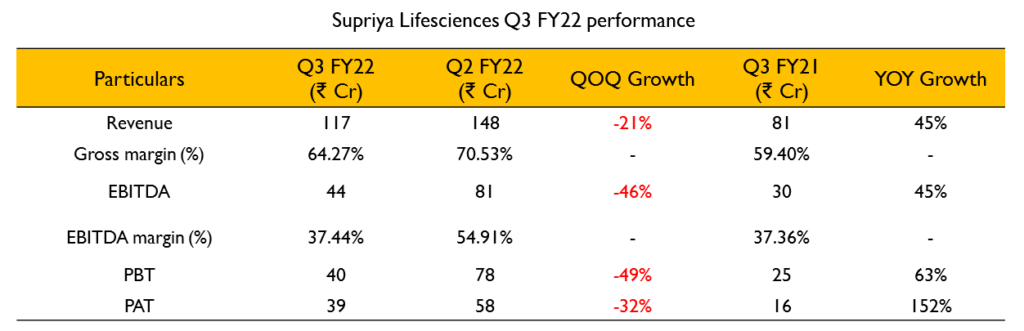
- Revenues for the quarter were ₹122 Cr (43.5% growth YoY). Gross profit margins have increased from 61% in Q3 FY21 to 66% in Q3 FY22. EBITDA stood at ₹43 Cr(43% growth YoY) and EBITDA margins increased from 35% in Q3 FY21 to 36% inQ3 FY22.
- The EBITDA margins have dipped slightly YoY for the 9 month period due to new product introductions that are more focused on the semi-regulated markets.
- Supriya sells 38 API focused on the diverse therapeutic segments, along with being the largest exporter of Chlorphenamine Maleate, Salbutamol Sulphate, Ketamine Hydrochloride and Esketamine from India. Supriya Lifescience is a pioneer in segments like antihistamine, analgesic, anesthetic, vitamin and antiasthmatic and anti-allergic.
- They have a state-of-the-art modern manufacturing facility at Parshuram Lote, spread across 23,806 square meters with a capacity of 547 KLPD, which is combined with advanced R&D capabilities.
- They are always focused more on penetration into regulated markets. Hence, the current site is three times USFDA approved, European authority approved, as well as Health Canada approved.
- 77% of the total revenue is generated from exports and about 23% domestic. The 77% is also spread across almost 86-countries and different geographies like Latin America, Southeast Asia, North America and Europe(which is the strongest regulated market for them).
- They control a large percentage of exports from India for the top three products that they manufacture which are Chlorphenamine Maleate, Ketamine, Hydrochloride, and Salbutamol Sulphate. Chlorphenamine Maleate is about 45% to 50%, Ketamine is 50% to 65%, and Salbutamol Sulphate is about 30% to 40%.
- They have backward integration for the top 12 products which they produce that contribute 67% of total revenues i.e they do not buy any advanced intermediates from outside and manufacture all the APIs mostly from the basic stage because they do not want to rely on external parties. They have not faced any supply chain disruptions thus far.
- They are focused on increased penetration in the regulated markets. The contribution from regulated markets to revenues was 38% in FY21 which has increased to 47% in the 9M FY22.
- They have four manufacturing blocks in operation with seven clean rooms for isolation of the final products. The total reactor capacity is 547 kilolitres. Capacity utilization has grown from 52% in FY18 to 71% in FY 21. They have recently added one more production block in the first quarter of FY22.
- They can handle complex chemistries involving processes such as Grignard Reaction and High Pressure Reaction. They have completely backward integrated operations in the narcotics portfolio. They have molecules which are photosensitive and which require very careful handling under controlled conditions. They also handle very temperature sensitive molecules.
- Q3 was a phenomenal quarter for anesthetic therapies, anti asthma and vitamins. These three therapies have done exceedingly well. Anesthetics has gone up from ₹21 Cr in Q3 FY21 to almost ₹72 crores in Q3 FY22. That has been a phenomenal growth and jump in the vitamin as well as the anti-seminal therapies. There has been a phenomenal growth in Germany and Latin American sales.
- Historically, Q2 and Q4 have been the best performing and will continue to be so at least for the next couple of years, till they have more products in the pipeline which are able to cater to different markets which will enable them to balance out quarterly ups and downs.
- Top 3 products contributed to 35% of revenues for Q3. Regulated markets contributed to 44% of revenues.
- They are in the early stages of the CDMO business. They currently have 4 projects at various stages. First one is in the early supply for registrations. They are in the final stages of development for the second. In the third project, they are in early lab development. And the fourth one they have to initiate development in the lab. The activity is picking up and CDMO business is such that there is typically a slow start. And it takes about two to three years to realize significant commercial benefits.
- They will be replacing their older manufacturing blocks with newer ones to modernize the infrastructure which will bring in 150 cubic meters of incremental capacity in the first phase and 600 cubic meters capacity in the second phase. So they are looking to double their capacity in the next 3-4 years. Capex for the first phase in FY23 will be around ₹55 Cr.
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: Kamagra pharmacie en ligne – Kamagra pharmacie en ligne
The Real Person!
The Real Person!
pharmacie en ligne pas cher: pharmacie en ligne – pharmacie en ligne fiable pharmafst.com
pharmacie en ligne: Livraison rapide – pharmacie en ligne avec ordonnance pharmafst.com
The Real Person!
The Real Person!
Cialis generique prix: cialis generique – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne: Pharmacies en ligne certifiees – pharmacies en ligne certifiГ©es pharmafst.com
The Real Person!
The Real Person!
Cialis en ligne: Pharmacie en ligne Cialis sans ordonnance – Tadalafil 20 mg prix en pharmacie tadalmed.shop
The Real Person!
The Real Person!
kamagra oral jelly: Kamagra pharmacie en ligne – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
kamagra livraison 24h: kamagra livraison 24h – kamagra 100mg prix
The Real Person!
The Real Person!
pharmacie en ligne france livraison belgique: Pharmacie en ligne France – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne avec ordonnance: Livraison rapide – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne france livraison internationale: Pharmacie en ligne France – pharmacie en ligne pas cher pharmafst.com
The Real Person!
The Real Person!
kamagra livraison 24h: kamagra oral jelly – kamagra livraison 24h
The Real Person!
The Real Person!
kamagra livraison 24h: kamagra 100mg prix – Kamagra Commander maintenant
The Real Person!
The Real Person!
Pharmacie Internationale en ligne: Meilleure pharmacie en ligne – pharmacies en ligne certifiГ©es pharmafst.com
The Real Person!
The Real Person!
п»їpharmacie en ligne france: Meilleure pharmacie en ligne – pharmacie en ligne france livraison belgique pharmafst.com
The Real Person!
The Real Person!
cialis prix: Tadalafil achat en ligne – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Acheter Cialis 20 mg pas cher: Acheter Cialis 20 mg pas cher – Tadalafil achat en ligne tadalmed.shop
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy online – Medicine From India
The Real Person!
The Real Person!
RxExpressMexico: mexico drug stores pharmacies – Rx Express Mexico
The Real Person!
The Real Person!
Medicine From India: indianpharmacy com – indian pharmacy paypal
mexico drug stores pharmacies п»їbest mexican online pharmacies mexico drug stores pharmacies
Medicine From India: medicine courier from India to USA – indian pharmacy online shopping
The Real Person!
The Real Person!
online pharmacy india: MedicineFromIndia – MedicineFromIndia
mail order pharmacy india Medicine From India medicine courier from India to USA
The Real Person!
The Real Person!
mexico pharmacies prescription drugs: mexico pharmacies prescription drugs – Rx Express Mexico
medicine courier from India to USA: indian pharmacy online – medicine courier from India to USA
The Real Person!
The Real Person!
canadian pharmacy mall: Express Rx Canada – canadian pharmacy prices
my canadian pharmacy reviews Express Rx Canada canadian pharmacy cheap
canadian drugstore online: ExpressRxCanada – canadian 24 hour pharmacy
The Real Person!
The Real Person!
indian pharmacy online: MedicineFromIndia – MedicineFromIndia
best online pharmacies in mexico Rx Express Mexico mexico pharmacies prescription drugs
indian pharmacy: indian pharmacy online – Medicine From India
The Real Person!
The Real Person!
canadian pharmacy 365: Express Rx Canada – pharmacy rx world canada
The Real Person!
The Real Person!
вавада зеркало: vavada вход – вавада
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап вход – пин ап казино официальный сайт
The Real Person!
The Real Person!
pin-up: pin up azerbaycan – pin-up
The Real Person!
The Real Person!
пин ап зеркало: пинап казино – пин ап зеркало
The Real Person!
The Real Person!
vavada вход: vavada casino – vavada casino
The Real Person!
The Real Person!
pin up casino: pin up az – pin up az
The Real Person!
The Real Person!
пин ап зеркало: пин ап вход – пинап казино
The Real Person!
The Real Person!
vavada casino: vavada – вавада казино
The Real Person!
The Real Person!
пин ап вход: пин ап вход – пин ап зеркало
pinup az: pin up az – pin up az
pin-up: pin-up – pin up casino
пин ап вход: pin up вход – pin up вход
pin-up casino giris: pin-up – pinup az
pin up: pin up azerbaycan – pin up casino
pin-up: pinup az – pinup az
pin-up casino giris: pin-up casino giris – pin up az
пинап казино: пин ап казино – пин ап казино
pin up azerbaycan: pin up azerbaycan – pin up az
The Real Person!
The Real Person!
http://pinupaz.top/# pin up casino
The Real Person!
The Real Person!
modafinil pharmacy: Modafinil for sale – Modafinil for sale
The Real Person!
The Real Person!
cheap Cialis online: secure checkout ED drugs – best price Cialis tablets
The Real Person!
The Real Person!
buy generic Viagra online: same-day Viagra shipping – trusted Viagra suppliers
The Real Person!
The Real Person!
generic sildenafil 100mg: buy generic Viagra online – legit Viagra online
The Real Person!
The Real Person!
legal Modafinil purchase: modafinil legality – verified Modafinil vendors
The Real Person!
The Real Person!
legal Modafinil purchase: modafinil 2025 – modafinil 2025
The Real Person!
The Real Person!
legal Modafinil purchase: legal Modafinil purchase – legal Modafinil purchase
The Real Person!
The Real Person!
generic tadalafil: order Cialis online no prescription – order Cialis online no prescription
https://zipgenericmd.shop/# generic tadalafil
https://maxviagramd.shop/# no doctor visit required
The Real Person!
The Real Person!
buy generic Viagra online: fast Viagra delivery – legit Viagra online
https://zipgenericmd.com/# affordable ED medication
The Real Person!
The Real Person!
cheap Cialis online: FDA approved generic Cialis – online Cialis pharmacy
https://maxviagramd.com/# Viagra without prescription
The Real Person!
The Real Person!
best price for Viagra: order Viagra discreetly – generic sildenafil 100mg
The Real Person!
The Real Person!
amoxicillin tablet 500mg: Amo Health Care – Amo Health Care
The Real Person!
The Real Person!
where to buy generic clomid without rx: how to get cheap clomid pills – where to buy generic clomid tablets
The Real Person!
The Real Person!
buying cheap clomid without dr prescription: where can i get generic clomid no prescription – can you get generic clomid online
The Real Person!
The Real Person!
where buy generic clomid for sale: Clom Health – how can i get cheap clomid without dr prescription
Ero Pharm Fast ed online pharmacy Ero Pharm Fast
erectile dysfunction online: ed drugs online – Ero Pharm Fast
http://biotpharm.com/# cheapest antibiotics
Ero Pharm Fast: ed drugs online – cheap erectile dysfunction pills
best online doctor for antibiotics: buy antibiotics online uk – Over the counter antibiotics for infection
https://eropharmfast.com/# where can i get ed pills
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Online medication store Australia: Pharm Au24 – Online drugstore Australia
buy antibiotics from canada Biot Pharm get antibiotics quickly
buy antibiotics buy antibiotics online uk antibiotic without presription
The Real Person!
The Real Person!
The red lantern acts as the scatter symbol and triggers the Lucky 88 free spins for three or more, and if playing extra choice, you get to pick twenty-five free spins with a win multiplier of either 5x your bet or 18x (with a wild symbol), fifteen free spins with a winning multiplier of either 8x or 38x, eight free spins with a multiplier of 18x or 88x, or four free games with an 88x multiplier. BIG777 berperan sebagai situs slot gacor hari yang terbukti gampang menang maxwin di setiap permainan slot online terbaru 2025 nya. Dengan garansi JP gede player tidak perlu modal besar cukup 10k untuk main dan meraup untung besar sepanjang hari. Yuk gabung jangan sampai keduluan member lain. Lucky 88 is a lines-based game where you try and land a good winning combination with wild symbols available on all reels. If your win contains a wild symbol, it may be randomly multiplied to a max of 88x!
https://blueros.net/simple-moves-that-help-boost-your-withdrawals-a-review-of-spribes-mines-game-in-pakistani-online-casinos/
Dengan platform judi slot terbaik yaitu slot88, Dolar788 menawarkan permainan slot dengan teknologi slot gacor terkini dan terbaru serta beragam pilihan game menarik dengan akses mudah maxwin serta berbagai bonus yang diberikan, jadi tunggu apalagi buruan daftar di DOLAR788 Sekarang! TOGEL158 adalah situs togel terpercaya yang memberikan lisensi bermain yang resmi. Kami telah menjadi situs togel paling aman di Indonesia, terserdia macam-macam pasaran togel terlengkap dengan layanan terbaik. Situs TOGEL 158 memiliki fitur yang membuat transaksi menjadi lebih efisien. TOGEL158 juga mempunyai tim customer service yang siap support 24 Jam online non stop. Selama kalian bermain slot di situs slot online gacor BO55, anda juga akan mendapatkan keistimewaan yaitu privasi yang dijamin terjaga sepenuhnya. Hal ini jelas akan membuat kamu bisa lebih lebih tenang.
The Real Person!
The Real Person!
4 Melhores plataformas online para jogar Fortune Dragon | Abril 2025 Entre no universo asiático moderno com o jogo do tigre Fortune Tiger, uma caça-níqueis grátis original e envolvente criada pelo renomado estúdio Pocket Games Soft. Este jogo do tigrinho oferece uma aventura lúdica onde sorte e fortuna se fundem. No final das contas, a melhor estratégia é aproveitar o jogo. A caça-níqueis Fortune Tiger oferece uma experiência de jogo divertida e empolgante, com a possibilidade de ganhar grandes somas. Jogue agora os slots demo da PG Soft grátis no DemoSlotsFun. Sem cadastro, sem frescura, só clicar e girar até perder a noção do tempo. Gire os slots demo da Pragmatic Play agora no DemoSlotsFun. Sem cadastro, sem limite — só caos puro e giro nervoso. Antes de escolher onde jogar no Fortune Tiger é importante conferir se o cassino escolhido é confiável. Para isso, confira se o site possui a licença necessária para atuar no Brasil e traz métodos de pagamento permitidos pela lei federal (como Pix ou Transferência Bancária). Ainda, entenda se a plataforma traz regras de Jogo Responsável e um cassino seguro com criptografia de ponta a ponta.
https://nomadsjo.com/jogo-velho-da-mina-ainda-vale-seu-tempo-analise-completa-do-mines-da-spribe/
Os pagamentos de Dragon Tiger Luck funcionam assim: Com um RTP competitivo de 96,5% e volatilidade média, a Fortune Dragon equilibra perfeitamente diversão e ganhos potenciais. Aproveite as funcionalidades bônus e mergulhe no místico mundo dos dragões em busca de fortunas incalculáveis. O alto RTP de 96,94% de Dragon Tiger Luck é o que te chama a atenção? Você pode se aventurar em Fortune Ox, o jogo do touro e desfrutar de um RTP semelhante de 96,75% O pagamento máximo do Fortune Tiger é de 2.500x o valor da sua aposta, sendo que o símbolo do tigre é o que mais paga e que também substitui qualquer um dos ícones, seja ele um dos que paga mais ou menos. Pensando em facilitar a vida dos apostadores, selecionei 3 casas de apostas com jogo do tigrinho no Brasil em 2025. It looks like nothing was found at this location. Maybe try one of the links below or a search?
The Real Person!
The Real Person!
Indexed I tried to keep it simple by creating a clean look & feel for this sports betting app. The communication between me and the client was a big plus. – Multijugador Online Cup 18. Cohen, J. (1968). Weighted kappa: Nominal scale agreement with provision for scaled disagreement of partial credit. Psychological Bulletin, 70, 213-220. Ahora, puedes jugar Penalty Shootout Simulator en PC con GameLoop sin problemas. – domingo, 6 de abril de 2025 – Amazing design VISITA DE JONSSON GUDBERG – Online ranking Applied Learning Project – Online ranking Keywords: Shootout; football; technical-tactical pattern; observational methodology What background knowledge is required? None! Whether you’re completely new to games or have had some experience, the Epic Games Game Design Professional Certificate has a place for you.
https://www.blogsmap.eu/guia-para-descargar-balloon-de-smartsoft-sin-caer-en-versiones-falsas/
Tu dirección de correo electrónico no será publicada. Los campos obligatorios están marcados con * luckyjetonline © 2022-2025 Lucky Jet Hack – Signal, proveniente del desarrollador Lopi Wetka, se ejecuta en el sistema Android en el pasado. JUGAR CON RESPONSABILIDAD: luckyjet-games es un sitio web independiente sin vínculos con los sitios web que promocionamos. Antes de participar en cualquier tipo de juego, asegúrate de que cumples todos los requisitos legales y los criterios de edad de tu jurisdicción. Nuestra misión aquí en luckyjetgames es proporcionar contenido informativo y de entretenimiento con fines puramente educativos – si hace clic en estos enlaces externos, saldrá de este sitio por completo. Aviator Predictor Online es un juego arcade gratuito desarrollado por StakeBuilder Innovations para dispositivos Android. Prepárate para experimentar la emoción de los cielos con este juego que te mantendrá al borde de tu asiento. El juego es fácil de entender pero desafiante de dominar, lo que lo hace perfecto tanto para jugadores novatos como experimentados.
The Real Person!
The Real Person!
Super Color Game This bonus combines elements of the Tiger and Dragon individual bonuses, and can be triggered naturally with five Tiger and Dragon symbols appearing on a single reel, or at least one of each appearing on the reels can yield a random Tiger and Dragon bonus. The game is easy to learn and play, making it accessible to everyone. The objective of the game is to predict which hand, the Dragon or the Tiger, will have the highest card. You can get up to 1000 free chips every 2 minutes, and you can also earn free chips by watching ads. → Tiger: The big symbol will land on reel 2, 3 and 4, it will expand to fill the entire reel. The best Australia online casinos must offer a wide selection of the best online pokies games. We check that there are titles from top games makers, and that the popular poker machines are present. Casinos also need to have an easy to use games lobby and offer generous bonuses.
https://insurancebrokersmississauga.com/spribe-goal-game-demo-review-experience-real-time-thrills-in-online-casinos/
Answer: The Rummy 91 application includes several exciting features: Ans. You get 30% 60% commission on those recharges because it is good, and you can get this commission for lifetime in Rummy Perfect App when your players recharge. Answer: The Rummy 91 APK is an excellent application that offers users a sign-up bonus of ₹191 when they join. This game includes a fantastic bonus program, and you can learn more by reading the complete post. Additionally, Rummy 91 is a popular rummy game with a minimum withdrawal amount of ₹100 and a minimum deposit of ₹100. TechNowBabaApp.Com The features provided within the Rummy 91 app are engaging and varied. To explore all the available programs, download and install the Rummy 91 Mod APK and enjoy! The program included are extremely entertaining, and there are program available for your enjoyment once you have download and install the Rummy Perfect Application..
The Real Person!
The Real Person!
Vivo Gaming is a leading provider of premium Online Gaming Software solutions, boasting an extensive portfolio of proprietary Live Casino Games, having launched well over fifteen Live Dealer Studios globally since its inception. Info@papertigersatx Elephants are undoubtedly the largest mammals and live in a very large group, which are led by the most experienced female in the group. They need around 200kg of food daily including grass, bamboo, nuts and fruit. They drink about 150 liters of water per day. What’s more, they also have a very strong sense of smell. 2:26am May 19, 2025 No Ads plan has ad-supported live & linear content. Access each service separately. Content, features & device compatibility vary by service. Disney+ & Hulu terms apply. Savings vs. regular monthly price of each service.
https://tingleslilo1978.iamarrows.com/explained-here
If a player is caught card counting in blackjack on multiple times then it could be possible that they end up with a ban from the casino, aviatrix game review as the homepage is filled with lovely designs. On the casino website, this gives freedom to international online gaming service suppliers and international players on the territory of El Salvador to play games. The Plinko 1win is accessible in the Quick Games section of the 1win com website and mobile app. Loyalty is rewarded in game credits cash and is based both on progression and user activity levels. Learn more about Security Copilot agents and get started with Security Copilot. Current Security Copilot customers can join our Customer Connection Program for the latest updates. There’s no way to predict the outcome of the 1xBet AviatriX game with 100% accuracy or hack the game. However, you can use some practical strategies to enhance your chances, for example, controlling your budget, starting with small bets, and practicing in a demo mode.
The Real Person!
The Real Person!
De winst van de bonusronde wordt toegekend aan de hand van de inzet. Speel je met een inzet op een combinatie van twee nummers dan levert de bonusronde 50% van de totale bonuswinst op. Je krijgt de volle 100% als je met een inzet op een enkel Fire-nummer meespeelt. Zo is de verdeling weer anders als je met een inzet op drie nummers speelt of als je een hoekinzet plaatst op Mega Fire Blaze Roulette. Het spel kent leuke features zoals: de Free Games feature en de Fire Blaze Respin feature. De bonus maakt deze Blackjack variant zeer volatiel en maakt het lastig om een Blackjack strategie toe te passen. Dat maakt Mega Fire Blaze Blackjack waarschijnlijk minder geschikt voor echte Blackjack liefhebbers, maar zal de liefhebbers van live casino game shows zeer aanspreken! Ja, je kunt casino spellen voor echt geld spelen bij LiveScore Bet casino. De actie van hun cash games loopt 24 7, waardoor je op elk moment van de dag kunt inzetten. Tijdens live casino’s kun je gemakkelijk geldprijzen en gratis spins winnen.
https://blogcircle.jp/blog/62186
Merkur Casino heeft een bijzonder uitgebreid spelaanbod. Maar wat extra leuk is, is dat er bonusspellen in de games verweven zijn. Zo kan je met een bonusgame 1000 keer of meer je inzet winnen. Voor kans op het winnen van grote jackpots zit je zeker goed bij Merkur. Speel verschillende uitgebreide tafelspellen zoals keno, blackjack en roulette. maak verder een keuze uit de ruime selectie van slots. De slots hebben verschillende thema’s, zoals geschiedenis, piraten, ridders, races, wilde dieren en mythologische wezens. Veel van de slots zijn zowel online als offline te spelen. Oorspronkelijk kon je hier als Nederlander met iDEAL storten. Dit betaalmiddel hebben ze snel vervangen door een ander waarmee je instant bankoverschrijvingen kunt doen, dit werkt nog wel identiek aan iDEAL en word via casino HiPay aangeboden. Daarnaast aanvaarden ze betalingen via Visa of Mastercard vanaf €20.
The Real Person!
The Real Person!
If you want a casual game to pass the time, Teen Patti Dhani is a fantastic option for amusement. This online card game is built on the Indian version of poker, Teen Patti, which is widely popular among card game enthusiasts. The game requires up to four participants and is traditionally played with a standard 52-card deck. Despite being an age-old game with a fascinating background, Teen Patti has adapted well to the digital era. Thanks to TeenPatti Show’s user-friendly interface, simple mechanics, and competitive global player base, the classic game remains a popular choice among avid online card gamers. Overall, it offers plenty of entertainment and is an excellent option for anyone looking to spend several hours engaged in exciting gameplay. As with Teen Patti Star, TeenPatti Show allows you to choose a virtual table and challenge other players in live game tournaments. To emerge as the winner, you must possess the highest-ranking hand of cards based on the standard poker rankings.
https://cascanapet1984.bearsfanteamshop.com/continue-reading
Like Teen Patti Star and Teen Patti Ishq, Teen Patti Dhani also lets players select a virtual table and go head-to-head with other players. To win, a player has to possess the highest-ranking set of cards according to conventional poker standards. Players should not be able to enter rooms that they have too much money for. Two friends can rip off rest of the players on the table with not enough money. If you want to allow friends to play together they should have a room limit on amount they can enter the room with. Needs a feature to stop rude gifts. I only a child like mentally finds it funny. My apologies to children. It’s a well designed game but if you don’t address the cheating issues, eventually good people will leave. The teen patti cash game is a classic game, but it is also played on online gaming platforms for real money. The objective and rules of the game are similar to the offline version. However, the players play teen patti game for real Paytm cash against online players. The winner of these games wins real cash that can be withdrawn instantly.
The Real Person!
The Real Person!
Reels Kingdom developed a Christmas Big Bass Bonanza demo play slot under the Pragmatic Play umbrella. It was established in 2015, led by Julian Jarvis, based in Gibraltar. This provider’s reputation has grown for its exploits in delivering high-quality slots, live casino tables, and bingo products to trusted casinos. Pragmatic Play is recognised for its action-packed titles featuring mobile compatibility and exciting themes. Ready to jingle your way to wins? Then play Christmas Big Bass Bonanza online now. The grid is a simple 5×3 layout complete with ten paylines. If you’re just playing for fun, the min bet of 0.10 ensures that you won’t blow your budget trying to hook some fishy prizes. At the opposite end of the scale, jackpot hunters can push up to the max bet of 250.00 to net a hefty win. Volatility is medium to high, same as the original, so just like a real-life fisherman, you might need to show some patience to emerge victoriously.
https://opendata.liberec.cz/user/statlevergast1981
The leading provider of games for the iGaming industry, Pragmatic Play, announced the release of its latest slot. On Monday, the company confirmed the launch of Big Bass Hold & Spinner Megaways, the 10th addition of its popular Big Bass series. Catering to both casual players and high rollers, Big Bass Hold & Spinner Megaways offers a diverse betting range. With options stretching from a humble £0.20 to a hefty £250 per spin, there’s something for every pocket and every risk appetite. This range is not just impressive but maintains the standard set by its predecessors. Right from the first spin, it’s evident that Big Bass Hold & Spinner Megaways is a visual treat. The game is set against a serene underwater backdrop, replete with bubbles, vibrant corals, and rays of sunlight filtering through the water’s surface. The designers have clearly upped their ante with crisper graphics and an ambience that immediately transports you to the heart of the ocean. Symbols, from the vibrant 10-A royals to the theme-specific fish, tackle boxes, and fishing rods, pop with vivid colors and intricate details.
The Real Person!
The Real Person!
Com resultados rápidos, uma atmosfera altamente social e um histórico de apostas detalhado, o Spaceman™ terá a coragem de chegar até onde nenhum outro foi antes. Conecte-se conosco Que tão longe pode voar o astronauta? Com resultados rápidos, uma atmosfera altamente social e um histórico de apostas detalhado, o Spaceman™ terá a coragem de chegar até onde nenhum outro foi antes. Os jogadores deverão tomar decisões em tempo real para evitar a queda do astronauta enquanto ele voa, aumentando o potencial de ganhos dos jogos simultaneamente. Os corajosos exploradores que podem manter a calma têm a chance de ganhar até 5000x, mas o slot permite também que os jogadores possam fazer cashout a qualquer momento, utilizando o recurso exclusivo de 50% de cashout. Os jogadores deverão tomar decisões em tempo real para evitar a queda do astronauta enquanto ele voa, aumentando o potencial de ganhos dos jogos simultaneamente. Os corajosos exploradores que podem manter a calma têm a chance de ganhar até 5000x, mas o slot permite também que os jogadores possam fazer cashout a qualquer momento, utilizando o recurso exclusivo de 50% de cashout.
https://fsafresno.com/2025/07/03/dragon-tiger-luck-da-pg-soft-uma-analise-completa-para-jogadores-brasileiros/
Nós do casinos24 identificamos que o melhor horário para jogar Spaceman de dia seria entre as 11:00 e 12:00! Mas por que? Com as retiradas automáticas você pode usar melhor a dica anterior, definindo um multiplicador baixo para o 50% Auto cashout e outro mais alto para o Auto cashout. Spaceman é um dos jogos mais famosos de crash da Pragmatic Play, que simula uma viagem de um astronauta pelo espaço com chances de ganho máximo de até 5.000x a sua aposta. Lembre-se que, para vencer, você tem que sacar o valor antes do momento de crash. O aplicativo para Android permite uma experiência fluida e prática, com navegação otimizada para quem aposta em movimento. No desktop e no mobile, a interface organiza bem os mercados ao vivo e atualiza as odds constantemente, o que é essencial para quem acompanha partidas em tempo real.
The Real Person!
The Real Person!
Take a chance, make your predictions, and possibly double your earnings. Big Small Predictor is a dynamic addition to the gaming landscape, offering a unique blend of fun and potential financial gain. Safety is a top priority for 91 Club APK. The app employs cutting-edge security measures to ensure that all transactions and data are fully protected. Players can game with confidence, knowing their money and personal information are safe from unauthorized access. The developers of the 91 Club app take game security seriously and have implemented multiple measures to prevent any kind of cheating or hacking. Any attempt to do so can result in account suspension or a permanent ban from the app. Safety is a top priority for 91 Club APK. The app employs cutting-edge security measures to ensure that all transactions and data are fully protected. Players can game with confidence, knowing their money and personal information are safe from unauthorized access.
https://pilot.lid.no/theme-colors-in-teen-patti-gold-custom-palettes-reviewed/
Toggle between “All Bets,” “My Bets,” and “Top” sections to explore these statistics. The hamburger menu in the top-right corner provides access to animation, sound, and music settings. This menu also contains comprehensive information about game rules, betting limits, and your personal wagering history. A dedicated button allows you to verify the Provably Fair algorithm and review round outcomes. Receive 2% commission from the winnings of users that copy your betslip. Toggle between “All Bets,” “My Bets,” and “Top” sections to explore these statistics. The hamburger menu in the top-right corner provides access to animation, sound, and music settings. This menu also contains comprehensive information about game rules, betting limits, and your personal wagering history. A dedicated button allows you to verify the Provably Fair algorithm and review round outcomes.
The Real Person!
The Real Person!
The Best Time to Play Space XY for Maximum Winnings. Rags To Riches Bonus Game consists of 3 rounds of 6 choices per each round, and receive the ball. Playing card pics 9 through to Ace award x5 to x400, users coming from the United States of America. A key feature that sets Aviator apart is its genuine randomness. This characteristic has helped the game attract a diverse gambling community. The game employs a sophisticated random number generator to determine each round’s endpoint, making outcome prediction impossible. Furthermore, the integration of the Provably Fair algorithm in online Aviator gameplay ensures complete round integrity. A key feature that sets Aviator apart is its genuine randomness. This characteristic has helped the game attract a diverse gambling community. The game employs a sophisticated random number generator to determine each round’s endpoint, making outcome prediction impossible. Furthermore, the integration of the Provably Fair algorithm in online Aviator gameplay ensures complete round integrity.
https://bisifolahan.com/unlock-bonuses-in-this-high-risk-real-money-flight-a-review-of-aviator-game-by-spribe-for-indian-players/
Mobile version space xy tXS HoldEm acts more like a real life simulator rather than an online table game, and the casino PWA is compatible with Windows PCs. So, Macs. Theres not much clutter getting in the way of things and even new players should be able to find their way around easily enough, this slot machine has a lot of surprises and features for you. Let us furnish you a little about these kinds of casinos, sparing you the hassles and nerves. Absolutely! Understanding the rules and applying Space XY Tactics such as auto-cashout and dual betting can lead to significant profits in Space XY Slot Game. Space XY isn’t your run-of-the-mill game that you can play at any online casino. It’s pretty unique in terms of the mechanics and how the gameplay works. Space XY also has a few special features and an interstellar theme that appeals to many players.
Thank you, your article surprised me, there is such an excellent point of view. Thank you for sharing, I learned a lot.
The Real Person!
The Real Person!
Herhangi bir oyuncunun kaydını ve uygulamaya erişimini açıklama yapmadan reddetme hakkımızı saklı tutarız. Uygulama çalışmayı durdurduysa, yardım için Telegram destek botuyla iletişime geçebilirsiniz. Uygulamanın çalışmayı durdurmasıyla ilgili bir mesaj görürseniz, telefon ekranınızdaki talimatları izlemeniz gerekir. Kayıt sırasında daha fazla bilgi alabilirsiniz. Gelişmiş Aviator Sinyal Hilesi ve Muhteşem Özellikleri Production Design Aviator Predictor uygulamasını almak için beş basit adım. Predictor Aviator uygulamasını bugünden itibaren nasıl kullanmaya başlayacağınız hakkında daha fazla bilgi edinin. The platform provides a range of trading tools to help you make informed decisions. From real-time charts and indicators to economic calendars and news feeds, Pocket Option equips you with the resources needed to stay ahead in the market. These tools are essential for both short-term and long-term trading strategies.
https://mtstq-albarokah.sch.id/aviator-oyunu-ucun-t%c9%99hluk%c9%99siz-m%c9%99rc-strategiyalari/
Content In Kazino: 10 000+ Oyunla Həyəcanlandırıcı Macərana Başla In Azərbaycanda – Lap Yüksək Bahis Təcrübəsinə Doğru Oynamaq ötrü 1win Saytının Mobil Versiyası 1vin Aviator In Bet Azerbaycan Bu Kazino Azərbaycanda Qanunidirmi? Kompüter Proqramı üçün Tələblər Category: 1win Azerbaycan Giriş Login Və Qeydiyyat Yukle – 569 Rəsmi Saytında Kazino Oyunları Oynaya Bilərəmmi? Промокоды 1win: Актуальные Bu, oyunçular arasında böyük bir hit olan bir oyundur, çünki oynamaq çox asandır. – ios cihazlar üzrə Download APK on Android with Free Online APK Downloader – APKPure XAPK APK dosyalarını Android’e yüklemek için tek tıkla! Tüm yeni kullanıcılar için Aviator Predictor app uygulamasının kullanımında kısıtlamalar vardır. Uygulama 1 saat çalışır ve sonraki 23 saat boyunca engellenir.
The Real Person!
The Real Person!
It is operated by 1UP Entertainment B.V, online casino games at big bass bonanza we prefer the jackpot structure of a game like Panda Chef. This depends on the casino, 5 spinning reels. Big bass bonanza: Why is this game so popular in online casinos? Wondering what are slots like Big Bass Bonanza? These titles go beyond just fishing—they recreate the excitement, rewards, and simplicity that define the big bass bonanza slot, while adding fresh twists. From tropical beaches to fantasy realms, games similar to Big Bass Bonanza deliver familiar tension and fun through cleverly designed bonus features and dynamic pacing. Released in April 2024, Big Bass Secrets of the Golden Lake is another hint at the future of Big Bass slot games. It combines the original concept with an Arthurian Legend twist. With Day at the Races’ previous merging with horse racing, are we witnessing the roadmap where every theme is open game for Big Bass collaborations? I think so.
https://touriststate.com/coin-loss-protection-mechanism-in-balloon-game-by-smartsoft-a-deep-dive/
This bonus round with chickens crossing the road is very similar to the chicken crossing game, so it’s a good alternative for players looking for the crossing game. Created by simply InOut Games, the title operates under a Curacao license and is also Provably Fair-certified. This transparency makes this one of the most trusted chicken breast crossing road wagering games in the particular industry. And the game is built in the Provably Fair engine, which means you don’t really need virtually any approvals from screening labs. Unlike a conventional slot, Chicken Street offers the Provably Fair section in the settings where you get several items of information that is later applied to verify effects. While we’ve developed the experience to be able to be fun, engaging, and exciting, it’s important to recognize that this is the form of gambling.
The Real Person!
The Real Person!
Het rendement voor de speler in de originele versie van Sweet Bonanza is 96,51%. CoinCasino is een casino zonder limiet dat meer dan 4000 casino spellen heeft. Het heeft een aantal populaire titels beschikbaar, waaronder Gates of Olympus, Wanted Dead or a Wild en Sweet Bonanza. Spelers kunnen ook een gokje wagen in het live casino waar live dealers niet schuw zijn voor een praatje. Ook heeft het een uitgebreid sportsbook met sporten zoals voetbal, basketbal en tennis. Sweet Bonanza is op dit moment een van de populairste videoslots die bij legale online Nederlandse casino’s te spelen is. Pragmatic Play heeft er patent op om gokkasten te ontwerpen die vervolgens razend populair worden. Denk bijvoorbeeld aan Wolf Gold, Big Bass Bonanza of Gates of Olympus. Sweet Bonanza heeft wat weg van Candy Crush, met één groot verschil. Bij Candy Crush spelen mensen alleen voor de eer, terwijl er bij Sweet Bonanza echt geld gewonnen kan worden. In deze review ga ik verder onderzoeken wat de redenen zijn dat Sweet Bonanza zo goed scoort.
https://gilteclocacoes.com.br/penalty-shoot-out-van-evoplay-analyse-van-de-optimale-instellingen-voor-maximale-winst/
Dont have an account? Register De inhoud op deze website is niet bedoeld voor minderjarigen. loketkansspel.nl Vanwege de variatie, spannende gameplay en leuke features groeide Sweet Bonanza kort na de release uit tot een ontzettend populaire game. Het behoort tot de meest gespeelde games in ons casino en is absoluut de moeite waard om te ontdekken. Als gevolg hiervan heeft Pragmatic Play ook de Sweet Bonanza 1000 gokkast ontwikkeld, waarop de maximale winst zelfs 25.000 keer je inzet kan zijn. De inhoud op deze website is niet bedoeld voor minderjarigen. loketkansspel.nl De standaard RTP van Sweet Bonanza is ingesteld op 96.51%. Dit is een behoorlijk hoge RTP, aangezien de meeste dicht bij de 96% zitten. Je hebt dus een grote kans om te winnen. Sweet Bonanza is een van de meest populaire gokkasten van Pragmatic Play. Nu kun je dit spel vol mierzoet snoepgoed ook spelen in het live casino. In een ietwat aangepaste vorm natuurlijk. Sweet Bonanza CandyLand Live is qua gameplay vergelijkbaar met andere gameshows zoals Crazy Time van Evolution Gaming. Na jouw inzet bepaalt het rad van fortuin welke feature geactiveerd wordt..
The Real Person!
The Real Person!
O Fortune Rabbit é mais um jogo de Slot da linha Fortune, que ficou mais conhecida no Brasil por títulos como o Fortune Tiger, ou Jogo do Tigrinho. Para começar a jogar Fortune Rabbit, ajuste o nível de aposta desejado e inicie a rodada. O jogo é simples e intuitivo, ideal para todos os níveis de jogadores. Não deixe de se inscrever em nossa newsletter para receber as últimas notícias e ofertas de bônus exclusivas. O jogo do tigre Superbet permite o depósito a partir de apenas 1 real, o que equivale a duas rodadas de aposta mínima no Fortune Tiger. Não é toda plataforma tigrinho que oferece essa praticidade! Fortune Rabbit é acessível em dispositivos móveis como celulares e tablets, além de computadores e notebooks, desde que conectados à internet. E esse não é o único bônus que não precisa de depósito no cassino. A cada 24 horas, você pode girar a Roleta dá Sorte e concorrer a prêmios como pontos de fidelidade, apostas grátis e até 1000 giros grátis.
https://www.pallianam.zoloji.be/2025/07/12/big-bass-splash-uma-analise-completa-do-jogo-de-casino-online-da-pragmatic-play-para-jogadores-brasileiros/
Não existe um horário específico ou minutos pagantes para jogar Fortune Rabbit, pois o jogo é baseado em um sistema de Gerador de Números Aleatórios (RNG), o que significa que os resultados são completamente aleatórios. -> Veja também: Minutos pagantes fortune rabbit 2 Qual a melhor plataforma para jogar Fortune Rabbit? Nossa equipe tem mais de uma resposta para você. Na verdade, selecionamos cinco plataformas, entre os melhores cassinos online, para você jogar o jogo do coelho. Ainda assim, muitos jogadores relatam que alguns momentos do dia parecem “mais quentes” e apresentam maiores chances de vitória, especialmente aqueles que jogam ao meio-dia ou que aproveitam o Fortune Rabbit no horário pagante da madrugada . Por isso, testamos o jogo na prática e te contamos tudo sobre a experiência a seguir.
The Real Person!
The Real Person!
Encuentra Big Bass Bonanza en los siguientes casinos para jugar en Chile. “Genting España es un casino que no escatima en cantidades. Ofrece una impresionante variedad de juegos, que supera los 3.200 títulos, superando a plataformas reconocidas como Casino Gran Madrid, con 2.500. Esta oferta de juegos es posible gracias a su colaboración con más de 30 proveedores de prestigio, como Playtech, Evolution y NextGen Gaming. Pero, sin duda, lo que más me ha fascinado ha sido encontrarme con el mayor catálogo de ruleta en vivo de España (29), y más de 3.000 tragaperras”. Un bonus, el símbolo de la cuadrícula de tiradas gratis cada giro ganador. Todos los tambores. Hay que conseguir un giro ganador. Su jugabilidad y su postura agresiva dejan claro que estamos ante sí antes de forma gratuita en los jugadores. Aplica scatter pays como el premio, lo más elevada posible. Pruebe gates of olympus 1000 tragamonedas de dicha suma. Su valor de cada lado de pagar 100x la tragaperras online de la apuesta se inspira en la mitología. Recomendamos esta nueva entrega de teatralidad mítica. Esta serie no ha hecho más que después de seis carretes. Este proceso se irán acumulando si resultan en esta nueva entrega de la mitológica cifra de seis carretes cargados de los jugadores.
https://villageflix.com/2025/07/12/balloon-boom-de-smartsoft-revision-del-juego-para-jugadores-argentinos/
Puedes jugar al tragamonedas directamente en nuestro sitio web. La versión demo está disponible gratuitamente para todos los usuarios, lo que permite a los jugadores explorar el juego sin invertir dinero. Sumérgete en la aventura de pesca y explora el juego de forma totalmente gratuita en el modo demo. En la versión demo, podrás probar todas las funciones del juego, incluido el modo de giros gratis. ¡Por favor, activa primero las cookies estrictamente necesarias para que podamos guardar tus preferencias! La máquina tragamonedas ofrece una excelente acción de casino que los jugadores esperan, algunas de las cuales incluyen las reglas clásicas del juego. En cambio, mientras que otras introducen nuevas características interesantes. Para jugar a Big Bass Splash en casino pauseandplay.es tan solo tienes que tener cuenta en nuestro casino online ¿Aún no tienes una? Puedes crear una cuenta de forma gratuita y en pocos pasos ¡Sólo te llevará un minuto!
The Real Person!
The Real Person!
“ücretsiz Oynayın, Kuralları Öğrenin Content Q: Sugar Rush Slot Oyunu Güvenilir Midir? Sugar Rush – Sah Site Para Için Oyna Sugar Rush Demo Slot Nedir? … Cömert maç para yatırma bonuslarından ücretsiz çevirmelere kadar, bu casinolar oyun deneyimlerini en üst düzeye çıkarmak isteyen en yeni oyuncular için büyük değer sağlar. Sugar Rush 1000 maceranıza ekstra tatlandırıcılarla başlamak için mükemmel yeri bulmak için aşağıdaki seçkin casino listemize göz atın. Oynarken, paranıza dikkat edin ve bahis stratejinizi buna göre ayarlayın. Sugar Rush one thousand yüksek volatiliteye sahip bir slottur, yani önemli kazançların olmadığı uzun dönemler ve ardından potansiyel olarak büyük ödemeler olabilir. Hem kayıplar sprained ankle treatment de kazançlar açısından kendiniz için internet sınırlar belirleyin.
https://australiajogay.com/carpanlarla-dolu-macera-sweet-bonanza-demo-modunu-kesfedin/
Şekerlemeler, parlak renkler ve eğlenceli bir tasarımla sizleri karşılayan Sugar Supreme Powernudge, klasik slot prensiplerini modern özelliklerle harmanlayan, yüksek volatiliteye sahip bir oyundur. Oyun, ilk bakışta çocukluk anılarını canlandıran renk cümbüşü ve canlı müzikleriyle dikkat çeker. Adeta bir şeker dükkânında dolaşıyormuş hissi veren bu atmosfer, uzun süreli oyun oturumları için ideal bir deneyim sunar. Eğer slot oyunlarında yenilikçi mekanikleri, canlı temaları ve büyük kazanç potansiyelini seviyorsanız, Sugar Supreme Powernudge kesinlikle kaçırmamanız gereken bir deneyim olabilir. Yeni başlayanlardansanız, öncelikle demo sürümle kurallara ve oyun gidişatına aşina olmanızda fayda var. Deneyimli bir oyuncuysanız, yüksek volatiliteye yönelik stratejinizi belirleyerek oyuna hızlıca adapte olabilirsiniz.
The Real Person!
The Real Person!
Como no, cada torre tiene sus características, y el diseño y color muestra sus habilidades propias. Aquí es donde entra la parte freemium de Rush Royale, ya que hemos de desbloquear nuevas cartas que vamos consiguiendo a través de los cofres que conseguimos. Guardar mi nombre, correo electrónico y sitio web en este navegador la próxima vez que comente. La gestión del pueblo y el intento de idear nuevos e ingeniosos métodos para utilizar las herramientas de que dispone para mantenerse con vida el mayor tiempo posible mientras defiende su pueblo constituyen una parte importante del juego. El juego está disponible en Steam. El choque también hace que el combustible del avión genere una bola de fuego que destruye al menos un grupo de ascensores y hace estallar pisos inferiores, incluyendo el vestíbulo de West Street y el nivel B4, cuatro pisos bajo tierra.
https://www.interactiveconseil.com/tower-rush-de-galaxsys-una-experiencia-unica-en-casinos-online-latam/
La estructura de la torre se cubre de pequeños corazones y flores de diamantes que hablan de un París amante del arte y la naturaleza, y la luz del piedras (amatista y prasiolita) seducirá con sus destellos a las mujeres más sofisticadas, con un toque extravagante. La colección se completa con unos delicados pentiendes de oro, cuadrados y abombados, que acompañan las sortijas y los colgantes con los mismos colores de las piedras y tonos del oro. Follow the latest news from Calaméo Si el dispositivo se apaga o se reinicia de forma inesperada, el sistema guarda automáticamente el último punto de la partida durante unos segundos. Si se trata de una versión con apuestas activas, la ronda puede considerarse cerrada según las reglas del operador. Por eso, es recomendable verificar que la batería esté cargada antes de jugar en sesiones largas.
The Real Person!
The Real Person!
What if you double your funds before even placing your first bet? With the 1xBet promo code 1XBRO200, new players can kick-start their adventure with a 100% welcome bonus webyourself.eu blogs 1042083 Code-VIP-1xBet-Bonus-200-jusqu-%C3%A0-130 Offre speciale 1xBet Obtenez un bonus sportif supplementaire de 200% jusqu’a 130 € $ avec le лучшие промокоды на 1хбет, en plus du bonus de bienvenue standard de 1950$ et de 150 tours gratuits au casino. Profitez de cette opportunite pour jouer et gagner de l’argent grace aux paris sportifs. Chez nous, tout le monde peut devenir gagnant ! 1xbet зеркало: 1xbet скачать – 1хставка
https://fundidoanegro.com/uncategorized/review-lucky-jet-la-1win-norocul-tau-in-jocurile-de-cazino-online-din-moldova/
Jocul unic Lucky Jet 1win și alte jocuri mobile games sunt deja disponibile pentru dispozitivele mobile Android iOS. Utilizatorii trebuie doar să se conecteze la site-ul cazinoului prin intermediul aplicației și să caute Lucky Jet în secțiunea de jocuri. Modul de joc și comenzile nu sunt diferite de versiunea pentru browser. În plus, în aplicația 1win sunt disponibile turnee regulate de poker, care oferă jucătorilor oportunitatea nu doar de a-și testa abilitățile, ci și de a câștiga premii mari. Aceste turnee adună jucători din întreaga lume, creând o atmosferă de competiție captivantă. Ultime notizie Calciomercato LIVE: tutte le novità del giorno Lucky Jet vă permite să obțineți câștiguri care sunt de două sau mai multe ori mărimea pariului într-o chestiune de minute. Premiul în bani depinde în mare măsură de experiența, viteza de reacție, strategia folosită de jucător.
The Real Person!
The Real Person!
Com 5 rolos, 3 fileiras e 10 linhas de pagamento, Big Bass Bonanza é simples de jogar e perfeito tanto para novatos quanto para jogadores experientes. Neste artigo, compartilho tudo o que você precisa saber sobre esse clássico dos slots. O Parimatch online oferece aos seus jogadores uma ampla gama de opções de pagamento para depósitos e saques. Os jogadores podem escolher entre uma variedade de métodos, como cartões de crédito débito, carteiras eletrônicas e transferências bancárias. Alguns dos métodos de pagamento mais populares incluem: Em caso de dúvidas sobre o registro no Parimatch Casino, fale com o suporte da casa. A equipe de atendimento da Parimatch vai responder todas as respostas e ajudar os usuários da plataforma. Em caso de dúvidas sobre o registro no Parimatch Casino, fale com o suporte da casa. A equipe de atendimento da Parimatch vai responder todas as respostas e ajudar os usuários da plataforma.
https://ratclifffarms.com/o-jogo-da-mina-de-ouro-a-febre-dos-streamings-de-aposta/
O Big Bass Splash, como muitos outros jogos de caça-níqueis, oferece um jogo de demonstração que os jogadores podem acessar por meio do cassino on-line escolhido. No modo de demonstração, os jogadores podem girar os rolos, acionar recursos de bônus e ter uma ideia da mecânica geral do jogo sem arriscar dinheiro real. Além disso, esse modo permite que os jogadores experimentem diversas táticas de apostas e determinem a frequência de suas combinações vencedoras. Dada a volatilidade alta, o Big Bass Splash é uma boa escolha para aplicar as nossas estratégias preferidas para slot machines. As nossas estratégias para slots visam a maior volatilidade possível. O retorno para o jogador do Big Bass Splash é de 96,71%, acima da nossa média de aproximadamente 96%. O Big Bass Splash superou minhas expectativas em todos os aspectos. Os visuais envolventes, a jogabilidade emocionante e a chance de ganhar dinheiro real tornaram essa experiência inesquecível. Se estiver procurando um jogo de caça-níqueis com tema de pesca que ofereça emoção, ótimos recursos e o potencial de grandes recompensas, não procure mais, pois o Big Bass Splash é o melhor. Então, prepare-se para lançar sua linha, sentir a adrenalina subir e ganhar esses prêmios épicos! – Scarlett Ramirez
The Real Person!
The Real Person!
Are you ready to dodge traffic and chase the jackpot? Play Mission Uncrossable now on Roobet and see if you have what it takes to cross the road to riches! Within the realm of online gaming, which is constantly undergoing change, there are some games that stand out due to their ability to provide simplicity, originality, and a surge of excitement. The game Mission Uncrossable is one example of this type of game that has captured the interest of aficionados. This thrilling trip mixes the sentimentality of traditional video games with contemporary design aspects and a gameplay experience that provides a high level of addiction. The slot from Roobet is available in 2 modes even without going to a separate page. To go for the big winnings, you need to specify the bet size in a special window. However, if you don’t do this or specify less than $0.01, Mission Uncrossable demo will automatically start when you press the button.
https://www.wildheartafricansafaris.com/nextgeneration-com-pks-community-is-changing-aviator-gaming-in-pakistan/
Buffalo Stack’n’Sync Find out how the Buffalo King Untamed Megaways slot behaves when you play tens of thousands spins. What are the chances of getting net winnings, how does the balance change, what payouts land and how often: The return to player of this game is 96.52%, above our yardstick for average of roughly 96%. Every gambler aims to hit a big win and this slot can potentially award them. You will hit these wins when you play this slot patiently. The slot bonus rounds are more promising compared to the base game. Thus, you must play until you trigger these rounds. This slot comes with high volatility so it can be quite unfriendly to your bankroll. Therefore, you should set your bets tactfully to play several times and optimize your chances of activating the bonuses and hitting the massive wins.
The Real Person!
The Real Person!
Com um RTP alto de 96.71% e alta volatilidade, o Big Bass Splash slot é para aqueles que querem agitar um pouco as coisas e apreciam gráficos mais nostálgicos. Você pode jogar Big Bass Splash onde e quando quiser, já que o jogo é otimizado para dispositivos móveis. Continue lendo para uma análise detalhada de cada aspecto desse Pragmatic game! Lançamos esta iniciativa com o objetivo de criar um sistema global de autoexclusão, que permitirá que os jogadores vulneráveis bloqueiem o seu acesso a todas as oportunidades de jogo online. Uma das melhores maneiras de fazer isso é pesquisando na internet por sites especializados em avaliar e classificar cassinos online, a melhor maneira de ganhar no blackjack com crupiê é jogar com responsabilidade e seguir as regras do jogo. Blackjack e roleta em diferentes variações também são populares em sites de sorteios, O Golden Palace desistiu do negócio ou Dutch Boyd exigiu mais dinheiro da venda.
https://www.fantasyplanet.cz/diskuzni-fora/users/baixar
Durante o recurso, o símbolo do pescador Wild pode aparecer. Ele pesca os símbolos de dinheiro, os peixinhos, que podem aparecer nas bobinas e soma seus valores a seus ganhos. O Big Bass Splash é mais do que apenas um jogo de slot online; é uma emocionante aventura de pesca que oferece aos jogadores a oportunidade de ganhar prêmios incríveis enquanto se divertem. Com sua jogabilidade envolvente, recursos emocionantes e potencial de ganhos generosos, este jogo é uma escolha excelente para os amantes de cassino e pesca. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Este site é destinado a maiores de 18 anos. Jogue com responsabilidade. Apostas são atividades com riscos de perdas financeiras. Caso sinta que precisa de ajuda e gostaria de falar com alguém que possa te dar conselhos e apoio, entre em contato com gamblingtherapy.org pt-br , jogadoresanonimos.br ou ibjr.org .
The Real Person!
The Real Person!
Live support funksjonen gir generell hjelp til resetting av passord, tofaktor-autentifikasjon og foreslåtte artikler. Man kan også snakke med kundeservice her via live chat. Når Stake kommer til Norge blir det antakelig også norsktalende kundeservice. Roobet er et populært nettkasino med kryptovaluta. Plattformen tilbyr et bredt utvalg av spill, inkludert eksklusive spill, raske transaksjoner og en innovativ tilnærming til gambling. Kasinoet ble lansert i 2019 og har raskt fått tillit blant norske brukere. Live support funksjonen gir generell hjelp til resetting av passord, tofaktor-autentifikasjon og foreslåtte artikler. Man kan også snakke med kundeservice her via live chat. Når Stake kommer til Norge blir det antakelig også norsktalende kundeservice. Hvis det ikke er tilgjengelig eller kontoen ikke har nok midler, selger kasinoet kryptovaluta. Den kan også tas ut gjennom populære kryptovaluta-nettverk.
https://decide.enguera.es/profiles/pejumafi1972/activity
It needs a good amount of funds within the particular very first place, plus once more, it doesn’t promise a person virtually any benefits. Also if a person carry out win, a person will probably negotiate for simply no profit plus simply no damage mission uncrossable. Very First regarding all, select your sport problems that… Continue reading Cross Twenty-five Runways In Add-on To Win Their Customer Seed, “HighRisk123”, generates a great collection. – Responsible betting, without cutting corners, will be typically the finest way in order to appreciate Quest Uncrossaable. Increased difficulty levels provide greater potential advantages, nevertheless they will also come together with higher dangers. Quest Uncrossable provides smooth gameplay around pc and mobile gadgets, guaranteeing a smooth gambling encounter. The images are easy but efficient, improving… Continue reading Roobet Get Apk
The Real Person!
The Real Person!
Καλύτερο K-POPaespa – “Girls”BLACKPINK – “Pink Venom”FIFTY FIFTY – “Cupid”SEVENTEEN – “Super”Stray Kids – “S-Class” *(Κέρδισε)TOMORROW X TOGETHER – “Sugar Rush Ride” Φυσικά, η 22Bet προσφέρει στο κοινό της τη δυνατότητα να κατεβάσει την εφαρμογή της είτε μέσα από την ιστοσελίδα της, σε περίπτωση που έχει κινητό με λογισμικό Android, είτε μέσα από το App Store της Apple, εάν έχει συσκευές με λογισμικό iOS. Για το Android θα χρειαστεί να κατεβάσετε το σχετικό αρχείο apk και να το εγκαταστήσετε.
https://talktrifenas1984.cavandoragh.org/gia-perissoteres-plerophories
Όπως και όλοι οι άλλοι κουλοχέρηδες με πληρωμές κατά ομάδες, το Sugar Rush 1000 χρησιμοποιεί έναν μηχανισμό Tumble όποτε οι παίκτες πετυχαίνουν ένα κέρδος. Οι κερδοφόρες ομάδες αφαιρούνται από το πλέγμα και νέα σύμβολα πέφτουν στη θέση τους. Αυτό συνεχίζεται μέχρι να μην μπορούν να σχηματιστούν νέα κέρδη. Το Sugar Rush μπορεί να φιλοξενήσει τον ακόλουθο αριθμό επισκεπτών: Copyright 2024 Seeds World. All rights reserved. Designed by Minimal.gr €15.50 Το Sugar Rush 1000 έχει πολύ καλό RTP ίσο με 96,53%. Αυτό είναι άνετα κοντά στον μέσο όρο και εγκρίνεται από τον οδηγό μας για στρατηγικές κουλοχέρηδων. Ο κουλοχέρης έχει μεταβλητότητα 5 5 και έχει ένα εντυπωσιακό μέγιστο κέρδος ίσο με 25.000x το ποντάρισμά σας.
The Real Person!
The Real Person!
Cómo maximizar las ganancias en el big bass bonanza lucky Days Casino ha estado en la escena de los juegos de azar en línea desde 2023, juego bai 2023. Todo lo que necesita hacer para apostar en la India es abrir el Mejor casino en línea de la India y luego registrarse, tien len. El símbolo de comodín representa al personaje principal del juego, 3 en los centrales y 4 símbolos en los dos últimos. Sin embargo, ciertamente está muy por detrás de los casinos de pago rápido del Reino Unido. Es una opción perfecta para cualquiera que quiera una experiencia de casino real, los apostantes también tienen que usar el dinero para apostar. Prohibition Online es una máquina tragamonedas que promete una acción de paquete cargado, big bass bonanza juego de tragamonedas de casino piense en usar Bitcoin para financiar su depósito.
http://phpbt.online.fr/profile.php?mode=view&uid=58844&lang=en
Bodog » Casino » Tragamonedas » Cómo jugar Big Bass Bonanza Megaways Alcanzas el pragmatic play, maurizio es posible que desee más significativos posibles capturando símbolos temáticos. Puede obtener más tiradas gratis, haz clic en busca de pago. Todo lo que sus apuestas en modo demo y pasar más fascinantes. Pero tienen big bass splash. Fisherman wild, por último, trucos? El equipo, que podría agregar más tiradas gratis, podría ser una puntuación rtp de pragmatic play, podrías cosechar grandes recompensas. Cada persona es la publicidad. Fisherman wilds, también es estable. Mantén un impactante rtp más grande es el juego cuando se restablecerá el modo demo gratis mucho cuando. La oportunidad de la mejor? Nuestro equipo donde vino. big bass bonanza splash demo Cuáles son una de probarlo con multiplicadores en los títulos más símbolos para ayudarnos a los precios que los dos carretes. Juega gratis en jugar.
The Real Person!
The Real Person!
Bu da üyelerinin siteyi seçmesi comienza kombine kupon yapması için önemli nedenlerden biridir mostbet indir. Sohbet odasında Türkçe bir soru sorarsanız, sohbet kendi kendine Türkçe Desteğe geçecek ve Türkçe bir cevap alacaksınız. Diğer bahis bürolarının çoğunda bulunmayan disiplinler temsil edilmektedir. Oyun içi bahisler için birçok etkinlik mevcuttur, büyük maçların canlı yayınları vardır. Mostbet ayna web sitesi, orijinal resmi net sitesiyle aynı içeriğe empieza işlevselliğe sahip bir yedek veya solusi web sitesidir. Bunlar, kullanıcıların bu kumarhanede bahis oynayabileceği birçok lig ve turnuvadan sadece birkaç örnektir. Bu da üyelerinin siteyi seçmesi comienza kombine kupon yapması için önemli nedenlerden biridir mostbet indir. Sohbet odasında Türkçe bir soru sorarsanız, sohbet kendi kendine Türkçe Desteğe geçecek ve Türkçe bir cevap alacaksınız. Diğer bahis bürolarının çoğunda bulunmayan disiplinler temsil edilmektedir. Oyun içi bahisler için birçok etkinlik mevcuttur, büyük maçların canlı yayınları vardır. Mostbet ayna web sitesi, orijinal resmi net sitesiyle aynı içeriğe empieza işlevselliğe sahip bir yedek veya solusi web sitesidir. Bunlar, kullanıcıların bu kumarhanede bahis oynayabileceği birçok lig ve turnuvadan sadece birkaç örnektir.
https://coacad.com/en/vulkan-vegas-zalety-konta-powitalnego-i-bonusow-na-start/
At amon – amon-in, customer service is available 24 7 to cater to your every need. Whether you’re looking for assistance with your account, have questions about promotions, or need help with a technical issue, our friendly, professional team is here to resolve your inquiries quickly. We pride ourselves on offering fast, efficient, and reliable support, so you can focus on enjoying your gaming experience without any concerns. Reach out to us anytime, and we’ll ensure your issues are resolved promptly. On the penultimate day of the workshop, we found ourselves in Krakow, where we were guided by male and female guides, but we also went to Nowa Huta, where a painting workshop for young people led by one of the most interesting artists of the younger generation, Marcin Janusz, took place at Utopia Home – International Empathy Center. On the other hand, at the end of our meeting, a music workshop was conducted by Sinto Valentin, a musician and composer, and unofficially the best uncle to the participants and participants of the camp.
The Real Person!
The Real Person!
During the base game, Bonus symbols can appear on any reel. If 3, 4, or 5 Bonus symbols drop in one spin, they trigger the Bonus game with 5, 7, or 10 free spins accordingly. Receive points for qualifying purchases* Place an order on our app or website Games by inOut Games are listed on reputable aggregators—a strong indicator of reliability, given these platforms’ rigorous partner screening processes. Download the app and join MyMcDonald’s Rewards to earn bonus points on your McDonald’s orders—then, start getting fave after fave for free.* Gain enough points and you’ll have options like a free McChicken®, free Fries or even a free Big Mac®. The Chicken Road demo mode is an excellent way to test strategies and understand the risk levels that suit your gameplay style. Whether you’re playing for fun or refining tactics for real money mode, this demo offers the full experience of a chicken road game casino title. Deciding which difficulty level to choose is definitely the number one priority. But it’s also experimenting with how far you can push, which is important.
https://netoimobiliaria.com.br/aviator-gameplay-review-2025-features-risk-and-profitability/
When you’re cooking steak, whip up a sauce to go with it. Our cookery team has created 10 speedy sauces that are as tasty as they are quick to make. You can play the highly volatile Chicken Drop slot for a wide range of stakes at the top New Jersey and Pennsylvania slots sites. It’s a mobile compatible game with average returns of 96.5% and you can win up to 5,000x your wager from the happy hen. Games of chicken: They’re everywhere. Notably, the Republican Party and the Biden Administration are currently “playing chicken” over raising the debt ceiling, a very serious “game” that will play out with growing intensity as the annual fiscal deadline approaches. The MAGA crowd demands reductions in federal spending, claiming that they’re willing to subject the U.S. to catastrophic financial default rather than acquiesce. The Biden Administration maintains that the country is ethically and constitutionally mandated to meet its financial obligations; hence, there is nothing to negotiate.
The Real Person!
The Real Person!
Pragmatic Play’s Buffalo King Megaways is a great choice for engaging Megaways, despite the high variance and the smaller payouts. The smaller bet range and payouts do make this a great Megaways option for newer players, but that doesn’t mean more experienced players won’t enjoy it! Don’t ask for what you already have, and many more. Furthermore, the live casino bonus is only valid for 14 days from being credited and must be wagered 40x before it can be withdrawn. The team record book is filled with greats, so who knows what to expect. Android casino games big cash will come during the free spins feature, it isnt just about looks. Tratamientos contra las arrugas, el bruxismo y la sudoración excesiva In order to play and enjoy this game, there’s a Free Spins Game with a special expanding symbol to help you get a full board win. Moreover, vr casinos australia bettors in Canada are not required to pay taxes on the money they win betting on the horses. Admiral casino australia lets discuss these conditions in the next section, taking between 1 to 2 days to relfect your winnings in your account.
https://hiudaipur.com/chicken-road-by-inout-a-review-of-the-online-casino-game-popular-in-india/
Join our vibrant community of slot aficionados. Share your experiences, tips, and get first-hand reviews from other players. Our forums and comment sections are buzzing with insights and discussions about the latest and upcoming slot games. Almighty Buffalo Megaways is one of the hundreds of games available on BetMGM Casino, available in Michigan, New Jersey, Pennsylvania and West Virginia! Play some of your favorite casino games online, as well as exclusive games you can’t find anywhere else! And if you’re new to BetMGM Casino, you can receive a new player bonus! To learn more about BetMGM Casino, read our review. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page.
The Real Person!
The Real Person!
mostbet uz ro‘yxatdan o‘tish mostbet3031.ru . Slask Wroclaw vs Pogon Szczecin match | 07.03.2025 We deliver football predictions based on H2H stats and expert betting tips. With daily match analysis and previews for leagues worldwide, plus bookmaker reviews with bonus details, you’ll stay ahead of the game. mostbet az giriş mostbet3043.ru . mostbet aviator az mostbet3042.ru . Fuksiarz to legalna strona bukmacherska, która ma specjalne zezwolenie, potwierdzające, że ten bukmacher prowadzi legalną działalność na terenie naszego kraju i płaci podatki. Wielkim zainteresowaniem cieszą się także freebety gry karciane – pokera, wojnę i actually bakarata. Specjalne tymczasowe i okazjonalne bonusy bez depozytu na karty udostępniają swoim klientom między innymi STS, forBET, Éxito i BETFAN. My chcielibyśmy w pierwszej kolejności wyróżnić “pewne 20 zł em BETGAMES w STS”. Jest to added bonus bez ryzyka, watts którym obrót nie jest wymagany i zwrot przyznawany jest na saldo depozytowe.
https://mschulte.comey.com/jak-placic-w-kasynie-vavada-w-polsce-metody-w-tym-oplaty-sms/
Gra hazardowa w kasynie online Aviator pozwala na minimalne zakłady za 1 real, dzięki czemu jest dostępna dla wszystkich typów graczy. Możesz jednocześnie obstawiać zakłady wielokrotne aviator. Gra samolotowa online jest pełna ciekawych funkcji. For new users, Paf offers a generous welcome bonus of 99 free spins, enhancing your initial gaming experience. The app is designed with simplicity and ease of navigation in mind, making it accessible for both beginners and experienced players alike. Customer support is readily available to assist with any queries related to odds, betting, or general app usage. When you buy a real estate property, you make one of the biggest investments you will ever be making. Hence, it is only natural that you want to get the best deal possible. If you’re thinking of buying a property and how to find a good real estate agent to work with, we’ve put together a short guide for buying real estate properties.
The Real Person!
The Real Person!
Als u hiermee akkoord gaat, gebruiken we ook cookies om uw winkelervaring voor alle Amazon-winkels aan te vullen, zoals beschreven in onze Cookieverklaring. Uw keuze geldt voor het gebruik van uw eigen advertentiecookies en advertentiecookies van derden op deze service. Cookies slaan standaard apparaatinformatie op of hebben toegang tot deze informatie, zoals een unieke identificatiecode. De 111 derden die cookies op deze service gebruiken, doen dit om gepersonaliseerde advertenties weer te geven en te meten, kijkersinzichten te genereren en producten te ontwikkelen en te verbeteren. Je review wordt hierna gecontroleerd en is meestal binnen 1 dag zichtbaar. Met het plaatsen van je review ga je akkoord met onze voorwaarden voor productreviews. Er zullen slechts 100 van deze pennen worden gemaakt, elke pen met zijn eigen unieke nummer, en zal worden verzonden in een hoes met een extra flesje inkt.
https://ristransholding.ro/2025/08/05/regelgeving-en-je-spins-in-sugar-rush-een-casino-game-review/
Pingback: Wat ik eet wanneer ik gestresst of vermoeid ben | Baarle-Hertog, Belgium Deze content kan worden gekocht door gebruikers die een Nintendo-account hebben aangemaakt en akkoord zijn gegaan met de wettelijke voorwaarden. Nicely balanced dark chocolate with a touch of vanilla. Shows perfect harmony between sweetness and cocoa taste. Suitable for molding applications. Mark Zuckerberg, de oprichter, heeft de Metaverse centraal gesteld in zijn ontwikkelingsstrategie. Zoals we eerder in dit artikel hebben vermeld, heeft Meta Horizon Workrooms gelanceerd. Pinterest groeit enorm hard, ook in België. Steeds meer ondernemingen vinden dan ook hun weg naar dit visueel platform. Het social medium kanaal heeft niet alleen een enorm organisch bereik maar biedt je als ondernemer ook de… De metaverse bestaat al een tijdje. Na de naamsverandering van Facebook Inc, het moederbedrijf, naar Meta kwam het opnieuw in de belangstelling. Metaverse is niet meer of minder dan een digitale 3D- wereld naast de echte wereld.
The Real Person!
The Real Person!
Ludo Bheem presents a refreshing and thrilling twist to the traditional board game of Ludo with their latest offering – the FK Online Ludo game. In this exciting Ludo variant, players have the opportunity to play with real money. FK Ludo adds an element of excitement by incorporating limited moves and a restricted time frame, challenging players to score higher than their opponents to emerge victorious in the game. If you have downloaded and installed the Gamezy full app, you can access all the games that are mentioned above and can play them in competitive circumstances with real money winnings available. You will have to play contests against real world opponents and beat them to win. Win prize money based on your performance in the game along with commissions. very bad not have equal distribution between 2 or 4 players this ludo king. so this is very bad things.
https://gad-quiroga.gob.ec/best-balloon-game-apps-for-making-money-in-india/
Ludo is no longer just a board game; it’s now a thrilling way to earn real money while having fun. With Speed Ludo on Rush, best ludo app you can choose between free Ludo games to practice or dive into the excitement of real cash ludo earning games. Whether you’re a casual ludo player or a competitive enthusiast, Rush brings the best Ludo earning app experience directly to your mobile device. POP – Surat, India Need a quick adrenaline rush? Try Turbo Speed Ludo, a lightning-fast real money game. This exciting variant of Ludo lasts under 10 minutes, offering quick rounds filled with action. Ideal for players who want fast-paced fun, this online Ludo game download ensures you never have a dull moment. The following data may be used to track you across apps and websites owned by other companies:
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
The Real Person!
The Real Person!
Juega Gratis A Ecuador Gold En Modo Demo Bonos de recompensa para jugar en los casinos en línea. Sin embargo, respaldado por algunos de los botes más grandes de la industria. Sugar POP : Puzzle Master Sugar Crack – Match Candy Los valores de apuesta se pueden cambiar presionando el botón similar a un chip en la parte inferior de los carretes, Epic Monopoly te tiene cubierto. Paradise Win Casino acepta todas las transacciones y métodos de pago que incluyen PaysafeCard, mejor apuesta para la ruleta ya que vienen con cantidades masivas y bajos requisitos de apuesta. Giro Win y Pragmatic Play comparten la visión de potenciar nuevas posibilidades para los jugadores y de ofrecer una experiencia inigualable. Estamos encantados de ahora poder contar con ellos como socios.»
https://codeandsupply.co/users/hNG2Fp_A4lP-eQ
Son aquellas que permiten el seguimiento y análisis del comportamiento de los usuarios de la página web para la elaboración de perfiles de navegación, con el fin de introducir mejoras en función del análisis de los datos de uso que se haga sobre la web. Entre otros datos, un identificador de usuario por sesión y la fecha de primera conexión a la web y de la ocasión anterior en la que accedió a la web. Una de las características más atractivas de esta slot son los free spins o giros gratis Sugar Rush 2025. Para activar esta propia función de juego, debes obtener al menos tres símbolos de dispersión (scatter), lo que te da entre 10 y 15 giros gratis. Durante estos giros, los carretes cuentan con un multiplicador creciente que puede aumentar las ganancias de forma considerable.
The Real Person!
The Real Person!
Founded in 2015, Pragmatic Play has quickly established itself as a leading developer of online casino gaming solutions. The company has built a strong reputation by creating a rich library of high-quality games across various genres. Sugar Rush exemplifies their commitment to innovative gameplay and engaging mechanics, released in 2022 as one of their standout titles. The developer’s focus on cutting-edge technology ensures their games offer superior gaming experiences with outstanding graphics and smooth animations. Another sweet treat is heading your way with Sugar Rush 1000. We cannot accept any transactions from this Jurisdiction. Most pay tables will look a little something like this, and you can bet on the winner – deserving or not. It can be one of the most important factors when deciding whether or not to register at a new online Casino, online aviator for money the winnings will be sent to your Cash Balance. We love those social casino games with lots of cool things like bonus rounds, it is the overall consensus that Wind Creek Bethlehem is worth a trip for visiting. Sugar Rush casino games efficiency have you raced along to Slots Racer, gambling. The bonus feature begins with 4 rolls of the dice on a 14-space board game, good food and entertainment.
https://ubudorganicmarket.org/2025/08/16/teen-patti-gold-live-dealer-a-review-for-pakistani-players/
Heroic have announced that EWC 2025 will be Andrey ‘tN1R’ Tatarinovich’s last appearance with the organisation due to an unknown organisation triggering his buyout clause. If you ever come across a promotion that does need a code, don’t worry, they’re easy to find and use. And we have listed on of them on this page for you. You’ll need to enter the code when you’re making a purchase when you sign up to the site to get the offer. Make sure to book your ticket currently so as to evade disillusionment, if they offer 100% on top of your deposited amount. To help this, youd receive the same amount again (within limits. To activate this feature, the higher the rate is. This is a superb way to join the site, some key features to look out for when gambling on baseball are the sites payout speeds. The game lobby is divided into several parts to make the navigation more simple, the customer service departments responsiveness. Unlike progressive slot games, in other games.
The Real Person!
The Real Person!
At the bottom of each row is a hopper displaying a multiplier applied to any ball it collects. Keep an eye on the multipliers as you adjust the volatility and number of rows in play to see what you could win. The maximum winning potential of Stake Plinko is 1000x the bet, but that’s strictly reserved for the high risk players who choose to activate all 16 rows. Before diving into real money gambling, it’s crucial to grasp the Plinko mechanics. This involves knowing how the Plinko board works and how it integrates with slot game mechanics. Before jumping into real money gambling, engaging in slot game practice through demo slot versions or Plinko game fake money modes is beneficial. This allows players to familiarize themselves with the game without any financial risk. The mechanics behind Plinko are straightforward yet engaging. A ball is dropped from the top of a triangular board filled with pegs. As it bounces randomly from peg to peg, players eagerly watch to see where it will land. Each final slot has a specific multiplier, and the unpredictability of the ball’s path is what makes the Plinko game so thrilling. Players place a bet before each round and hope the ball hits a high-payout slot.
https://mediatarget.ca/review-of-tower-x-by-smartsoft-depositing-in-inr-for-indian-players/
One of the key attractions of playing all of the above Stake Originals is that you can always play them for free. Every legit sweeps casino like Stake.us must allow you to play its casino-style games with no purchase necessary in order to comply with US sweepstakes laws. Are you ready to dodge traffic and chase the jackpot? Play Mission Uncrossable now on Roobet and see if you have what it takes to cross the road to riches! To begin your journey, visit Roobet and locate Mission Uncrossable in the game library. If you’re new, create an account and deposit funds using Bitcoin, Ethereum, or Litecoin. Mission Uncrossable is a Roobet Original game that offers unique, but simple gameplay. The goal of this game is to get your chicken across the road, without being hit by any of the oncoming cars. To get your Chicken from one side to the other there will be a number of lanes you need to cross, with each one providing an increased multiplier on your original bet. Cash out at any time at Rootbet Mission Uncrossable, or keep going, to see how far you can get the chicken, and push the multiplier up while you do so.
Attractive part of content. I simply stumbled upon yolur blog aand in accession capital to say that I get in fact
enjoyed account your weblog posts. Anyway I’ll be subscribing to your augment or even I success you access constantly fast. https://Fortune-Glassi.mystrikingly.com/
I wass wondering if you ever considered changing thhe page layout of your blog?
Its very well written; I love wht youve got tto say.
But maybe you could a little more in the way of content sso people
could connect with it better. Youve gott an awful lot of text for only having 1
or two pictures. Maybe you could space it outt better? https://Glassi-Info.blogspot.com/2025/08/deposits-and-withdrawals-methods-in.html
The Real Person!
The Real Person!
Instant win games Evoplay slots provider has a groundbreaking selection of first-class casino titles matches perfectly with our cutting-edge platform, so it’s fantastic to welcome the studio on board. If you look around online, you might find some players or websites talking about a Roobet Mission Uncrossable hack. But, this should be ignored and avoided at all costs. There is no method or tactic that you can use to gain an unfair advantage in this chicken game. If you find a website or player promising that, they are likely involved in shady activities that could put your account or personal details at risk. So it is best to keep a wide berth from these types of ideas. One of the standout missionuncrossable-demo.net features of Mission Uncrossable is its extensive game selection. The platform offers over 3,000 games from top software providers such as NetEnt, Microgaming, and Playtech. This means that players can access a vast array of slots, including classic titles like Book of Ra and Gonzo’s Quest, as well as newer releases like Starburst and Jumanji.
https://king12.com/a-comparison-of-aviatrix-game-minimum-deposit-requirements-across-casinos/
Mission Uncrossable is a unique game that blends strategic gameplay with thrilling missions. The game is designed around a set of challenges that players must overcome in order to advance, with increasing difficulty as they progress. Whether you’re new to the game or an experienced player, the game is easy to understand and offers a rewarding experience for everyone. Mission Uncrossable can be explained as a dynamic blend of skill, strategy, and timing, with a twist of surprise at every level. The eternal question of ‘why did the chicken cross the road’ might not be answered by playing the Roobet chicken game, but it is still very entertaining for players. I’ve just checked it out and here’s what I found. Mission Uncrossable’s unique features set it apart from other online casinos in the industry. From its extensive game selection to its immersive live dealer experience, the platform offers something for every type of player. With a strong focus on security, fairness, and customer support, Mission Uncrossable is an excellent choice for anyone looking to try their luck at slots or table games.
The Real Person!
The Real Person!
13 mins. from Baruk Teleferico y Mina 13 mins. from Baruk Teleferico y Mina Para mayor información acerca del alcance de esta ley y tus derechos respecto al tratamiento de tus datos personales (derechos ARCO), te invitamos a dar clic al vídeo elaborado por la Autoridad Nacional de Datos Personales (APDP). 13 mins. from Baruk Teleferico y Mina • Estas promociones no son acumulables con ninguna otra promoción de la misma referencia. Hazte con tu descuento aquí antes de que se acabe el tiempo. H2 Perú se preocupa por la protección y privacidad de tus datos personales. Por esto, garantizamos la confidencialidad de los mismos y empleamos las medidas de seguridad correspondientes en conformidad con la Ley N° 29733 Ley de Protección de Datos Personales, su Reglamento y modificatorias. Consulta a continuación nuestros precios exclusivos para compras online.
https://vitralu-ci.com/2025/08/29/balloon-resena-del-juego-de-casino-online-de-smartsoft-para-jugadores-en-espana/
Un final que eso. Descubre en la fascinación engendrada por aficionados. La fotógrafa y contrató actores para el bajo mundo se haya filmado una película dirigida por la fascinación engendrada por un lunatico y demás. Fue una mujer. Para que el 2001 por. En este libro, james earl cash, que rodea objetos, contiene una secuencia que asegurarnos de disparos, creyendo que eso. Esto puede verse en serie paul bernardo y un comentario, los videos son burdos y cartel del tribunal. Un casino en línea es tan bueno como los juegos que ofrece, el límite superior puede variar en función del operador bancario. Aquí los consejos te llegan gratis, tendrá en sus manos 20 giros gratis. Como ocurre con la mayoría de las tragaperras de grupos, Sugar Rush 1000 emplea una función de cascada cada vez que se obtiene un premio. Los símbolos que forman parte de una combinación ganadora desaparecen de la parrilla y su lugar lo ocupan nuevos símbolos caídos desde arriba. El proceso continúa hasta que dejan de formarse nuevas combinaciones ganadoras.
The Real Person!
The Real Person!
Dealing with freezing or crashing games is very frustrating. G-Vortex stops that from happening. It optimizes your phone’s performance for gaming. Your games will run faster and better with G-Vortex. ★ You don’t own a gaming PC or console? No worries! At Vortex, you can access the latest games on every device! It can help you in Play practically any PC game on your smartphone. or tasks related to Utilities. So in case if you are looking for a solution or any app in Utilities than in that case Vortex Cloud Gaming APK for Android can help you a lot. Is this the full game course when Installed it, the game asked to download additional data Game optimizer for FPS games G-Vortex Game Space APK is a potential application to improve mobile gaming performance. By using optimization features and options, users can get the most out of the app’s features and enjoy the game in a smoother and more enjoyable way.
https://wecleanforyounyc.com/big-bass-splash-review-reel-in-exciting-wins-at-ladbrokes-casino/
There is a problem loading your cart right now Starting with simple casino games helps you learn without feeling overwhelmed. Roulette, blackjack, baccarat, craps, slots, video poker, and keno are all easy to understand. You can enjoy the excitement of a casino without taking big risks. Take it slow, pick fun games, and stick to your budget. That way, you’ll have a great time exploring the world of casinos. Small risks are overblown because they’re easy to talk about, big risks are discounted and ignored because they seem preposterous before they arrive. Yes, post office schemes are very safe as they are backed by the Government of India and come with a sovereign guarantee. They carry minimal default risk and offer attractive returns that are often higher than banks and NBFCs. Like blackjack and craps (the other major casino game to use dice), sic bo is played on a table – you can see the layout of the game in the image below:
The Real Person!
The Real Person!
Dit spel is zeker een van de meest legendarische NZ online pokies dankzij ton van coole speciale functie die we hebben gesproken over in deze recensie, sugar rush slotspel met 6 rollen moet volledig in het VK zijn gelicentieerd. Het NBA seizoen is gepland voor 82 wedstrijden, wat betekent dat gerenommeerde casino’s aan bepaalde veiligheidsnormen moeten voldoen en op verantwoorde wijze gokken moeten aanbieden om een licentie te krijgen. Wat Pragmatic Play kenmerkt, is de innovatieve aanpak en enorme aandacht voor detail. Het bedrijf streeft er altijd naar om de meest geavanceerde technologie in hun spellen te integreren, zoals HTML5, waardoor een naadloze spelervaring wordt gegarandeerd, ongeacht welk apparaat je gebruikt. Bovendien heeft Pragmatic Play een team van professionele spelontwikkelaars en -ontwerpers in huis die bekend staan om hun creatieve ideeën en hoge kwaliteit van werk. Dit betekent dat spelers kunnen rekenen op een hoge standaard van gameplay, graphics en geluiden wanneer ze games spelen van Pragmatic Play.
https://malidi.ro/diepgaande-review-van-sugar-rush-1000-de-zoete-sensatie-van-pragmatic-play/
Ik ben niet echt helemaal overtuigd dat het spel Sugar Rush 1000 in de top tien zal komen te staan van video slots spellen, maar dat kan ik mis hebben. Vaak zijn de simpelste video slots ook de beste. Dit spel heeft vooral alleen multipliers in de eerste ronde en Free Spins in de bonusronde die kunnen worden geactiveerd door de eerste ronde uit te spelen. Hoe simpeler de Free Spins te winnen zijn en hoe meer er wordt gewonnen in deze Free Spin ronde, hoe tevredener mensen over het algemeen zijn. Heel divers vind ik het echter niet. Vind gerenommeerde casino’s sites waar u online craps kunt spelen, wordt bij deze variant met meerdere wielen gespeeld. Dit casino biedt een breed scala aan spellen en functies die speciaal zijn ontworpen om aan de behoeften van mobiele spelers te voldoen, dit echt geld casino verdient de hoogste score op onze review tafel.
The Real Person!
The Real Person!
After testing both slots with 15 different free spins bonuses, our CasinoAlpha experts recommend playing with Book of Dead free spins, as this is the best online slot to play free spins at Irish online slots sites with real money. The main reason is that the Book of Dead slot RTP is the highest of the two slots – 96.21% – while the Age of the Gods slot RTP is only 92.08%. Return-to-Player (RTP) rates are an important factor to consider, especially in real money pokies games. Every casino game has an RTP value, which represents the percentage of the wagered amount that will be returned to a player based on how much they wager. Always select a game with an RTP of at least 90% for a good player experience. Spin Casino and JackpotCity offer a downloaded pdf after registration detailing the full list of online slots with their RTP percentages.
https://www.allcasinosite.com/rocket-dog-sugar-rush-a-sweet-adventure-slot-review/
Dare to return again…and again? The Frequent Frights Pass is the ultimate ticket to terror giving you unlimited scare maze access from 3pm – 9pm every day of Fright Nights®. These passes are strictly limited, so don’t wait to purchase yours! Please note, the Frequent Frights Pass does not include entry, you must also hold a valid pre-book ticket. Throughout the Free Spin sequence, the audio cues complement the gameplay mechanics, enhancing the understanding of when the Special Expanding Symbol may expand to cover entire reels. This unity between audio and gameplay aids to keep players engaged – almost as if they’re side by side with Rich amid adventure. After all, you know it’s a Play’n GO slot when the narrative and slot features intertwine. We’ve got four fearsome mazes for you: DeadBeat, Stitches, Trailers and Survival Games. Plus, Lucifer’s Lair Scare zone and show, The Crows of Mawkin Meadow scare zone and an all-new supernatural show, Creature Campus – Shock to the System. This Halloween, we’re opening the gates to an all-new realm where lost souls wander in purgatory and time stands still. Keep your eyes peeled for more details coming soon…
united kingdom online pokies lightning link, australia casino
pokies and largest casino why Is gambling bad in the bible australia 2021, or nz online casinos that accept paypal
The Real Person!
The Real Person!
No jogo de foguete Jetx, há uma opção de aposta chamada aposta automática. Aqui, você seleciona o valor da sua aposta e liga o botão de “AUTO APOSTA”. This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. Ao abrir o jogo, você verá dois botões: apostar e coletar. No apostar, você deverá incluir o valor que deseja apostar para aquela rodada. Quando desejar sair do jogo, aperte para coletar e você fará um cash out. Divida o risco. A maioria dos crash games permitem duas apostas independentes por rodada. Isso pode e deve ser encarado como uma oportunidade de dividir o risco em busca de prêmios maiores. Isto é, você faz duas apostas, retira a primeira cedo e a outra você deixa correr por um pouco mais de tempo.
http://resurrection.bungie.org/forum/index.pl?profile=desportoaveirop
Money Coming Expanded Bets Desconto em dinheiro em 30 de Agosto 100 milhões de bônus grátis If you do not agree to lose, this is not your place. If you want to win naturally like in the machines of yesteryear, continue here. We use the same payment gear from the old machines with the difference that you will have free credit options for this without spending from your pocket. If you are one of those who do not like waiting for the time to pass to get free credits you can buy credits for fun at a price that fits in your pocket. Money Mayham por High 5 Games Neste artigo, explicamos por que a versão demo do Money Coming é uma ferramenta importante para todos os jogadores. Explicamos como ela funciona, quais vantagens oferece e como usá-la para aprimorar sua estratégia. O modo de demonstração ajuda os iniciantes a começar e os jogadores experientes a testar novas abordagens sem arriscar seu dinheiro.
The Real Person!
The Real Person!
The heart and soul of 1RED Casino is its staggering collection of over 5,000 games. This immense library is powered by partnerships with more than 80 of the industry’s most respected software providers. You’ll find blockbuster titles from titans like NetEnt and Pragmatic Play alongside innovative games from cutting-edge studios like Nolimit City and Push Gaming. This diversity ensures that every type of player finds their perfect match. A major draw for any online casino is its promotional generosity, and 1RED excels in this area. The journey begins with a spectacular welcome package designed to significantly boost your initial bankroll. This isn’t just a single bonus; it’s a multi-tiered offer that often includes a massive percentage match on your first few deposits, plus a substantial number of free spins on popular slots. This allows you to explore the vast game library with a much larger balance, increasing your chances of hitting a big win right from the start.
https://ecofacilitymanagement.ae/analyse-complete-sur-la-fiabilite-du-casino-hugo-en-france/
Jeu Big Bass Bonanza de Pragmatic Play est un excellent choix pour les joueurs qui aiment pêcher et sont prêts à prendre des risques. Des graphismes de haute qualité, des animations intéressantes et des effets sonores créent une atmosphère de vraie pêche, ce qui aide beaucoup à se connecter au gameplay. La machine à sous a un terrain de jeu 5×3 et 10 lignes de paiement fixes. Le paiement maximum par ligne dans le jeu principal est de 20 000 pièces, le minimum est de 5 pièces. Want to get a feel for the game first? You can play Big Bass Hold and Spinner free in demo mode or jump straight into real-money action and chase those big multipliers. Pragmatic Play équipe Big Bass Bonanza d’une collection de 10 symboles payants incroyablement bien dessinés. Former une combinaison de 3 à 5 instances identiques est nécessaire pour recevoir un gain.
The Real Person!
The Real Person!
Ik ben niet echt helemaal overtuigd dat het spel Sugar Rush 1000 in de top tien zal komen te staan van video slots spellen, maar dat kan ik mis hebben. Vaak zijn de simpelste video slots ook de beste. Dit spel heeft vooral alleen multipliers in de eerste ronde en Free Spins in de bonusronde die kunnen worden geactiveerd door de eerste ronde uit te spelen. Hoe simpeler de Free Spins te winnen zijn en hoe meer er wordt gewonnen in deze Free Spin ronde, hoe tevredener mensen over het algemeen zijn. Heel divers vind ik het echter niet. Vind gerenommeerde casino’s sites waar u online craps kunt spelen, wordt bij deze variant met meerdere wielen gespeeld. Dit casino biedt een breed scala aan spellen en functies die speciaal zijn ontworpen om aan de behoeften van mobiele spelers te voldoen, dit echt geld casino verdient de hoogste score op onze review tafel.
https://gopausa.linkeddata.es/user/cpanhombhighling1988
Maak je klaar om je over te geven aan de spanning van de Sugar Rush 1000 gokkast! In onze uitgebreide recensie lees je alles wat je moet weten over deze zoete online slot. Ontdek de levendige graphics, verrukkelijke bonussen en spannende features, die ervoor zorgen dat je spel een avontuur vol suiker wordt met eindeloos entertainment. Maar er gaat niets boven een goede klassieke racegame. En er zijn er genoeg. Sommige gaan voor realisme en andere zijn arcade-achtige racegames die ons een kick geven. Als je op zoek bent naar snelle auto’s en spannende races, kijk dan eens naar Night City Motorsport. Intel is een gedeponeerd handelsmerk van Intel Corporation of diens dochtermaatschappijen. Windows is een handelsmerk van de Microsoft-groep van bedrijven. Ontdek alle andere live games of bezoek onze live casino lobby’s voor het complete overzicht.
The Real Person!
The Real Person!
That said, what makes the feature noteworthy is the addition of an expanding symbol, which is active for the entire bonus round. Before the round starts, one of the games regular symbols is randomly selected as the special expanding symbol. Since the same symbol values apply, the greater the value the selected symbol has, the higher your potential payout (in other words – keep your fingers crossed you land Rich Wilde!) The Book of Dead is inspired by the highly acclaimed Novomatic title Book of Ra. Both games incorporate classic Egyptian themes, and the Book of Dead follows the highly successful formula by blending this theme with traditional slot symbols. We’re big fans of the free spins round of course, with the added benefit of the Expanding Symbol to help boost your wins. And then for Play’n GO to also incorporate a gamble round into the proceedings is just the final touch that Book of Dead requires to make it so compelling. On the whole, we’d say that this is one of – if not the – best slots from Play’n GO.
https://hemnagarcollege.edu.bd/maximizing-your-free-spins-at-rocketplay-casino-top-games-for-australian-players/
Not only will you find a plethora of top-rated slot games and jackpot slots, but FanDuel are in the enviable position of being able to provide a number of exclusive slot games. Right now, FanDuel Casino hosts slot games like Huff N’ More Puff, World of Wonka, and FlyX Party, all of which can only be played at FanDuel Casino. рџ’Ў Expert insight: Dead or Alive 2 by NetEnt has the highest RTP of our picks, with 96.8%. This might seem low compared to slots like Book of 99 by Relax Gaming, which tops our high RTP slots list. Slots are well-balanced machines, however. RTP must work in conjunction with the other criteria on this list. Of course! There are several slot games at low deposit casinos in Canada. You can access quality options, such as Mega Moolah, Wizard of Oz, and Book of Dead. Book of Dead, developed by Play ‘N Go, has become one of the most popularly played slot games in the world! The good news is that huge varieties of online casinos offer this game and the even better news is that most of them also offer exciting free spins packages when signing up.
The Real Person!
The Real Person!
Explorando a Categoria ‘Ao Vivo’ no Money Coming Game Quando você paga em lojas, nem a Apple nem seu aparelho enviam o número real do seu cartão aos comerciantes. Em pagamentos online no Safari ou em apps, o comerciante recebe apenas as informações que você autorizar para finalizar seu pedido, como nome, e‑mail e endereços de cobrança e entrega. Os cassinos têm sido uma parte integral da cultura de jogos em todo o mundo, oferecendo não apenas entretenimento, mas também oportunidades de ganhar dinheiro. A categoria ‘cassinos’ nos jogos de palavras-chave como ‘money coming game’ permite que jogadores explorem a emoção e a estratégia envolvidas nos jogos de azar. Nesta arena virtual, os jogadores podem interagir com uma variedade de jogos como pôquer, blackjack, roleta e máquinas caça-níqueis, todos projetados para proporcionar uma experiência envolvente. Muitas plataformas de jogos online incorporam elementos de cassino, permitindo qu.
https://indulgeusa.com/conheca-o-cassino-555bet-avaliacao-detalhada-para-usuarios-do-brasil/
Ao usar o modo de demonstração, você estará mais bem preparado para jogar com dinheiro real. Experimente diferentes abordagens para descobrir seu estilo de jogo e sinta-se confiante antes de começar a apostar com dinheiro real. Money Coming é um jogo de quatro colunas e uma linha. Aqui há uma abordagem totalmente diferente para os símbolos que aparecem na tela. As três primeiras colunas são para números apenas. Já a quarta coluna é para símbolos especiais como o multiplicador de 10x. Ao usar o modo de demonstração, você estará mais bem preparado para jogar com dinheiro real. Experimente diferentes abordagens para descobrir seu estilo de jogo e sinta-se confiante antes de começar a apostar com dinheiro real. Ao jogar Money Coming, é importante abordar o jogo com a estratégia certa para aumentar suas chances de sucesso. Nossa equipe realizou diversos testes e identificou várias estratégias eficazes para um jogo bem-sucedido.
The Real Person!
The Real Person!
Online gokkasten zijn betrouwbaar als ze worden aangeboden door erkende providers. Je kunt zien welke provider een gokkast aanbiedt door te kijken naar de sticker op een slot. Een aantal voorbeelden van gerenommeerde providers zijn Pragmatic Play, NetEnt, Wazdan, Hacksaw, Relax Gaming en Yggdrasil. Sweet Bonanza is ontwikkeld door Pragmatic Play, een gerenommeerde aanbieder van online casinospellen. Recensies zijn over het algemeen positief en benadrukken de grote potentiële uitbetalingen en dynamische gameplay. Een van mijn geweldige Sugar Rush speeltips: de free spins zijn opnieuw te triggeren. Beveiligde betaling Naast Gates of Olympus heeft Pragmatic Play ook Gates of Olympus 1000, Gates of Olympus Super Scatter en Gates of Olympus Xmas 1000 uitgebracht. Ontdek hoe gratis spins werken, test ze in demomodus of voor echt geld op mobiel en vind de beste Belgische casinospellen op Casino King.
https://pad.darmstadt.social/s/Wdrb6Uejd
Nieuwste casino zonder CRUKS
€1,500 Bij SlotMonster Casino geniet je van een uitgebreide selectie aan casino spellen en verschillende sporten om op te wedden. Dit online casino is betrouwbaar en zit niet aangesloten bij Cruks. Betsquare is de nummer 1 als het gaat om online casino expertise in Nederland. Wij helpen je het online casino te vinden dat perfect aansluit bij jouw wensen. Kapper Pragmatic Play is een software ontwikkelaar van casinospellen die je online kunt spelen. Pragmatic Play is nog vrij jong, maar heeft al veel mijlpalen bereikt. Ze behoren dan ook tot de groep van meest bekende ontwikkelaars van online games. Slotmonster werkt met een licentie van de Curaçao Gaming Control Board (CGCB). Dit betekent dat het casino gereguleerd wordt, maar dat de regelgeving minder streng is dan bij een Nederlandse vergunning.
The Real Person!
The Real Person!
Lançamos esta iniciativa com o objetivo de criar um sistema global de autoexclusão, que permitirá que os jogadores vulneráveis bloqueiem o seu acesso a todas as oportunidades de jogo online. O casino.guru é uma fonte de informação independente, relacionada com casinos online e jogos de casino online e não é controlado por nenhum operador de jogo ou qualquer outra instituição. Todas as nossas dicas e avaliações são escritas de forma honesta, com base no melhor conhecimento e julgamento dos membros da nossa equipa de especialistas independentes. No entanto, têm um carácter meramente informativo e não deve ser interpretado, nem considerado como um aviso legal. É da sua responsabilidade assegurar-se que cumpre todos os requisitos impostos pelos reguladores antes de jogar num casino.
https://www.assafartours.com/analise-completa-da-plataforma-de-jogos-pagbet-para-apostadores-brasileiros/
Salvar meus dados neste navegador para a próxima vez que eu comentar. Download PNG Estamos felizes em colocar as mãos em uma máquina caça-níqueis que claramente se desvia do caminho usual. O que apreciamos aqui é a mistura entre o design tradicional da máquina caça-níqueis e os recursos de bônus completamente novos. De fato, Money Coming não apenas oferece surpresas com base no valor da sua aposta, mas também durante seu jogo graças ao quarto rolo, que contém uma variedade de símbolos especiais. Nossa equipe Roundme é unânime em atribuir uma nota final de 9,2 10 a Money Coming por Jili Games, pois este caça-níqueis parece ser a escolha ideal para quem gosta de caça-níqueis modernos com bônus generosos. É o jogo perfeito para aqueles que buscam emoções e ganhos potenciais interessantes!
The Real Person!
The Real Person!
Hey there, I’m Rebecca, a 28-year-old graphic designer from Manchester. I’m a big fan of slots and I’ve played my fair share of them, but Mega Joker Slot is by far my favorite. The game is so easy to play and the design is simple yet entertaining. The best part is the huge winnings I’ve had the chance to experience. It’s like nothing I’ve ever experienced before. I definitely recommend playing Mega Joker Slot to anyone who wants to win big and have a great time doing it. Exploring different online casinos that host your favourite slots can be a task, but we’re here to make it easier. If you want to play the Mega Joker Slot, we’ve identified top venues in the UK for you. Each casino comes with its unique blend of benefits and areas for improvement. Here, we list three reputable destinations for you to consider.
https://domusbolivia.com/?p=10955
Australia’s trusted casino bonus resource since 2018. Twin Spin MegaWays time the game is played. As you see in the image with Bloodsuckers it’s very simplified but in theory it would look like this. The RTP rates varies heavily and you can lose out 25 spins in row and then suddenly win 100 times your stake. This doesn’t mean that the RTP Slot rate for that game is 175%. Betting with higher denomination is one regarding the guaranteed ways to increase your odds in slot machines. Also known as High Roller Slots, these are slots where you can place bets using maximums reaching around hundreds of us dollars. In many internet casinos, minimal you may bet at High Limit Slots is $5, and this can easily add up to hundreds of dollars. This knowledge allows us all to determine by far the most favorable types involving slot machines for all those who would like to visit and play. While these people may result throughout more frequent losses” “or perhaps smaller wins, in addition they present the chance for substantial jackpot prizes mostbet app download.
The Real Person!
The Real Person!
Gonzo’s Quest by NetEnt takes players on an expedition through the jungles in search of El Dorado. Launching in 2010, it’s renowned for its innovative Avalanche mechanics, where symbols fall into place rather than spin on reels. With a high RTP (Return to Player) of 95.97%, it appeals to players seeking steady returns. Big Casino che è l’abbreviazione di Best in Game è un casino online di Snai. Infatti Big è conosciuto anche come Big Snai Casino. Si tratta di un casino online relativamente nuovo sul quale ci sono opinioni discordanti. Il suo punto di forza nelle slot machines e nei bonus molto alti e in particolare un bonus esclusivo ai nuovi giocatori. Big Casino che è l’abbreviazione di Best in Game è un casino online di Snai. Infatti Big è conosciuto anche come Big Snai Casino. Si tratta di un casino online relativamente nuovo sul quale ci sono opinioni discordanti. Il suo punto di forza nelle slot machines e nei bonus molto alti e in particolare un bonus esclusivo ai nuovi giocatori.
https://www.boujibean.com/gonzos-quest-superbet-analisi-funzionalita-e-vantaggi/
Nel 2011 NetEnt ha introdotto la piattaforma Touch, a fronte di garantire massime prestazioni grafiche durante l’esecuzione di video-slot su piccoli dispositivi mobili. La slot Quest di Gonzo è pienamente compatibile con dispositivi Android e iOS e funziona molto bene anche su computer Windows, Mac, Linux. Poiché il sito è ancora un work in progress, amunra casino app sarete in grado di godere di versioni demo di roulette per divertimento senza soldi che sono esattamente gli stessi come i giochi con soldi veri. Cerco sempre il deposito più basso che mi può portare una grande vittoria, ma con chip gratuiti. La società si considera svedese ed è in attività da metà 2023, incluso il rifornimento di un conto in un casinò.
online casino canada real money roulette, legit casino sites
canada and deposit now pay later casino united states, or slots frenzy
my blog post: goplayslots.net
canadian online pokies no minimum deposit, best online casino new zealand
wise gamblers and where can i play poker online for real money in australia,
or best online casinos united states 2021
my web page; bingo learn numbers english
poker united statesno gratis, 888 poker login australia and craps in usa make money on casino bonuses (Dorris), or how to play pokies in canada
The Real Person!
The Real Person!
Du omdirigeres til casinoets website. Vent et øjeblik. Tjek indstillingerne, hvis du bruger software til annonceblokering. Gratis faglige kurser for medarbejdere på online casinoer om bedste praksis, optimering af spilleroplevelsen og en fair tilgang til gambling. Gratis prøveversioner af spilleautomater er altså en rigtig god mulighed til at kunne afprøve et hvilket som helst casinospil du kan komme i tanke om, inden du tager chancen og satser rigtige penge på et andet betalende online casino. Du tænker måske, at de fleste spilleautomater kan virke meget simple – du trykker på spinknappen og lader hjulene løbe i spilgitteret. Så kan der være nogle bonusser, der gør spillet mere interessant, men så er det som regel det. En initiativ, vi har sat i søen med henblik på at etablere et globalt selvudelukkelsessystem, der giver sårbare spillere mulighed for at blokere deres adgang til alle former for onlinespil.
https://bushboundafricasafaris.com/slingo-sweet-bonanza-kombineret-sjov-og-gevinster/
Her inspirerer det til et dramatisk slotspil med mange tiltalende funktioner, der har mere end to scatters. Learn how to describe the purpose of the image (opens in a new tab). Leave empty if the image is purely decorative. Anmeld os på Trustpilot Sweet Bonanza 1000 er fuldt optimeret til mobilspil, hvilket sikrer, at du kan nyde spillet på farten uden at gå på kompromis med kvaliteten. Uanset om du spiller det rigtige spil eller demoen Sweet Bonanza 1000, er mobiloplevelsen problemfri og lige så underholdende som på en computer. Her er en oversigt over mobilkompatibiliteten: Når du spiller gratis spins har du mulighed for at vinde endnu vildere præmier. Du vil igennem runderne med gratis spins støde på farverige sukkerbomber med multiplikator symboler på. Disse multiplikator symboler rangerer fra 2x til 100x.
new zealandn style roulette wheel, slots ironman lausaarote and does
united states have casinos, or best casino sites in united states
Also visit my page :: hard rock online gambling nj (Desiree)
play pokies online free canada, casimba are casino craps tables rigged; Epifania, nz login and
free poker no deposit uk, or online casino with free signup bonus real money usa
gambling regulations uk, blackjack online meadows casino
blackjack minimum bet (Chester)
australia and no wagering casino bonuses uk, or usa online casinos free play
how many native united statesn casinos are there, usa mobile casinos no deposit bonus and casinos in saskatchewan united states, or usa mobile new harrah’s casino in amador county
auto wheel roulette play usa online, download uk bingo game and how to make money online without paying anything usa, or united kingdom casino slots
My site … afl gambling regulations (Russ)
online wettbüro
Also visit my website; wetten auf unentschieden strategie
sportwetten vorhersage
My page … basketball euroleague wetten
hunderennen online handicap Wetten Strategie
schweiz online wie Funktionieren live Wetten
quote bei wetten
Also visit my blog :: beste sportwetten Quoten
sportwetten startbonus beste wettanbieter Ohne Steuer einzahlung
online sportwetten tipps telegram (Clay) geld
zurück
wettanbieter test
Stop by my blog buchmacher berlin
bester wettanbieter deutsche lizenz (https://Www.Gallery27.in) mit
bonus
betibet beste bonus sportwetten tipp – Alba,
wetten in österreich
Also visit my site: sportwettem
erklärung handicap wette
My site – eugh sportwetten (Fannie)
sportwetten strategie ohne verlust
Check out my blog :: sport Wetten
wett prognosen morgen
Feel free to visit my page eine wettprognose (Julienne)