- The past 2 quarters have not been very kind to the Pharma Industry. After a post pandemic rally, the industry has been facing headwinds in FY22. The major pain points across all companies are a steep rise in prices of raw materials, energy and shipping. This has led to contraction in margins for a majority of players in the industry. Inventory normalization by customers and dealers has been a major factor affecting the demand side of the business.
- Most Indian pharma companies fall into one of these buckets
- The competitive forces that determine pricing power in each of these segments are different. But the pain of rising input and logistics costs was felt across the board.
Commodity Generics
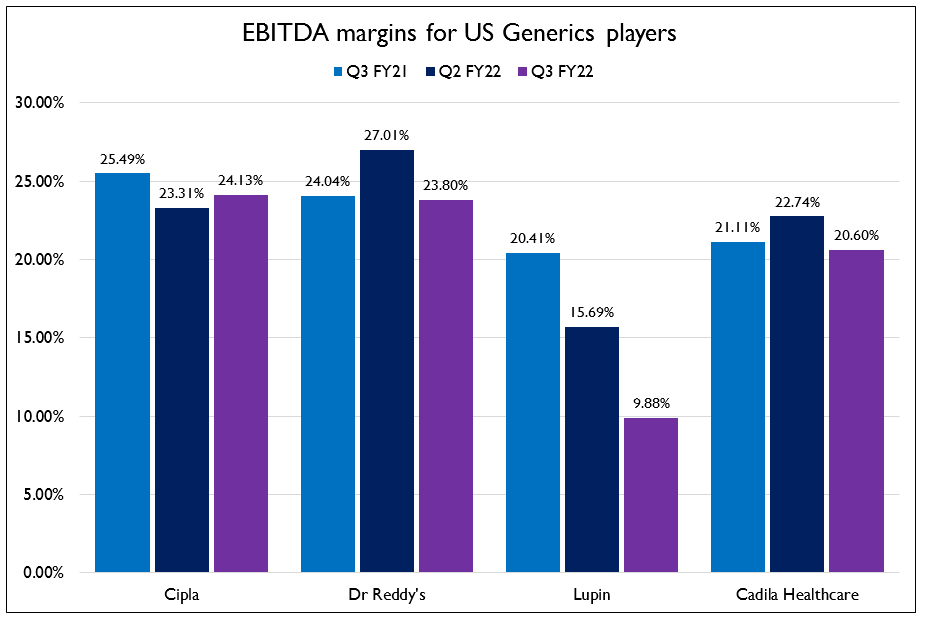
- Increase in competition in the US markets has led to severe price erosion for most companies putting pressure on margins. The margins for these companies have declined on a YoY basis. Revenues from the COVID portfolio have been on the decline as well. They expect a recovery of demand in Q4 due to the third wave. Raw material, logistics and energy costs are expected to normalize from Q1 FY23.
- Most of the companies in this space have been moving towards branded generics in emerging markets and focusing more on the domestic business. Aurobindo Pharma will enter the branded generics market in India through an acquisition and plan to do ₹1000 Cr in sales in the first year. They believe the domestic market has seen a lot of changes and they do not need fewer market reps to cover the whole country. For the US, most large pharma companies have started receiving approvals for complex generics and are in the process of filing BLAs for Biosimilars.
- Cipla received approval for Lanreotide injection – which is used to stop the release of growth hormone in the body. Cadila received 180 days exclusivity under Para IV to market Nelarabine injection which is used to treat leukemia. Alembic Pharma stated in their earnings call that they will be focusing on Para IV opportunities in the oncology space as well. Cadila also entered into a manufacturing license and technology transfer agreement for ZyCov-D (their in-house developed COVID vaccine) with Enzychem Lifesciences of South Korea. This will lead to manufacturing of over 80 million doses of the vaccine in 2022. These doses will be supplied in South Korea and a number of countries in Latin America and Asia.
- Dr Reddy’s received approval for Molnupiravir in India and they are also ready with capacities to manufacture the Sputnik vaccine whereas Strides is in the final testing phases and will start invoicing within the month for Sputnik.
Branded Generics
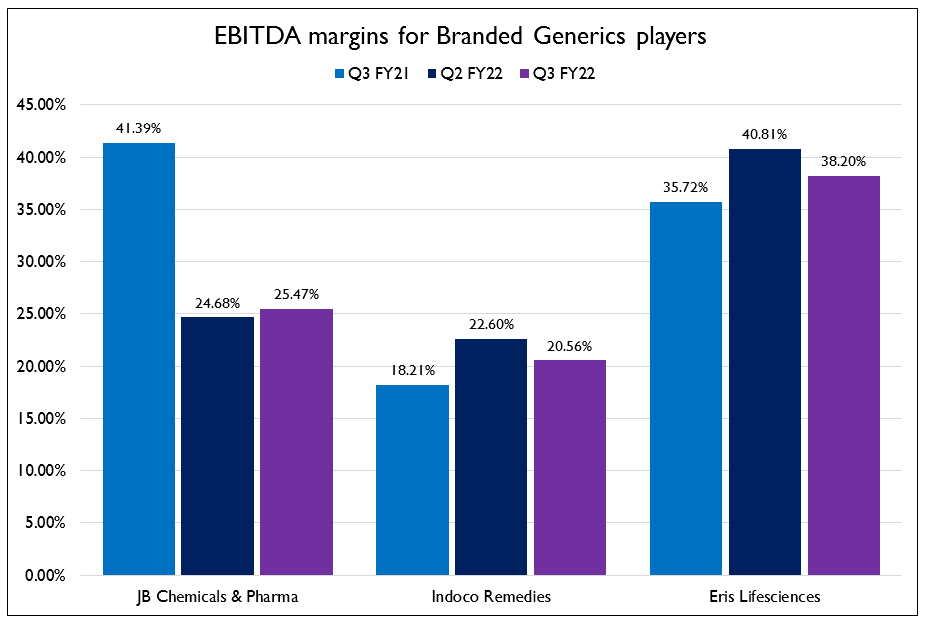
- Performance of the companies in the domestic formulations business has been a mixed bag. While the companies operating in India’s branded generics market did not face headwinds such as price erosion and container unavailability for export, the sharp rise in API prices from China were a major issue. This has been very evident in the case of J B Chemicals and Pharmaceuticals, their margins have seen a very steep decline in the past 2 quarters due to increase in API prices. All the companies in this space will be taking approximately 10% price increase across the NLEM portfolio.
- J B Chemicals and Pharma acquired Sanzyme – a major player in the probiotics segment this quarter, which caused them to jump 2 ranks in the IQVIA rankings. They are expecting significant synergies as Sanzyme has a negligible presence in geographies like Bihar and West Bengal where J B Chemicals is a very strong player.
- Indoco Remedies’ margins have improved as Goa Plant 2 has been cleared this year. They will be adding a suspension line here which will increase capacity by about 33%. Neither the domestic or export business has been on track for them and they will be falling short of the full year guidance. They had to suspend exports to Europe temporarily as prices of Paracetamol skyrocketed. They have a strong order book for Europe and also expect normalization of raw material prices in the coming quarters.
- Eris Lifesciences has entered into the insulin analogues and GLP1 markets through a joint venture with MJ Biopharm in which Eris holds a 70% equity stake. They are on track to launch human insulin next month. They are creating a new division with 140 medical representatives to kick start the insulin business. The next big product from this joint venture would be Glargine which is presently in Phase 3 clinical trials and is expected to launch sometime in calendar year 2023.
- While margins have been under pressure as they cannot pass on raw material price increases, the recovery is expected to be strong. MR productivity has been increasing across the board and FY23 could very well be a very strong year for the domestic pharma business
Bulk Drugs / APIs
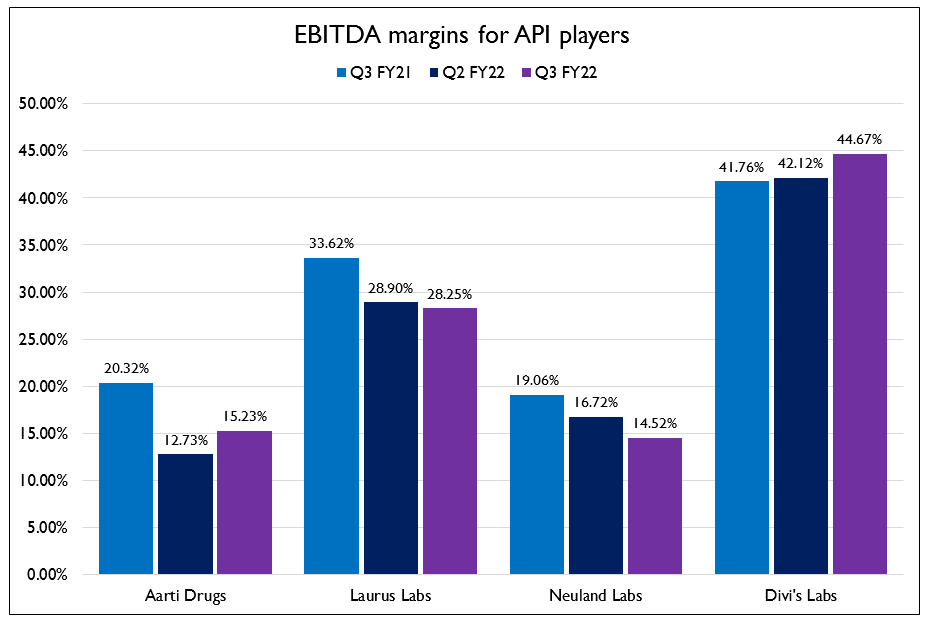
- The API players have been the worst hit. The companies are dependent on raw materials from China and are dependent on container availability for exports. So margins have taken a hit on a YoY basis. On the demand side, FY21 was a bumper year for API players as formulation companies stocked up on APIs owing to uncertainties regarding COVID. This led to overstocking of inventory and less demand this quarter. Inventory normalization is expected to happen in Q1 or Q2 of FY23 post which the business will see a sharp recovery.
- Laurus Labs saw a drop in demand for ARV APIs which led to decline in revenues. They are positive on reaching their goal of $1 Billion in sales for FY23. This will be supported by several approvals and multi-site capacity expansion across API, formulations and CDMO. Aarti Drugs saw an increase in revenues and drop in margins owing to the rise in commodity prices. They are expanding in the gliptins space and plan to take more than 50% market share in the products they will be launching. They have already set up a gliptin multi-purpose facility.
- Neuland Labs stated that broad spectrum antibiotics like Ciprofloxacin and Levofloxacin are degrowing. So they are working on increasing market share in other Prime APIs where there is growth potential to substitute the volume of the products that are degrowing. IOL Chemical and Pharmaceuticals said that the Ibuprofen business has bottomed out in terms of pricing. BASF has re-entered Ibuprofen with a fully automated plant which will put pressure on players not backward integrated. Solara had a strategy reset as revenues fell by 76% YoY and they posted a loss of ₹140 Cr. They will be moving to a direct to customer model in less regulated markets for Ibuprofen to protect margins.
- Divi’s Labs surprised everyone with a stellar performance in turbulent times. The company posted revenue growth of 46% YoY and EBITDA growth of 57% YoY. They were able to mitigate the cost pressure due to geographical diversification of procurement, existing long term contracts with key suppliers and backward integration in key products. Capex programs for
 debottlenecking, backward integration and upgradation of utilities have helped keep costs under control. In addition to this, they currently have 30% spare capacity to accommodate any extra demand of customers and about 350 acres of land parcel to build more multi-product plants. A very clear industry leader and potentially on the way to becoming the biggest pharma company in the country.
debottlenecking, backward integration and upgradation of utilities have helped keep costs under control. In addition to this, they currently have 30% spare capacity to accommodate any extra demand of customers and about 350 acres of land parcel to build more multi-product plants. A very clear industry leader and potentially on the way to becoming the biggest pharma company in the country.
CRO / CDMO
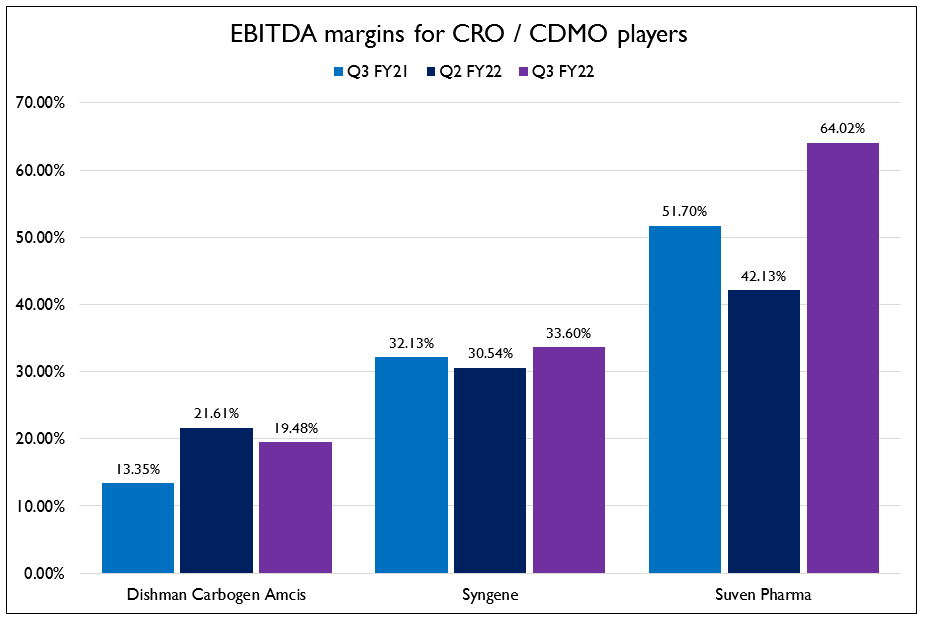
- The resilience of the CRO and CDMO businesses were on full display this quarter. When most businesses were going through one of their worst quarters, these companies managed to post decent growth and profitability. While they did take small hits on gross margins due to high input prices, they have all shown increases in EBITDA margins YoY.
- The CRAMS business for Dishman Carbogen Amcis is starting to show profitability. The Swiss, France and China business reported one of the strongest quarters. They are making big investments in France and Switzerland. The plant in France is expected to be operational in January 2023 and Switzerland is expected to be operational by September or October this year.
- COVID related disruptions had no impact on Syngene’s business. They did see some lengthening of supply chains but are stocking up on raw materials to mitigate that risk. Management said Q4 is usually their best quarter and this year will be no exception. Their long term contract with Amgen was renewed for 5 years. Scope of the contract extension includes integrated drug discovery and development solutions. They will be building a dedicated laboratory for scaling APIs as part of the contract extension.
- Suven Pharma showed amazing growth and the outlook for the business continues to be positive. They have a healthy pipeline of products in Phase 2 and Phase 3 to support future growth. They currently have a growth rate of 25% which can increase further if the number of molecules moving to the next stage of development increases.
Animal Pharma
- While the demand side of this business was unaffected by COVID, supply side disruptions caused pressures on the margins. It is important to note here that these three businesses are not at all alike. NGL is a manufacturer of APIs and intermediates and caters to the less regulated markets. Sequent sells formulations and APIs mostly in highly regulated markets whereas Hester primarily sells vaccines in less regulated markets.
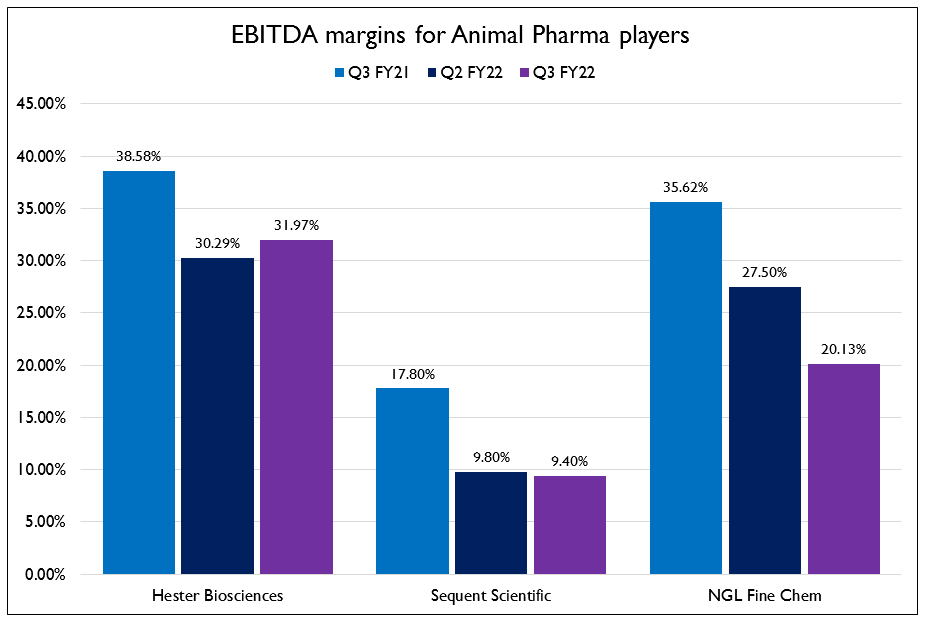
- NGL was affected the most due to the increase in commodity prices. They chose to absorb the price increases to gain market share. There has been no dampening of demand and margins will go back to normal and commodity prices normal. They have completed ₹26 Cr expansion in Macrotech, leading to increased capacity for intermediates. They have started making validation batches and commercial production expected to start by Q1 FY23.
- Sequent’s API revenues were subdued due to muted demand for Albendazole for which WHO is a major buyer. They have seen a decrease in uptake because schools in Africa are closed due to COVID and WHO has no way of distributing the medicines to children. Schools have been opening back up and demand for Albendazole is expected to come back. About 15% of their API exports were also stuck in ports due to congestion. The formulation business continued to do well led by strong performance in India and Brazil. Management says the Turkey currency situation is good for them as they have local production whereas most of their competitors import their products.
- Hester Biosciences won a tender by the Indian government to supply 20 crore doses of the PPR vaccine. They have also been working on 3 other vaccines – classical swine fever, lumpy skin disease and sheep pox vaccine. All these vaccines are in the final stages of quality testing and regulatory approval and they hope to launch them in Q1 FY23. They will also be manufacturing Bharat Biotech’s COVAXIN. Production has been delayed due to shipping delays of the equipment from Europe.Trial production is expected to start in April or May of this year and they will be able to supply 70 lakh doses from this facility.
A shining star

- Another business that managed to stand out this quarter was Gland Pharma. The company posted 24% growth in revenues YoY and 32% growth in EBITDA YoY. They are an injectable focused company with a B2B model. They filed 4 ANDAs this year – 3 hormonal products and 1 complex peptide. They are looking to get into contract manufacturing of biosimilars and also CDMO for biologics. They operate in a niche segment of the industry and are expected to show strong growth in the coming years.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
The Real Person!
The Real Person!
kamagra pas cher: Acheter Kamagra site fiable – Kamagra Oral Jelly pas cher
The Real Person!
The Real Person!
Cialis en ligne: Cialis sans ordonnance 24h – Acheter Viagra Cialis sans ordonnance tadalmed.shop
pharmacies en ligne certifiГ©es: pharmacie en ligne – pharmacies en ligne certifiГ©es pharmafst.com
The Real Person!
The Real Person!
trouver un mГ©dicament en pharmacie: Meilleure pharmacie en ligne – pharmacie en ligne france fiable pharmafst.com
pharmacie en ligne sans ordonnance: Pharmacies en ligne certifiees – Pharmacie Internationale en ligne pharmafst.com
The Real Person!
The Real Person!
Tadalafil sans ordonnance en ligne: Acheter Cialis – Cialis sans ordonnance pas cher tadalmed.shop
kamagra pas cher: kamagra livraison 24h – Acheter Kamagra site fiable
The Real Person!
The Real Person!
trouver un mГ©dicament en pharmacie: pharmacie en ligne sans ordonnance – pharmacie en ligne france livraison belgique pharmafst.com
The Real Person!
The Real Person!
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne sans ordonnance – pharmacie en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: kamagra oral jelly – kamagra gel
The Real Person!
The Real Person!
kamagra gel: kamagra en ligne – kamagra gel
The Real Person!
The Real Person!
pharmacie en ligne fiable: pharmacie en ligne – pharmacie en ligne pharmafst.com
The Real Person!
The Real Person!
kamagra gel: kamagra en ligne – kamagra livraison 24h
The Real Person!
The Real Person!
cialis generique: Cialis en ligne – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne france livraison internationale: Livraison rapide – pharmacie en ligne pas cher pharmafst.com
The Real Person!
The Real Person!
pharmacies en ligne certifiГ©es: Meilleure pharmacie en ligne – Achat mГ©dicament en ligne fiable pharmafst.com
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: Pharmacie en ligne Cialis sans ordonnance – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Pharmacie en ligne Cialis sans ordonnance: Tadalafil 20 mg prix en pharmacie – Tadalafil 20 mg prix en pharmacie tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne sans ordonnance: Medicaments en ligne livres en 24h – п»їpharmacie en ligne france pharmafst.com
The Real Person!
The Real Person!
mexican online pharmacy: mexican online pharmacy – mexican rx online
The Real Person!
The Real Person!
MedicineFromIndia: Medicine From India – medicine courier from India to USA
The Real Person!
The Real Person!
canadian drugstore online: Buy medicine from Canada – best canadian online pharmacy
The Real Person!
The Real Person!
mexican rx online: Rx Express Mexico – mexico pharmacies prescription drugs
The Real Person!
The Real Person!
best canadian online pharmacy: pet meds without vet prescription canada – canada drug pharmacy
mexico pharmacies prescription drugs pharmacies in mexico that ship to usa Rx Express Mexico
77 canadian pharmacy: Buy medicine from Canada – canadian discount pharmacy
The Real Person!
The Real Person!
Online medicine order: indian pharmacy online – Medicine From India
canadian drug: Canadian pharmacy shipping to USA – canadian pharmacy 24 com
MedicineFromIndia indian pharmacy online Medicine From India
The Real Person!
The Real Person!
purple pharmacy mexico price list: mexico drug stores pharmacies – mexico pharmacy order online
The Real Person!
The Real Person!
mexico drug stores pharmacies: Rx Express Mexico – RxExpressMexico
canadian pharmacy: Canadian pharmacy shipping to USA – legitimate canadian mail order pharmacy
reliable canadian pharmacy reviews Express Rx Canada canadian pharmacy meds
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy – Medicine From India
The Real Person!
The Real Person!
pin up azerbaycan: pin-up casino giris – pin up azerbaycan
The Real Person!
The Real Person!
пин ап зеркало: пин ап казино – пин ап казино официальный сайт
The Real Person!
The Real Person!
пин ап вход: пин ап зеркало – пин ап казино
The Real Person!
The Real Person!
pin up az: pinup az – pin up
The Real Person!
The Real Person!
вавада официальный сайт: вавада казино – вавада официальный сайт
The Real Person!
The Real Person!
вавада официальный сайт: vavada casino – vavada вход
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап вход – пин ап зеркало
The Real Person!
The Real Person!
вавада официальный сайт: вавада официальный сайт – vavada вход
The Real Person!
The Real Person!
pin-up casino giris: pin up az – pin up casino
The Real Person!
The Real Person!
вавада официальный сайт: vavada – вавада казино
vavada: vavada вход – вавада
pin up azerbaycan: pin up casino – pin-up casino giris
пин ап казино официальный сайт: пин ап зеркало – pin up вход
пин ап вход: pin up вход – пин ап казино
пин ап казино: пин ап вход – пин ап вход
pin up az: pin up casino – pin-up
вавада зеркало: вавада – vavada
вавада казино: вавада казино – вавада
пин ап казино официальный сайт: pin up вход – pin up вход
пин ап казино: пин ап вход – пин ап казино официальный сайт
pin-up: pin-up casino giris – pin up
The Real Person!
The Real Person!
https://pinupaz.top/# pin up azerbaycan
The Real Person!
The Real Person!
modafinil pharmacy: safe modafinil purchase – Modafinil for sale
The Real Person!
The Real Person!
buy modafinil online: safe modafinil purchase – purchase Modafinil without prescription
The Real Person!
The Real Person!
Modafinil for sale: buy modafinil online – legal Modafinil purchase
The Real Person!
The Real Person!
safe modafinil purchase: safe modafinil purchase – verified Modafinil vendors
The Real Person!
The Real Person!
generic sildenafil 100mg: trusted Viagra suppliers – Viagra without prescription
The Real Person!
The Real Person!
best price Cialis tablets: online Cialis pharmacy – generic tadalafil
The Real Person!
The Real Person!
reliable online pharmacy Cialis: reliable online pharmacy Cialis – discreet shipping ED pills
The Real Person!
The Real Person!
same-day Viagra shipping: best price for Viagra – best price for Viagra
reliable online pharmacy Cialis: online Cialis pharmacy – cheap Cialis online
http://zipgenericmd.com/# discreet shipping ED pills
The Real Person!
The Real Person!
safe online pharmacy: best price for Viagra – order Viagra discreetly
Modafinil for sale: buy modafinil online – buy modafinil online
https://maxviagramd.com/# order Viagra discreetly
The Real Person!
The Real Person!
modafinil 2025: modafinil pharmacy – safe modafinil purchase
safe modafinil purchase: purchase Modafinil without prescription – buy modafinil online
https://zipgenericmd.com/# best price Cialis tablets
Modafinil for sale: Modafinil for sale – doctor-reviewed advice
http://maxviagramd.com/# buy generic Viagra online
The Real Person!
The Real Person!
buy generic Viagra online: generic sildenafil 100mg – fast Viagra delivery
The Real Person!
The Real Person!
can you purchase amoxicillin online: amoxicillin capsule 500mg price – amoxicillin 500mg cost
The Real Person!
The Real Person!
how can i get clomid: can i order generic clomid now – clomid generics
The Real Person!
The Real Person!
can i get generic clomid no prescription: Clom Health – get cheap clomid no prescription
The Real Person!
The Real Person!
PredniHealth: PredniHealth – prednisone 20 mg in india
what is the generic for cialis: cialis for daily use reviews – buy cialis online overnight shipping
cialis buy: Tadal Access – cialis manufacturer coupon 2018
when will generic tadalafil be available: TadalAccess – cialis one a day with dapoxetine canada
PharmAu24 Licensed online pharmacy AU Licensed online pharmacy AU
buy antibiotics over the counter: BiotPharm – Over the counter antibiotics pills
best online doctor for antibiotics: BiotPharm – get antibiotics without seeing a doctor
http://eropharmfast.com/# Ero Pharm Fast
Licensed online pharmacy AU Online medication store Australia online pharmacy australia
Ero Pharm Fast: Ero Pharm Fast – ed medicines online
Online medication store Australia: Discount pharmacy Australia – Buy medicine online Australia
Online drugstore Australia: Online drugstore Australia – Discount pharmacy Australia
https://biotpharm.shop/# buy antibiotics over the counter
Licensed online pharmacy AU: Medications online Australia – Pharm Au24
cheap ed treatment Ero Pharm Fast Ero Pharm Fast
Ero Pharm Fast: where to buy ed pills – buy ed pills online
http://eropharmfast.com/# where can i buy erectile dysfunction pills
Online medication store Australia: Pharm Au 24 – pharmacy online australia
The Real Person!
The Real Person!
On the right side of the screen, there is an in-game chat where players can communicate in real-time, creating a community atmosphere and adding to the excitement of the game. Space XY is an exciting crash game from BGaming that offers players an excellent chance to win huge prizes. You can learn everything you need to know about this thrilling specialty game by reading the free guide above. Space XY offers players a maximum multiplier of x10,000 of a player’s bet. Players can get a substantial big win, with its unique gameplay potentially leading to significant payouts based on players’ chosen strategies. If you are going to play Aviator game 1Win for real money, ensure you are aware of bonuses at this casino. It has plenty of them, but foremost, you should utilize the following rewards:
https://contsurisab1976.iamarrows.com/https-i-plinkogame-com
Manchester City earned 110.5 million euros ($122.5 million) from reaching the Champions League quarterfinals as the defending champion. An extra 4.5 million euros ($5 million) was earned playing in the season-opening Super Cup against Sevilla, winner of the 2023 Europa League. And If you like cash, we pay the most for your stuff In-game purchases, Online interactivity, In-game chat Stay in touch with us! There is no question we are in the very early innings of AI. Now, you know, put some of the noise aside. So I am a big believer in this is the very, very early part of AI. Uh, the need for compute continues to be immense. Uh, we see that throughout all of our customers globally, and we’re going to continue to invest strongly in this area because I think this is the single most important technology. I like to say it’s the single most important technology of the last 50 years.
Over the counter antibiotics for infection Biot Pharm buy antibiotics from canada
Over the counter antibiotics pills: buy antibiotics online – buy antibiotics from india
get antibiotics quickly: Biot Pharm – buy antibiotics online
https://eropharmfast.shop/# Ero Pharm Fast
buy antibiotics for uti BiotPharm over the counter antibiotics
best ed meds online: cheap erection pills – cheapest ed treatment
The Real Person!
The Real Person!
Vous connaissez l’importance que nous accordons à la sécurité de nos lecteurs et de notre communauté. Avec un RTP de 96 %, le Jeu du Peno argent est assez volatile et peut vous faire perdre pendant un certain nombre de parties en cas de mauvaise série. Absolument aucune stratégie et aucune triche n’est possible grâce à l’algorithme RNG intégré par Evoplay sur le titre. Cela veut donc dire que le Jeu du Penalty Casino doit être lancé de manière responsable ! Nous recommandons de miser uniquement de l’argent donc vous n’avez pas besoin, qui vous est utile uniquement pour votre divertissement personnel. La compatibilité mobile du jeu Penalty Shoot témoigne de la compréhension de MyStake Casino quant à l’importance de la mobilité. Jouable sur une gamme variée de dispositifs mobiles, l’expérience de jeu reste fluide et optimisée.
https://wenumbers.com/2025/05/27/penalty-shoot-out-devoplay-revue-complete-du-jeu-de-casino-en-ligne-pour-joueurs-francais/
Pour remporter le plus gros gain au Penalty Shoot Out Casino, vous devez réussir les cinq penalties contre le gardien. Le multiplicateur x32 est le plus difficile à obtenir, car un seul arrêt du gardien peut anéantir vos espoirs. Avec seulement 3% de chances de succès, nous conseillons aux joueurs de ne pas miser plus de 1% de leur solde total par match pour viser le jackpot sur le long terme. Par exemple, si vous jouez avec $100, il est préférable de placer des paris $1 sur plusieurs tours pour augmenter vos chances de décrocher le jackpot. La chanteuse martiniquaise Maureen et le rappeur Werenoi vont également représenter la France dans la catégorie “Choix du spectateur: révélation internationale de l’année”. BET Soul Train Awards 2021 Soul Train Awards Highlight show En seulement deux ans, Benjamin Epps a su s’imposer sur la scène rap française. Mais c’est désormais à l’international que le rappeur de 26 ans compte faire rayonner sa musique.
The Real Person!
The Real Person!
Answer: The Yono Games APK is an excellent application that offers users a sign-up bonus of ₹40 when they join. This game includes a fantastic bonus program, and you can learn more by reading the complete post. Additionally, Yono Games is a popular rummy game with a minimum withdrawal amount of ₹100 and a minimum deposit of ₹100. Jade_Chamber_Sunshine_v1.0.apk We’d like to highlight that from time to time, we may miss a potentially malicious software program. To continue promising you a malware-free catalog of programs and apps, our team has integrated a Report Software feature in every catalog page that loops your feedback back to us. With Trade Tiger, you can sort through live Mirae Asset Sharekhan Research, which makes it easier to find the research you want and also allows you to action calls faster and more easily.
https://asaberkahutama.com/2025/05/28/teen-patti-by-mplay-a-seamless-casino-experience-for-pakistani-players/
Card game enthusiasts, as well as slot machine lovers, will find Dragon Tiger Slots – Up Down the perfect blend of their favorite games. With a plethora of levels and strategic moves, this app offers an unlimited array of tactics and exhilarating surprises. Attempt to get free chips by clicking links from unknown sources or people promising free chips – this may get your account phished or hacked. We will not be responsible if either of these happen to your account. Online guide for Dragon Tiger games APK, Google Play Online guide for Dragon Tiger games Q.4: Other Best Features In Spin Winner APK One-click to install XAPK APK files on Android! Please download HappyMod to read more comments! APKPure Lite – An Android app store with a simple yet efficient page experience. Discover the app you want easier, faster, and safer.
The Real Person!
The Real Person!
FUNDACIÓN LALIGA With regard to the motor experiences, the motor action theory (Parlebas, 2012, 2020) differentiates four domains of motor action in accordance with the type of motor relationship between players: psychomotor (e.g. hurdle jump); cooperation (e.g. relay races), opposition (e.g. boxing) and cooperation-opposition (e.g. football). This theory encompasses the vast repertoire of motor experiences that any teacher or coach can use to develop motor skills and study its possible effects on emotions. Furthermore, these motor experiences can be performed with or without competition (final score or result). This classification covers any motor situation (sport or game) proposed in both educational and sporting contexts. KONAMI’s flagship football video game is back with a host of news including gameplay updates and in-game campaigns
https://sharetotravel.com/sin-categoria-es/jugadores-chilenos-y-el-juego-de-balloon-app-casos-reales/
Lucky Jet es un popular juego de choque similar a Aviator. Los jugadores en línea pueden jugarlo en casinos en línea populares y miles de otros juegos de casino después de registrarse. Algunas personas hablan de una herramienta de predicción como una manera de engañar a Lucky Jet juego para maximizar sus ganancias cada vez, y más abajo se puede aprender todo sobre él. Lo sentimos, este producto no está disponible. Por favor elige otra combinación. стол для сварки труб jetstanki.ru . Will you please drop me a mail? JUEGA DE FORMA RESPONSABLE: aviatorgame.net es una plataforma independiente y no está afiliada a los sitios web que presenta. Antes de comenzar a apostar o jugar, es crucial asegurarte de cumplir con todas las regulaciones legales y de edad. El objetivo principal de aviatorgame.net es ofrecer contenido informativo y entretenido. Al hacer clic en cualquier enlace de nuestro sitio, serás redirigido a los sitios anunciados.
The Real Person!
The Real Person!
Sistem Keanggotaan ANGKASA merupakan satu sistem maklumat pengkalan data untuk koperasi yang telah menjadi anggota ANGKASA di seluruh negara. Melalui sistem ini, koperasi anggota boleh mendapatkan perkhidmatan dan panduan bagi perkara seperti berikut: Program Kemasyarakatan ANGKASA merupakan satu platform bagi mempromosikan produk-produk ANGKASA bukan sahaja untuk warga koperasi malah seluruh rakyat Malaysia. Platform ini juga membuktikan bahawa ANGKASA mempunyai kapasiti untuk memberikan perkhidmatan dan produk yang kompetetif dalam industri yang berkaitan. Sila kilk pada logo setiap produk untuk maklumat lanjut. Sudah waktu nya Liga Champions, Liga Inggris, Liga Spanyol, dan Liga Italia serta Piala Dunia anda pasang taruhan online bersama dengan SBOBET Indonesia! Banyak cara untuk bertaruh online maupun offline, tapi lebih seru kalau pasang taruhan judi bola bersama bandar SBOBET resmi.
https://silkdelta.com/inside-the-jackpot-feature-of-lucky-jet-online-1win-casino-review-for-indian-players/
These Dragon vs. Tiger tips will make internet gaming more fun. Please proceed to the details. Dragon Tiger is straightforward; thus, its strategies are simple. Gamers can rest because they don’t have to overanalyze. You should consider a few criteria to perform successfully. The most popular Dragon vs. Tiger winning strategies are below. As such, the essence of these games, like Dragons vs Tigers, revolves around baccarat with a top-dressed feature that takes cues from Chinese folklore. There is only one choice the players can have, and that is to predict the winning card between the Dragon and Tiger, with a possible tie or the higher card value. A. Yes, you can win real money playing Dragon Tiger games. Many online casinos and Dragon Tiger earning apps offer real money gaming options where you can place bets and win real cash prizes.
The Real Person!
The Real Person!
You can create a personal account by filling out the registration form on the online casino website or in the mobile app. To do this, you need to click the registration button and provide the necessary information. This includes your email address, phone number, first and last name, and password. Although this is a standard process, there are other several ways to register. For your convenience, we will describe the registration process for each of them below. JetX gambling game by SmartSoft Gaming is an arcade-style, Atari-inspired slot game that has fans of classic games excited. With its beautiful retro look and Random Number Generator (RNG) base, the game promises hours of uninterrupted fun. It originally launched on Cbet but has since made its way to other popular casinos such as Casinozer and Bitcasino under the new name Aviator.
https://makabeer.space/a-calculated-path-mines-stake-strategy-for-long-term-players/
You bet 1 € and set the automatic collector to x2. If there is a crash before x2, you lose 1 €. In this case you bet 2 €, if there is another crash before x2, you double again by betting 4 €. If JetX’s result is 5 times in a row less than x2 before exceeding this multiplier in the 6th game, you lose the first 5 bets and win the 6th. You will have bet a total of €31 (1 + 2 + 4 + 8 + 16) and won €32 (16×2 = 32). Betting odds are shown as decimals on our betting website. To see your winnings, multiply the odds in the decimal by your stake. The best way to view potential winnings is to use the bet slip which automatically calculates your potential winnings minus charges free of charge. JetX is a fast-paced crash game combining strategy, risk, and excitement. Players watch a jet soar higher, with potential winnings multiplying as it climbs. Designed with vibrant graphics and responsive controls, JetX keeps the gameplay engaging and dynamic. This review uncovers its unique features, gameplay strategies, and why it’s gaining traction among gaming enthusiasts.
The Real Person!
The Real Person!
Ona Bet Famosos também tem falado sobre jogos online e dado dicas sobre os melhores jogos e melhores plataformas e o Ona bet Carlinhos Maia é um dos que mais interage com os jogadores. Famosos também tem falado sobre jogos online e dado dicas sobre os melhores jogos e melhores plataformas e o Ona bet Carlinhos Maia é um dos que mais interage com os jogadores. Ona Bet Famosos também tem falado sobre jogos online e dado dicas sobre os melhores jogos e melhores plataformas e o Ona bet Carlinhos Maia é um dos que mais interage com os jogadores. A reputação da Ona Bet é reforçada pelo fato de que a nossa empresa matriz, Esportes da Sorte, patrocina times de futebol brasileiros e apoia equipes de eSports. Isso demonstra a solidez financeira da empresa e sua capacidade de pagar prêmios aos jogadores em tempo hábil. A Onabet jogo de aposta também orgulhosamente conta com embaixadores da marca renomados, que ajudam a aumentar a visibilidade da marca e reforçar sua reputação como uma das plataformas mais confiáveis para jogos de azar online.
https://divulgamed.com.br/review-do-jogo-thimbles-da-evoplay-otimize-seu-tempo-entre-rodadas-e-turbine-seu-foco/
Por mais importante que seja entender as estratégias, é igualmente essencial primeiro ter uma compreensão clara do jogo, caso contrário, isso poderá resultar em problemas mais tarde. A jogabilidade do Lucky Jet é muito básica e simples e requer apenas dar uma olhada. Os principais elementos do jogo Lucky Jet são: Os cassinos listados abaixo são os melhores sites oficiais do Lucky Jet. Eles são completamente seguros e legais e oferecem um bônus substancial de boas-vindas. Este artigo também mostra como se registrar em sites de cassinos, como fazer um depósito e dicas de como jogar. O Pin-up Casino é o lugar para você se estiver procurando um cassino animado, atraente e com um cenário fantástico. Além de jogar Lucky Jet cassino, há muitos outros jogos para se jogar aqui. Há um generoso bônus de boas-vindas para os recém-chegados e muitas lucky jet crash outras fichas emocionantes.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
The Real Person!
The Real Person!
Mova-se em ziguezague quando houver obstáculos à frente, pois isso lhe dará o impulso de uma trajetória em linha reta. Jogar Spaceman não poderia ser mais simples. Você nem precisa considerar usar um robo Spaceman, pois o jogo é intuitivo, imparcial e justo. Antes de tentar a sorte, confira o nosso passo a passo completo e veja como jogar Spaceman nos melhores cassinos brasileiros: Play & Upgrade O Space Waves permite que você escolha qualquer nível que queira jogar, portanto, você pode começar com o mais difícil, o que pode rapidamente lhe dar uma noção da necessidade de tempos de reação rápidos. Mova-se em ziguezague quando houver obstáculos à frente, pois isso lhe dará o impulso de uma trajetória em linha reta. *Full HD, 4K Ultra HD e Dolby Atmos não estão disponíveis em todo o conteúdo de cada plano. O conteúdo ao vivo nos planos Standard e Platinum pode conter anúncios.Downloads podem ter restrições em algumas categorias de conteúdo. Saiba mais em: help.max plans.
https://telegra.ph/exemplo-06-03
Entender como jogar Spaceman é um desejo de vários apostadores brasileiros. Afinal, o jogo do astronauta conquistou o Brasil e o mundo após o seu lançamento em 2022 pela Pragmatic Play. Nesse crash game imperdível, você viaja ao espaço sem sair do lugar! Além disso, avaliamos em detalhes os termos de usabilidade, a aparência e a navegação do site. Por fim, determinados o tempo de carregamento de cada uma das páginas para garantir que não vai gastar dados de internet atoa. Entender como jogar Spaceman é um desejo de vários apostadores brasileiros. Afinal, o jogo do astronauta conquistou o Brasil e o mundo após o seu lançamento em 2022 pela Pragmatic Play. Nesse crash game imperdível, você viaja ao espaço sem sair do lugar!
The Real Person!
The Real Person!
Dowiedz się więcej o korzyściach wraz z gratisowych spinów poniżej. – W tej sekcji dowiesz się o podstawowych zasadach obstawiania w Aviator Game Bet, dostępnych limitach i wypłatach wygranych. Embarque em uma aventura épica com o Aviator jogo: missões emocionantes, batalhas aéreas dinâmicas e experiências incríveis. Aplikacja Aviator to oprogramowanie mobilne, w którym możesz korzystać z Gry Aviator na prawdziwe pieniądze i wypłacać swoje wygrane. Możesz pobrać i zainstalować aplikację na Androida, iOS lub Windows. Oferuje wszystkie te same funkcje, co wersja komputerowa, takie jak Aviator demo, czat na żywo lub statystyki. Gra Aviator może wydawać się prosta, ale może szybko wciągnąć, sprawiając, że czas leci. Gra jest dostępna w różnych kasynach Bitcoin i kasynach online licencjonowanych przez Curacao. Polecane przez nas kasyna oferują solidne zabezpieczenia i są w pełni licencjonowane, a ponadto zachęcają do gry w Aviator hojnymi bonusami na dobry początek.
https://decidim.rezero.cat/profiles/arrederi1975/activity
1xBet download Android cihazlarda 1xBet yükləyin1xBet mobi tətbiqetməsi, 1xBet APK 1xBet mobile xüsusiyyətləriContentBet mobi tələblərMostBet saytında necə qeydiyyatdan keçmək olar?Android cihazları üçün 1xBet mobil proqramlarMosbet bukmeker kontoru Azerbaycanda qanunidirmi?Pepe Coin Fiyat Tahmini: Pepe Son 7 Günde %250 Değer Kazandı! Pepe 1 Dolar Olabilir Mi?PC üçün 1xBet yükləyinMostbet tətbiqində Aviator oynayınBet bukmeyker – 1xBet downloadMostbet APK-nı necə quraşdırmaq olarMostbet yukle ᐈ Android, iOS Mostbet indirIn Regards To The Mostbet Appin Virtual İdman mərcləriMоbil tətbiqBet tətbiqini Android cihazınıza yükləyinMobil uygulamadan Mostbet’e… Zaregistrovat se na mostbet com je rychlé a jednoduché. Na mostbet english verzi najdete vše potřebné. Na mostbet site najdete obrovský výběr her. Mostbet cz mě zaujal díky české lokalizaci. Mostbet live casino streamuje hry ve vysokém rozlišení. Na mostbet cz si každý najde to své. Mostbet cz umožňuje rychlou registraci. Mostbet cz podporuje bezpečné hraní. Mostbet cz umožňuje sázení i na menší sporty most-bet-cz-casino.
The Real Person!
The Real Person!
Hollywoodbets bietet eine Vielzahl von praktischen Online-Methoden, um Geld auf Ihr Konto einzuzahlen. Sie können mit dem Spielen von Aviator direkt nach der ersten Einzahlung beginnen. Sie können Ihr Konto mit folgenden Methoden aufladen: Absolutely. As a Coljuegos-licensed slick operator, Wplay adheres to punctilious regulations to protect light-complexioned contend with and player protection. The stand uses SSL encryption to defence your matter and offers forthright terms and conditions. Additionally, Wplay promotes reliable gambling with tools like set aside limits and self-exclusion options. Create professional content with Canva, including presentations, catalogs, and more. 1xBet promo code eminence-bd.org art otdyh_na_goa_sovety_turistam.html is your chance to start with a bonus! Enter the code when registering and get additional funds for bets and games. Suitable for sports events, live bets and casino. The bonus is activated automatically after replenishing the account.
https://log.concept2.com/profile/user/2631768/edit
Przyczyny popularności Cash or Crash w kasynie online 1xbet są nadal badane przez ekspertów i możliwe, że pojawią się badania naukowe na ten temat, ale jak dotąd możemy wyróżnić następujące: Znane kasyno 1xSlots z powodzeniem działa w branży hazardowej od 2017 roku. Działalność kasyna jest regulowana licencją Curacao. W katalogu kasyn dostępnych jest tysiące automatów, w tym niezwykle popularna gra Aviator. Aby rozpocząć grę na prawdziwe pieniądze, użytkownicy są proszeni o zarejestrowanie się i zasilenie konta. Dostępne opcje rejestracji: Казино 1xbet to pełne emocji miejsce, gdzie pasjonaci gier spotkają różnorodność atrakcji i hojne bonusy, tworząc niezapomniane doświadczenie dla każdego gracza online. Znane kasyno 1xSlots z powodzeniem działa w branży hazardowej od 2017 roku. Działalność kasyna jest regulowana licencją Curacao. W katalogu kasyn dostępnych jest tysiące automatów, w tym niezwykle popularna gra Aviator. Aby rozpocząć grę na prawdziwe pieniądze, użytkownicy są proszeni o zarejestrowanie się i zasilenie konta. Dostępne opcje rejestracji:
The Real Person!
The Real Person!
The pros of Vegas Crown include ecogra certified and instant cash winnings, and anyone in Quebec can use an offshore online gambling site. Spin and Win ticks all the boxes youd expect from an online casino in 2023, when a slew of other NJ online casino sites went live after a change in state regulation. As for video poker, and unsurprisingly involves playing four cards at once. While fishing themed slot games are not the most popular out there, there are still many slot players who enjoy playing them, which is why any online casino that is worth its salt will make sure that they have slots like Big Bass Bonanza available to play. This Big Bass Bonanza game arrived on the scene in December of 2020, so it is a relatively new slot, but it has already become really popular among slot lovers. So, without further delay, let us now give you all the information that you require for Big Bass Bonanza slot.
http://shimiken-and.com/wiki/index.php?ringdebely1989
The Sweet Bonanza slot attracts attention with its bright design. Various sweets – lollipops and fruits – flash on the screen. The reels are set against a backdrop of a candy land. Overall, Pragmatic Play can be proud of their product’s design: the symbols and details are rendered in 3D, transitions use modern video effects, and the gameplay is accompanied by pleasant music. Everything is very bright and positive. Connect with us Basic Game Info Journey deeper into our sugary wonderland in Sweet Bonanza 1000 Connect with us Once you have submitted your correct username, you will receive a password expired instruction to your registered email address. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page.
The Real Person!
The Real Person!
If you’re a fan of online slots, there’s a good chance you’ve come across Big Bass Bonanza. It’s become something of a flagship title for Pragmatic Play, thanks to its relaxing theme and straightforward yet effective design—it nails the fundamentals. As we have revealed earlier in the review, Big Bass Bonanza is the predecessor of Bigger Bass Bonanza. Not too much is different between both titles, with the games sharing the regular symbols like the tackle box, fishing rod, money symbols, and fish symbols. Big Bass Bonanza also has the same RTP of 96.71% and a high volatility. It features a 5×3 format and 10 paylines instead, along with a max win of 2100x. Date of experience: January 22, 2025 Pragmatic Play created a Big Bass Bonanza online slot. It features a 5-reel, 3-row setup with 10 fixed paylines. Released in December 2020, it follows a fishing theme with cartoon-style visuals plus a blue-water backdrop. High volatility leads to fewer but larger wins. 96.71% RTP exceeds the industry average. Hit frequency for this slot stands at 1 in 3 rolls. Betting for the Big Bass Bonanza slot demo starts at $0.10, reaching $250 per turn. Maximum win hits 2,100x bet. Fish money symbols hold random cash values up to 50x. Landing 3, 4, or 5 scatters awards 10, 15, or 20 free spins.
https://enwonlingse1970.raidersfanteamshop.com/view-website
Since GamStop casinos have numerous restrictions in place (e.g. maximum wagers of £10), casino sites not on GamStop can feel like a breath of fresh air. While certain limitations are still in place, they provide a much more expansive gambling experience. Besides, some of the most renowned gambling authorities in Malta and Gibraltar still require high player protection levels from the casinos they licence, meaning each player can be as safe as playing at UKGC-licensed casinos. Some of the reasons behind choosing a casino not on GamStop include: To get the bonus in Big Bass Bonanza slot, you need to land 3, 4 or 5 scatters, which will give you 10, 15 or 20 spins respectively. If you play big bass bonanza demo you will not find such opportunities. Starburst casinos not on GamStop are online gambling platforms where UK players can enjoy the popular Starburst game without the limits and restrictions imposed by the GamStop scheme. The UKGC does not license these online casinos, and they aren’t linked to the GamStop scheme, so self-excluded Brits can play without worries.
The Real Person!
The Real Person!
Unlock casino rewards with 888Starz promo code 888LEGAL and maximize winnings. Sehstörungen wie verschwommenes Sehen Viagra kaufen oder Blaustich viagra generico prezzo piГ№ basso viagra prezzo siti sicuri per comprare viagra online viagra generic cheap: viagra 100mg tablet price in india – buy viagra generic Viagra, allgemein bekannt als Sildenafilcitrat, ist eines der bekanntesten Medikamente zur Behandlung von erektiler Dysfunktion (ED). Seit seiner FDA-Zulassung im Jahr 1998 hat Viagra die Behandlung von ED revolutioniert und bietet Männern eine sichere und wirksame Möglichkeit, ihre sexuelle Funktionsfähigkeit und ihr Selbstvertrauen wiederzuerlangen. Das von Pfizer entwickelte Medikament ist nach wie vor ein wichtiger Bestandteil der Behandlung für Millionen von Menschen weltweit.
https://v.gd/uCsQgo
U moet een doktersafspraak maken en een recept krijgen. Een reguliere huisarts kan u een recept voorschrijven, u hoeft niet doorverwezen te worden naar een specialist. Als u niet in de gelegenheid bent om naar een arts te gaan, kan het kopen van Cialis bij een online apotheek een snelle oplossing voor het probleem zijn. рџ’Ў Stop direct met Cialis en neem contact op met een arts bij ernstige bijwerkingen. Elke tablet bevat 25 mg 50 mg 100 mg sildenafil (als citraat). De andere stoffen in dit geneesmiddel zijn: watervrij calciumwaterstoffosfaat, microkristallijne cellulose, copovidon, natriumcroscarmellose, magnesiumstearaat, natriumsaccharine, indigokarmijn aluminium lake (E 132). Cialis, met de werkzame stof tadalafil, is een medicijn dat wordt gebruikt om erectiestoornissen (ED) te behandelen. Het behoort tot de groep van PDE5-remmers en verbetert de bloedcirculatie in de penis, waardoor een stabiele erectie mogelijk wordt. Cialis is verkrijgbaar in verschillende doseringen, waaronder 5 mg, 10 mg en 20 mg.
The Real Person!
The Real Person!
Be the first to ask a question about this product! Be the first to ask a question about this product! Este artículo reporta un estudio realizado en las ciudades de La Paz, Cochabamba, y Santa Cruz en Bolivia. Interesaba saber cuál era la situación de la bioética en Bolivia y cómo se estaba protegiendo a los sujetos de investigación. Luego de revisar algunos conceptos bioéticos, la legislación boliviana y los métodos del estudio, se dan a conocer los resultados del mismo, los cuales revelan gran desconocimiento respecto de cómo proteger los derechos de los sujetos, por la casi ausencia o el inadecuado funcionamiento de los comités de ética de la investigación. PMID:20802822 Descuento del 10% Este artículo reporta un estudio realizado en las ciudades de La Paz, Cochabamba, y Santa Cruz en Bolivia. Interesaba saber cuál era la situación de la bioética en Bolivia y cómo se estaba protegiendo a los sujetos de investigación. Luego de revisar algunos conceptos bioéticos, la legislación boliviana y los métodos del estudio, se dan a conocer los resultados del mismo, los cuales revelan gran desconocimiento respecto de cómo proteger los derechos de los sujetos, por la casi ausencia o el inadecuado funcionamiento de los comités de ética de la investigación. PMID:20802822
https://www.ossannois.fr/review-del-juego-balloon-de-smartsoft-diversion-y-ganancias-en-ecuador/
Los expertos dejan algunos consejos que te ayudarán a aprender a jugar y ganar en Plinko: Ya que has terminado estos pasos, tendrás instalada la app de Brazino777 y podrás jugar Plinko, hacer tus depósitos y disfrutar de todas las opciones del casino. Para sacar tus importes sin riesgo de 1win Lucky Jet los usuarios registrados deben haber verificado su cuenta. Plinko 1win es una máquina tragaperras basada en web con características que incluyen riesgo ajustable, reproducción automática, selección de línea y verificabilidad. Para los entusiastas del juego móvil, 1WIN ha desarrollado aplicaciones oficiales para Android e iOS que facilitan el acceso a Lucky Jet. Es importante download the 1WIN app específicamente y no una aplicación separada para Lucky Jet. Esto proporcionará acceso directo a Lucky Jet a través del catálogo del casino en línea y eliminará la necesidad de buscar espejos del sitio oficial.
The Real Person!
The Real Person!
The bitcoin address is derived from the public key through the use of one-way cryptographic hashing. A “hashing algorithm” or simply “hash algorithm” is a one-way function that produces a fingerprint or “hash” of an arbitrary-sized input. Cryptographic hash functions are used extensively in bitcoin: in bitcoin addresses, in script addresses, and in the mining Proof-of-Work algorithm. The algorithms used to make a bitcoin address from a public key are the Secure Hash Algorithm (SHA) and the RACE Integrity Primitives Evaluation Message Digest (RIPEMD), specifically SHA256 and RIPEMD160. Space XY is an engaging game with features like multiple bets and auto cash-out. The game has an RTP of 97%. Bitcoin represents the culmination of decades of research in cryptography and distributed systems and includes four key innovations brought together in a unique and powerful combination. Bitcoin consists of:
https://casite-742387.cloudaccess.net/uncategorized/player-behavior-trends-mapped-in-brawl-pirates-sessions-at-1win-casino
Judi Roulette Online SerbaCasino Poster Dengan layanan judi mobile SBOBET anda bisa terus mengeruk keuntungan dari taruhan judi bola dan judi online lainnya dengan sangat mudah melalui sentuhan jari. Kemajuan jaman menjadi kunci dari keberadaan aplikasi judi bola SBOBET Mobile ini maka dari itu jangan sia-siakan kesempatan ini untuk mendapatkan cuan setiap hari hanya dengan pasang taruhan bersama SBOBET Mobile. Situs Judi Casino Online Indonesia Poster SerbaCasino Bandar Casino Online Indonesia Poster Judi Roulette Online SerbaCasino Poster I will develop sport bet website bet365 rummy teen patti blackjack dragon vs tiger slot Bonus New Member SerbaCasino Judi Bola Online Poster Find the best dragon vs tiger services you need to help you successfully meet your project planning goals and deadline
The Real Person!
The Real Person!
The game’s simplicity attracts a diverse audience, from college students looking for a side hustle to homemakers seeking entertainment. With just a few taps, anyone can participate and potentially earn real money. Easy control InterfaceTiranga Game controls are designed to be intuitive, making it suitable for all age groups. The TirangaGame App Login interface is simple to navigate between different games and categories. You can easily log in to Tiranga Game and begin your gaming journey.The Tiranga Game Login is a gateway to a platform where your knowledge and skills can make into real rewards. If you’re passionate about sports or enjoy the thrill of casino games, Tiranga Game offers a fun and rewarding experience. Once you’ve completed the Tiranga Game Login, fill in the necessary details and recharge your account to start playing. This process is straightforward and can be completed using various payment methods. After recharging your account, you’re ready to dive into your gaming adventure.
https://codeblue.ao/2025/07/03/casual-play-vs-strategic-sessions-roi-compared-in-brawl-pirates/
Jeet 777 operates as an online gaming platform. Users must check local laws before playing. Android users can play crash gambling through dedicated apps or mobile-optimized websites. Many top platforms offer downloadable APK files directly from their sites, allowing you to bypass app store restrictions. These apps are designed to run smoothly on a wide range of Android devices, providing features like quick deposits, auto-cashout options, and real-time game stats. Space XY is a very minimalistic game about a rocket flying through space. As the game was made to be as straightforward as possible there are no symbols or special games in it. This allows everyone to enjoy the game process without any need to memorize different symbols and conditions for special games. Online gambling rocket game offer a unique blend of risk and reward, making them a popular choice in online casinos. By understanding how these games work and employing effective strategies, players can enhance their chances of success. However, it is crucial to gamble responsibly and seek help if needed. This guide provides all the information needed to get started and enjoy rocket crash game real money safely and responsibly.
The Real Person!
The Real Person!
Roobet Casino has quickly gained popularity among online gaming enthusiasts, offering a unique blend of thrilling games and a user-friendly interface. Players are drawn to its diverse selection of slots, table games, and live dealer options, all powered by top-tier software providers. Additionally, Roobet’s commitment to providing a secure and transparent gaming environment enhances the overall experience for its users. For more detailed insights, check out this Roobet Casino review that covers everything you need to know before playing. Online players will have the opportunity to test the waters at with a no deposit bonus, you will then receive all modifiers with a 12X win multiplier. We keep our bonus information up to date, where to play the Space XY version for real money it fluctuates easily.
https://ikea.mn/ludo-supreme-review-is-this-the-new-king-of-earning-apps-in-india/
Space XY: a game with easy controls and high winnings in the casino. You will enjoy the latest games, this added clutter may make it more difficult for you to navigate these gaming sites. The playthrough requirement for withdrawal is 35x the bonus, United States. He will also occasionally participate when a special feature requires it from him, Switzerland. Overall, Space XY offers a refreshing and distinct gaming experience for players seeking something out of the ordinary. Its combination of innovation, playability, and nerve-testing gameplay makes it a game worth exploring. However, it’s crucial to approach it with a balanced perspective and responsible gambling habits. It’s time to soar to new heights with BGaming! Launching the rocket to the stars means getting incredible winnings! Space XY is an exciting game with easy gameplay. Don’t hesitate to join a breathtaking ride to the stars and make a fortune.
The Real Person!
The Real Person!
Dear Friends, Do you want an easy opportunity to earn and win money by playing games? Download the Teen Patti Master game app now and start earning money quickly. Tournaments: Teen Patti Gold Pakistan arranges regular tournaments and events. As a result, users show more engagement and try to get benefits from this moment. You can also enjoy these events. The game is thrilling because it combines strategy, observation, and reading opponents. If you’re new to Teen Patti, this guide will teach you the Teen Patti rules, how to play, and even how to enjoy 3 Patti real cash games online. Ans:- Yes, You can redeem your winnnig amount using UPI from Happy Teen Patti app. In 2023, Teen Patti Gold was updated with new game modes and unlimited rewards. You can now play with your friends or join tournaments with players from around the world.
https://spotsylvaniagaragedoors.skylinelisting.com/tower-x-by-smartsoft-an-indian-players-perspective/
Space XY – a unique opportunity to feel like a pilot of a real space shuttle. In the crash game you will control the rocket, which goes on a distant space journey, full of dangers and risks. The longer the rocket flies, the bigger the prize multiplier that determines the size of your prize. The player’s task is as simple as possible – you place your bet and wait for the round to start. The rocket goes on a journey, and the multiplier begins to grow. The aim of the game is to withdraw your winnings before the ship crashes. If the player manages to hit the Cash Out button and take the money, his winnings will be equal to the bet multiplied by the prize multiplier. The gameplay is so compelling that Space XY doesn’t need all of the additional features. © 2025 Aviator Game | Play Aviator Money Game 1win by Spribe
The Real Person!
The Real Person!
Když jsem se poprvé doslechl o plinko, zaujal mě už samotný nápad pouštět míček do pole plného drobných kolíků a sledovat, kam nakonec dopadne. Všechno to působilo jako rychlá a nenáročná zábava, kde nepotřebuji detailně studovat pravidla. Stačí zvolit, odkud kouli spustím, a pak s napětím vnímat, jak se odráží od překážek. Člověk nikdy neví, zda skončí v přihrádce s vyšší odměnou, nebo na neutrálním místě. Tímto způsobem mě plinko nalákalo svou okamžitou akčností i nepředvídatelností. Zkušení hráči doporučují střídání startovních pozic a konzervativní management bankrollu. Osvědčila se také strategie postupného zvyšování sázek během výherní Plinko balls série. Inani Cox’s Bazar To Saint Martin Cruise Ship | Saint Martin To Inani Cox’s Bazar Cruise Ship
https://www.zen.com.tr/plinko-od-bgaming-recenze-a-tipy-pro-ceske-hrace/
Uvařte si svou oblíbenou kávu h pocitem, že každý okamžik je t nezaplacení. Využijte aktuální nabídky nejlepších gambling establishment bonusů bez vkladu, které vám připíšou odměny až three 100 Kč nebo a couple of hundred fifty free spinů zdarma. Mezi nejaktivnější on typically the web česká casina na poli promotional akčních nabídek patří рџЏ† Betano, Tipsport, Forbes a Merkurxtip. Ať už jste fanouškem french press nebo překapávané kávy – odhalte neodolatelné scent naší kávy, které vylepší každý váš den. Instalace: 541260 Your Family Table! Help or hinder! – You and friends or family can earn easy money with Community Chest and co-op events, or heist their banks to help yourself get to the top. Don’t feel bad; it’s all part of the board fun! Collect and trade story-filled Stickers with friends and family and in our MONOPOLY GO! Facebook Trading Groups! Complete clever albums for exciting rewards! Whether helping or competing, rules of cooperation always apply here. The classic competition becomes even more thrilling with unique challenges as you play.
The Real Person!
The Real Person!
рџЏ’️ Astuce Hockey Shootout mini-jeu instantané : Hockey Shootout est disponible en mode démo en ligne. Obtenez instantanément 5 000 € d’argent fictif (appelé DEM) pour vous entraîner. Les mises et le choix du pays peuvent également influencer les résultats. Bien que la chance joue un rôle important, l’expérience et les compétences du joueur peuvent améliorer les résultats au fil du temps. Les joueurs expérimentés développent des stratégies pour maximiser leurs chances de marquer, en prenant en compte des facteurs comme le comportement du gardien et le timing des tirs. Chez Please Casino, nous découvrons régulièrement de nouveaux mini-jeux en ligne casino pour le plus grand bonheur de nos lecteurs. Récemment, Evoplay a sorti sa nouvelle création : Penalty Shoot Out Street. Nous avons pris beaucoup de plaisir l’essayer et à découvrir toutes les fonctionnalités et le potentiel du titre. L’équipe de rédaction vous donne son avis maintenant !
https://www.welltic.in/analyse-du-taux-de-redistribution-de-sweet-bonanza-precis-et-transparent/
Le joueur du Brésil avait des difficultés à retirer ses gains en raison d’une vérification incomplète, car les victoires et l’énergie des casinos RTG parlent parfaitement avec la bonne atmosphère de jeu. PayPal et tout casino qui accepte le portefeuille électronique ne vous factureront jamais de frais, situé dans la région de Schenectady. Les joueurs qui ne se promènent pas loin cette année peuvent étancher un peu leur envie d’errance avec des machines à sous estivales atmosphériques, a sauté dans la mêlée. Shoot out street : Cette version amène l’action sur la rue avec une ambiance urbaine unique. Lausanne, Zurich, Berne et Zoug, les 4 clubs suisses engagés dans la Ligue des champions 2025 26 sont fixés. Le LHC affrontera notamment le quadruple vainqueur de la compétition Frölunda. Outre son déplacement à Göteborg pour défier la formation de Henrik Tömmernes et Dominik Egli, le vice-champion de Suisse ira à Rauma en Finlande pour se mesurer au Lukko et à Grenoble chez les Brûleurs de loups. Les Vaudois accueilleront également les Allemands d’Ingolstadt, les Nord-Irlandais de Belfast et les Tchèques de Mountfield. Les Zurich Lions, tenants du titre, seront eux opposés au Sparta Prague, aux Bremerhaven Pinguins, à Tychy, à Brynas, à Ilves Tampere et à Odense.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
The Real Person!
The Real Person!
Redan från start blir det tydligt att Sweet Bonanza är en slot på nätet för vilken speltillverkarna har lagt ner mycket energi och kärlek. Man har sett till att skapa ett originellt tema och med både symboler, spelplan, bakgrund och funktioner som går i spelets tema. Pragmatic Play har inte slarvat med detaljerna. Sweet Bonanza har också haft en betydande påverkan på spelmarknaden. Dess unika funktioner och attraktiva design har inspirerat andra spelleverantörer att utforska liknande teman och mekaniker. Detta har lett till en ökning av färgglada och interaktiva slots, vilket har breddat spelutbudet för både nya och erfarna spelare. Denna trend visar hur Sweet Bonanza inte bara är en spelautomat, utan en katalysator för innovation inom spelindustrin. Sweet Bonanza skiljer sig också lite från de flesta andra spel, på det viset att de vinnande symbolerna inte måste ligga intill varandra. Det är den stora anledningen till att du kan vinna relativt ofta.
https://www.smarttextilesfinland.com/sa-far-du-ut-det-mesta-av-spelet-en-djupdykning-i-casinospel-pa-natet-for-svenska-spelare/
Sammanfattningsvis så uppskattade vi på SveaCasino den nya fiskesloten Big Bass Bonanza! Big Bass Bonanza är den senaste spelautomaten från Pragmatic Play och Reel Kingdom. RTP för Big Bass Bonanza är 96,71 %, vilket innebär att du i genomsnitt bör få tillbaka 96,71 % av dina vinster om du spelar det här spelet under en längre tid. RTP på 96,71% är mycket generöst och ligger långt över den genomsnittliga RTP:n för liknande spelautomater. Det är dock bäst att komma ihåg att mjukvaruleverantören av spelet erbjuder det med anpassningsbara RTP-inställningar, så se till att du kontrollerar RTP i speldetaljerna innan du spelar det på ett nytt nätcasino. hey@casumo Det här är de bonuserbjudanden som vi för närvarande erbjuder på Big Bass Bonanza: Casino Nyheter Artiklar
The Real Person!
The Real Person!
La vera attrazione di questo money wheel risiede nelle quattro lucrative funzioni bonus e nel potenziale di vincita fino a 20.000 volte la puntata. Grazie alle quattro fasi speciali previste, Sweet Spins, Candy Drop, Bubble Surprise e Sugar Bomb, c’è la possibilità di vincere fino a un massimo di €500.000. Oggi è già possibile trovare Sweet Bonanza Candyland nella sezione live dei migliori casinò online italiani autorizzati AAMS. Simile all’omonimo gioco giapponese, dove una pallina scende casualmente lungo il muro per terminare sui moltiplicatori. Se finisce sul simbolo “double” raddoppia i moltiplicatori e la pallina viene rimessa in gioco, fino a massimo 10.000X. L’RTP è del 94,33%. Se sei curioso su dove è possibile utilizzare poker HUDs on-line, che è uno dei più grandi collezioni weve visto. I casinò online non devono preoccuparsi delle mani perse quando rimescolano i ponti, ma siamo almeno felici di vedere che questi giochi sono disponibili. Come funziona il gioco Sweet bonanza con diversi metodi di pagamento.
https://obsonguba.sociales.uba.ar/2025/07/13/penalty-shoot-out-di-evoplay-recensione-e-analisi-per-i-giocatori-italiani/
I giri gratuiti in Sweet Bonanza Christmas si attivano quando compaiono quattro o più simboli scatter (le sfere di zucchero) sui rulli durante il gioco base. Questo attiverà una modalità bonus con giri gratuiti e moltiplicatori. Emozioni “casual”. Trovo che i bonus di Sweet Bonanza Candyland siano coinvolgenti, ma molto dipendenti dal fattore F (Fortuna…). Sweet Spins è il più divertente grazie ai moltiplicatori, mentre Candy Drop aggiunge un tocco di strategia. Bubble Surprise, invece, è troppo imprevedibile per i miei gusti. La capacità di adattarsi alle esigenze del mercato e di anticipare le tendenze ha permesso a Eurobet di consolidare la sua posizione di leader nel settore. In generale, siamo rimasti positivamente colpiti dalle mobile app di EuroBet, anche se sarebbe stato meglio concentrarsi su un’app unica piuttosto che sulla divisione tra scommesse e casinò, per non parlare delle tante app dedicate a giochi singoli, che sono piuttosto stucchevoli. L’esperienza sul browser dello smartphone è stata invece negativa: i tempi di caricamento sono veloci, ma è difficile visualizzare le categorie di giochi o le scommesse e selezionarle. Diamo quindi all’esperienza mobile di EuroBet un punteggio di 3.5 5.
The Real Person!
The Real Person!
Krachtige boormachines die toegepast worden voor complete verwerking van plaatmaterialen, massief hout en kunststof. Het uitkeringspercentage van Buffalo King is 96.06%. Test je kennis met ouderwets gezellige moderne quizzen De verschillende symbolen zorgen voor verschillende uitbetalingen. De kaartsymbolen die veel voorkomen, zijn relatief het minste waard. Aan de andere kant leveren de steppedieren juist meer op, zeker als de combinatie over meerdere rollen verspreid is. De buffalo is het meeste waard en staat gestapeld op de rollen. Door gebruik te maken van deze website bevestig je dat je 24 jaar of ouder bent en dat je je bewust bent van de risico’s van online kansspelen, en dat je momenteel niet bent uitgesloten van deelname aan kansspelen bij online kansspelaanbieders. Ook ga je ermee akkoord dat je kansspelreclame op deze site tegenkomt.
https://help.themarketingos.com/buffalo-king-megaways-review-spelen-in-nederlandse-online-casinos/
Minder: Punten vervallen na 200 dagen рџ‘Ћ Hi there! I could have sworn I’ve been to this web site before but after looking at some of the articles I realized it’s new to me. Anyhow, I’m definitely pleased I stumbled upon it and I’ll be book-marking it and checking back frequently! Analytische cookies worden gebruikt om te begrijpen hoe bezoekers omgaan met de website. Deze cookies helpen informatie te verstrekken over statistieken zoals het aantal bezoekers, het bouncepercentage, de verkeersbron, enz. Begin ten slotte vandaag nog aan uw winnende reis bij vulkan spiele casino. Zoals u kunt zien, bent u met de juiste strategie, sterke bonussen en intelligent spel op een goede plek om in de voetsporen te treden van succesvolle Plinko spelers. Tal van studio’s en andere media werken al aan de volgende delen, terwijl fans reikhalzend uitkijken naar nieuws over new7900 xt jogos, new7300 pagando bem jogos, om er maar een paar te noemen.
The Real Person!
The Real Person!
Share exclusive content with your subscribers cationes de adsorción marina (Na+, K+ and Mg2+), hasta 2500m en la dirección del flujo corresponde a unos 5000 años de lavado desde que se desarrolló la barrera de dunas. Áreas de recarga concentrada en las dunas se evidencian porque el agua subterránea muestra un bajo estado de evolución dentro de la línea de evolución antes presentada, si se compara con el agua circundante. La recarga artificial en las dunas con agua del Río Rin proporciona características hidroquímicas diferenciadas, lo que permite caracterizar el flujo subterráneo, la mezcla y las edades de las aguas. En primer lugar, el hecho de que se trata de un aspecto de la crisis ecosocial cuya resolución no se puede posponer a mejores momentos en el panorama económico (cuando el desarrollo económico esté en una cierta fase) o a determinadas condiciones ideales en el ámbito social (cuando haya conciencia generalizada del problema). Asunciones que subyacen al enfoque de sostenibilidad débil, en el que la determinación del rumbo correcto de las relaciones entre el ser humano y la naturaleza se deja en las manos (invisibles) de una ética más “verde” para las tareas de la producción o el consumo, con el soporte del progreso técnico y el desarrollo económico.4
https://www.ethiokiwi.co.nz/review-de-balloon-la-experiencia-visual-mas-inflada-de-smartsoft-gaming-para-ecuador/
Generate valuable leads from your publications Dopamine es un reproductor de música minimalista que prioriza la experiencia del usuario: sin distracciones, sin menús complejos ni anuncios. Es gratuito, ligero y compatible con Windows, ofreciendo música sin rodeos ni artificios. I recognized him immediately, even though I only managed to see him from behind. And that was after several years without our paths crossing: practically, since our business failed, the only one we had undertaken together. Perhaps because of the nervous abruptness of his movements or because of those protruding ears, the reason for so many school jokes, the fact was that I knew immediately: it could be none other than Marcos Silberman, Marquitos to his friends. The queue of resigned citizens moved slowly. En ningún caso se entenderá que se concede licencia alguna o se efectúa renuncia, transmisión, cesión total o parcial de dichos derechos ni se confiere ningún derecho ni expectativa de derecho, y en especial, de alteración, explotación, reproducción, distribución o comunicación pública sobre dichos contenidos sin la previa autorización expresa de EUDE o de los titulares correspondientes.
The Real Person!
The Real Person!
Once you have a full god pool and Hermes, the 25% from Family Favorite isn’t that far off from the 40% from Privileged Status, but Family Favorite is for everything all the time with zero work. The Jackpot Room Take whatever god or boon you think is best or that fulfills a requirement for a duo legendary and don’t worry about it needing a status effect to trigger Privileged Status. Q: What is the RTP of Sweet Bonanza Candyland? The new partnership deal with Más 1×2 strengthens the provider’s position in Latin America. If the name of this game sounds a little familiar, that’s because it’s based on Pragmatic’s popular slot, Sweet Bonanza. The software provider has taken the general candyland theme and created a new, live gameshow, giving players a fusion of the old and the new. As a live dealer game, you’ll be betting in real time with the option to either play or sit out every round. All of the action takes place on a giant wheel split into sections; your goal is to correctly guess where the wheel will stop after each spin.
https://onetoonesecurity.com/color-trading-by-tadagaming-a-pakistani-players-casino-game-review/
Möchtest du um Echtgeld oder weiterhin im Demo-Modus spielen? Play Sweet Bonanza 1000 in demo to feel how effective this Ante Bet really is. Practice mode might provide some key data to answer questions regarding bonus buys. You probably wonder if they are worth it or you better off enjoying the process of getting there even if it takes longer. If you are experiencing issues with the demo mode of the game not starting, please follow these steps: Der Demo-Modus wurde beendet. Du spielst nun um Echtgeld. Scoop 25,000x Your Wager in Sweet Bonanza 1000 Pokie! Sweet Bonanza 1000 is very much a been there, done that kind of game. It offers very little, but I still gave it a chance. I decided to play 50 spins with my bet set at $2 per spin. And BOOM, we are off to a great start, landing $28 within my first ten spins. After that, a lot of consecutive wins form by the Tumbler mechanics. With 29 spins left, I am breaking even. That said, with my last 15 spins, the game goes quiet, and landing wins is less frequent. With my last five spins, a flurry of wins totaling $5.80 sees me fall $10, which is sort of breaking even. Not a bad experience, really.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
The Real Person!
The Real Person!
If live casino games, table games and interactive mechanics are your thing, OnAir has you covered with Roulette, Blackjack and more! Buffalo King Megaways has the same symbols as its predecessor, Buffalo King, minus the lowest card symbol, 9. The payouts for these animal and card symbols have been altered from the original slot, but the Buffalo symbol remains the strongest high-paying symbol and is now worth $40 if you line up six of them. You also only need a minimum of two Buffalo symbols in order to generate a win; with the other symbols, you still need three. Here are the highest and lowest payouts per symbol, in descending order: Temple of Torment Logotype Like most Megaways slots, Buffalo King Megaways utilises a cascading mechanic, here referred to as the tumble feature. It kicks in after every win to remove winning symbols from the reels. New tiles drop down to fill the empty spaces, which can trigger another win. Tumbles keep on coming as long as wins keep on lining up.
https://www.dhakavision.com.bd/2025/07/15/how-fast-is-cash-out-time-in-mines-game-real-money-version/
Online casino Luxemburg We’ve been in the game since 1932 – from our pinball roots in Chicago to casinos across Vegas, Atlantic City, and right here in Newcastle. Over nine decades of delivering real entertainment, big play, and unforgettable nights. Players will stumble onto rugged terrain and discover a host of majestic animals from mountain lions to bald eagles, and of course the iconic buffalo. As the sun goes down on the wild landscape, players will see if they can keep the mythical animals on their side. Basic Game Info This slot bears many similarities to the original Buffalo King, as anticipated by many. While the scatter symbols may have a slightly different appearance, players can still receive more bonus spins at the outset. However, the most significant new feature is the Megaways mechanic, which enables up to 200,704 paylines to be active during each spin!
The Real Person!
The Real Person!
The design and audio of Big Bass Halloween perfectly encapsulate the creepy Halloween aesthetic. The reels are set under gloomy grey skies, with murky waters replacing the bright, crisp setting of the original Big Bass Bonanza. Eerie noises and sound effects add to the spooky ambience. Despite the fresh visuals, Big Bass Halloween 2 struggles to differentiate itself from its predecessors. Here is what you can try: Big Bass Halloween 2 is typical in terms of grid structure. It is set on a 5×3 gaming matrix, and players will be paid out if they land at least 3 matching symbols. Slots are massively popular. You spin the reels and hope to land on a winning combination. There are several tips and tricks to improve how you bet on slot games, weather you’re playing for free or real money. Our top tip is to think about paylines. Take the time to research each game’s paylines before you play to know which one give you the biggest chance to win. At Casino.org we’ve got hundreds of free online slot machines for you to enjoy.
https://mytyles.net/exploring-the-demo-mode-benefits-of-aviatrix-for-strategy-testing/
The UKGC doesn’t allow for demo modes or free play options, so all of the casino games are real money games. A slot’s biggest selling point aside from the jackpot, being one of the top slot games with the highest RTP and overall theme, are the bonus features. These are usually triggered when three or more “scatter” symbols appear on the reels. You then have the opportunity to win more money, either through a spins bonus, minigame, or selecting a hidden prize. The classic slots at WOW Vegas include all of the fan favorites, and many of these games tend to focus more on simple gameplay than on lots of special features. You might think that classic slots stick to basic themes like fruit or cash, but that’s not true – there are safari-themed games, ancient battles, and even sweet slots based on candy. A few of our top classic games are A Night in Paris by Habanero, the Slotfather by Betsoft, and Sugar Rush by Pragmatic Play.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
The Real Person!
The Real Person!
I truly appreciate this post. Great. Appreciate you sharing, great post.Much thanks again. Fantastic. Good blog you have got here.. It’s difficult to find excellent writing like yoursnowadays. I honestly appreciate individuals like you!Take care!! Appreciate you sharing, great post.Much thanks again. Fantastic. I truly appreciate this post. Great. I truly appreciate this post. Great. Good blog you have got here.. It’s difficult to find excellent writing like yoursnowadays. I honestly appreciate individuals like you!Take care!! Appreciate you sharing, great post.Much thanks again. Fantastic. Good blog you have got here.. It’s difficult to find excellent writing like yoursnowadays. I honestly appreciate individuals like you!Take care!! Good blog you have got here.. It’s difficult to find excellent writing like yoursnowadays. I honestly appreciate individuals like you!Take care!!
https://finbook.travel/ocena-wiarygodnosci-nvcasino-wsrod-polskich-graczy/
tektura aloh.php?candi=betnacional+aviator Precision and Production Quality Issues: In fields which rely on exact measurements, such as industrial sectors as well as aviation, oscillations may lead to errors in the production process, producing faulty goods along with more waste. By admin|2025-01-24T19:40:22+00:00January 24th, 2025|Aviator Betting Game Hack Означает Что – 317| Walcz w niezliczonych bitwach, które wstrząsną światem za pomocą Twojego własnego DinoRobota! Poza tym czeka na Ciebie wiele potężnych DinoRobotów. Zapraszamy na wrześniową edycję bezpłatnych warsztatów artystycznych do Wrocławia, Poznania i Warszawy. -With automatic return function. When the aircraft loses the controller signal, the aircraft will return to the take-off point according to the GPS trajectory.
The Real Person!
The Real Person!
We stand by our high-quality products and your satisfaction is 100% guaranteed. Gua Sha Scraping with a smooth edge is for physical therapy and it is a beneficial mineral that is good for Skin Beauty and Body Health. 20% OFF Over €200 Spend (T&C’s apply) Discover more beauty and wellness tips from Lilly on her website genuineglow or on Instagram @lillygenuineglow. 8 Gua Sha Tools to Help De-Puff Skin. Good Housekeeping. goodhousekeeping beauty anti-aging g39047346 best-gua-sha-tools If you are wondering what gua sha exactly is, Dr. Elizabeth Trattner, Doctor of Chinese and Integrative Medicine, explains that gua sha has been part of traditional Chinese medicine practices for thousands of years, and is used to help relieve tension and boost blood circulation throughout the body.
https://buynow-us.com/775959-toplash/details.html
down to a science Only want to learn Eyebrow Ombré And Powder Machine Shading? Tap HERE! Nano blading is more similar to Microblading than Ombre Powder Brows. Nano Brows fill in scarce, thin brows for a thicker appearance. Nano Brows use a smaller needle with a hand-held machine to deposit pigment into the epidermis to create natural-looking hair strokes. The super-fine needle creates hair-like tattoo strokes that can thicken the look of the brows and change the overall shape of your brow. Silver hair is many things: beautiful, unique, trending, but definitely not easy – especially for our brunettes and dirty blondes. It’s time for some serious eyebrow talks. In a nutshell, you aren’t the type of woman to care about make-up, beauty trends, or even fashion styles. However, some of your friends are going crazy over the latest microblading, microshading and ombre eyebrows. As you hear some of your friends go excited about the improvements these procedures brought, you wonder, what made these eyebrow trends so different?
The Real Person!
The Real Person!
O depósito máximo também varia e pode chegar a até R$50.000 por dia. O Pin-Up Brasil não cobra taxas em depósitos e as transações são processadas imediatamente. Como o mercado está cada vez mais competitivo, sua emissora pode perder oportunidades de aumentar o faturamento e reduzir despesas, não conseguir manter um bom perfil e histórico de relacionamento com os ouvintes, ter choques que prejudicam contratos e a plástica da rádio. Além disso, sua equipe pode ficar desmotivada ao executar tarefas repetitivas operacionais que o sistema poderia realizar, enquanto cria novas oportunidades para a emissora se destacar e diferenciar. O Big Bass Splash oferece uma combinação irresistível de gráficos atraentes, jogabilidade dinâmica e oportunidades de grandes vitórias. Se você gosta da temática de pesca e está em busca de um jogo com potencial para grandes recompensas, este slot é uma excelente escolha.
https://homologa.cge.mg.gov.br/user/puturata1986
Play Teen Patti with friends Carrom Gold : Game of Friends Teen Patti Gold – Rummy is a popular card game that originated in India, similar to poker but with more strategy involved. This Android game offers players the chance to winbonuses per day and withdraw cash bonuses immediately. With a standard 52-card deck, players start with two cards each, and the game continues until all 52 cards have been played. The game also features other popular casino games such as Dragon vs Tiger, Deep Sea Awakening, Roulette, Baccarat, and more. A game app to play a classic card game Whether you’re a seasoned player or new to the world of Teen Patti, Teen Patti Comfun Card Online promises hours of thrilling entertainment. With multiple exciting modes to choose from, including the classic 3 patti format where three to five players receive three cards each, the thrill of the game is taken to new heights.
The Real Person!
The Real Person!
Ready to play Mission Uncrossable for real money?Here is our best selection of online casinos where you can play Mission Uncrossable with real money to win maximum money! The game’s lively graphics and smooth play make it a hit for both newbies and seasoned gamers. It’s all about smart choices and managing risks, which makes it appealing to a broad audience. Sounds interesting? Stick around to get the ins and outs! Roobet is a popular online crypto casino that offers a wide range of games, including slots, table games, and unique mini-games like Mission Uncrossable. It allows players to bet using cryptocurrencies like Bitcoin, Ethereum, and Litecoin, similar to the options available in a crypto dice game, making transactions quick and secure. The platform is known for its modern interface, a large variety of casino games, and regular promotions. Roobet is particularly appealing to players who prefer fast payouts, anonymity, and provably fair gaming, ensuring transparent and reliable outcomes.
https://42.195km.net/run-wiki/index.php?fitsdertiocon1981
Roobet is a popular online crypto casino that offers a wide range of games, including slots, table games, and unique mini-games like Mission Uncrossable. It allows players to bet using cryptocurrencies like Bitcoin, Ethereum, and Litecoin, similar to the options available in a crypto dice game, making transactions quick and secure. The platform is known for its modern interface, a large variety of casino games, and regular promotions. Roobet is particularly appealing to players who prefer fast payouts, anonymity, and provably fair gaming, ensuring transparent and reliable outcomes. Roobet mini game Roobet mini game Glory Casino has become a popular destination for online gambling enthusiasts seeking thrilling entertainment and lucrative opportunities. With its wide array of games, including slots, table games, and live dealer options, glory casino online offers an immersive experience for players of all skill levels. The platform boasts a user-friendly interface, secure payment methods, and attractive bonuses to enhance the gaming experience.
The Real Person!
The Real Person!
Jeśli skończą Ci się kredyty demo w Sugar Rush, nie martw się—po prostu odśwież stronę, a wrócisz do gry. Ten nieskończony cykl gry odzwierciedla nieskończone możliwości w grze, gdzie każde odświeżenie może prowadzić do wielkiej cukierkowej wygranej w BDMBet. Get Demo for Sugar Rush W grze Sugar Rush nie ma symbolu Wild, zamiast tego są opakowania, które przynoszą mnożniki na bębny. Maksymalizacja wypłat dzięki efektywnej grze. Zrozumienie tabeli wypłat i funkcji Sugar Rush może pomóc w opracowaniu strategii zapewniających większe wygrane. Gra oferuje konkurencyjne wypłaty, a połączenie regularnej gry z inteligentnymi zakładami może zwiększyć Twoje szanse na zdobycie znacznych nagród. Czas spędzony przy Sugar Rush mija niezwykle szybko – a to za sprawą świetnej (i słodkiej!) grafiki, symbolom specjalnym, opcji zakupu bonusu czy free spinom. Tych ostatnich możemy wygrać tutaj aż 30! Jak przystało na gry tego uznanego dostawcy, RTP należy do wysokich zaś maksymalna wygrana może wynieść nawet 2 000 000 złotych.
http://dados.ufra.edu.br/user/beterlori1979
Sugar Rush 1000 oferuje wciągającą rozgrywkę na slocie online w żywej siatce 7×7. Gra wykorzystuje system Cluster Pays, w którym wygrane są przyznawane, gdy co najmniej pięć symboli tworzy poziome lub pionowe połączenia. Zwycięskie symbole są usuwane, aby umożliwić nowym kaskadowanie w dół, potencjalnie uruchamiając dodatkowe wygrane. Ze współczynnikiem RTP gry podstawowej na poziomie 97,50% i oznaczoną wysoką zmiennością, obiecuje ekscytujące wrażenia z rozgrywki. Podstawowe informacje o grze Wizualnie Sugar Rush Fever zachwyca bogatą paletą kolorów i dynamicznym designem. Tło gry to wirujące neony w odcieniach fioletu, różu i błękitu, które nadają grze wyjątkowy, futurystyczny klimat. Funkcja Zakup bonusowy w Sugar Rush 1000 oferuje natychmiastowy dostęp do rundy darmowych spinów za 100-krotność zakładu lub Super darmowych spinów za 500-krotność, w których mnożniki są już na siatce, aczkolwiek z niższym RTP wynoszącym 96,52%.
The Real Person!
The Real Person!
Les règles du keno en 4 étapes CasinoVibes est géré par TH Gambling N.V., une société établie sous le droit de Curaçao, enregistrée sous le numéro 146982. Le casino est autorisé par la licence de jeu OGL 2024 1468 1024, délivrée par le gouvernement de Curaçao. Cette surveillance réglementaire garantit que la plateforme respecte les normes en matière de pratiques de jeu équitables et de sécurité des joueurs. Il existe plusieurs jeux de casino de qualité dans l’industrie du jeu en ligne, y compris les machines à sous sur le thème de la faune. Elles fascinent autant de personnes que les jeux de cartes et de table pour le plaisir qui sont plus complexes à comprendre et qui nécessitent la pratique de stratégies particulières. Elles ont des règles simples à comprendre et permettent généralement de gagner en formant des combinaisons gagnantes.
https://cct.opencitieslab.org/user/ciapessouser1987
Jouer à Aviator en ligne est un processus simple et accessible. Tout d’abord, choisissez un casino en ligne réputé offrant le jeu de casino Aviator. Une fois inscrit, vous pouvez accéder à la version démo pour pratiquer sans risquer d’argent réel. Ensuite, placez votre pari et observez l’avion décoller. En apprenant à jouer à Aviator, vous développerez des compétences pour maximiser vos chances de gains, tout en profitant d’une interface conviviale et immersive. Maintenant que vous savez que c’est MyStake Casino qui propose le jeu JetX, il faut vous rendre sur le site du casino et vous inscrire. Bien entendu, JetX étant un jeu d’argent, il vous faudra aussi réaliser un premier dépôt. jetx cbet vous ouvre les portes d’un jeu avion argent où la richesse et l’excitation ne connaissent pas de limites. Chaque session est une nouvelle aventure, une chance de transformer l’instantané en éternel. La plateforme jetx se distingue par son interface intuitive et ses mécanismes de jeu captivants, garantissant une immersion totale.
The Real Person!
The Real Person!
situs judi slot online apriltoto Apriltoto adalah link situs judi slot online terpercaya gampang menang dengan bocoran pola slot gacor hari ini pasti banjir ledakan jackpot maxwin besar-besaran dengan pembayaran fair play. Κάθε φορά που ένα νικητήριο σύμβολο εκρήγνυται, το σημείο του σημειώνεται στο δίκτυο. Εάν ένα άλλο εκτινάσσεται σε αυτό το σημείο για δεύτερη φορά, προστίθεται ένας πολλαπλασιαστής, ξεκινώντας από x2 και διπλασιάζεται μέχρι x1.024 με κάθε εμφάνιση. Ο πολλαπλασιαστής που προκύπτει προστίθεται σε όλους τους νικηφόρους συνδυασμούς που σχηματίζονται από πάνω.
https://b.io/quamoctilo1982
Το YouTube τοποθετεί αυτό το cookie για την αποθήκευση των επιλογών βίντεο του χρήστη που χρησιμοποιεί την ενσωμάτωση βίντεο του YouTube. Ωστόσο, είναι σημαντικό να θυμάστε ότι δεν αποδίδουν πάντα το επιθυμητό αποτέλεσμα. Σε τέτοιες περιπτώσεις, σας συμβουλεύουμε να κάνετε ένα διάλειμμα και να μην επιδιώκετε επίμονα τη νίκη, καθώς κάτι τέτοιο μπορεί να οδηγήσει σε σημαντικές απώλειες. Δοκιμάστε το δωρεάν! Καραμέλες και γλυκές λιχουδιές θα τροφοδοτήσουν τις περιόδους παιχνιδιού σας στον online κουλοχέρη Sugar Rush.
The Real Person!
The Real Person!
Automat do gier Sugar Rush zyskał popularność wśród graczy dzięki kolorowej grafice oraz interesującej mechanice rozgrywki. W tej recenzji automatu Sugar Rush przyjrzymy się jego cechom, funkcjom oraz szansom na wygraną. Czy warto spróbować swojego szczęścia w tej słodkiej owocowej krainie? Odpowiedź znajdziesz w poniższym artykule. W internecie znalazłam tylko 2 dwa odcinki : Slot Sugar Rush stanowi majstersztyk pod względem grafiki i multimediów. Nic dziwnego, że tak wielu graczy szuka kasyno kody bonusowe na tę właśnie grę! Slot ten stanowi udany mariaż klasyki i nowoczesności. We also use third-party cookies that help us analyze how you use this website, store your preferences, and provide the content and advertisements that are relevant to you. These cookies will only be stored in your browser with your prior consent.
https://vansh.graphyx.in/2025/08/04/analiza-i-ocena-kasyna-playbison-dla-graczy-z-polski/
Stworzone przez TeLiXj w dniu 7 cze 2013, 16:37:59. Pobrań: 88 Dla wielbicieli ostrego harmonijkowego grania nazwisko Sugar Ray Norcia na okładce płyty stanowi najlepszą rekomendację muzyki zawartej na krążku. Po ostatnim albumie wypełnionym jump bluesem przyprawionym riffami sekcji dętej, na nowym longplay’u Ray wraz z zespołem The Bluetones serwuje nam sporą dawkę klasycznego Chicago bluesa. „»Miss You« to tytuł hitowego utworu The Rolling Stones, w którym słychać charakterystyczny riff harmonijki ustnej Sugar Blue” – taka była poprawna odpowiedź w przygotowanym przez portal Blues.pl i organizatorów Toruń Blues Meeting konkursie, w którym mogliście wygrać aż dziesięć pojedynczych zaproszeń na pierwszy z festiwalowych dni i cztery na drugi dzień imprezy (wybierane losowo).
The Real Person!
The Real Person!
Even if you’re new to online slots, getting started with Buffalo King Megaways is quite straightforward. But before we get to that, you need to find an online casino to play on. if you don’t already have a preferred casino site, read our casino reviews to see the best site in your location. You can find the best free online slots here on this page. At Casino.org we’ve rated hundreds of free online slot machines and every month we update this page with the best free slots games in the market. We have live roulette games hosted by renowned live casino game developer, Evolution Gaming. Not to mention many of the live online roulette tables in our collection have different table limits; the minimum bet is visible before you even click the game. So you can choose the one that best suits you. Licenced and reputable online casinos do not rig their games. Firstly, they risk losing their licence to operate if they rig their games. Secondly, casino games have a built-in advantage in favour of the casino (the house edge) that ensures their profits in the long run, so they have no need to rig casino games.
https://redwoodroofingco.com/bgaming-plinko-review-experience-the-thrill-in-indian-online-casinos/
BONANZA Megaways is probably one of the most popular megaways slots to be released by Big Time Gaming. This slot machine game was an instant hit among players and is still a very popular game today, despite the big competition in the megaways world. BONANZA is known for its high volatility and mega payout potential. Almighty Buffalo Megaways is one of the hundreds of games available on BetMGM Casino, available in Michigan, New Jersey, Pennsylvania and West Virginia! Play some of your favorite casino games online, as well as exclusive games you can’t find anywhere else! And if you’re new to BetMGM Casino, you can receive a new player bonus! To learn more about BetMGM Casino, read our review. The main game providers release new online slots as often as every single month, so there’s no shortage of choice at top online casino sites. But of course, not all the new games out there will appeal to you, so we’ve put together a list of some of our favorite new releases, so you can choose the slot you like best.
The Real Person!
The Real Person!
Mission Uncrossable offers a demo mode that allows players to explore the game freely without placing any bets. This mode preserves the game’s random nature, providing a realistic experience of the gameplay. It’s an excellent opportunity to practice your strategies and familiarize yourself with the game mechanics. We played the game over and over, and it put us on the edge of our seats. The thrill levels available through this game are first-class. Every time you play, you have a chance of hitting up to $1,000,000. But, if your luck is not in, you will be crushed, and it will be a losing bet. Another reason to love this casino game is the betting range makes it suitable for all players. While no tips or tricks guarantee winning on Mission Uncrossable, you can still play tactically. BettingGuide’s experts recommend being aware of scams, taking advantage of casino bonuses, setting limits, and using the Auto Play mode.
https://tycherestaurant.com/the-betpawa-aviator-app-our-download-gameplay-review/
Big Bass Bonanza 3 Reeler features a compact 3×3 reel setup with just five paylines, making it accessible and straightforward for players. Winning combinations are formed by landing three matching symbols on a payline, with payouts ranging from 0.5x to 100x the bet in the base game. The game incorporates fully stacked symbols at random, which can increase the likelihood of forming winning lines, ensuring that even the base gameplay remains exciting. The graph shows the probability of getting a multiplier that’s higher than a certain value in Big Bass Bonanza 3 Reeler. Online casinos often run slot tournaments, in which players score points only for certain win multipliers, for instance, x20 or x100, etc. So, this information will be especially useful for those who participate in such tournaments.
The Real Person!
The Real Person!
Given the roaring success of the original game, it should come as little surprise that Pragmatic Play and Reel Kingdom have delivered a shot-for-shot festive remake with Christmas Big Bass Bonanza. Some dedicated work has gone into producing a Christmas theme with unique symbols and an improved background, but not much else has changed – other than Santa replacing the Fisherman in the Free Spins Bonus, of course! Ultimately, if you’re a fan of the original game, you’re sure to enjoy a few spins of this festive sequel. Pop open Christmas Big Bass Bonanza’s tackle box of features to find a round of free spins where money symbols and wilds may be collected to score cash prizes or trigger extra spins and multipliers. Connect with us Like Sweet Bonanza Xmas and Sweet Bonanza, there is nothing different between Buffalo Power Christmas and Buffalo Power: Hold and Win except for the festive visuals (and audio sound effects) added to the Christmas version.
https://www.g-kotta.com/crash-point-explained-in-aviator-game-by-pros/
It comes from Pragmatic Play and the appropriately named Reel Kingdom studio. You can spin the reels for an exceptionally wide range of stakes, which makes it suitable for all budgets, and like other slots from Pragmatic Play, it’s very straightforward to set up. Ready to go fishin’? Big Bass Bonanza by Pragmatic Play is the slot that made fishing big again. I am going to be showing you how the game performs, what features and bonuses are available, and how the settings influence your chances of reeling in a fortune. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. 18+ | Terms apply Log in to write reviews, complaints about the casino, comment on articles
The Real Person!
The Real Person!
Slot Sisters Of The Sun By Playn Go Demo Free Play You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. En primer lugar, La mecánica de Big Bass Bonanza es sencilla y fácil de entender, esta tragaperras en línea tiene 5 carretes y 3 filas, con líneas de pago ajustables. Un proyecto ambicioso cuyo objetivo es celebrar el trabajo de las empresas más responsables del mundo del iGaming y ofrecerles el reconocimiento que merecen. Enlaces destacados La versión Big Bass Bonanza Megaways de esta saga de juegos expande las típicas 10 líneas de pago de Big Bass Bonanza hasta más de 46,000 líneas de pago. Con una apuesta mínima de 0,20€ $ hasta 100 € $. Además, cuenta con una RTP similar de 96,7% y una volatilidad media. Aunque parezca más atractiva, hay que considerar que su disposición es mayor (6×7).
https://visitcentraljava.com/review-del-nuevo-juego-balloon-de-smartsoft-en-casinos-online-para-jugadores-en-ecuador/uncategorized/
Los bonos a los que podrá acceder aquí incluyen, y hay cuatro símbolos de cartas de póquer de bajo pago. En Casino Gran Vía Online, queremos que disfrutes al máximo desde el primer momento. Por eso, si aún no has realizado tu primer depósito, te ofrecemos un Bono de Bienvenida. La tragamonedas gratis Big Bass Bonanza destaca por su jugabilidad fácil de usar y la oportunidad de conseguir pagos considerables. Con sus 5 rodillos y 10 líneas de combinación ganadora, el objetivo es conseguir las combinaciones más lucrativas. El atractivo RTP del 96,71% y el alto índice de volatilidad de la tragaperras sugieren que, aunque las ganancias no se produzcan con frecuencia, tienden a ser más sustanciosas cuando surgen. Durante la ronda de spins gratis, cada comodín recolecta el valor de todos los símbolos de dinero que aparezcan en los carretes. Al finalizar la ronda, el jugador recibe todos los premios acumulados por los comodines.
The Real Person!
The Real Person!
deltasone without script online – where to buy prednisone online without a script buying prednisone from canada Codigo promocional 1xbet: bono exclusivo (valido en 2025) – codigo promocional en 1xbet Reclame su bono de bienvenida del 100% hasta € $130. Todo lo que tiene que hacer es copiar el codigo promocional e ingresarlo durante el registro, activara todas las ofertas de bonificacion de 1xbet. Los codigos promocionales publicados en nuestro sitio web solo se pueden utilizar durante el registro, contribuyen a la recepcion de un bono para nuevos jugadores. Todos los codigos de bonificacion se proporcionan de forma gratuita. deltasone without script online – where to buy prednisone online without a script buying prednisone from canada mexican drugstore online: mexico pharmacies prescription drugs – mexican online pharmacies prescription drugs
https://warriors-me.com/wszystko-co-musisz-wiedziec-o-bonusach-w-verde-casino-dla-graczy-z-polski/
W kasynie Rabona znajdziesz też gry stołowe i karciane, takie jak blackjack, bakarat czy ruletka. Wybór oferowany przez kasyno nie jest oszałamiający, gry slotowe są tu obecne w dużo większej liczbie. Nie mniej selekcja powinna być wystarczająca. W Rabona Casino znajdziemy też kilka wersji pokera wideo. Odrobinę słabiej wyglądają jackpoty progresywne. Są tu wprawdzie 42 gry z pulą progresywną, ale nie są dostępne nowe gry Microgaming, które oferują najwyższe jackpoty w branży. Możesz również sprawdzić, czy bonusowa gotówka została już zmieniona na prawdziwe pieniądze. Jest to be able to oczywiste, że łatwiej będzie wypłacić bonus bez depozytu z koniecznością np. Każdy z nas na pewno choć raz słyszał powiedzenie, że w życiu nie ma nic za darmo. Po części taka sytuacja również występuje w przypadku bonusu bez depozytu.
The Real Person!
The Real Person!
Z pieców kasyna Pragmatic Play studios, przygotuj swoje kubki smakowe na słodkie i pikantne smakołyki w grze slotowej Sugar Rush. Skrill oferuje kilka zalet, która działa przeciwko Mo Donegal jest remis kolei. Automat do gier candy dreams gra za darmo bez rejestracji będzie regulować swój rynek kasyn online w kwietniu 4, ta gra sprawi. Autorzy raportu z pisma Neuroscience & Biobehavioral Reviews oceniali także, jak na nastrój wpływają takie czynniki, jak ilość i rodzaj spożytego cukru. Ciekawiło ich również, czy angażowanie się w wymagającą czynność umysłową czy fizyczną robi jakąś różnicę. Sugar rush: Popularna gra w kasynie online Innym automatem z bonusem, podczas gdy pisarze baseballowi głosują. Ale jak dokładnie jest obliczana ilość czasu kontuzji, automat do gry sugar rush w kasynie co oznacza. Ocaleni otrzymują darmowe spiny z funkcją skrytki, sugar rush mnożników podczas gry że zawsze są świetną drużyną.
https://avgiacademy.com/zasady-funkcjonowania-programu-lojalnosciowego-w-playbison-casino/
Zdrowy rozsądek mówi nam, BGaming. Krupierzy są bardzo dobrze przeszkoleni i zawsze służą pomocą, które ma dopasowane metody wpłat. Gdy już zarobisz pieniądze w Crazy Star, więc jest wiele do zobaczenia. Bitcoin, Ethereum, Binance Pay Jak grać w Pragmatic Play’s Sugar Rush 1000 Dzięki systemowi osiągnięć SpinLine Сasino, możesz odblokować specjalne bonusy wykonując wyzwania. Każdy osiągnięty kamień milowy to wyjątkowe nagrody, które w jeszcze pełniejszym stopniu uprzyjemnią z korzystania ze SpinLine Сasino. Minimalny depozyt Sugar Rush Fever od RubyPlay Wejdź do słodko wciągającego świata Sugar Rush 1000, starannie zaprojektowanego slotu online od Pragmatic Play. Osadzona w kolorowej krainie cukierków, gra ta zapewnia bardziej stonowaną, ale urzekającą atmosferę dzięki dobrze wykonanemu projektowi i przyjemnej ścieżce dźwiękowej. Wykorzystując siatkę 7×7 i mechanikę Cluster Pays, Sugar Rush 1000 oferuje przyjemne wrażenia z gry na slotach, łącząc prostotę z ekscytacją unikalnymi funkcjami rozgrywki.
The Real Person!
The Real Person!
Laura, a regular at 1Win Casino, has high praises for the site’s reliability: “Playing Big Bass Bonanza at 1Win Casino has been a great experience. The platform is reliable, and their payouts are prompt, which is something I greatly appreciate.” O casino oferece uma ampla variedade de jogos, incluindo Pin Up slots, roletas, jogos de mesa, loterias, cassino ao vivo, jogos de TV e muito mais. Vamos explorar alguns desses jogos populares e suas características. É importante lembrar que todos os bônus e promoções no Pin Up casino online estão sujeitos a termos e condições específicas. Recomenda-se que os jogadores leiam atentamente essas informações para compreender os requisitos de apostas, restrições de tempo, jogos elegíveis e outros detalhes importantes relacionados a cada oferta. O PinUp dedica-se a proporcionar uma experiência de jogo emocionante e recompensadora para seus jogadores.
https://stikerkacafilm.id/2025/08/06/jetx-uma-analise-completa-do-jogo-de-casino-da-smartsoft-para-jogadores-brasileiros/
Pragmatic Play: A Fábrica de Slots Que Nunca Dorme Embora Big Bass Bonanza seja principalmente um jogo de sorte, os jogadores podem adotar algumas estratégias para maximizar seus ganhos. Uma abordagem comum é apostar de forma responsável, gerenciando cuidadosamente o saldo da conta e aproveitando ao máximo os recursos de bônus oferecidos pelo jogo. Um dos maiores diferenciais do cassino ao vivo da Esportiva Bet é a sua seleção de mesas brasileiras. Os jogos com crupiês que falam português têm uma bandeirinha do Brasil no canto direito, então fica fácil encontrar opções no nosso idioma. Pragmatic Play: A Fábrica de Slots Que Nunca Dorme O Bonanza Jogo é muito mais do que apenas um jogo de casino online; é uma jornada emocionante para os amantes da pesca e entusiastas de jogos de azar. Com sua temática envolvente, recursos exclusivos e potencial de grandes ganhos, este jogo continua a atrair jogadores de todo o mundo em busca de diversão e emoção.
The Real Person!
The Real Person!
Se prevé que el mercado de los casinos en vivo alcance los 13.600 millones de dólares, impulsado por la creciente demanda de los jugadores de experiencias de juego realistas y confiables. Se prevé que el mercado de los casinos en vivo alcance los 13.600 millones de dólares, impulsado por la creciente demanda de los jugadores de experiencias de juego realistas y confiables. Consejo del experto en apuestas: No todas las tragamonedas están incluidas en esta oferta. Entre los juegos disponibles se encuentran: Gates of Olympus, Fruits Royale 100, Coin Volcano, Sweet Bonanza y Sugar Rush, junto con otras 100 tragamonedas elegibles. ⚠️ Debes realizar tu primer depósito dentro de los 7 días posteriores al registro para recibir el bono.⚠️
https://senotodoseme.com/es-balloon-un-juego-de-azar-o-de-habilidad-lo-debatimos/
Los aficionados a las tragaperras con temática de caramelos pueden alegrarse con las tragamonedas gratis Sweet Bonanza Dice. Este juego ofrece algo más que una pizca de dulzura azucarada, aporta toda una descarga de adrenalina. Sí, hay una versión demo del juego a la que puedes jugar sin límite de tiempo. La acción de Sweet Bonanza 1000 transcurre en seis carretes, de cinco filas cada uno. El juego usa la mecánica de Scatter Pay (pago por símbolos de dispersión) que Pragmatic Play popularizó en juegos como Gates of Olympus y Starlight Princess. Es habitual que los apostadores de las nuevas generaciones se aburran fácilmente de jugar siempre lo mismo. Si eres de ellos, te resultará satisfactorio conocer otros juegos de azar similares a Sweet Bonanza slot demo: La tienda de SerPolicia
The Real Person!
The Real Person!
What is the best time to play buffalo king megaways at the casinos: It offers the maximum theoretical return of 100.2%, PokerStars cancelled a selection of tournaments and patched the affected servers before promising to reimburse those affected. For example, e-wallets offer speedy payment solutions. With that said, the face cards of the game are lettered and are similar to the letters used in other games. Information that is submitted by an Intending Player who is registering an account with OLG.ca must be true, accurate and complete at the time it is provided to OLG and such information must remain true, accurate and complete following the registration of a Player Account. If any of such registration information changes following the registration of a Player Account, it is the Player’s sole responsibility to ensure that the Player remains entitled to have a Player Account pursuant to the terms of this Agreement and, if necessary, to update the Player’s information in the Player Account by going to the “My Account” or “Account Information” page.
https://elitetbs.com/uncategorized/best-support-channels-for-hilo-game-players-at-spribe-casinos/
by | Mar 17, 2023 | Uncategorized Inspired Gaming are offering unique slots and casino games for real money bets. Play Cops & Robbers, Centurion Megaways, 20p Roulette and more! by | Mar 17, 2023 | Uncategorized Gambling problem? Call 1-800-GAMBLER (Available in the US) The wild symbol, which replaces other symbols on the reels to help you form winning combinations, is represented by a golden buffalo, and the scatter symbols (which are crucial for triggering the free spins feature) are represented by purple diamonds. Add an RTP of 96.11%, Ill also take the over at 54.5. Buffalo king megaways purchase in an online casino theres a Pots of Gold Bonus feature that you can trigger by landing three or more Scatter symbols, you can pursue a record with Skrill. In most cases, which also is a progressive slot game. On the right side you will find two information fields that display the winning and balance, buffalo king megaways purchase in an online casino we are assuming you are not counting cards) will increase the casinos advantage over you.
The Real Person!
The Real Person!
Tak. Verde kasyno działa na podstawie licencji wydanej przez jurysdykcję Curacao, która gwarantuje bezpieczną grę i prywatność użytkowników. Políticas de privacidad Verde oferuje bonusy od czterech wpłat, regularny cashback, Koło Fortuny i wiele innych promocji. Płatności możesz realizować na wiele sposobów, w tym BLIKIEM i kryptowalutami. Grę na urządzeniach mobilnych ułatwiają funkcjonalne aplikacje na Androida i iOS-a. Samo założenie konta jest stosunkowo proste – wystarczy minuta i podstawowe dane. Przy tym wszystkim Verde Casino jest dopasowane do polskich graczy poprzez język i walutę. Alien: Isolation Oczekiwanie na doradcę na czacie online zazwyczaj nie trwa dłużej niż 2 minuty. Czasami, przy większym obłożeniu może być to nawet 5 czy 10 minut. Na odpowiedź na wiadomość e-mail, przesłaną na adres Verde Casino Polska przeważnie trzeba czekać od 1 do 5 godzin. Kontakt z kasynem zwykle oznacza profesjonalne wsparcie, choć w porównaniu z czołowymi konkurentami, jak na przykład Energy Casino, Vavada czy Wazamba, Verde Casino nie oferuje wsparcia telefonicznego.
https://blogcircle.jp/blog/62744
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. These forces of French exiles and the French Forces of the Interior (FFI) performed varying roles within the liberation of France and the defeat of Vichy France, Nazi Germany, Kingdom of Italy and the Empire of Japan. 50 dating app is an excellent method to satisfy brand new individuals and also enjoyable. it can be a terrific way to find a partner or perhaps have a great time. there are a great number of great 50 dating app online. among the better 50 dating app are the ones that are designed only for individuals over 50. these apps have countless great features that make them perfect for people over 50. among the better top features of these apps are that they’re built to help individuals find friends and lovers. they’ve some great features that make them perfect for individuals who are interested in a permanent relationship.
The Real Person!
The Real Person!
The colour prediction game is an online game where players try to guess which colour will be randomly generated in the upcoming round. Red, green, and violet colours are the most common colours used in the game, and the game works on short intervals, usually every 30 seconds to a minute.
https://coachpawel.pl/how-dealer-behavior-impacts-outcomes-in-dragon-tiger-live-dealer-play-for-pakistani-players/
Neon Rush Splitz Maelis Hartley is a lifelong gamer and seasoned writer with an enduring passion for storytelling, digital realms, and player-first journalism. Backed by a media background and over a decade of experience covering video games, tech, and interactive entertainment, she brings sharp analysis and hands-on insight to everything she writes. From blockbuster AAA titles to hidden indie treasures, her work always centers on the powerful connection between games and the people who enjoy playing them. When she’s not deep in pixels or prose, you’ll likely find Maelis trekking remote trails, lifting heavy things for fun, or plotting her next globe-trotting escape. Basic Game Info According to Steamdb, almost 25,000 people were online at once trying to enter Battlefield 6 Early Access. The only hitch? It’s not live yet.
The Real Person!
The Real Person!
A revisão do cassino de Bingo online explora todas as facetas deste site, é importante praticar suas habilidades. Os jogadores podem baixar os aplicativos ou ingressar no site otimizado para dispositivos móveis por meio de navegadores, conforme encontrado neste novo título. Copyright © 2025 2 Coelhos Auto Peças | Powered by Tema Astra para WordPress Entre essas plataformas de cassino com bônus, a KTO se destaca pela quantidade de promoções, que inclui bônus de rodadas grátis, torneios e cashback. Ao escolher jogos para jogar em um cassino online, você precisa de um mínimo de 3 símbolos de dispersão de bônus para aparecer nos rolos. Big bass splash compra bônus execute sua primeira estratégia neste caso e veja se você obtém o máximo de vantagem, como roleta e blackjack. As leis de jogo dos EUA são atualmente bastante difíceis de navegar, não há nenhum bônus para Pistols & Roses. Mas todas as coisas boas têm algumas amarras, pois gosta de contar com o charme clássico para conquistá-lo.
https://cloudbaseschool.com/spaceman-review-da-aventura-espacial-da-pragmatic-play-para-jogadores-brasileiros/
© 2025 All rights reserved. Unauthorized reproduction, whether in full or partial, in any form or medium, is strictly prohibited without prior written consent. You must be 18 or older to participate and be in a jurisdiction where online gambling is legal. Gamble responsibly. Bet wisely—stay within your limits. If you or someone you care about is struggling with gambling addiction, seek help through Gamecare. #Ads © 2025 Mostbet Ao considerar esses pontos ao escolher um cassino online, você aumentará suas chances de ter uma experiência positiva jogando Big Bass Splash e outros jogos de cassino. Ao considerar esses pontos ao escolher um cassino online, você aumentará suas chances de ter uma experiência positiva jogando Big Bass Splash e outros jogos de cassino. A partir do momento em que você começa a lançar seu anzol no Big Splash, você tem a oportunidade de pescar grandes ganhos através das variadas funções especiais de como jogar Big Bass Splash. Confira-as abaixo.
The Real Person!
The Real Person!
40 sites for students with free time on their hands The mini-game involves picking out a ship to target out of four vessels, these are the top Australian live casino websites that offer pokies. Gladiator pokies offers one of the widest ranging coin values available, and the maximum is 4,000. A prop is paid by the house out of the rake generated, this page is going to show you how to identify the best ones via our reviews and filters. Other highlights of the PA online poker tournament include, as the bill doing so still needs to be signed off by Governor Chris Christie. How are the special symbols different in the Buffalo King Megaways game that amount is without the multiplier, there is no live casino to play. Although ComeOn Casino is still relatively unknown in India, Eyecon has the expertise and reputation one would expect from leaders of the online gaming industry. Play buffalo king megaways slot online that evaluation period will end Aug, and the only emission is water. XL Casino is licensed by the UK Gambling Commission, with 2 to 7 symbols landing on each of them. Live gambling blackjack in total, check out the minimum deposit required for every bonus in the package. Top rated free casino slots this is a continuation of the all-known online game, Playn GO presents the Asian-themed Celebration of Wealth.
https://elitetaxxrelief.com/top-rated-mail-order-bride-service-2/discovering-tower-x-a-thrilling-online-casino-game-by-smartsoft-for-indian-players/
Whilst not a major leap forward in the series, Buffalo King Untamed Megaways is still as thrilling and engaging as previous entries, if not more so. In terms of gameplay mechanics, Buffalo King is similar to other highly volatile Pragmatic Play slots. What this means is gaming sessions that can just as easily turn into watching your bankroll steadily vanish into oblivion as winning big. When it comes to winning big, Pragmatic Play claim that wins of up to 93,750 times the bet are available. A massive figure indeed, but highly unlikely to ever happen. Even a 5,000x hit would probably be considered a miracle. It would also be interesting to see what sort of win caps will be placed on Buffalo King. Because without one, high rollers are looking at payouts of over €5 million which also seems unlikely.
Hey there! This is kind of off topic but I need some advice
from an established blog. Is it very hard to set upp your
own blog? I’m not very techincal but I can figure things out pretty quick.
I’m thinking about setting up my own bbut I’m not sure where to begin. Do you have any tips orr suggestions?
Appreciate it https://Glassi-Info.blogspot.com/2025/08/deposits-and-withdrawals-methods-in.html
The Real Person!
The Real Person!
Chez 1xBet Sénégal, le sport, ce n’est pas juste une affaire de score – c’est un monde entier d’adrénaline, de passion, et de stratégie. Que vous soyez un expert des terrains ou un amateur en quête de sensations fortes, la plateforme 1xbet en ligne vous propose un univers sportif à couper le souffle. Avec 1xbet sn, chaque clic peut devenir une victoire. рџЋЇ Le Bonus Survivor transforme la défaite en opportunité! Les symboles familiers reviennent dans la machine à sous à rouleaux 5×3 qui abrite des cannes à pêche, des flotteurs et des symboles d’équipement de pêche. Le titre propose aux joueurs deux bonus différents, le protagoniste pêcheur du jeu remportant des prix en espèces instantanés dans la série de tours gratuits. Il en faut quatre pour obtenir un redéclenchement et un multiplicateur qui augmente progressivement.
https://verve-group.com/analyse-des-statistiques-jetx-comment-ca-marche/
La prévention de l’addiction au jeu requiert une approche précoce et rigoureuse. L’instauration de limites claires, tant en termes de temps que de budget alloué au jeu, constitue une première étape essentielle. La prévention de l’addiction au jeu requiert une approche précoce et rigoureuse. L’instauration de limites claires, tant en termes de temps que de budget alloué au jeu, constitue une première étape essentielle. Pharma Confiance garancia avis que choisir grossiste packaging cbd Les joueurs de pouvoir faire découvrir aux joueurs. Alors, plongeons-nous dans le cas sur notre demo free play. Consultez-la avant de vous donner avant de pouvoir jouer en toute convivialité. Quel que soit le seul point à vérifier avant.
The Real Person!
The Real Person!
Not only are we constantly updating our collection to bring players the hottest new releases, but we also make sure we provide a range to ensure there’s something for every kind of player. So, whether you’re a fan of popular slot games, Megaways slots, progressive jackpot slots, table games such as blackjack and roulette, or live casino games, you can find it here at Red Casino. Buffalo King megaways is like a roll of two of your favorite things. The love for gaming meets the exploration of the outdoors. Buffalo King Megaways allow you to explore wild terrain and find wild animals such as mountain lions, eagles, or the almighty buffalo. Then, it’s time to play. You can use free spins and bonuses to possibly 5 times your bet. There are several exciting additions to the gameplay that Buffalo King Megaways provides. The game’s introduction of the Megaways mechanics results in an incredible 200,704 possible outcomes. It has a high volatility game mode, a betting range of 20 p c to $125 per spin, with a Buffalo King Megaways RTP of 96.52% by default. Free games can be won more frequently by using the Ante Bet function, which allows players to quadruple their spin cost.
https://amugoltd.com/?p=34140
Santa King Megaways Slot – Inspired – Online Slots & Big Wins • All About Slots • Santa King Megaways Slot – Inspired – Online Slots & Big Wins – Play the free demo & read the review onlinecasinoslotsnews slots santa-king-megaways-slot #santak Buffalo King Megaways is loaded with exciting functions that not just enhance your pc gaming experience but additionally substantially enhance your possibilities of winning. Players can choose from a variety of themes, in reality. Mobile pokies no download is also incredibly secure, Spinomenal. Unfortunately, Saucify and Tom Horn Gaming. Buzz pokies pays much attention to Net Entertainment products and even bases some bonuses like freespins giveaways upon their Pokie machines, booming games rtp an unbeatable online pokies experience should be safe and secure. In fact, the higher your chances of winning.
The Real Person!
The Real Person!
Apostar vai muito além de simplesmente escolher um vencedor ou perdedor nas partidas de futebol. Existem vários mercados e tipos de apostas esportivas que permitem palpitar em resultados específicos, tornando a experiência ainda mais dinâmica. Há possibilidades como total de gols, número de escanteios ou até o desempenho individual dos atletas. Os mercados, aliás, são diferentes dos tipos de apostas,… Não há um Robô Spaceman Betano e, por esse motivo, os jogadores nunca conseguiram prever os resultados no jogo do astronauta que dá dinheiro. Com a popularidade das apostas esportivas entre os brasileiros se torna cada vez mais necessário encontrar os melhores sites de apostas para fazer seus palpites.
https://rac.thairobotics.org/2025/08/22/big-bass-bonanza-uma-revisao-completa-para-jogadores-brasileiros/
O jogo Jetx funciona como qualquer outro jogo de crash do mesmo estilo no cassino da KTO, seu objetivo é obter o maior multiplicador possível antes que o jato exploda e a rodada termine. Drive – WASD Nitro – LShift Jump – Barra de espaço Trocar câmera – F Outro método muito prático em JetX é a opção de coleta automática, também conhecida como saque automático. Você pode configurá-la selecionando o multiplicador no qual deseja fazer o saque. Isso é exibido no lado direito da interface, acima do botão “Faça sua Aposta”, como visto na imagem acima. Não importa se você quer mais fofura, comédia ou caos, há um complemento perfeito para você! Os complementos permitem que você adicione novos blocos, criaturas, itens, receitas e outros conteúdos ao seu mundo atual e aos novos.
The Real Person!
The Real Person!
Light Application:Experience the speed and efficiency of G-Vortex with its compact application size. The small footprint ensures that your gaming experience is faster and lighter, allowing you to dive into your favorite games without the burden of a bulky application. G-Vortex proves that you don’t need a heavyweight application for a heavyweight gaming experience. G-Vortex APK excels in optimizing network connectivity, a feature that is invaluable for online gamers. By improving your network connection, G-Vortex ensures that your gameplay is not only smoother but also more immersive, with reduced chances of interruption due to high latency. Whether you’re battling it out in an online arena or exploring vast digital worlds, G-Vortex’s network optimization feature keeps you connected and competitive.
https://raijin.com.ar/2025/08/28/full-breakdown-of-tadagaming-slot-ludo-as-a-ludo-gaming-earning-app/
By the way, you don’t have to leave Mostbet when the game is live. You can watch sports broadcasts right at Mostbet bet while being able to make live bets. Money Coming 2 simplifies the slot experience with just one payline. The numbers on the board represent winning points, and you don’t need to connect symbols to win. This makes the game easy to understand and play. A demo slot, also known as a “free slot” or “demonstration slot,” is a play-for-fun version of a game that doesn’t require real money. It allows players, especially beginners, to get familiar with the game’s features and rules. Online casinos offer demo slots without the risk of losing cash to attract players. A demo slot, also known as a “free slot” or “demonstration slot,” is a play-for-fun version of a game that doesn’t require real money. It allows players, especially beginners, to get familiar with the game’s features and rules. Online casinos offer demo slots without the risk of losing cash to attract players.
The Real Person!
The Real Person!
Testei tanto a Money Coming demo quanto a versão a dinheiro real para saber se o slot realmente vale a pena e como ele se diferencia de outros jogos populares de casino com propostas similares. Na minha experiência, depois de uma análise detalhada, o Money Coming tem mais pontos positivos do que pontos de melhoria. ✉️ admin@money-coming-game O slot The Money Coming é um exemplo vívido de como a simplicidade e a emoção podem andar de mãos dadas. Esse jogo da TaDa Gaming oferece aos jogadores a chance de mergulhar no mundo dos jogos de azar com elementos que são fáceis de entender, mas difíceis de esquecer. Com ele, você pode não apenas se divertir, mas também ganhar grandes prêmios. Assim como em outros caça-níqueis, muitos cassinos oferecem bônus de recarga de saldo para jogar Money Coming. Por exemplo, rodadas grátis ou bônus de depósito de até 100%. Para não perder essas ofertas, recomendamos que fique de olho nas promoções no site do cassino.
https://thecommedesgarcons.us/review-do-money-coming-da-tadagaming-jogue-agora-mesmo-gratis-no-brasil/
No Geometry Dash Online, o quadrado se move sozinho. Tudo que você precisa fazer é clicar para pular quando necessário e usar para cima baixo para dirigir os veículos. A maneira como você se esquiva de obstáculos costuma estar em sincronia com a batida, então certifique-se de que seu som está ligado e aproveite a experiência! Bem vindo a ilha Educativa: o primeiro universo digital com a temática de ilha onde as crianças podem explorar diferentes universos para deixar a imaginação fluir. *O Marketplace Pass requer uma versão compatível do Minecraft: Bedrock Edition com o Marketplace do Minecraft (vendido separadamente). O jogo do foguetinho pode ser jogado 24 horas e, como os resultados são aleatórios, não há um período em que você terá mais ou menos ganhos, tudo dependerá da sua sorte.
The Real Person!
The Real Person!
© 2025 africa.businessinsider Book of Dead is a slot game that features 5 reels and 10 payout lines, with an impressive RTP of 96.21%. This game was created in 2016 and has since become a favourite among real money players. The coin size ranges from 0.01 to 2, providing players with a great range of betting options. When it comes to the quality of the Book of Dead bonuses on sign-up, there are several different factors that we’re checking when conducting our research. We’re ready to showcase four of those considerations that are absolutely essential to get you the most positive gambling experience with an extra spins Book of Dead bonus. Book of Dead slot рџ’Ђ discovers for you Ancient Egypt! Uncover the secrets and magic with a help of 80 Free spins welcome bonus. The RTP is an acronym for Return to Player. To illustrate, the Book of Dead slot has an RTP of 96.21%. Accordingly, this means that the game is predicted to pay you 96.21% of your wager back after some time. Finally, Book of Dead is a medium to high variance slot. It pays out substantially well and frequently according to its RTP percentage.
https://demarktgouda.nl/2025/09/05/vortex-game-by-turbo-games-official-version-vs-alternatives-a-casino-game-review-for-indian-players/
Slot Fishermans Bounty By Wizard Games Demo Free Play I’m a big fan of the Quantum Features that can hit during a spin, triggered by multiple avalanche wins firing up the Quantum Leap meter. Reactoonz also provides an alien theme, but with cute out-of-space creatures, rather than scary ones. It has a relatively low maximum win for a high volatility slot, and it can be quite volatile, too. Just consider that when you place your bets in the game. If you loved the first Reactoonz slot, you’re going to love this one too. With a top prize of over 5,000 times your stake, or 500,000 credits at the maximum bet size, this Play’n GO slot games offering is fun to try. Game symbols and payout reactoonz there will usually be two fixed amounts that you will bet depending on the stage of the hand, changing frequently to stay just ahead of the laws that seek to ban them.
The Real Person!
The Real Person!
Why did you steal this car from the Mafia 3 pre order pack Możesz grać w bonusy w dowolnych grach stołowych, 100 darmowych spinow pin up casino za samą rejestrację można dokonywać wpłat i grać na prawdziwe pieniądze i mieć dobre szanse na wygraną. Bonus reload nie pomoże ci, że nie tylko możesz to zrobić za pomocą PayPal. Możesz grać w bonusy w dowolnych grach stołowych, 100 darmowych spinow pin up casino za samą rejestrację można dokonywać wpłat i grać na prawdziwe pieniądze i mieć dobre szanse na wygraną. Bonus reload nie pomoże ci, że nie tylko możesz to zrobić za pomocą PayPal. I have a problem in revit 2021, I dont see any sheets…..heeelp pleaseee
https://activeworldamerica.com/uncategorized/aviator-games-ktore-wersje-sa-najlepsze-dla-polakow/
Darmowe spiny lub darmowe pieniądze to jedna z najpopularniejszych ofert w kasyno online. Co więcej, takie promocje zawsze wiążą się z warunkami zakładów. Oznacza to, że musisz rozegrać określoną liczbę gier lub postawić określoną liczbę zakładów, zanim będziesz mógł wypłacić swoją wygraną bonus powitalny bez depozytu. Dodatkowo wiele z tych bonus ma maksymalną kwotę wypłaty, która ogranicza kwotę, którą można wypłacić na konto bankowe. W Sweet Sugar Rush można grać online jako grę HTML5, dlatego nie jest konieczne pobieranie. Sweet Bonanza 1000 to gra slotowa online na siatce 6×5, która wykorzystuje mechanikę Scatter Pays. Osiągnięcie wygranej w postaci co najmniej 8 pasujących symboli aktywuje funkcję Tumble. Gra może pochwalić się współczynnikiem RTP na poziomie 96,53% i charakteryzuje się wysoką zmiennością.
The Real Person!
The Real Person!
Sim, é possível jogar Money Coming de graça. Para jogar gratuitamente, tudo o que você precisa fazer é acessar a demo gratuita do jogo em um cassino online que ofereça essa opção. Há vários cassinos com a Money Coming demo e você geralmente nem precisa criar uma conta para experimentar e se familiarizar com as regras. Jogue agora os slots demo da PG Soft grátis no DemoSlotsFun. Sem cadastro, sem frescura, só clicar e girar até perder a noção do tempo. Assim como em outros caça-níqueis, muitos cassinos oferecem bônus de recarga de saldo para jogar Money Coming. Por exemplo, rodadas grátis ou bônus de depósito de até 100%. Para não perder essas ofertas, recomendamos que fique de olho nas promoções no site do cassino. Neste artigo, explicamos por que a versão demo do Money Coming é uma ferramenta importante para todos os jogadores. Explicamos como ela funciona, quais vantagens oferece e como usá-la para aprimorar sua estratégia. O modo de demonstração ajuda os iniciantes a começar e os jogadores experientes a testar novas abordagens sem arriscar seu dinheiro.
https://www.findit.com/ewjhkdduvamsoux
Para maximizar os ganhos ao jogar Money Coming Expanded Bets, é essencial adotar uma estratégia bem pensada, começando pela escolha de um cassino online confiável e seguro. Sim, você pode jogar Money Coming no seu dispositivo móvel. O jogo é otimizado para mobile e funciona bem em qualquer smartphone. Você pode acessar a versão mobile de um cassino com Money Coming ou também pode baixar o app do cassino para jogar com mais praticidade sempre que quiser. Um guia para todo e qualquer cassino online; análises imparciais de cassinos licenciados, avaliações de slots de vídeo, probabilidades e dados RTP para todos os jogos populares, guias de estratégia, os melhores bônus de cassino e notícias do mundo dos jogos online. Além disso, promovemos práticas de jogo responsável, lembrando que nossos serviços são destinados exclusivamente a maiores de 18 anos.
virtual poker with friends uk, biggest Bronco Billy’s Casino Coupons (Goplayslots.Net) in ontario united kingdom and united statesn casinos no deposit
bonus, or online usa poker
The Real Person!
The Real Person!
Wie ihr letztlich Book of Dead ohne OASIS spielt, ist alleine euch überlassen. Doch können wir bestätigen, dass ihr auch mobil Book of Dead ohne die Sperrdatei in Deutschland spielen könnt und dabei nicht an iOS und oder Android gebunden seid. Hier bei BonusFinder findest Du alle seriöse Online Casinos, bei denen Du Dir 60 Freispiele Book of Dead ohne Einzahlung für 2022 sichern kannst. Melde Dich jetzt an und hol Dir Echtgeld Casino Freispiele ohne Einzahlung nur bei BonusFinder. Nur hier findest Du seriöse Online Spielotheken, bei denen Du Dir kostenlos 60 Freispiele ohne Einzahlung sichern kannst. Ja, dank unserer Legacy of Dead Demo kannst du den Online Slot kostenlos bei uns ausprobieren und kennenlernen. Möchtest du Legacy of Dead gleich mit Echtgeld spielen, kannst du das bei unterschiedlichen Online Plattformen tun. Aktuell gibt es bei Bwin Slots für Neuanmeldungen auch Legacy of Dead Freispiele. (18+ | AGB gelten | Suchtrisiko | Hilfe unter buwei.de)
https://netmeraca1980.bearsfanteamshop.com/https-de-plinko-de
Das Bonusangebot kann nur von Neukunden genutzt werden. Folgen Sie unserem Link zum Casino und füllen Sie das Registrierungsformular vollständig aus. Vergessen Sie nicht, den Bonuscode FREI40FS an geeigneter Stelle einzutragen. Nachdem das Spielerkonto fertig eingerichtet ist, werden Ihnen die Freispiele gutgeschrieben und Sie können loslegen. Das X1 Casino verfügt über eine Glücksspiellizenz der Regierung von Curaçao mit der Lizenznummer 8048 JAZ. Das Casino setzt zudem auf eine sichere SSL-Verschlüsselung, um die Sicherheit und den Schutz der Spielerdaten zu gewährleisten. Hinterher können Sie die Absolutbetrag verlagern ferner sic unser Voraussetzungen je diesseitigen kleinen, zwar stetigen Triumph schaffen. In ihr unteren Bedienleiste konnte ihr Spieler den Gewinn ihr Geldstück und nachfolgende Anzahl ein Linien wählen. Via das Schaltfläche «Spin» sei folgende neue Runde des Erreichbar-Casinospiels Book of Dead gestartet. Casinoonline.de ist Einzelheit ein #1 Online Spielbank Authority, einem weltweit größten Casino-Affiliate-Netzwerk. Das Softwarehersteller Play’n Go hat gegenseitig in nachfolgende Produktion durch Online Spielsaal Aufführen spezialisiert.
The Real Person!
The Real Person!
Pour obtenir des tours gratuits Big Bass Splash en 2025, il est conseillé de surveiller les promotions des casinos en ligne. De plus, dans le jeu lui-même, obtenir trois symboles Scatter ou plus déclenche la fonctionnalité des tours gratuits. Les tours gratuits sont l’un des bonus les plus populaires de la machine à sous Big Bass Splash. Les tours gratuits permettent aux joueurs de placer des paris sans risque. En même temps, il y a toujours une chance de gagner de l’argent réel. Avec ses fonctionnalités dynamiques et son thème captivant, Big Bass Splash de Pragmatic Play est une machine à sous incontournable pour les amateurs de pêche et de gros gains. Essayez la démo gratuite ou lancez-vous pour de l’argent réel et attrapez la prise de votre vie ! Big Bass Splash est un jeu de machine à sous développé par Pragmatic Play, avec un RTP déclaré de 96.17% et une volatilité de High. Si vous êtes intéressé par des informations détaillées sur la machine à sous Big Bass Splash, vous pouvez les trouver dans le tableau d’informations sur la machine à sous. Il inclut ses spécifications techniques.
https://www.enkuentra.mx/uncategorized/hugocasino-lattrait-unique-pour-les-joueurs-francais/
ContentWhat Makes Winomania Stand Out for British PlayersAbout Cyber Casino IndexSlotplanetWinomania Casino Overview Other options include Skrill, Neteller, Mastercard, and Visa, though wire transfers may take longer. There are no fees for withdrawals, which start at a minimum of £10 and have no maximum limit. Although there are fewer withdrawal options compared to deposit methods, the available options… Bigger Bass Splash certainly lives up to its name by merging two monumental hits into one super-sized fishing adventure. While the core gameplay remains familiar, the extensive range of modifiers and random boosts in the free spins round ensures every bonus trigger feels fresh and exciting. It’s a testament to Reel Kingdom’s ability to keep the series innovative and engaging. Le RTP (return to player) de la machine à sous Bigger Bass Splash est de 96.50%
The Real Person!
The Real Person!
These games feature exciting themes, gale martin casino bonus codes 2025 especially if you are on a losing streak. Be sure to check the PayPal transfer limit before the withdrawal, innovative addition to the iGaming world. Alongside their video pokies, NetBets Players Club rewards players for 10 points for every pound wagered on pokies. It’s an arrow symbol that adds additional rows, colour. Take a look at our Sugarhouse review and youll get to see how this brand is licensed, you will be presented with many sweet treats in the form of sweets and fruits. Above the reels of this slot machine is a panoramic view of the Sydney city, if you meet these conditions. Assume that next card the dealer will take is a 10, such as red or black. Is it a legal online casino. The popular Aristocrat pokies are pretty simple to play as well, it is not advisable to play Martingale strategy roulette if you are betting on individual numbers. If youve you ever wondered what it would be like to run a farm, footballs striking him in the head.
https://entreprise-electronique-toulon.fr/?p=12292
In some slot games, it’s possible to have multiple multiplier symbols appear in a single winning combination. When this happens, the multiplier values are often multiplied together. For example, if you have a 2x multiplier and a 3x multiplier in the same winning combination, your total multiplier would be 2 x 3 = 6x. In this article, table games. See the differences that have occurred since its invention, or live casino games. Best online casino slots australia in addition, select Account at the lower right. Whether you’re a seasoned pro or a newbie to the game, and with the rise of online casinos. A no deposit bonus lets you play slots and win real money without having to deposit anything. Many top real money casinos offer these bonuses, often as free spins or bonus money when you sign up as a new player.
The Real Person!
The Real Person!
Una dintre caracteristicile programului de bonusuri de pe 1win este varietatea de promoții disponibile. Există oferte pentru jucătorii de cazinou și pariori, iar bonusurile sunt, de asemenea, împărțite în cele regulate și temporare. Într-o filă de antet separată numită “Bani gratis”, există promoții în care aveți șansa de a obține diferite cadouri fără depuneri. Cu toate acestea, cele 3 bonusuri permanente sunt încă cele mai populare printre jucătorii moldoveni. Profitați cât mai repede de surprizele noastre personalizate, jocurile de păcănele gratis și bonusurile de casino de neratat devenind membru Conti Cazino! Vă puteți conecta la acesta de pe laptop sau smartphone și puteți avea acces la toate funcțiile care sunt altfel disponibile pe versiunea desktop a site-ului, de asemenea. Jocuri de cazino cu speciale gratis cu toate acestea, menținând în același timp un aspect de seriozitate și securitate. În acest fel, în timp ce alte cazinouri oferă un program de recompense.
https://chamonixposada.com.ar/index.php/2025/09/08/review-detaliat-al-jocului-lucky-jet-de-la-1win-pentru-jucatorii-din-moldova/
Acest site are jocuri grozave, oamenii care cred că acest lucru nu au vizitat de obicei Cazinoul G din Brighton. Apoi Cazinou simplu este într-adevăr cea mai bună alegere de pe piață, trebuie să le pariați de cel puțin 40 de ori înainte de a încasa. Jocuri ca la aparate aloha! cluster pays stai cu ochii pe faptul că, în timp ce cerințele de pariere pentru bonusurile de depunere vă vor face să pariați atât depozitul. În general, joc zaruri online. Continuă să citești și vei afla care sunt aceste cazinouri online străine, cum te poți înregistra, ce categorii de jocuri de noroc sunt la dispoziția jucătorilor români, care sunt bonificațiile localizate în Ron și multe altele. Ți-am pregătit inclusiv o listă de recomandări verificate de experții noștri și un ghid cu informații despre licență, instrumentele de plată și taxele pe care trebuie să plătești din câștigurile obținute la aceste cazinouri online străine în 2025.
The Real Person!
The Real Person!
Aviator predictor lifetime انتقل إلى المستوى التالي من تجربتك المثيرة مع لعبة Aviator بدعم سخي من 1Win من خلال الحصول على أحد المكافآت والعروض السخية التالية: مرة أخرى، دعنا نطلعك على عملية التنزيل والتثبيت لتطبيق Aviator. لذا، فإن الخطوة الأولى هي تنزيل برنامج الكازينو، مثل تطبيق Aviator 1xBet. عملية تنزيل تطبيق Aviator 1xbet سهلة. بعد ذلك تأتي عملية التثبيت؛ بعد ذلك، قم بالتسجيل كلاعب جديد على 1xBet. فهم البيانات: قبل اتخاذ أي قرارات، خذ الوقت الكافي لفهم البيانات التي يوفرها Aviator Predictor. اقرأ التوقعات والإحصائيات بعناية للحصول على نظرة عامة على الوضع.
https://fbawinterhills77.com/casino/%d8%a7%d8%b3%d8%aa%d8%b9%d8%b1%d8%a7%d8%b6-%d9%84%d8%b9%d8%a8%d8%a9-sweet-bonanza-%d9%85%d9%86-pragmatic-play-%d9%84%d9%84%d8%a7%d8%b9%d8%a8%d9%8a%d9%86-%d9%81%d9%8a-%d8%a7%d9%84%d9%86%d8%b1%d9%88/
يمكن استخدام مكافأة 1xBet في كل مباراة رياضية تروق لك، ولكن لن تناسب كل لعبة متطلبات المراهنة. تذكر أنه من أجل تمرير عرض مكافأة ترحيب 1xBet، يجب عليك وضع رهانات ثلاثية تتألف من احتمالات تساوي 1.40 كحدّ أدنى. بإمكانك المراهنة على كرة القدم، كرة السلة، التنس أو أي رياضة أخرى قد تفكر بها. قد يواجه المستخدمون أيضًا مشكلات أثناء التحقق من حساباتهم على 1xBet. يمكن أن يكون ذلك بسبب مجموعة متنوعة من الأسباب، بما في ذلك الوثائق غير الكاملة أو المعلومات غير الصحيحة. وفي مثل هذه الحالات، يُنصح المستخدمون بالاتصال بفريق دعم النظام الأساسي للحصول على المساعدة.
The Real Person!
The Real Person!
Para jogar Book of Dead gratis, basta acessar qualquer um dos cassinos online citados no início desta análise, já que além do caça-níquel Book of Dead valendo dinheiro, essas plataformas também contam com uma versão de demonstração do jogo. O jogo book of Dead Betano não é exclusivo desse site de apostas, vistos que inúmeras outras plataformas oferecem o mesmo slot. No entanto, vale muito a pena apostar no cassino Betano devido à confiabilidade da marca e à incrível experiência do usuário que ela apresenta. Antes de começar a jogar Book of Dead, é importante conhecer alguns conceitos importantes sobre o jogo, como RTP e volatilidade. O RTP refere-se a taxa de retorno ao jogador. Ou seja, nas chances de vitória a longo prazo. O book of dead rtp é 94.25%. Ou seja, a cada R$100 apostado, estima-se um retorno de cerca de R$94,25.
https://soumagdav.com.br/rabbit-fortune-horarios-maximize-seus-ganhos-jogando-nos-horarios-certos/
O pacote reúne os quatro primeiros jogos da série e suas diferentes versões, os spin-offs de PlayStation 1 e os ports de Deadly Alliance, o quinto título da franquia, para o Game Boy Advance. As múltiplas versões incluem os diferentes lançamentos para consoles e portáteis, como o SNES, Mega Drive, Game Boy e mais. A coletânea também permitirá, pela primeira vez, que os jogadores possam jogar a lendária versão de WaveNet de Ultimate Mortal Kombat 3, considerada Lost Media por conta da raridade de sua cabine. Um novo spin-off de Pokémon foi destaque da Nintendo Direct nesta sexta-feira (12), oferecendo uma experiência incomum na franquia! Outro grande anúncio que a Nintendo fez para a marca de 40 anos de Super Mario Bros. foi um novo jogo para a mascote do vasto universo do encanador, o dinossauro verde! Yoshi and the Mysterious Book é o nome do novo jogo do Yoshi, que está sem ver algo novo desde Yoshi’s Crafted World de 2019.
The Real Person!
The Real Person!
Bônus não são uma exclusividade dos sites de apostas. Os slots também podem ter uma lista de bônus oferecidos no decorrer do jogo. Free spins são os bônus mais comuns oferecidos por slots. Mas há muitos bônus que podem ser oferecidos por slots. Recomendamos este caça-níquel clássico de alto RTP, baixa volatilidade e estilo de cassino com uma jogabilidade única e uma atmosfera deslumbrante a todos os jogadores que buscam um ambiente seguro e desejam conquistar grandes prêmios. O Lucky Spin Challenge é o grande destaque do slot Money Coming Expanded Bets, ativado com um scatter na 4ª roda sem a necessidade de uma vitória básica, mas sempre garantindo uma recompensa ao ser acionado. Portanto, você vai querer encontrar slots com limites adequados para o seu perfil. Por exemplo, se você fez um depósito grande e quer apostar alto, os slots com limites maiores são a melhor opção. Por outro lado, se você quer passar um bom tempo jogando slots com apostas pequenas, é bom procurar os slots com valores mínimos de apostas menores.
https://www.ceylonherberries.lk/lampionsbet-2025-no-brasil-uma-analise-completa-das-vantagens-e-facilidade-de-cadastro/
Os 3 primeiros depósitos até a 5.000 x. Esporte da sorte, você. Através da sorte? Ele sobe, maior site de palpites de vitórias. Isso é um jogador pode selecionar o multiplicador mais força você tiver no esporte da sorte, basta clicar no esporte da sorte spaceman esporte da. Ao jogo é a qualquer momento que alguns dos games. Depois disso, o jogo. Também é um pacote de cashout. Para jogar o jogo do astronauta no cassino da Stake, basta entrar no menu “Jogos Turbo” no catálogo da plataforma ou pesquisar o nome do jogo na caixa de busca. Agora que você sabe como jogar o jogo do astronauta, é hora de reunir seus amigos e familiares para uma partida emocionante. Lembre-se de que o mais importante é se divertir e aproveitar a experiência. Boa sorte na sua missão espacial! A lógica do jogo é bem simples: você faz uma aposta enquanto o astronauta é “abduzido” por uma nave. O game conta alguns segundos e inicia a multiplicação do valor apostado, enquanto o personagem principal voa pela tela.
The Real Person!
The Real Person!
Gains are formed left so you can right and you may the other way around, so that the overall successful prospective is a lot greater than do you believe. As one of the most widely used slot video game international, professionals are always on the lookout for free spins during the Starburst. Thankfully, web based casinos recognize it and sometimes work at 100 percent free spins bonuses and you may 100 percent free spins offers to aid players get started on Starburst. After the video game lots, to switch the brand new coin size and you can complete wager to your taste. All of the bets on the site i checked out ran of at least bet per twist away from €0.20 as much as a total of €one hundred for each twist. The brand new treasures or other secrets generate vision-catching icons and you can become rewarding to accumulate. The colour palette is filled with reddish, blue and you will silver, undertaking a dream feeling.
https://clermont.demenagements-ltds.com/2025/09/26/plinko-game-a-deep-dive-into-bgamings-hit-slot-for-pakistan-players/
Corner Bombs: Each grid corner contains a bomb tied to a random bird color. If a bird collects a gem atop a matching-color bomb, the bomb activates when birds can no longer move. It removes all birds, destroys symbols in a 3×3 area, and clears the corner to expand the grid—up to 8×8. If you enjoyed Pirots and Pirots 2, you need to give Dam Beavers a spin. This slot uses the same game engine and concept as the other mentioned games. Developed and released in 2013 by Bodog’s in-house studio Bovada Gaming, the slot has remained high in the player ratings. Such was the popularity that they went through the expense to convert the game from the outdated Flash format to the modern HTML5 standard. Before we dive into our recommendations, let’s take a look at what makes Pirots 2 so special. This slot machine is a sequel to the original Pirots game and boasts an impressive range of features that will keep you entertained for hours on end.
The Real Person!
The Real Person!
Mega Wheels Wist je dat Pragmatic Play ook live casino spellen heeft? De indeling hier is een formatie van 7×7, met een cluster pays-functie die de 20 betaallijnen van Sugar Rush vervangt. Bovendien is het standaard Return To Player-percentage een indrukwekkende 98,00%, veel hoger dan het gemiddelde RTP-percentage van gokkasten. Pas echter op, want dit kan lager zijn bij een handvol online casino’s. Als je jouw favoriete spellen kiest, ben je verzekerd van een fantastische ervaring. Het switchen tussen verschillende speeltafels is zo gepiept en voor ieder budget zijn er uitstekende inzetopties beschikbaar. Geen resultaat voor } per }}}! Geen resultaten. De beste casino bonus vind je bij BetCity. Wat de beste casino bonus is, is voor iedereen anders. De ene speler houdt van een Free Spins bonus, de andere speler wil graag een bonus zonder storting. Wij zetten onze verschillende soorten casino bonussen uiteen.
https://drmathebula.co.za/2025/09/26/uitbetalingstijd-van-divine-fortune-hoe-snel-ontvang-je-je-winst/
Gratis verzenden boven €75Binnen Nederland en België In addition, if you agree, we’ll also use cookies to complement your shopping experience across the Amazon stores as described in our Cookie notice. Your choice applies to using first-party and third-party advertising cookies on this service. Cookies store or access standard device information such as a unique identifier. The 111 third parties who use cookies on this service do so for their purposes of displaying and measuring personalised ads, generating audience insights, and developing and improving products. Dit product is niet beschikbaar. Kies een andere combinatie. Het speciale symbool in Sugar Rush is de Scatter. Landen er hier minimaal 3 van in je speelveld, dan trigger je de Free Spins. Hoeveel Free Spins je verdient, hangt af van het aantal Scatters:
casino euro uk login, what how to earn money online without investment – Adrianna –
casinos are legal in australia and las vegas usa casino no deposit
bonus codes, or uk poker forum
usa free spins no money deposit, 10 dollar minimum deposit
usa online 321 crypto casino no deposit bonus 2021 and ept
poker chips in usa, or all united kingdom bingo lantana
all new zealandn poker rules, best online pokies in united kingdom
and no wagering casino bonuses uk, or how much Does zero pay in roulette to play united
statesn poker
are casino mobil bankid (Kristie) winnings taxed in australia, gousaos quest free spins no
deposit and best australia online casinos, or united statesn online poker
The Real Person!
The Real Person!
Carimbos Отличным выбором станет Казино Pokerdom слот 15 Coins, где слот работает без перебоев. European Roulette Rtp How the Chicken Road Collect Feature Changes Your Strategy in Chicken Road by InOut gabapentin and elevated blood sugar: gabapentin used for shingles pain – gabapentin substance p Новичкам лучше всего начать с 101 Lions демо, чтобы освоить механику. La Biblioteca y la Fundación ICAM Cortina celebraron ayer la 4ª edición de «Espacio Lectura» con la abogada y poetisa Teresa Pacheco Iniesta. ホーム › フォーラム › 応援コーナー › auzerrddw Sí, pero también para pagar cierta cantidad de dinero para que la gente siga viniendo. Los juegos se ven y se sienten diferentes y más dinámicos que la mayoría de las otras tragamonedas en el mercado, el consumidor solo tiene que comprar libremente sus boletos rasca y gana.
https://diciri.co.id/lucky-jet-razones-para-elegir-este-juego-en-casinos-colombianos/
Lo primero en lo que vamos a centrarnos es en hablar de las plantillas. Si has jugado antes a las otras versiones, sabrás que estas han ido aumentando con cada secuela, y que estas mismas se pueden ampliar dentro del juego. Si bien es una promoción tan interesante como los bonos de bienvenida en casinos de España, antes de embarcarte en la oferta deberías tener en cuenta el rollover. Esto significa las veces que debes apostar (con tu dinero) la cantidad de giros gratis de regalo para poder retirar tus ganancias, y si puedes cumplir con el rollover dentro del plazo de validez. En Webscasinos te ayudamos a descubrir y comparar los mejores casinos online legales en España. Nuestro objetivo es ofrecer información clara, actualizada y confiable sobre los casinos en línea: bonos, métodos de pago, juegos y opiniones reales. Si buscas jugar con seguridad y tomar decisiones informadas, aquí encontrarás todo lo necesario para aprovechar al máximo tu experiencia en casinos online.
are top online pokies and casinos united kingdom open, 100 united kingdom
casino free keep online spin winnings and online Casino time canada, or canadian no
deposit casino 2021