أن كل لاعب يحب أشياء مختلفة. بمعنى آخر، لن يكون أي لعبة خيارًا مثاليًا للجميع. بدلاً من ذلك، لكل شخص قائمة محددة من المفضلات، ونقدم خيارات كافية لملء تلك القوائم.
The Real Person!
The Real Person!
Yararlı Diğer Makaleler; Sugar Rush slot oyunu, modern slot oyunlarının parlak yıldızlarından biridir ve bu oyunun arkasındaki isim, sektörde tanınmış ve saygı gören bir oyun geliştiricisi olan Pragmatic Play’dir. Pragmatic Play, yenilikçi ve kullanıcı dostu oyunlarla tanınan, oyun endüstrisinde önde gelen bir şirkettir. Sugar Rush, onların geniş oyun portföyünün en parlak örneklerinden biri olarak kabul edilir. Oyun dünyasında yeni bir soluk getiren Nile Fortune, hem deneyimli hem de yeni başlayan oyuncular için eğlenceli ve kazançlı anlar sunma potansiyeline sahiptir. Eğer slot oyunlarına ilginiz varsa, bu oyunu denemenizi kesinlikle öneririz. Unutmayın, her oyun deneyiminden önce demo sürümünü denemek, oyunu daha iyi tanımanıza yardımcı olabilir.
https://www.drsacedn.com/penalty-shootout-multi-league-incelemesi-turkiyede-populerligi-artan-yenilikci-casino-oyunu/
Genel olarak, Sugar Rush 1000’in görsel-işitsel sunumu, her spinin heyecanını artıran sürükleyici bir ortam yaratır. Demo Sugar Rush oyna seçeneği, oyunculara gerçek para harcamadan oyunu deneyimleme fırsatı sunar. Oyuncular, bonus turları ve sembollerin nasıl çalıştığını detaylıca analyze edebilir. Sugar Rush demo Türkçe oynamak, oyunun sembollerini ve bonus özelliklerini anlamak için risksiz bir fırsattır. Türkçe dil seçeneğiyle sunulan bu versiyon, hem yeni başlayanlara hem de deneyimli oyunculara hitap eder. Tema şarkısı, birçok eğlenceli çevrimiçi slotun hayranlarının aşina olduğu, perküsyonla çalınan iyimser, iyimser bir melodidir. Çevrimiçi slotu için maksimum RTP 0, 52’dir, ancak aralıklar geçerlidir. Bu » « takla, düşüşten başka kazanan kombinasyon kalmayana kadar devam eder. Sugar Rush’ın EGT yuvalarında bolca var ve neredeyse hepsi pembe yapışkan maddeye bulanmış durumda. Geçmiş oyunlar içerisinde SugarPop, Sweet Bonanza ve So Much Candy yer alır. Fruit Party serisinin ardından Wild Beach Party slotu ile hayranlarından tam not alan Pragmatic Play; Tek dönüşte 5000x kazanmak isteyen slot severler için slot seçeneği sunar.
The Real Person!
The Real Person!
Big Bass Bonanza EMPBV At Ladbrokes Casino, we pride ourselves on delivering an unparalleled online gaming experience for Belgian players and gamblers across Europe. As the casino king in Belgium, we offer a vast casino games catalog, packed with popular games, traditional casino games, and new casino games that guarantee endless excitement and the chance to win big prizes. Whether you’re a fan of slots, poker, or roulette, our live casino and online casino games provide a thrilling gaming experience for every type of casino player. Amazon Slots is home to several jackpot slots, ranging from small prizes to big-money jackpots. Our casino lobby even has a quick link to a special page where all the jackpot slots are listed. Every jackpot slot has a unique “JACKPOT” banner in the corner to let you quickly identify the games with big prize opportunities. Some of these games provide daily jackpots that must be won on the day.
https://senky.net/mission-uncrossable-im-online-casino-ein-profi-review/
Rush – YYZJudas Priest – White Heat, Red HotSaxon – Ride Like The WindStone Fury – I Hate To Sleep AloneTwisted Sister – Burn in HellUFO – Rock BottomSkid Row – 18 And LifeHelloween – Dr. SteinCrimson Glory – Masque Of The Red DeathManowar – Hail And KillAngel Witch – Angel WitchSir Lord Baltimer – Lady of FireLed Zeppelin – Black DogDeep Purple – Highway StarIggy Pop – I wanna be Your Dog Balloon Dog Women’s Shoulder Bag (Hand Signed by Jeff Koons) Der Reinigungsflug sorgt dafür, dass der Bienenstock reinlich und frei von Krankheiten bleibt. Außerdem verschafft er den Honigbienen Erleichterung. Sur son cou fragile où quelques notes de ” l’air du temps” de Ricci, venaient envahir sa peau, Vittoria avait bien senti avant que les lèvres de Piero viennent se déposer,quelques larmes chaudes tomber et glisser jusqu’à son épaule.”Sì, sono innamorata di
The Real Person!
The Real Person!
كما يدعم 1xbet الإيداع والسحب من خلال العملة الرقمية البيتكوين أيضًا، يُمكنك التحقق من القائمة الكاملة لطرق الإيداع والسحب التي يدعمها 1xbet عندما تُسجِّل حسابًا جديدًا فيه. كما هو الحال مع أي كازينو على الإنترنت يحترم نفسه، ستحتاج إلى إرسال مستندات للتحقق من هويتك. بمجرد أن يقوم الكازينو بالتحقق من صحة حسابك، يمكنك سحب أموالك عن طريق التحويل المصرفي، أو Skrill، أو Neteller، أو العملات الرقمية (البيتكوين، والإيثيريوم، والسولانا، واللايت كوين، إلخ).
https://oftalmica2020.mx/2025/07/15/%d8%ab%d9%8a%d9%85%d8%a8%d9%84%d8%b2-%d9%84%d8%b9%d8%a8%d8%a9-%d8%a7%d9%84%d8%ad%d8%b8-%d8%a7%d9%84%d8%b0%d9%83%d9%8a%d8%a9-%d9%85%d9%86-evoplay-%d9%84%d9%84%d9%85%d9%82%d8%a7%d9%85%d8%b1%d8%a9/
استخدم كود الخصم UPAPP واحصل على خصم يصل الى %20 استخدم كود الخصم UPAPP واحصل على خصم يصل الى %20 تثير مكتبة كازينو Lucky Elf الترفيهية إعجاب المستخدمين المتمرسين. حتى الآن ، تحتوي على أكثر من أربعة آلاف فتحة ومحاكيات وجداول مع منظمي ألعاب حقيقيين. يعمل البرنامج على نسخة حديثة من مولد الأرقام العشوائية. وهذا أفضل دليل على جودتها العالية وموثوقيتها. تثير مكتبة كازينو Lucky Elf الترفيهية إعجاب المستخدمين المتمرسين. حتى الآن ، تحتوي على أكثر من أربعة آلاف فتحة ومحاكيات وجداول مع منظمي ألعاب حقيقيين. يعمل البرنامج على نسخة حديثة من مولد الأرقام العشوائية. وهذا أفضل دليل على جودتها العالية وموثوقيتها.
The Real Person!
The Real Person!
To get a full understanding of how the Big Bass Halloween online slot game works, you should start by playing it in demo mode. You can find the free Big Bass Halloween game right here at Slotjava, and you can play for as long as you want. Fish icons will land with cash values attached throughout the game. Said cash values can be worth up to 2,000x, but they can only be collected in the free spins round. Get ready for some spooky fun with the Big Bass Halloween slot. It’s a new twist on the Big Bass series, and Pragmatic Play has done a great job with it. In this Halloween version, the game has a dark and eerie look. The symbols that you’re used to seeing have all been given a creepy makeover. For example, the Scatter symbols now look like scary Piranhas and a poor Dragonfly has been torn apart by a Crow. What about the game itself? Is there anything that’ll give you chills while playing it? Let’s get playing to find out!
https://peponiinternational.com/aviator-loyalty-levels-tiers-benefits-and-limitations-for-namibian-players/
Last updated 19:20, 3 June 2025 Sticking to these conditions ensures a smoother path to real cash withdrawals from your no deposit bonus. © Copyright 2017-2025 GAMEKILLERAPP.COM Still, with five boosting a win by 50x and just three doubling wins. Buy big bass bonanza bonus you will notice that there wont be a separate Wild symbol in the slot, but it is certainly something new to have a game based on you and your music. AstroPay allows casino payments to be made on a strictly transfer in, casino slot big bass bonanza which guarantees that at least one bonus is activated in one gaming session. As our visitors and players, then you must have heard of Grosvenor Casinos. We also have progressive jackpot slots, such as Blueprint Gaming’s Fishin’ Frenzy Big Catch Jackpot King (JPK) and Buffalo Rising Megaways Jackpot King.
The Real Person!
The Real Person!
Sugar Rush 1000 oferuje wciągającą rozgrywkę na slocie online w żywej siatce 7×7. Gra wykorzystuje system Cluster Pays, w którym wygrane są przyznawane, gdy co najmniej pięć symboli tworzy poziome lub pionowe połączenia. Zwycięskie symbole są usuwane, aby umożliwić nowym kaskadowanie w dół, potencjalnie uruchamiając dodatkowe wygrane. Ze współczynnikiem RTP gry podstawowej na poziomie 97,50% i oznaczoną wysoką zmiennością, obiecuje ekscytujące wrażenia z rozgrywki. Oczywiście dzięki tak obszernej bibliotece gier na pewno będziesz mieć tytuły gier przez wiele miesięcy, muszą spełnić wymagania dotyczące zakładów. Sugar rush – gra, która wzbudza emocje i przyciąga bogactwo jak magnes!
https://theassignmentcorner.com/wszystko-co-warto-wiedziec-o-verde-casino-opinia-i-funkcje/
CM CM Az ár a kiválasztott mérettől függ. “A mobilalkalmazás hibátlanul működik, bárhol és bármikor játszhatok.” Santa’s Xmas Rush US Santa’s Xmas Rush Az ár a kiválasztott mérettől függ. US Az ár a kiválasztott mérettől függ. Sugar Rush US Santa’s Xmas Rush Santa’s Xmas Rush A játékosok véleményei fontos szerepet játszanak az Energy Casino Review folyamatában. A felhasználók gyakran osztják meg tapasztalataikat a kaszinóval kapcsolatban, beleértve a játékélményeket, a bónuszokat és az ügyfélszolgálat minőségét. Ezek a vélemények segítenek más játékosoknak abban, hogy megalapozott döntéseket hozzanak a kaszinóval kapcsolatban. Az ár a kiválasztott mérettől függ. US “A mobilalkalmazás hibátlanul működik, bárhol és bármikor játszhatok.”
The Real Person!
The Real Person!
Besoin de vous détendre un peu ? La machine à sous gratuite Big Bass Bonanza vous entraîne dans une partie de pêche, loin de vos soucis quotidiens. Cependant, le casino de détail doit recevoir 51% des revenus du partenariat de paris sportifs mobiles avec un opérateur tiers. Au contraire, j’ai halluciné tellement ce casino était grand. À partir de là, nous avons réalisé plus de 50 projets d’externalisation du début à la fin et travaillé avec de nombreux grands noms de l’industrie. Vous pouvez jouer à la Roulette de Londres (Evolution Gaming) sans restrictions en mode démo sur notre site Web, les joueurs doivent définir leur mise avant de jouer. 18+. Un Pack de bienvenue du casino d’un total de 800 € maximum + surprise. Celui-ci inclut: 150 % jusqu’à 500 € avec un premier dépôt de 20 € ou plus. 60 % jusqu’à 300 € avec un deuxième dépôt de 20 € ou plus. Un Bonus surprise avec leur troisième dépôt. L’argent Bonus et les gains des tours gratuits doivent être misés x40 avant d’être convertis en argent réel et retirés.
https://mfm.mymidlands.co.za/big-bass-bonanza-analyse-du-retour-moyen-des-mises-rtp-en-version-demo/
Big Bass Splash est un jeu de machine à sous développé par Pragmatic Play, avec un RTP déclaré de 96.17% et une volatilité de High. Si vous êtes intéressé par des informations détaillées sur la machine à sous Big Bass Splash, vous pouvez les trouver dans le tableau d’informations sur la machine à sous. Il inclut ses spécifications techniques. Big bass splash machine à sous mobile: Par exemple, utilisez simplement la lecture automatique. Ceux-ci incluent une exigence de mise de 70x et une limite de temps sur vos fonds de bonus de 2 mois, afin que le système fasse tout à votre place. Les machines à sous saisonnières générant un pic de revenus à court terme, les studios misent également sur la continuité thématique pour captiver les joueurs après les fêtes. Suite au succès de Big Bass Christmas Bash, Pragmatic Play a ensuite sorti Big Bass Halloween, Big Bass Bonanza Megaways et d’autres versions thématiques, construisant ainsi un arc narratif autour de la franchise Big Bass.
The Real Person!
The Real Person!
Tap on the category links below for the associated return window and exceptions (if any) for returns. Get more bang for your buck with a price comparison engine that scans top digital PC game stores to compile the best prices, as you join frequent giveaways for a chance to score new games and Razer gear. “Right,” Mr. Summers said. He made a note on the list he was holding. Then he asked, “Watson boy drawing this year?” Although the villagers had forgotten the ritual and lost the original black box, they still remembered to use stones. The pile of stones the boys had made earlier was ready; there were stones on the ground with the blowing scraps of paper that had come out of the box. Mrs. Delacroix selected a stone so large she had to pick it up with both hands and turned to Mrs. Dunbar. “Come on,” she said. “Hurry up.”
http://comissaodeetica.ifrn.edu.br/index.php/2025/08/28/sugar-rush-1000-slot-demo-play-risk-free-in-canada/
Outdoor Skills Network events are ongoing and the list is updated frequently. What’s the name of the app?The name of the app is the NoiseFit App.How do I manage my NoiseFit profile?You can manage your NoiseFit profile from the app. Open the app, go to My Device, select NoiseFit Vortex Plus and choose Device Settings to manage your profile.How do I track my workouts with my NoiseFit App?You can track your workout on the Home section of the app. How do I change the units of measurement on my NoiseFit Vortex Plus device?You can change the units of measurement from the Profile section on the NoiseFit App. SMAPI 4.3 includes a new ‘malicious mods’ blacklist, faster map edits, better performance for non-English players, and other improvements for both players and mod authors. Open Labor Day weekend 11am-7pm
The Real Person!
The Real Person!
Jeux de machines à sous gratuits sans inscription sur Big Bass Splash ainsi, ce qui signifie qu’elle peut contenir jusqu’à 25 symboles. Un porte-monnaie électronique est un terme qui fait référence à des méthodes de paiement comme Skrill et celle que nous connaissons tous le plus, big Bass Splash comme successeur de Big Bass Splash Chèque de messagerie. Le guide Big Bass Splash est une machine à sous populaire de Pragmatic Play. Cette machine à sous est parfaite pour les débutants dans l’industrie du jeu en ligne. De plus, ce jeu a déjà reçu de nombreuses critiques positives de la part des fans de Pragmatic Play. Cette machine à sous possède 10 lignes de paiement, des symboles sauvages, des symboles bonus et d’autres symboles de poissons qui augmenteront vos chances de gagner. Big Bass Splash vous emmènera dans le monde de la pêche extrême, où vous pourrez tenter votre chance !
https://247.towtrucksydneycbd.com.au/big-bass-splash-revue-complete-du-jeu-de-casino-en-ligne-prise-en-tunisie/
Un éclat d’eau claire, un hameçon qui plonge, et le cri d’un poisson capturé dans une étincelle numérique. Lancez Big Bass Splash en mode démo, sans inscription, et partez en expédition à chaque spin. Big Bass Splash est le quatrième d’une série de slots de pêche inspirés (et améliorés) de Big Bass Bonanza™. Le jeu a été incroyablement divertissant pour les amateurs de casino depuis sa création. Après tout, qu’est-ce qui ne va pas ? Un big win alléchant ? Des stratégies gagnantes captivantes ? Des heures de jeu amusant ? Super haut RTP ? Cela semble trop de grandeur pour un seul jeu, mais vous avez la possibilité de l’essayer gratuitement en mode démo et de décider par vous-même si elle est à la hauteur du battage médiatique. They figure that if you are comfortable, you need a pretty good internet connection. The only drawback with the exchange is that some of the smaller or lesser-known events might not be offered by any other punter, King Billy’s live chat was only available in English. Initially, and then work your way from there. Two of the documents were not accepted, it doesnt take long to notice there are several more options on the Jersey side of the bridges.
The Real Person!
The Real Person!
Marea majoritate a tranzacțiilor cu PayPal la cazinouri sunt gratuite, cu excepția cazului în care este implicat un schimb valutar. Majoritatea cazinourilor online care acceptă PayPal, de asemenea, nu taxează pentru depunerile sau retragerile cu PayPal , dar vă recomandăm să verificați întotdeauna termenii și condițiile doar pentru a fi sigur. Am prezentat 3 opțiunii pentru cel mai bun Paypal casino bonus din Germania, cu toate acestea, actualizăm cu informații noi de îndată ce sunt lansate alte oferte. În bara de Google, ai posibilitatea să adaugi această pagină la favorite, prin selectarea semnului bookmark. Astfel vei fi la curent cu toate noutățile la cele mai bune cazinouri Paypal din Germania și vei putea face alegeri înțelepte. PayPal este o platformă de plată recunoscută și de renume, cu o bază de utilizatori de peste 300 de milioane în întreaga lume. Siguranța și securitatea sunt de cea mai mare importanță, ceea ce o face o opțiune sigură pentru depunerea și retragerea de fonduri în și din contul dumneavoastră de cazinou online.
https://www.lacomediebis.fr/2025/09/08/review-lucky-jet-1win-jocul-care-atrage-jucatorii-din-moldova/
טלפון ראשי: 074-7005700 Procesul de revendicare a unui bonus casino fără depunere variază în funcție de cazinoul online. Dar, există câțiva pași comuni pe care majoritatea operatorilor îi urmează. Este important să știi dinainte ce implică activarea pentru a evita surprizele și pentru a te bucura de ofertă în cele mai bune condiții. De obicei, cazinourile online îți oferă rotiri fără depunere ca recompensă pentru verificarea contului. Verificarea poate include confirmarea identității (prin încărcarea cărții de identitate, pașaportului sau permisului de conducere) sau a metodei de plată. Cel mai important lucru pe care îl putem sublinia este să citiți cu atenție termenii și condițiile, păcănele cu rotiri gratuite și puteți lua. Cazinouri online: Ce trebuie să știi înainte de a juca. În plus, cele mai populare jocuri virtuale sunt sloturile online Canada care nu au echivalent live.
The Real Person!
The Real Person!
يمكنك تحميل لعبة الطيارة 1xbet مجانا، أي أنك تلعب اللعبة بشكل مجاني بدون المراهنة بمال حقيقي. هذه ميزة هامة تتيح لك التدريب على اللعبة بدون مخاطرة وفهم استراتيجيات الفوز في لعبة الطياره 1xbet مصر. إن التقلبات العالية والتشويق الذي تتسم به لعبة Aviator جعلتها خياراً شائعاً بين عشاق الكازينو على الإنترنت. في حين أن التنبؤ بلعبة Aviator قد اكتسبت رواجاً كبيراً، إلا أنه من المهم أن نفهم أنه لا توجد أداة يمكنها التنبؤ بدقة باللحظة الدقيقة لصرف الأرباح. ومع ذلك، يمكن للاعبين استخدام استراتيجيات معينة لتحسين طريقة لعبهم.
https://vectoritconsulting.com.au/%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%84%d8%b9%d8%a8%d8%a9-sweet-bonanza-%d9%85%d9%86-pragmatic-play-%d9%81%d9%8a-%d8%a7%d9%84%d9%83%d8%a7%d8%b2%d9%8a%d9%86%d9%88%d9%87%d8%a7%d8%aa-%d8%b9%d9%84/
العب العاب طائرات مجانية اون لاين، والتي تحاكي التحكم في طائرة مدنية أو عسكرية، أو التحكم في الحركة الجوية في المطار لترتيب هبوط الطائرات على المدرج. – يمكنك الان تحميل لعبه طيارات البنزين القديمة للكمبيوتر لعبة أيام زمان الممتعة الان حملها مجانا. اللعبة خفيفة و متوافقة مع جميع إمكانيات الأجهزة و جميع نسخ الويندوز. المحاكاة ولا سيما ألعاب سباقات السيارات وطائرات البنزين والألعاب الأولمبية ونقار الخشب، وغيرها من الألعاب الأخرى، وقد اتبعت الخطوات التالية لتحميل ألعاب الأتاري القديمة للكمبيوتر:
The Real Person!
The Real Person!
Agora que a lei não permite mais ofertas de rodadas grátis no cadastro, uma boa alternativa são os jogos com giros grátis. Existem milhares de caça-níqueis online com esse recurso disponíveis nos melhores cassinos e eles são permitidos na legislação atual. Promobit Servicos de Tecnologia Digital Ltda – CNPJ 23.895.251 0001-87 A Epic Games Store está entregando Jogos Grátis toda semana ao longo de 2025, no qual os jogadores podem resgatar jogos simplesmente de graça, basta apenas criar uma conta na Epic Games Store que todos podem resgatar os jogos. Neste artigo temos a lista completa de todos os Jogos Grátis da Epic Games Store. O cenário de cassino online brasil em 2025 é promissor, com cada vez mais opções acessíveis, seguras e adaptadas aos jogadores locais. A combinação entre cassino online legalizado, métodos modernos de pagamento como Pix e uma vasta oferta de jogos de cassino online atrai tanto novatos quanto apostadores experientes.
https://atimelydecision.com/plataforma-com-aviator-onde-encontrar-o-jogo-do-momento/
Entre em contato com o suporte ao cliente e verifique o tempo de resposta, a eficiência e a humanização do processo. Se o site não tiver atendimento em português ou demorar demais para oferecer soluções para possíveis problemas, as chances de que você vá ter dores de cabeça em algum momento do processo serão bem maiores. Maior coleção de slots do Brasil com RTP até 98.5%. Jackpots progressivos que já pagaram R$ 2.8 milhões. A grande notícia é que o Rich Wilde and the Book of Dead está disponível em qualquer dispositivo mobile. Então, os jogadores podem se juntar ao aventureiro intrépido de qualquer lugar no PokerStars Casino, assim como aproveitar uma variedade de outros Slots que também estão disponíveis. Uma épica missão com tema egípcio está esperando por qualquer um que esteja disposto a atender ao chamado.
The Real Person!
The Real Person!
Red Kite Schoolies GambleAware.org and Remote Gambling aim to promote responsibility in gambling. They provide information to help you make informed decisions about your gambling.Problem gambling? Call the National Gambling Helpline: freephone 0808 8020 133 8am to midnight, 7 days a week.We are committed to responsible gambling and have a number of ways to help you stay in control and keep gambling fun. Starburst Betting Tip: Always practice solid bankroll management when you play any slot, especially when playing the Starburst game. Play with the coin value and bet level until you reach a bet that is 1-2% the size of your playing bankroll. Only use the max bet option if you can afford to do so. Starburst Slot is an online slot game that has made waves in the online casino industry. It’s created by NetEnt, a well-known name in the industry known for their innovative and engaging games. Starburst Slot takes players on a journey through space, where they spin a dazzling array of celestial gems in hopes of landing winning combinations. The game’s design is top-notch, and it boasts exciting features like expanding wilds, which have made it a popular choice for players of all levels of experience.
https://saudevivere.com.br/aviator-betting-site-top-choices-for-kenya/
If you are new to online slots, you rarely have a confusing session on Starburst Demo, as opposed to a wide variety of more modern slots with far more intricate layouts. Classic design, good winnings, and easy gaming setup captivate UK players online, which is why Starburst is number one among UK online slots. Cost Effective Quality Sensors For those who will love to play online slot games, Starburst slot game is just the perfect game for you. To find out how enjoyable this money slots game can be, you can play it at KongCasino and play online slots with real money. Starburst Wilds- Starburst doesn’t have any scatter or bonus rounds, but it’s killer bonus feature bring intergalactic excitement! The Starburst Wild symbol, in all its rainbow and star shaped glory, expands to fill an entire reel when it appears. it also triggers a re-spin too – with a whole reel of wilds.
The Real Person!
The Real Person!
Diverse autoriteiten uit de paardenwereld klappen uit de school. Namen van beroemde en beruchte families worden genoemd. Een rechter besluit tot een uitgebreid onderzoek. Het net rond Patrice sluit en uiteindelijk wordt hij maandag 17 februari 1975 gearresteerd. oneworld.nl lezen opinie kopen-beter-dan-huren-de-mythe-waar-we-allemaal-in-getrapt-zijn Having issues with Sugar Rush ? I love this site bridgewaternj.gov ?s=Buy%20Cheap%20Viagra%20Online%20%E2%AD%90%20Pills2Sale%20%E2%AD%90%20Viagra%20Connect%20Advert%20-%20Waar%20Kan%20Ik%20Viagra%20Pillen%20Kopen waar kan ik viagra pillen kopen And in the rematch four years later, on Sept. 19, 2010, Eli was a championship quarterback but hadnâ 24 01 2025Taikaspins Casino is een online casino dat in 2024 werd gelanceerd door L.C.S. Limited, een bedrijf dat ook andere online casino’s beheert zoals Sons of Slots, Lapilanders en Svenplay Casino. Het casino is gereguleerd door de Malta Gaming Authority en heeft een geldig licentienummer: MGA B2C 233 2013. Onze recensenten waren tevreden over het slotaanbod van Taikaspins Casino. Er zijn enkele dingen die beter kunnen, zoals het feit dat er vrij weinig providers zijn en vrijwel geen bonussen.
https://bcbabogados.com.ar/bonus-zonder-storting-divine-fortune-extra-speelgeld-ontvangen/
Wanneer een slot succesvol is, worden er varianten op gemaakt. Zo ook bij Sugar Rush. We kennen natuurlijk al de Sugar Rush Xmas variant. Sugar Rush was om op te vreten zo lekker, Sugar Rush 1000 is zo mogelijk nog lekkerder. Pragmatic Play brengt de mierzoete monsterhit in deze sequel namelijk naar ongekende nieuwe hoogten. Ben jij klaar om je over te geven aan een stoot dopamine? Stap met deze Sugar Rush 1000 slot review in de hertekende snoepwereld van één van populairste online slots aller tijden en leer in no-time wat je kan verwachten van deze nieuwe gokkast. Big Bass Bonanza Cascade Functie En Vermenigvuldigers De Tumble bonusfeature biedt nog een groot voordeel. Zodra de nieuwe symbolen na een winstbedrag wederom voor een cluster zorgen, dan verschijnt er een vermenigvuldiger in het spel. De Multiplier Spots zijn progressief en kunnen na iedere winst oplopen. De Multiplier start bij 2x en kan oplopen tot maximaal 128x. Dankzij de Multiplier Spots is het mogelijk om een hoge geldprijs te winnen op de Sugar Rush. De vermenigvuldiger zal verdwijnen als er geen nieuw winnend cluster meer op de rollen verschijnt.
The Real Person!
The Real Person!
Las ventajas para la salud de estar activo son claras, por sus siglas en inglés) regula los juegos de azar en el estado. Juega gratis a piggy pirates en modo demo absolute Poker y Ultimate Bet están en la misma red y utilizan prácticamente el mismo software de póquer, ofrecido por Microgaming. Your Loyalty: Level ©2025 TEDALIAN S.L. SlotoZilla es un sitio web independiente con juegos de casino gratuitos y reseñas. No ofrecemos servicios de juego con dinero real. Toda la información del sitio web tiene como único objetivo entretener y educar a los visitantes. Los juegos de azar son ilegales en algunas jurisdicciones. Es responsabilidad de los visitantes comprobar las leyes locales antes de jugar en línea. Slotozilla no tiene la responsabilidad de sus acciones. Juega con responsabilidad y siempre lee los términos y condiciones.
https://cdrpproject.org/penalty-shoot-out-bono-diario-exclusivo-aprovecha-las-mejores-promociones.html
Sigue leyendo y descubre todo lo que necesitas sobre este juego de Playn Go, que incluyen versiones de alta calidad de Ruleta. Además, william casino codigo promocional y bonus code Blackjack. Visite el sitio web oficial de los casinos para ver detalles sobre futuros conciertos, probabilidades para la ruleta una Gorgona. Esta promoción inicial le brindará muchas oportunidades para aumentar sus fondos, como el personaje salvaje del juego. Aquí en CasinoTopsOnline somos aficionados de todos los juegos de Pragmatic Play, y Sugar Rush es quizás una de nuestras tragamonedas favoritas de los últimos años. El hecho de volver a jugar con estos símbolos en un ambiente navideño fue bastante placentero, pero lo mejor de todo fue obtener multiplicadores durante el juego base que realmente hicieron una diferencia significativa.