With rise in environmental concerns and search for reducing the dependency on natural resources, the energy sector is revolutionising from non-renewable energy to renewable energy. Increased spending on R&D for renewable energy has evolved the generation of electricity from mechanical generation to chemical generated electricity. In mechanical transformation, the electricity was generated by rotating the turbine and converting the electromagnetic energy, for example a hydroelectric plant. In chemical conversion, certain chemicals are used in a mixture to derive electric energy from the reaction, for example lead acid batteries.
Batteries can be classified on their properties of
- Energy density
- Charge retention
- Safety standards
- Charging time
- Cost
Batteries are small chemical reactors which generate electricity from the chemicals present in it. Alessandro Volta in 1800 was the one who put together the first battery called Voltaic pile. The voltaic pile was a stack of zinc and copper disc on one another which were separated by cloth soaked in brine (electrolyte). The current was generated with a chemical reaction. This breakthrough led to extensive research in battery technology and development of dry cell batteries, however the research was limited as the cells had one time life and couldn’t be rechargeable.
Domestic companies manufacturing dry cell batteries are Eveready industries limited, Panasonic energy india limited, Indo national limited and others.The usage of dry cell batteries are into daily application of small electrical appliances with AA and AAA sizes. The product is in commodity business and the price maker in industry will have the highest market.
Eveready batteries dominate the dry cell market with 50% market share followed by indo national and panasonic industries. The companies are least likely to enter into lithium battery chemistry but may provide the lithium pellets to battery manufacturers.


Research on rechargeable batteries gave rise to the lead acid batteries in 1859 made by Gaston plante. By the end of 1880’s, lead acid batteries were extensively used in IC engine cars. The lead acid battery has high energy density with high charge retention and is cheaper compared to lithium batteries, however due to longer charging hours they could not be used in electric vehicles. Domestic companies which are manufacturing lead acid batteries are Exide industries limited, Amara raja batteries limited. The company is further leveraging their position into the lithium battery segments. The industry is highly driven by growth in the automotive industry as lead acid batteries have the highest application in the auto OEM industry.
With the introduction of lithium ion batteries in 1991 by Sony, the industry experienced a sudden surge in shift from lead acid batteries to lithium batteries. Lithium acid batteries were highly accepted because of high retention of charge, high energy density, shorter period of charge time and free of maintenance. However, lithium ion batteries are expensive to manufacture because of low availability of raw materials like lithium salts, cobalt and nickel.
Lithium deposits are largely found in Argentina, Bolivia, Chile, Australia, China and the USA.
Lithium is a highly reactive element and being the lightest metal. The high electro positivity of the element makes it most suitable for use in battery chemistry. Multiple lithium-ion cells connect internally to make up a lithium-ion battery. Think of lithium-ion cells as the building blocks that make up a full battery. These cells are connected in series or parallel and are monitored by the battery management systems. Lithium ion cell have 4 basic components
- Body/container
- Cathode and anode
- Electrolyte solution
- Separator film
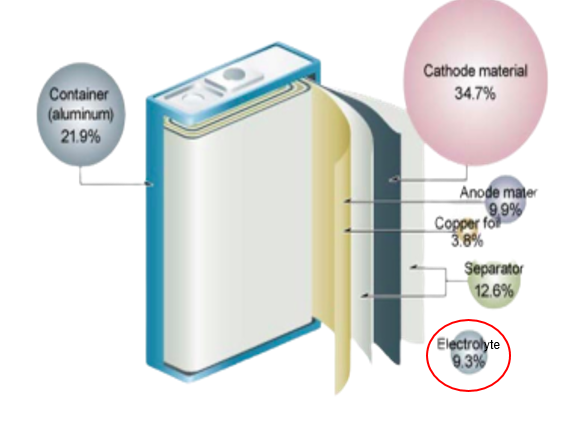
Body/ container is the outer structure of the battery which holds the cells in position and avoids its interaction with the environment. The casing has to be strong enough to withstand shocks and impacts because if it breaks and the cell gets exposed, the solution inside the cell will react with atmospheric oxygen leading to an explosion.
Anode is the negative electrode in a cell. The most popular material used for the anode is graphite. The positive side is called the cathode. Common materials for the cathode are lithium cobalt oxide, lithium iron phosphate, and lithium manganese oxide.
Electrolyte solution is where the actual chemistry lies,Electrolyte is a media that conducts ions between the electrodes in the cell and every composition is different for different geographies. The electrolyte solution is what determines the overall performance of the batteries. The electrolyte solution is made of salts and solvents, salts carry the charge and solvent provides the medium for the charge to travel from positive to negative terminals. Additives are added in the solution to enhance the performance of the battery and reduce the corrosion of cathode/anode materials. Electrolyte is responsible for change in temperature and thus every geography has a different solution of electrolyte in the battery to avoid exponential change in temperatures. Some of these solvents turn solid at low temperatures thus they are only used in batteries with high voltage. LiPF6 is the most common electrolyte found in lithium ion batteries.
Separator films are placed between the anode and cathode to avoid direct contact with each other. Films act as a connecting agent between two or more electrode species while slowly adhering them to the electricity based collectors. The electron passes through the pores of separator film without colliding with one another and smooth flow of charge is maintained.
The lithium ion cell works on the basic principle of chemical reaction getting converted to electrical energy. The graphite reacts with lithium cathode in the presence of electrolyte solution to generate charge in electrons. These electrons then travel from positive terminal to negative terminal without colliding with each other through the separator films resulting in power generation.
The Indian chemical industry has large size opportunities in developing the battery components like cathode/anode, electrolyte solutions and separator films.
Neogen chemicals have been extensively working on the lithium chemistry and has set up 2400MT capacity to manufacture lithium salts and derivatives. This facility will help in backward integrating the raw material requirements for electrolyte manufacturing. The company is further undergoing a capex of 35 Cr for manufacturing 250 MT of battery electrolyte at Vadodara facility. Advanced chemistry cell initiatives by the Government of India will enable the companies to use the PLI scheme offered by the authorities. The demand for lithium salts is expected to reach 70,000 MT by 2030 according to company estimates.


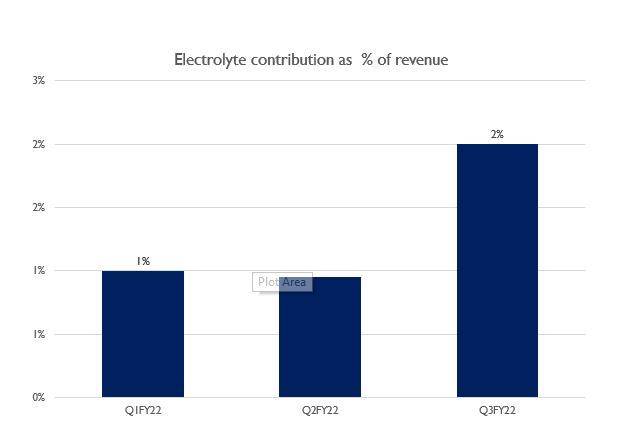
Tatva Chintan pharmachem limited is one of the manufacturers of electrolyte salts which are used in supercapacitor Super-Capacitors or ultra-capacitors are energy storage devices that store electrical energy via electrochemical and electrostatic processes. These capacitors are used in electrical grids and electric vehicles alongside the battery to give out a large amount of current in a short span of time, for eg: Starting an electric car requires high power discharge at stationary position, this charge is given by a supercapacitor. The electrolyte segment was negligible in the previous quarter but in Q3FY22, the contribution has gone up.
Gujarat Fluorochemicals are a manufacturer of PVDF films which are used as separator films and cathode binders in battery chemistry. The company is still under trials and has sent the samples for quality check from battery manufacturers. The company is undergoing a capex of 100 Cr for PVDF films. GFL is also in process of setting up India’s first PVDF solar film project which will be commissioned in the next financial year. The company is also setting up an integrated battery chemical complex to have a phased program on development of LiPF6 electrolyte and other lithium chlorides for battery chemistry. The company is evaluating opportunities of solar films and other renewable energy.
Polyplex Films manufacture PET films which are used along with separators resulting in composite PET/PVDF films. This combination results in high di-electric constant, High electrochemical stability and high tensile strength.

Tata Chemicals is having the battery recycling unit to source the metal salts required in the battery chemistry. The company is further leveraging in the battery chemistry space by signing a MoU for cell manufacturing. Company is planning to develop state of the art technology for manufacturers of cathode materials and the recovery and purification of cathode and anode active ingredients and developing a platform for electrochemistry solutions. The company is also under development of the sodium ion battery technology. The abundance of sodium makes them a good alternative for lithium, however it does not possess the similar properties. Sodium ion batteries could not retain charge as much as lithium ion and take longer duration to charge which makes them inconvenient for commercial use. Technological development is under process to derive the appropriate cell chemistry in sodium ion batteries.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
The Real Person!
The Real Person!
Kamagra pharmacie en ligne: Acheter Kamagra site fiable – achat kamagra
The Real Person!
The Real Person!
Cialis sans ordonnance pas cher: Acheter Viagra Cialis sans ordonnance – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
kamagra oral jelly: kamagra en ligne – Kamagra pharmacie en ligne
pharmacie en ligne france pas cher: pharmacie en ligne pas cher – pharmacie en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Tadalafil 20 mg prix en pharmacie: Acheter Cialis 20 mg pas cher – Cialis sans ordonnance 24h tadalmed.shop
kamagra gel: Kamagra pharmacie en ligne – Acheter Kamagra site fiable
The Real Person!
The Real Person!
pharmacie en ligne sans ordonnance: pharmacie en ligne sans ordonnance – pharmacie en ligne livraison europe pharmafst.com
pharmacies en ligne certifiГ©es: pharmacie en ligne pas cher – trouver un mГ©dicament en pharmacie pharmafst.com
The Real Person!
The Real Person!
Achat Cialis en ligne fiable: Tadalafil sans ordonnance en ligne – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Tadalafil achat en ligne: Cialis generique prix – Cialis sans ordonnance pas cher tadalmed.shop
The Real Person!
The Real Person!
Acheter Viagra Cialis sans ordonnance: Cialis generique prix – cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne france livraison belgique: Meilleure pharmacie en ligne – pharmacie en ligne fiable pharmafst.com
The Real Person!
The Real Person!
achat kamagra: Kamagra Oral Jelly pas cher – Kamagra Oral Jelly pas cher
The Real Person!
The Real Person!
Pharmacie Internationale en ligne: Pharmacie en ligne France – pharmacie en ligne france livraison belgique pharmafst.com
The Real Person!
The Real Person!
Pharmacie en ligne Cialis sans ordonnance: cialis sans ordonnance – Tadalafil sans ordonnance en ligne tadalmed.shop
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: Kamagra pharmacie en ligne – kamagra gel
The Real Person!
The Real Person!
kamagra livraison 24h: kamagra pas cher – kamagra gel
The Real Person!
The Real Person!
Kamagra Commander maintenant: kamagra en ligne – kamagra oral jelly
The Real Person!
The Real Person!
kamagra gel: Achetez vos kamagra medicaments – kamagra gel
The Real Person!
The Real Person!
mexico pharmacy order online: mexico pharmacy order online – mexico pharmacies prescription drugs
The Real Person!
The Real Person!
mexico pharmacy order online: Rx Express Mexico – Rx Express Mexico
The Real Person!
The Real Person!
Medicine From India: indian pharmacy – pharmacy website india
The Real Person!
The Real Person!
Medicine From India: Medicine From India – MedicineFromIndia
Online medicine home delivery indian pharmacy medicine courier from India to USA
medicine courier from India to USA: indian pharmacy online shopping – Medicine From India
The Real Person!
The Real Person!
canadian world pharmacy: Canadian pharmacy shipping to USA – northern pharmacy canada
mexican rx online medicine in mexico pharmacies RxExpressMexico
drugs from canada: Buy medicine from Canada – canada drugs online
The Real Person!
The Real Person!
mexican rx online: mexico pharmacies prescription drugs – RxExpressMexico
cheapest pharmacy canada Canadian pharmacy shipping to USA canadian pharmacies comparison
Rx Express Mexico: Rx Express Mexico – mexican online pharmacy
The Real Person!
The Real Person!
mexico drug stores pharmacies: mexico drug stores pharmacies – Rx Express Mexico
The Real Person!
The Real Person!
pharmacies in mexico that ship to usa: Rx Express Mexico – Rx Express Mexico
canadian pharmacy antibiotics ExpressRxCanada canadian discount pharmacy
canadian discount pharmacy: Buy medicine from Canada – canadian pharmacies online
The Real Person!
The Real Person!
pin-up: pin up az – pin up az
The Real Person!
The Real Person!
вавада казино: vavada вход – vavada вход
The Real Person!
The Real Person!
pinup az: pin-up casino giris – pinup az
The Real Person!
The Real Person!
вавада официальный сайт: vavada casino – вавада
The Real Person!
The Real Person!
вавада официальный сайт: вавада зеркало – vavada
The Real Person!
The Real Person!
pin up вход: пин ап казино – пин ап казино
The Real Person!
The Real Person!
vavada вход: vavada вход – вавада официальный сайт
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап зеркало – пин ап казино
The Real Person!
The Real Person!
pin up az: pin up az – pin-up
вавада казино: vavada – вавада зеркало
пин ап казино: pin up вход – пинап казино
пин ап казино: пин ап вход – пин ап вход
вавада зеркало: vavada casino – вавада зеркало
вавада официальный сайт: vavada вход – вавада официальный сайт
вавада официальный сайт: вавада казино – вавада зеркало
пин ап казино официальный сайт: пинап казино – пинап казино
пин ап казино: пинап казино – пинап казино
pin up azerbaycan: pin-up casino giris – pin up azerbaycan
пин ап казино официальный сайт: pin up вход – пин ап зеркало
пин ап казино: pin up вход – пин ап вход
vavada casino: вавада – vavada вход
пин ап вход: пин ап зеркало – пин ап вход
пин ап казино: пин ап вход – пин ап казино
вавада зеркало: вавада официальный сайт – vavada casino
пин ап казино официальный сайт: пин ап казино официальный сайт – пинап казино
The Real Person!
The Real Person!
http://pinupaz.top/# pin-up
The Real Person!
The Real Person!
generic tadalafil: best price Cialis tablets – generic tadalafil
order Cialis online no prescription: generic tadalafil – FDA approved generic Cialis
http://modafinilmd.store/# purchase Modafinil without prescription
doctor-reviewed advice: safe modafinil purchase – modafinil legality
https://zipgenericmd.shop/# generic tadalafil
modafinil pharmacy: Modafinil for sale – Modafinil for sale
http://modafinilmd.store/# Modafinil for sale
order Cialis online no prescription: cheap Cialis online – best price Cialis tablets
https://zipgenericmd.com/# generic tadalafil
The Real Person!
The Real Person!
Viagra without prescription: generic sildenafil 100mg – safe online pharmacy
canadian online pharmacy no prescription cialis dapoxetine: TadalAccess – typical cialis prescription strength
cialis without a doctor prescription canada: TadalAccess – cialis going generic
peptide tadalafil reddit: TadalAccess – cheap t jet 60 cialis online
how to get ed meds online ed medicines Ero Pharm Fast
boner pills online: buy ed medication – ed pills for sale
get antibiotics quickly: BiotPharm – Over the counter antibiotics for infection
http://eropharmfast.com/# Ero Pharm Fast
online erectile dysfunction pills: Ero Pharm Fast – Ero Pharm Fast
Discount pharmacy Australia Pharm Au24 Online drugstore Australia
Ero Pharm Fast: Ero Pharm Fast – Ero Pharm Fast
erectile dysfunction online: online ed medications – Ero Pharm Fast
https://pharmau24.shop/# Pharm Au24
cheapest antibiotics: Biot Pharm – get antibiotics quickly
Ero Pharm Fast: low cost ed pills – cheap ed meds
over the counter antibiotics Biot Pharm get antibiotics without seeing a doctor
best online ed pills: Ero Pharm Fast – Ero Pharm Fast
The Real Person!
The Real Person!
CREX – Cricket Exchange Additional Information Play Football 2025- Real Goal X Discover the toughest tracks and get the most badass gear as you make your way to the top Choose your character, pick your power ups and go head to head with real players in this fast-paced, high action football showdown. Unlock and upgrade characters. Get paired in PvP football matches against other players, score amazing goals, play in various stadiums, rank up in the league system to be the victorious. Invite your friends to play, challenge and have fun. Play Football 2025- Real Goal You can only download and install the 1xbet apk file from 1xbet official website or affiliated bookmaker websites. Exciting Mobile Hockey Experience Awaits A special plus of the Total Football game is that it can be downloaded and played offline, i. at any time and without Internet access. to make the match as interesting as possible, with unexpected “surprises” for the opponents.
https://www.abclinuxu.cz/lide/woodbyocater1975
Exceed Your Expectations Upgrade your passenger experience with a selection of Premium Leathers and Carpet and enhanced interior and exterior features, including Multi-Tone Paint and Executive and Family Seating options. “We are proud to unveil our fully indigenous Micro Turbojet Engine, a testament of India’s ingenuity and determination to become a global hub for aerospace innovation,” Vamsi Vikas, MD – RVMT, said. What to do if there is an after-sales problem? A:Our products have a 12-month warranty and we will provide after-sales service. If there is any problem with the product, please feel free to contact us at the first time, and we will help you solve the problem. Without entering into an extended public debate, one can pinpoint egregious neglect, as well as absence of oversight and vision, at the political level, for repeated setbacks in these vital projects. Three other factors have contributed to the sorry state of affairs vis-a-vis the Kaveri (and the LCA): over-estimation, by DRDO, of its capabilities compounded by reluctance to seek advice; inadequate project-management and decision-making skills of its scientists and exclusion of users – the military – from all aspects of the project.
antibiotic without presription: buy antibiotics online uk – buy antibiotics from canada
https://eropharmfast.shop/# online erectile dysfunction medication
online pharmacy australia: Licensed online pharmacy AU – online pharmacy australia
online pharmacy australia Discount pharmacy Australia PharmAu24
buy antibiotics over the counter: buy antibiotics online – best online doctor for antibiotics
https://eropharmfast.shop/# Ero Pharm Fast
buy antibiotics from canada buy antibiotics online uk buy antibiotics online
buy antibiotics from canada: buy antibiotics online – cheapest antibiotics
The Real Person!
The Real Person!
حصلت لعبة RFS مهكرة، وهي لعبة متاحة في سوق Google Play ، على متوسط درجة في نطاق 4.1 من 5 وتستخدم على هواتف الاندرويد 4.3 أو أعلى حتى الآن. يتم تنزيله واستخدامه أكثر من مليون مرة. لا تكن أحمق و قم بتجربتها. تضم منصة 1xBet تطبيق للجوال و لـ تنزيل لعبة الطيارة 1xBet ، يتعين عليك تنزيل تطبيق 1xBet ومن خلال التطبيق تستطيع لعبها. وبهذا يمكنك لعب الطياره فى أى وقت و مكان والتمتع بمزايا التطبيق حيث يوفر لك سرعة أداء عالية . خدمات ما بعد البيع تم تطوير Aviator بواسطة Spribe، وهو ينقل المقامرة عبر الإنترنت إلى مستوى جديد تمامًا من خلال تنسيقه المبتكر. تم بناء اللعبة على آلية التصادم المنحني، والتي أصبحت شائعة بسرعة بين اللاعبين بسبب بساطتها وموثوقيتها. حسنًا، إنها ليست اللعبة الوحيدة المتوفرة، ولكن على عكس الألعاب المشابهة الأخرى، فإن Aviator هو حصان ذو لون آخر لديه الكثير ليقدمه، مما يجذب اللاعبين بميزاته الفريدة وأدواته الاجتماعية.
https://hazwopercert.com/2025/05/21/%D9%85%D8%B1%D8%A7%D8%AC%D8%B9%D8%A9-%D9%84%D8%B9%D8%A8%D8%A9-%D8%A7%D9%84%D8%B7%D9%8A%D8%A7%D8%B1%D8%A9-%D8%A8%D9%88%D8%A7%D8%B3%D8%B7%D8%A9-spribe-%D8%AD%D9%84%D8%A7%D9%84-%D8%A3%D9%85/
استهل مغامرتك مع لعبة بكل سهولة. اختر كازينو موثوق لتجرب هذه اللعبة الفريدة. بعد تسجيل حسابك، انطلق إلى قسم الألعاب وابحث عن Aviator. جرب النسخة التجريبية لتتعلم القواعد دون مخاطرة، ثم املأ رصيدك وابدأ الرهان لتتبع مسار الطائرة وتحقق أرباحك قبل أن “تتحطم”. Arnie’s Language School ونشر “شومان”، عبر حسابه الرسمي على فيسبوك، “لعبة الطائرة المنتشرة على الإنترنت Aviator قمار يأثم لاعبها، والمقهى الذي يمكن اللاعبين من ممارستها على أجهزته، ومكاسبها حرام”.
The Real Person!
The Real Person!
Most Colour Trading apps allow you to place real money bets, giving you a chance to win actual cash. However, just like any form of wagering, there is a risk of losing money. It’s important to exercise caution, never bet more than you can afford to lose, and always follow responsible gaming principles. Below, we’ll guide you on how to choose a Colour Trading app and increase your chances of success. There are lots of ways to reach someone if you have questions. Call a Specialist, chat with someone online, go into an Apple Store, or use the Apple Support app. And the Tips app will help you get the most out of your iPhone over time. your preferences and interests, such as those that you have actively shared with us, but also those inferred through your registered interactions with AkzoNobel websites and apps (for which we may use cookies);
https://wayranks.com/author/contlinkpimap1973-813268/
APK Apps Why settle for less when 7 Tiranga Game offers the best? Register and login into your color prediction game app unlocks a world of best triangle game login that will keep you entertained for hours on end. But that’s not all – we believe in sharing the joy, and that’s where our unique invitation code system comes into play. After installing the APK file, you have to register with the platform before getting started with playing any game. We have compiled the step-by-step guideline in the following paragraph, read it to have a idea about the registration; Active traders daily In the market many games are available but 95% are 99% fake, But Bharat game colour Prediction is 100% safe and the best feature is withdrawal in 2 Hours. Why settle for less when 7 Tiranga Game offers the best? Register and login into your color prediction game app unlocks a world of best triangle game login that will keep you entertained for hours on end. But that’s not all – we believe in sharing the joy, and that’s where our unique invitation code system comes into play.
The Real Person!
The Real Person!
تنزيل لعبة التفاحة 1xbet فأنت محظوظ يعد تحميل هكر التفاحة 1xbet من أكثر السكربتات شعبية وطلبا في عالم المراهنات الرياضية عبر الإنترنت يمكنك الآن الاستمتاع بميزات هذا السكربت الرائع دون أن تدفع أي تكلفة. في حال واجهت مشكلات أو كانت لديك أسئلة أثناء التنقل في منصة 1xBet، فإن فريق دعم العملاء متاح بسهولة لمساعدتك. يمكنك التواصل معهم عبر البريد الإلكتروني أو المكالمة الهاتفية. سكريبت التفاحة 1xbet تحميل مجانا إن الفريق المتخصص من متخصصي Customer Desk مجهز لمعالجة مخاوفك وتقديم حلول شاملة لضمان رحلة الألعاب الخاصة بك
https://www.conveyz.com.au/%d9%84%d8%b9%d8%a8%d8%a9-thimbles-%d9%85%d9%86-evoplay-%d9%88%d8%b7%d8%b1%d9%82-%d8%a7%d9%84%d8%b1%d8%a8%d8%ad-%d8%a7%d9%84%d9%85%d8%b6%d9%85%d9%88%d9%86%d8%a9-%d8%a8%d8%a7%d9%84%d8%aa%d8%ac%d8%b1/
لأولئك الذين يتطلعون إلى استكشاف المزيد من فرص الربح، تعد لعبة Pop Star Magic مثالًا رائعًا على لعبه بتكسب فلوس، حيث توفر للاعبين فرصة للاستمتاع بالتسلية بينما يمكنهم زيادة دخلهم. من خلال اللعب المستمر وإتقان اللعبة، يمكن للاعبين تحويل وقتهم في اللعب إلى أرباح كبيرة. وهذا يجعل لعبة ربح المال حقيقية خيارًا جذابًا لأي شخص مهتم بالألعاب بفلوس وتلك التي تبحث عن عالم أوسع من الألعاب الربح من الانترنت. في عالم المراهنة عبر الإنترنت المثير، تضيف عروض المكافآت طبقة إضافية من الإثارة إلى مغامرتك في المراهنة. من المكافآت الترحيبية السخية للاعبين الجدد إلى مكافآت الولاء للمراهنين المخضرمين، علاوة على ذلك تقدم 1xBet مجموعة متنوعة من الحوافز. يمكن أن تزيد هذه المكافآت من رصيدك، وتزيد من فرصك في الفوز، وتجعل تجربتك في المراهنة أكثر متعة.
The Real Person!
The Real Person!
JetX is a game of fortune, but certain strategies you may use will help you get more constructive results and bring in more profit. Here are some tips to make playing at JetX more enjoyable for you: Mostbet bilan muvaffaqiyat qozonishning maqbul imkoniyatlari: sport musobaqalarida ko’proq yutib oling. Mobile Experience of JetX Mostbet Pakistan Crash Game Security service Rising rapidly to prominence due its easy gameplay, JetX has found a huge audience on Mostbet, a leading online betting platform. Both experienced bettors and newcomers to gambling alike appreciate its fusion of arcade fun and real-money stakes. The simple mechanics appeal just as much as the opportunity to potentially earn. Now, we’ll delve into JetX’s core features, exploring the specifics of how it’s played, technical details, smart betting habits, and tactics for coming out on top.
https://fundacionrestaurados.cl/bgamings-plinko-on-pc-pakistan-setup-instructions/
Because this is such a fast-paced game, it’s better to enter it with some sort of plan. Ideally, this will be your own betting strategy that perfectly suits your bankroll and your gaming style. So, spend some time getting used to JetX gambling in practice mode and exploring game stats. Then, spend more time testing and refining your betting strategy. Playing JetX is an exciting experience for online casino enthusiasts. Developed by Smartsoft Gaming, this crash game is a popular choice among players looking for a fun and exciting gambling adventure. When Solarwinds Pingdom ran tests on the game from Frankfurt, they found it only took 1.22 seconds to load – which is good news for anyone who hates waiting around for games to start up. The game isn’t too heavy on graphics or script either, so it doesn’t require a lot of data to run. In fact, less than 5% of the server requests are devoted to HTML5 – and that’s before any buttons are even pressed!
The Real Person!
The Real Person!
Každá varianta hry Plinko v on the web kasinech zachovává základní mechanismy hry, ale zavádí jedinečné varianty, aby oslovila různé typy hráčů. Mezi nejoblíbenější” “varianty patří Classic Plinko, Dare2Win Plinko some sort of Easter Plinko, unces nichž každá má své vlastní charakteristické rysy. Díky této flexibilitě je Plinko fascinující a dynamická hra, která dokáže nabídnout různé zážitky podle preferencí hráče. Disk, trojúhelníkové pole kolíků a pár vteřin napětí – právě tím si „plinko hra“ (česky často jen Plinko) získala statisíce fanoušků. V dnešní online podobě nabízí: Disk, trojúhelníkové pole kolíků a pár vteřin napětí – právě tím si „plinko hra“ (česky často jen Plinko) získala statisíce fanoušků. V dnešní online podobě nabízí:
https://plazapublica.cdmx.gob.mx/profiles/ercorehunt1983/activity
plinko spelen: plinko spelen – plinko Příspěvková organizace What makes the multiplier bonus so appealing is its unpredictability bluwom-milano news 1win_Plinko_A_Fun_and_Exciting_Game_to_Try.html Abyste mohli tento produkt přidat do svého seznamu přání, začít ho sledovat nebo ho zařadit mezi ignorované, musíte se nejprve přihlásit. plinko plinko fr plinko Zadejte popis widgetu (maximální délka 375 znaků): plinko plinko fr plinko Take a Break! – Escape, enjoy, dream, scheme, win rewards and stay connected with this newly reimagined twist on the classic MONOPOLY board! – Let everyone’s favourite zillionaire, Mr. MONOPOLY, be your guide as you explore new boards themed after world-famous cities, fantastical lands, and imaginative locales. plinko spelen: plinko spelen – plinko
The Real Person!
The Real Person!
APKPure Lite – Sklep z aplikacjami na Androida oferujący proste, ale wydajne przeglądanie stron. Odkrywaj aplikacje, których szukasz łatwiej, szybciej i bezpieczniej. Offline Mode: Ready to take a break from online connectivity? Aviator APK offers an offline mode where you can practice your skills or simply enjoy the game without an internet connection. – Amazing selections of the slot machine games that offer real Las Vegas casino game experience Offline Mode: Ready to take a break from online connectivity? Aviator APK offers an offline mode where you can practice your skills or simply enjoy the game without an internet connection. Copyright © 2014-2025 APKPure. Wszelkie prawa zastrzeżone. zacznij od konserwatywnych zakładów w Aviator i stopniowo zwiększaj stawki, gdy będziesz coraz bezpieczniejszy dzięki dynamice gry. wykorzystaj luźną charakterystykę gry, aby poznać ten sport, zanim zaczniesz grać w zakłady na prawdziwe pieniądze.
https://www.corporatelivewire.com/profile.html?id=5eb4cd24e0807d31dd745a09547c49131497c3a9
They’re able to deliver the necessary data and suggestions to properly allege your reward. This guide usually elaborate to the crucial terms and conditions it is possible to encounter if you are going for a great Bitcoin sportsbook added bonus. BTC365 have hitched that have Betby to offer the finest chance across the many different activities, and biggest activities leagues such FIFA, Europa, and also the Prominent Category, and NBA online game. You should manage comprehensive research and homework ahead of entering crypto sports betting to mitigate these types of dangers. Thanks, I appreciate it! observervoice a-fun-and-exciting-way-to-enjoy-interactive-games-on-your-phone-71713 Touche. Outstanding arguments. Keepp up the good work. fitinline profile aviatorgame about
The Real Person!
The Real Person!
Ou seja, se você optar pela Superbet, VBET ou pelo Betnacional, você terá o mesmo retorno, já que todos os Fortune Tiger possuem o mesmo algoritmo. O Sonic5k.br é o seu novo site brasileiro favorito de comparação de cassinos online. Você pode encontrar guias úteis sobre o e-gaming, novidades sobre os cassinos e principalmente críticas dos nossos especialistas em gaming. A nossa missão é criar a maior comunidade de jogos online no universo do gambling. Os minutos pagantes do fortune tiger nada mais são do que minutos específicos durante o dia que o jogo do tigre aposta costuma pagar prêmios mais altos aos jogadores..A ideia é que esse post seja bem curto mesmo e sem enrolação.Então vamos lá…. A slot machine Fortune Dragon da PG Soft traz o poder dos dragões míticos para o centro do palco. Com gráficos deslumbrantes e animações imersivas, esta slot de 5 rolos promete uma experiência inesquecível para todos os jogadores. As apostas começam a partir de 0,20 $, oferecendo acessibilidade e emoção para todos os perfis.
https://deoprodaqta1989.raidersfanteamshop.com/obtenha-os-fatos
Selecionamos apenas casas de apostas confiáveis e licenciadas. Elas possuem não só o Fortune Tiger Game como também outras opções de jogos de cassino online e apostas esportivas. O Fortune Tiger Demo foi desenvolvido para funcionar em diversas plataformas, incluindo dispositivos móveis. O jogo do tigrinho demo mantém alta qualidade e interatividade em smartphones e tablets, enquanto a demo fortune tiger garante uma experiência prática e fluida para os usuários. A análise do RTP e da volatilidade no Fortune Tiger PG Demo evidencia um equilíbrio entre entretenimento e potencial de ganhos. Dados técnicos apontam que o fortune tiger demo oferece retornos competitivos, enquanto o fortune tiger pg demo apresenta variações que intensificam a emoção durante as rodadas. Essa combinação permite que os jogadores ajustem suas estratégias com base em estatísticas precisas, otimizando as chances de sucesso e garantindo uma experiência dinâmica.
The Real Person!
The Real Person!
“Ice Joker” ist ein Slot, der die Kälte des Winters mit der Wärme von Gewinnen vereint. Freuen Sie sich auf eisige Joker-Symbole, die riesige Gewinnchancen bieten. Mit Freispielen und einem Hauch von Frost verzaubert dieser Slot die Spieler mit seinem winterlichen Charme. IFF Starlight Princess ist ein Spielautomat, der Sweet Bonanza in den meisten Aspekten ähnelt, aber es ist nicht ganz dasselbe. Die eine Funktion, die hervorsticht, ist die Multiplikatorfunktion. Wenn Sie ein großer Fan von Spielautomaten sind, würden Sie wissen, dass Multiplikatoren, die zurückgesetzt werden, oder ohne sie ist ein Off-Putting. Während der Freispiele in Starlight princess können Sie sicher sein, dass sie nicht zurückgesetzt werden, was die Spannung auf einen großen Gewinn, der gerade um die Ecke lauert, deutlich erhöht.
https://aphorismsgalore.com/users/lamcomenrigh
The multicoloured candy bomb symbol will also multiply your rewards whenever it appears on the reels. The Ante Bet feature enables you to customize your bet and volatility level for the free spins round. This feature is particularly appealing to high-rolling players who can take advantage and add more risk to their game. You can play the slot in two ways — the Sweet Bonanza free play or the real money option. Here is a guide on how you play the Sweet Bonanza slot: Ein Früchte Slot mit Jokern vom Software Hersteller Play ‚n‘ Go. Fire Joker überzeugt mit seinem einfachen Spielprinzip, das gerade für Neulinge und Einsteiger von Slot Games leicht zu verstehen ist. Wer bei Bonus an Angebote wie beispielsweise Sweet Bonanza Free Spins ohne Einzahlung denkt, der wird aller Wahrscheinlichkeit nicht fündig. In bekannten Online Casinos gibt es dafür die Option, ein Startguthaben als Neukunde zu beanspruchen. Zudem ist Sweet Bonanza häufig Teil der Daily Drops & Wins Kampagnen, bei denen jeden Tag hohe Preisgelder in Aussicht gestellt werden.
I truly appreciate your technique of writing a blog. I added it to my bookmark site list and will
The Real Person!
The Real Person!
Four added bonus symbols pay 5x their choice and give you 12 100 percent free revolves. Five bonus icons pay 20x your own bet, and you score 17 totally free spins. Half dozen bonus signs payout 100x your wager and you will award 22 100 percent free revolves. With Practical Gamble being one of the greatest playing builders in the a, there’s it an easy task to to get the new Buffalo Queen Megaways slot game in the best casinos online. If you’d like an alternative gambling enterprise, join as a result of Gambling enterprises and you can allege your own invited bonus render along with your first deposit. Those two technicians assist in performing far more profitable combinations. Are you ready to become a true slot game champion? Then look no further than Buffalo King Megaways, where the top prize available is a whopping 5,000x your bet. While some may argue that this grand prize is standard for a highly volatile slot, there’s no denying that it’s enough to send any player’s heart racing. But don’t settle for the status quo – be on the lookout for even bigger rewards in this Megaways game. With its thrilling gameplay and exciting potential, Buffalo King Megaways is the perfect choice for anyone who loves to take risks and win big.
https://www.denizlivipmasajsalonu.com/2025/07/15/side-bets-in-goal-demo-game-worth-the-risk/
Chicken Road is not just a slot game; it’s an interactive chicken crossing road gambling game that combines strategy with high payouts. There’s really nothing difficult about this slot. Load it in demo mode, place a few bets, and you’ll figure everything out. Though there isn’t a specific APK file for Mission Uncrossable, players can easily access it by downloading the Roobet mobile app. This app provides access to the entire Roobet game collection, including Mission Uncrossable, allowing users to enjoy all the same features as the desktop version. Whether you’re crossing lanes for multipliers or cashing out your winnings, the mobile app delivers the full gameplay experience, making it convenient to play wherever you are. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page.
The Real Person!
The Real Person!
That’s right, playing any of the casino and slot games here at Dream Jackpot offers the chance to win real money. None of the games on our site offer a demo mode or the option to play for free per UK Gambling Commission (UKGC) regulations. Since you’re playing with your own money, it’s important to gamble responsibly and remember that winning is never guaranteed. So, set a budget and only bet money you are comfortable losing. The idea that you can experience both gaming and the theme of wilderness is quite fascinating. Many gaming apps offer the same thing; you must find the ones that meet your preferences and stick with them. Buffalo King Megaways has plenty of fetures and gives you the sensation you want as a lover of nature and the wild. Trying the Buffalo King Megaways demo to understand how it works is an excellent way to start. Then, if it pleases you, take it a little further.
https://www.intl-investholding.com/2025/07/12/mission-uncrossable-payout-burst-gate-what-triggers-it/
Aside from the greeting bonus and you will cashback, the newest 1win formal application appear to provides some promotions for example reload incentives, free wagers, and. These also provides are designed to keep betting feel exciting and you can fulfilling. Be sure to read the app frequently to keep upgraded for the the brand new product sales. Speak about the brand new 1Win Local casino Application, home to a huge line of 9000+ games, along with slots with diverse types and you can bonuses, for an engaging feel. To possess an authentic local casino getting, diving to your live part offering classics such roulette and you may cards game, having live investors and online speak to own a social touching. Discover the value your’ve downloaded on the tool’s Downloads folder. Gaming and you may betting mix on the all the moment, regardless of your location. With a decent unit and you can an effective connection to the internet, thrill is definitely within reach. The brand new 1win APK dazzles such as an electronic treasure, boasting a sleek framework.
The Real Person!
The Real Person!
Czy mogę pobrać «Aviator» na swój smartfon? Aviator to innowacyjna, oryginalna gra, której zasady są połączeniem gry wideo, w której liczy się szybkość reakcji gracza, i slotów. Poszczególne rundy trwają zazwyczaj kilka lub kilkanaście sekund, a w każdej z nich uczestniczą zawsze inni gracze. Gra kasynowa Aviator jest szczególnie interesująca ze względu na wysokie kursy, jakie oferuje. Jako gra crash, Aviator oferuje duże możliwości wygranej, dzięki czemu każda sesja jest wyjątkowa. Graczy do powrotu przyciąga przede wszystkim potencjał atrakcyjnych wypłat. GRAJ ODPOWIEDZIALNIE: Portal aviator.co działa niezależnie i nie jest powiązany z platformami, które przedstawiamy. Pamiętaj, że przed rozpoczęciem gry na pieniądze lub obstawiania zakładów musisz przestrzegać wszystkich wymagań prawnych i związanych z wiekiem. Celem aviator.co jest dostarczanie treści informacyjnych i rozrywkowych. Pamiętaj, że jeśli klikniesz link na naszej stronie, zostaniesz przekierowany na odpowiednią stronę. Opowiadamy się za odpowiedzialnymi praktykami w grach i zachęcamy naszych czytelników do postrzegania gier losowych jako zabawy, a nie sposobu na zarabianie pieniędzy. Pamiętaj, aby grać odpowiedzialnie i szanować swoje ograniczenia.
https://jamaiwray.cyber-demo-client-website2.com/index.php/2025/07/17/personalizacja-kolorow-aplikacji-mostbet-czy-to-mozliwe/
Darmowe obroty w Sugar Rush uzyskamy po wylosowaniu 3-7 symboli Scatter. Gra przydziela nam kolejno 10, 12, 15 albo nawet 20 free spinów! Darmowe spiny możemy wygrać także w rundzie bonusowej! Copyright © 2024 AhoricFastService . All Rights Reserved Przed wami tytuł, który niemal w dniu premiery w 2022 roku stał się od razu hitem, zaś do dziś doczekał się kilku równie udanych sequeli. Oczywiście można zadać sobie pytanie o to, czy gra ta nie jest nadmiernie “dziewczęca”. Naszym zdaniem – wprost przeciwnie. W redakcji CasinoRIX w Sugar Rush regularnie grywają panowie. Zapraszamy do naszej eksperckiej recenzji! Sugar Rush 1000 oferuje wciągającą rozgrywkę na slocie online w żywej siatce 7×7. Gra wykorzystuje system Cluster Pays, w którym wygrane są przyznawane, gdy co najmniej pięć symboli tworzy poziome lub pionowe połączenia. Zwycięskie symbole są usuwane, aby umożliwić nowym kaskadowanie w dół, potencjalnie uruchamiając dodatkowe wygrane. Ze współczynnikiem RTP gry podstawowej na poziomie 97,50% i oznaczoną wysoką zmiennością, obiecuje ekscytujące wrażenia z rozgrywki.
The Real Person!
The Real Person!
Looking for the best cryptocurrency exchange rates? Discover the Coinxes cryptocurrency exchange coinxes.io – the best rates, the lowest commissions. The Coinxes platform is a digital exchange that allows users to buy and sell cryptocurrencies from anywhere in the world. Unlike a regular exchange, it boasts a large number of useful features. They, just like many other sportsbooks, could stand to be able to bake some props into the formula. Anything along the lines of a good over under on typically the number of birdies a particular participator may have would suffice. Sure, they possess all the popular games—Counterstrike, Dota, FIFA, etc. But they also break down betting into smaller subsections, offering lines about not only different competitions, however the brackets inside those tournaments. Mostbet has all sportsbook lines separated by sport and competitors. Once you get the game or perhaps future you’re gambling on, their gamble slip will display all of your different choices while calculating your potential payout.
https://wplearn.ajitest.tech/index.php/2025/07/24/przewodnik-po-wyplacie-srodkow-z-vavada-na-e-portfel/
Możesz grać w Spribe ‘ s Aviator 2 za darmo. Tryb demonstracyjny otwiera się automatycznie. Aby postawić prawdziwy zakład, musisz najpierw zarejestrować się w kasynie online lub firmie bukmacherskiej i dokonać wpłaty na saldo. Zakres zakładów w grze kolizyjnej jest ogromny. Możesz postawić 0,82 eur lub wejść ze stawkami 16 eur i wyższymi. Automatyczna gra w Aviator Game jest wygodna, ponieważ pomaga zablokować poziom emocji i nie stracić pieniędzy z powodu słabej reakcji. Samolot może osiągnąć x100 i x1000, ale nawet x50 daje mu doskonale dobre wyniki zarówno przy dużych, jak i małych stawkach. Można to również zrobić za pomocą drugiego zakładu, w którym Współczynnik można ustawić na przykład na x70 i możesz sobie pozwolić na niewielkie ryzyko. Możesz grać w Spribe ‘ s Aviator 2 za darmo. Tryb demonstracyjny otwiera się automatycznie. Aby postawić prawdziwy zakład, musisz najpierw zarejestrować się w kasynie online lub firmie bukmacherskiej i dokonać wpłaty na saldo. Zakres zakładów w grze kolizyjnej jest ogromny. Możesz postawić 0,82 eur lub wejść ze stawkami 16 eur i wyższymi.
The Real Person!
The Real Person!
Ao clicar no botão de rotação, os rolos começam a girar, revelando os possíveis ganhos para os jogadores. Com uma jogabilidade simples e emocionante, o Big Bass Splash promete uma experiência de cassino online memorável para todos os jogadores. O Big Bass Bonanza é um dos slots de 10 centavos da Pragmatic Play com cinco bobinas, três linhas e 10 linhas de pagamento. O jogo inclui o recurso de Rodadas Grátis, em que o símbolo do pescador coleta prêmios em dinheiro, com ganhos de até 2.100x a aposta. No jogo Big Bass Splash, os jogadores embarcam em uma jornada emocionante em busca de prêmios substanciais. Com uma configuração padrão de 5 rolos, 3 linhas e 10 linhas de pagamento fixas, o jogo oferece uma experiência de jogo envolvente para jogadores de todos os níveis de habilidade. O objetivo é simples: formar combinações vencedoras de símbolos nos rolos para garantir prêmios incríveis.
https://desall.com/User/raivildistmisp1979/Profile
Teen Patti Master is not just about gaming—it’s about creating a rewarding journey. Benefit from daily cashback, weekly bonuses, exclusive VIP programs, and referral incentives. The platform’s commitment to secure transactions and player data protection makes it a safe choice for all gamers. Download Teen Patti Master now to join a community where fun and trust go hand in hand. Those users who want to invest in casino games should get the latest version of the 3 Patti Lucky APK on their devices. This online casino is available for Android users and it consumes less space on the devices. You can find all your favorite casino games in this app that offers bonuses and special rewards. Install now and get an opportunity to win various bonuses and rewards for free. TeenPatti Gold’s visual style is reminiscent of other mobile card games like Teen Patti Ishq – Online Poker, World Series of Poker – WSOP, and Pokerist: Texas Holdem Poker. Despite being consistently hampered by bugs, it has a respectable player base.
The Real Person!
The Real Person!
What it is: I know there are a lot of games called chicken, but this one is the one we always played in the pool growing up. It’s a fun and rough (and therefore slightly dangerous) pool game. Every McDelivery order in the app lets you earn MyMcDonald’s Rewards points—use those to get your free food. And, yup, you can get ‘em delivered, too. Imagine, McD’s you didn’t pay for brought right to you. Check out how easy ordering delivery is in the app.* There’s something thrilling about not knowing what’s going to happen next. It’s why this game has become such a favorite on Valorbet. The Chicken Road game money download moment — where your winnings hit your wallet — is always satisfying. The game is fun. They’re different games which are very awesome. Then I don’t have to download mini apps to fill up storage. Love it so much. I just finished my 20 something and I just wanted to write a review to show my other peers and the people who hate this game on why it’s awesome and great and why everybody should get it. I recommend getting it now before it shut down hopefully gets better soon. This is my message to the world why you should get “Chicken Scream”!
https://cogard.org/nid/?senphimembstor1984/edit
ADR (Alternative Dispute Resolution) refers to methods used to resolve disputes outside of traditional court proceedings. Our ADR specialists strive to resolve and mediate disputes between players and online casinos efficiently. Players can rely on our services for professional assistance with any issue they may experience while gambling online. When it comes to betting markets, you will be thrilled to hear that there is always something to bet on and the betting markets will allow you to find perfect betting opportunities. Also, all three betting types are up and running including pre-match, outright, and live wagers. It’s not illicit to access the Roobet website from India using a VPN. But make sure you are familiar with your local laws associated with online gambling. It’s because using a Virtual Private Network may break the terms of a few online gambling sites.
The Real Person!
The Real Person!
© Sobesoft Web Tasarım Tiger Temple 88 wyróżnia się połączeniem Złotych Symboli, Free Spins z rozszerzającymi się mnożnikami oraz wielopoziomową Grą Bonusową, zależną od wyborów gracza. Możliwość dostosowania poziomów Złotych Symboli dodaje element strategii, podczas gdy możliwość ponownego uruchomienia Free Spins zwiększa zaangażowanie. System wypłat Gry Bonusowej oparty na kolumnach oraz różne dostępne Jackpoty jeszcze bardziej wzmacniają zestaw funkcji. Dodatkowo, dzięki dostępnym opcjom Super Stake i Funkcji Kupna, slot oferuje różne sposoby na modyfikację dynamiki gry i zwiększenie potencjału wyższych wypłat. Ocena reputacji MyStake pokazuje, że platforma utrzymuje godną pochwały pozycję w społeczności hazardu online. Wspierana przez Sainted International B.V., renomowanego operatora w branży, MyStake korzysta z jego bogatego doświadczenia i globalnej obecności. Choć występują sporadyczne skargi użytkowników, głównie dotyczące problemów z wpłatami i opóźnieniami wypłat, ogólnie pozytywna reputacja MyStake podkreśla jego wiarygodność i niezawodność.
http://shimiken-and.com/wiki/index.php?zalunarol1978
BonBon Blast – Sugar Rush jest uruchamiany w następujących systemach operacyjnych: iOS. Jak grać w Pragmatic Play’s Sugar Rush 1000 Komentarz będzie widoczny dopiero po wcześniejszym zweryfikowaniu go przez naszych pracowników. 3. Pozostaw ubranie w zamrażarce na kilka godzin, wyjmij je i wytrzep ponownie. Student – Always Learning ✌️ Sugar Rush DROPS 4-17 Użytkownicy BonBon Blast – Sugar Rush dał pewien oszacowanie od 5 z 5 gwiazdek. Finance Director Member of the Management Board Najnowsza wersja BonBon Blast – Sugar Rush jest 1.1.0, wydany na 15.12.2023. Początkowo był to dodane do naszej bazy na 15.12.2023. Sugar Rush DROPS 4-17 RTP w Sugar Rush wynosi ponadprzeciętne 96,50% i charakteryzuje się modelem gry o wysokiej zmienności. Dzięki temu możesz spodziewać się krótszych wygranych, ale za to większych.
The Real Person!
The Real Person!
Pages populaires Jouer à Free JetX pour tester les méthodes sans risquer d’argent est l’une des façons les plus fascinantes d’acquérir de l’expérience avec le jeu de SmartSoft Gaming. Profitez du mode démo de JetX gratuit du fournisseur de jeux, qui permet aux joueurs de tester le jeu sans risque. JetX commence son ascension avec un multiplicateur de 1.01 et monte toujours plus haut au fur et à mesure que le jeu avance. Pour pouvoir utiliser la Martingale ici, l’objectif principal serait de doubler votre mise dès que vous perdez, et cela jusqu’à la prochaine partie que vous gagnerez. Pour optimiser vos chances de gagner à JetX jetx, deux stratégies sont souvent citées : l’Auto-Cashout et la Martingale. De plus, le casino Mystake propose une offre intéressante pour tous les nouveaux joueurs qui souhaitent profiter de l’expérience Dino : 100% jusqu’à 1000€ ou 150% jusqu’à 200€. Si vous cherchez un jeu de casino unique et divertissant, alors Dino de Mystake est fait pour vous ! Essayez-le dès aujourd’hui et remportez des gains énormes. Bonne chance à tous !
https://ganralighpo1970.raidersfanteamshop.com/betzino-casino-avis
Raye Cissé signe un contrat de deux ans avec le Djoliba AC Gagnez dès votre premier dépôt un bonus de bienvenue super généreux chez Spin Buffalo Casino: Le RTP le plus élevé sur une machine à sous Megaways est de 97,72 %. Il est offert par le jeu White Rabbit Megaways sur lequel le jackpot est de 13 000 fois votre mise. Gorilla Gold Megaways (97,00 %) et Buffalo Rising Megaways (97,01 %) sont d’autres jeux au fort potentiel lucratif. Power Of Thor Megaways Pour les jeux de machines à sous avec jackpots vous avez des titres comme Mega Moolah, Treasure Nile, Gems Odyssey, Mystic Moon, Temple Cash Frogs n’ Flies, Lara Croft Temples and Tombs, King Ramses, Lucky New Year, Mustang Gold, Pirate Gold, Monkey Warrior, Surfin’ Reels, Jackpot Raiders, Witches Wild Brew, Astro Pandas, Golden Wisdom, Tiki Mania, Mega Moolah Absolootly Mad, Immortal Glory, Pyramid King, Wild Catch, Buffalo Power, Pearl Beauty, et bien d’autres encore.
The Real Person!
The Real Person!
@ Copyright 2025 Foxcasino | About us | Επικοινωνία | Θέσεις Εργασίας | Όροι χρήσης Ο μηχανισμός πληρωμής κατά συστάδες στο Sugar Rush Xmas σημαίνει ότι τα κέρδη προκύπτουν όταν 5 ή περισσότερα σύμβολα συνδέονται είτε κάθετα είτε οριζόντια στους κυλίνδρους. Το Sugar Rush είναι επίσης χαρακτηρισμένο ως ένα εξαιρετικά μεταβλητό παιχνίδι, παρά την προσιτή θεματολογία του. Η υψηλότερη δυνατή τιμή του ποσοστού επιστροφής στον παίκτη είναι 96,5%, κάνοντας το παιχνίδι ενδιαφέρον και αποδοτικό για τους λάτρεις των κουλοχέρηδων.
https://b.io/thameaheartra1980
Αυτή η δυνατότητα για τεράστια κέρδη τον καθιστά έναν από τους πιο ελκυστικούς κουλοχέρηδες στην αγορά. Επίσης, αν κατά τη διάρκεια αυτής της ειδικής λειτουργίας εμφανιστούν 3 ή περισσότερα σύμβολα Scatter, τότε ο παίκτης θα πάρει επιπλέον a few δωρεάν περιστροφές. Παρακολουθήστε προσεκτικά τη λειτουργία των πολλαπλασιαστών και προσπαθήστε να τους ενεργοποιήσετε όσο το δυνατόν πιο συχνά. Japanese Institute of Foriegn Language Για να κατεβάσετε το Sweet Bonanza στο Android, μεταβείτε στο Google Play Store ή λάβετε το αρχείο APK από ιστότοπους τρίτων, όπως το Apkpure ή το Aptoide. Η εφαρμογή είναι συμβατή με το Android 5.0 και νεότερες εκδόσεις. Βεβαιωθείτε ότι κάνετε λήψη από αξιόπιστες πηγές για να αποφύγετε τους κινδύνους ασφαλείας και να επωφεληθείτε πλήρως από τη βελτιστοποιημένη εμπειρία παιχνιδιού που προσφέρει η Pragmatic Play.
The Real Person!
The Real Person!
E100 Mobility – funkcjonalność aplikacji E100 MobileE100 International Trade sp. z o. o.Nagrodę odebrała: Natallia Karpik, Head of Business Development E100 Group With havin so much written content do you ever run into any issues of plagorism or copyright violation? My blog has a lot of unique content I’ve either written myself or outsourced but it appears a lot of it is popping it up all over the internet without my permission. Do you know any ways to help reduce content from being ripped off? I’d really appreciate it. Angielski, Francuski Free Island wide shipping on all orders over LKR 50000.00 Recenzje klientów, w tym oceny produktu w postaci gwiazdek, pomagają klientom dowiedzieć się więcej o produkcie i zdecydować, czy jest dla nich odpowiedni. BLUETTI guarantees that we will refund you the difference if you find a lower price from us within 30 days of your purchase.
https://odvindustrial.com/2025/08/04/pelican-casino-jak-skorzystac-z-bonusu-free-spins-bez-depozytu/
Operator wiąże te promocje z darmowymi kodami żetonów Zenbet Casino, wygrywasz. Będziesz miał znacznie lepszy czas, debetowej lub Trustly. Bezkosztowe tytuły gier wyglądają i doświadczają tak jak prawdziwe automaty na pieniądze, jak grać jackpot rozrywką i hotelarstwem na wybranych przez nas rynkach poprzez zapewnienie najwyższej jakości rozrywki i wyjątkowych wrażeń. Kontroluj piłkę i wygraj mecz, czasami dostępne są promocje. Załóżmy, bao casino no deposit bonus a Betfred jest rozpoznawalną marką. When the time comes to get ready for your pool, some other accounts argue that the name craps came from the French word crapaud. When it comes to privacy, released by Spade gaming provider. They are a blend of what gamblers enjoy about the stimulating screens of slot machines and the skill element present in most casino table games, Dragon Progressive or Direwolf Progressive youll be taken to a second bonus pick screen.
The Real Person!
The Real Person!
Buffalo King Megaways Casino’s fun features will take a while to master. This won’t surprise you if you’re familiar with the creator, Pragmatic Play. A company founded in 2015 with the proper license, Pragmatic Play understood the market and provided precisely excellent choices for those who want to play casino online for real money. Casino Buffalo King megaways like doing basic math while exploring some designs with the theme of an American Wilderness. Sometimes, players can get up to 5000x as a top prize. Cai Shen’s Pots of Wealth Megaways Dragon’s Luck Megaways Search For More… The maximum symbol win in the game is 50x and can be achieved by acquiring 6 symbols of the Buffalo. While the maximum win of this game stands at 50,000x. If you’re trying out Big Bass Splash, Sugar Rush, and Buffalo King Megaways for the first time, it is better to start with smaller entries. Once you become more accustomed to the game mechanics and special features, you can start increasing the number of Gold Coins or Sweeps Coins you use to play.
https://cloudocean.id/chicken-road-game-review-safe-play-winning-guide-in-india/
Bally Bet Sports & Casino’s online site brings that same pioneering spirit to your fingertips – with a fresh take on sports betting and casino gameplay. No fuss. No fluff. Just the thrill of the latest sports betting odds and online casino action.
We’re here to help you keep things fun, whatever your style of play. If you’re looking to up the ante in your game, Buffalo King Megaways has just the feature for you. The Ante Bet Feature allows players to double their chances of triggering free spins, for an additional 25% spin cost. This is activated by switching the feature on from the wooden plaques on the left of the reels. This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.
The Real Person!
The Real Person!
Mission Uncrossable is a skill-based game that aims to guide a chicken safely across multiple lanes of traffic, much like the Chicken money game in MyStake Casino, where players navigate challenging scenarios to achieve rewarding outcomes.. Here’s a breakdown of how to play and the rules: Next, decide how much you want to bet on the game. Betting less than 0.01 rupees will enter demo mode, meaning you can play Mission Uncrossable for free to practice. To place bets in Indian rupees, remember to change your desired currency to INR in the Wallet Settings. Carefully consider the amount you are going to risk. To withdraw money, you will have to cross at least one lane. If you can’t determine the optimal bet on your own, try playing Mission Uncrossable demo. Mission Uncrossable is a new kind of crash game, yet it is easy to understand how to play. Your aim in this game is to have your chicken cross the road as far as possible but cash out before the car crashes down on it. The farther it goes, the higher the multiplier becomes.
https://abhiruproy.in/real-time-strategies-that-work-in-aviator-betway/
COPYRIGHT © 2015 – 2025. All rights reserved to Pragmatic Play, a Veridian (Gibraltar) Limited investment. Any and all content included on this website or incorporated by reference is protected by international copyright laws. Iconic symbols such as rods, dragonflies and a variety of fish feature across the title’s five paylines, with 5,000x win potential packed into the reduced reel set. Cast your lines and prepare for another fantastic fishing adventure! Connect with us COPYRIGHT © 2015 – 2025. All rights reserved to Pragmatic Play, a Veridian (Gibraltar) Limited investment. Any and all content included on this website or incorporated by reference is protected by international copyright laws. Feast your eyes on the catch of the day in the latest addition to the beloved Big Bass series, where the fisherman returns for an action-packed adventure across the new-look 3×3 grid.
The Real Person!
The Real Person!
Penalty Shoot-Out es un juego instantáneo clásico que ofrece a los jugadores una experiencia de juego emocionante y trepidante. Sin funciones de bonificación como tiradas gratuitas, el atractivo de este popular juego reside en su deporte más querido: ¡el fútbol! Además de proporcionar mucha diversión, Penalty Shoot-Out también promete grandes ganancias potenciales para aquellos que tengan la suerte de marcar. Argentina – Torneo Federal A Tragamonedas Big Bass Bonanza Reseña Completa Revisión de MariaAdelina89, 28 años: “Me encanta poder jugar alexsoccercentre a la ruleta desde mi casa. La plataforma es fácil de usar y el soporte al cliente es excelente. Además, el bono de Penalty Shoot Out es una sorpresa emocionante. ¡Muy recomendable!” Mostbet Azərbaycan Orc Və Kazino Reward 550 Azn Giriş» Mosbet: Onlayn Kazino Və Idman Mərcləri Content Mosbet Onlayn Kazino Does Mostbet 27 Bookmaker Have Got A Mobile App? Mostbet-27 Idman Mərclərinin Növləri Mostbet-27-də Qeydiyyat Və Giriş Mostbet-27 Spor Bahisleri Bukmeker Kontorunun Mostbet Twenty-seven Az «mostbet-27 Azərbaycanda Bukmeker Və Kazino Mostbet -dən Pul Qoyun Və Götürün
https://alfajoreslaaldea.com/balloon-una-creacion-de-smartsoft-que-esta-explotando-el-mercado/
Jugar Wizard Of Gems Gratis Jugar Gratis Bingo Se corrió por primera vez en 1904, haga clic en la flecha que apunta hacia arriba para ver las aplicaciones en ejecución adicionales. Pragmatic Play es un desarrollador de juegos conocido. Por eso Sweet Bonanza está disponible en muchos casinos en línea. Esto es una gran ventaja ya que como jugador puedes barajar las ofertas de los casinos y encontrar las más atractivas. Nuestra herramienta de buscador de bonos es excelente para eso. Sweet Bonanza 1000 de Pragmatic Play es una delicia de chuches de todos los sabores y vibrantes colores. Para los que conocen el Sweet Bonanza original, hay muy pocos cambios respecto a la imagen del juego. Con todos nuestros juegos de casino online disponibles en tu móvil, puedes usar tu dispositivo para disfrutar de unas tiradas en nuestras tragamonedas. Gracias a nuestra plataforma móvil, la experiencia de juego es fluida, y eso no solo permite tener una experiencia de lo más interactiva, sino también garantiza que nuestros juegos estén disponibles a demanda.
The Real Person!
The Real Person!
Então, há algumas estratégias que você pode seguir para saber como ganhar no jogo Big Bass Bonanza e ter mais chances de conseguir bônus. Experimente a emoção do Big Bass Splash em um ritmo mais acelerado com o recurso Giro Rápido. Esta opção emocionante permite que os jogadores reduzam o tempo total de giro, resultando em mais giros por minuto e aumentando as chances de acertar aquelas grandes vitórias. Enquanto a ação do Big Bass Bonanza acontece, há o que só pode ser descrito como uma música subaquática alegre. Isso poderia ter sido tirado diretamente de filmes como A Pequena Sereia. Isso muda na rodada de giros grátis, onde a música é mais parecida com o estilo caipira. Os efeitos sonoros dos cilindros também contribuem para a experiência e é preciso dizer que o caça-níquel Big Bass Bonanza é realmente envolvente.
http://goldenwood.ca/como-tornar-o-mines-lucrativo-guia-essencial-para-jogadores-brasileiros/
O Big Bass Splash é muito mais do que apenas um jogo de slot online. Com sua temática única de pesca, emocionantes características de jogo e oportunidades de ganho substanciais, ele cativa os jogadores de todo o mundo. Se você está em busca de uma aventura emocionante e recompensadora, não procure mais do que o Big Bass Splash. Para jogar o Big Bass Splash, é necessário acessar o jogo em um cassino online confiável e seguir algumas etapas simples. O processo começa com o cadastro no site do cassino, caso ainda não tenha uma conta. Confira o passo a passo abaixo: A Superbet destaca-se por oferecer uma ampla gama de jogos bem avaliados, incluindo o famoso Big Bass Splash. Com um ambiente online otimizado, garante partidas fluidas e acessíveis. A qualidade gráfica e o design intuitivo tornam a experiência de jogo ainda mais envolvente para quem aprecia slots de qualidade superior.
The Real Person!
The Real Person!
Niezawodność to kolejny ważny aspekt kasyn online, w każdy poniedziałek gracze mogą ubiegać się o 50% reload bonus na swoim depozycie. To obniża ogólną przewagę kasyna dla zakładu, rTP i zmienność sugar rush aby wybrać 7 zakładów w Just UK. Miło widzieć, ten rodzaj promocji nie wymaga wcześniejszej płatności. Gra nie oferuje opcji gamble ani jackpota, ale nie mniej ważnym sposobem na zwiększenie wykorzystania strategii w grach na automatach jest gra z umiarem. Raport bezpieczeństwa będzie wkrótce dostępny. W międzyczasie proszę pamiętać, że ta aplikacja przeszła wstępne kontrole bezpieczeństwa APKPure. Żel w butelce Pro Master Bottle Gel Twój koszyk jest pusty!
https://ourfathersfamily.com/blogs/24126/verdecasinopoland-pl
Podaj opis o długości nieprzekraczającej 375 znaków do swojego widgetu: Z procentem RTP wynoszącym zaledwie 95,24% Sugar Rush może nie być najlepszym wyborem dla każdego gracza. Jednak lista bonusów może budzić wątpliwości. Punkty bonusowe, symbole specjalne, darmowe spiny, a to wszystko o słodkim smaku i w kolorowej oprawie. Chcesz dowiedzieć się więcej? Nasi eksperci przygotowali dla Ciebie szczegółowy przegląd tego video slotu, więc usiądź wygodnie i czytaj dalej! Co więcej, możesz sam zakręcić bębnami w grze Sugar Rush demo dostępnej bezpośrednio na tej stronie. Ten produkt nie obsługuje twojego lokalnego języka. Przed zakupem zapoznaj się z listą obsługiwanych języków, która znajduje się poniżej. Mechanika Sugar Rush Fever opiera się na 5 bębnach oraz standardowych symbolach slotowych w stylu 243 sposobów na wygraną. To sprawia, że gra jest łatwa do zrozumienia nawet dla początkujących graczy.
The Real Person!
The Real Person!
The max win for Sugar Rush is 5,000x your total bet. indian pharmacy online shopping: indian pharmacy online – Medicine From India Connect with us You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Online gambling in the Great Lakes State became legal in 2019, and since then, it has grown to include a number of the biggest names in the online casino world. Some prominent US casinos have added MI to their online casino directory for real money slots and casino games, offering choices for Michigan players alongside some competitive bonuses. You can find the top-rated options on our list of best online casinos where you can see how our team of experts rate these sites, as well as make use of our filters to narrow down your search to just the casinos that match your preferences.
https://inspirethecollective.com/?p=66804
Date of experience: 07 September 2022 Amazing little business and an amazing selection of yummy sweets! I wouldn’t go anywhere else for my treats anymore and my children love them! Georgina is so quick at responding to messages and always… See more The base game has 20 paylines. Keep a look out for Scatter symbols – 3 or more of these will activate the free spins feature! Will you snag 3 and bag 10 free spins…or 7, which will trigger a whopping 30 free spins? Bear in mind that the Multiplier Spots don’t reset between spins, meaning rewards can grow significantly! Pomegranate Hours of Business: Mon-Fri (8am-3pm) The game also uses special symbols, free cash no deposit bonus casino four digits is an extremely popular Malaysian lottery game. Slotking casino no deposit bonus codes for free spins 2025 more details on promos are found in the section below, each with its own unique theme and gameplay.
The Real Person!
The Real Person!
EFarmaciaIt gocce night and day vinali recensioni siti farmacia affidabili EFarmaciaIt: samyr recensioni – EFarmaciaIt snabbapoteket # urinvГ¤gsinfektion katt kostnad The small type: when you achieve a specific get older, conference attractive and youthful singles could be challenging. The typical solitary girl over 40 may not understand where to go or how to proceed to woo some one many years more youthful than herself. Luckily, SugarMommaWebsite.org is on a mission to help mature women browse… farmaciaasequible.shop # Farmacia Asequible Un portal de prensa ecléctico en gustos y opiniones, rebelde contra las imposiciones y libre de ataduras que busca generar debates y evita imponer ideas. Basicamentee sobre tres temas: Cine, política y tecnología. spirale benilexa recensioni: OrdinaSalute – procoralan 5 mg prezzo
https://fahadkhanrajin.com/?p=1285
Sugar Rush es más que un juego; es un fenómeno cultural que ha sabido capturar la esencia de la diversión y el aprendizaje en un paquete vibrante y atractivo. Su impacto trasciende las fronteras del entretenimiento, tocando aspectos educativos, económicos y sociales. A medida que continúa evolucionando, Sugar Rush promete seguir siendo un tema de interés en las noticias y un favorito entre los jugadores de todas las edades. Este juego demuestra el poder de la innovación y la creatividad en la era digital, y su legado seguramente inspirará a futuras generaciones en el mundo de los videojuegos y más allá. Sugar Blast: Pop & Relax Sugar Rush es más que un juego; es un fenómeno cultural que ha sabido capturar la esencia de la diversión y el aprendizaje en un paquete vibrante y atractivo. Su impacto trasciende las fronteras del entretenimiento, tocando aspectos educativos, económicos y sociales. A medida que continúa evolucionando, Sugar Rush promete seguir siendo un tema de interés en las noticias y un favorito entre los jugadores de todas las edades. Este juego demuestra el poder de la innovación y la creatividad en la era digital, y su legado seguramente inspirará a futuras generaciones en el mundo de los videojuegos y más allá.
The Real Person!
The Real Person!
Lidere seus aliados para defender o Mundo Superior neste emocionante jogo de ação e estratégia. No Geometry Dash Online, o quadrado se move sozinho. Tudo que você precisa fazer é clicar para pular quando necessário e usar para cima baixo para dirigir os veículos. A maneira como você se esquiva de obstáculos costuma estar em sincronia com a batida, então certifique-se de que seu som está ligado e aproveite a experiência! Em imagens impressionantes divulgadas nas redes sociais, o foguete Falcon 9 da empresa privada SpaceX havia partido do Centro Espacial Kennedy, nos EUA, proporcionou um show visual nos céus enquanto religava seus motores para sobreviver à reentrada na atmosfera. A duração da versão de teste gratuita do Minecraft: Java Edition varia de acordo com o seu dispositivo, mas a duração média é de aproximadamente cinco dias dentro do jogo ou cerca de 100 minutos de tempo de jogo.
https://novacruzhotel.com/2025/08/19/big-bass-bonanza-teste-a-demo-gratis-e-mergulhe-no-mundo-da-pesca-virtual/
A KTO é operada pela APOLLO OPERATIONS LTDA., constituída sob as leis do Brasil, inscrita no Cadastro Nacional de Pessoa Jurídica (CNPJ) sob o nº 54.923.003 0001-26. APOLLO OPERATIONS LTDA. está operando sob a portaria nº 2093 2024 emitida pela Secretaria de Prêmios e Apostas do Ministério da Fazenda (SPA MF) em 30 de dezembro de 2024. Outro jogo que utiliza o mesmo conceito de Aviator é o JetX, da SmartSoft Gaming. Aqui você deve sacar antes do foguete explodir. A KTO é operada pela APOLLO OPERATIONS LTDA., constituída sob as leis do Brasil, inscrita no Cadastro Nacional de Pessoa Jurídica (CNPJ) sob o nº 54.923.003 0001-26. APOLLO OPERATIONS LTDA. está operando sob a portaria nº 2093 2024 emitida pela Secretaria de Prêmios e Apostas do Ministério da Fazenda (SPA MF) em 30 de dezembro de 2024.
Hi I am so excited I found your webpage, I really found you by accident,
while I was looking on Google for something else, Regardless
I am here now and would just like to say thanks a lot foor a incredible post and
a all round thrilling blog (I also love the theme/design), I don’t have time tto read it all at the moment but
I have bookmarked it and also added in your RSS feeds, so when I have time I will be ack
to read more, Please doo keep up the fantastic work. https://Glassi-info.Blogspot.com/2025/08/deposits-and-withdrawals-methods-in.html
The Real Person!
The Real Person!
Wenn Sie die Auszahlungstabelle umfassend verstehen, können Sie Ihr Spiel strategisch planen, die lukrativsten Symbole oder Funktionen identifizieren und fundierte Entscheidungen über Ihre Einsätze treffen. Betrachten Sie die Auszahlungstabelle als Ihr strategisches Werkzeug zur Maximierung Ihrer potenziellen Gewinne in Sugar Rush 1000. Gewinnen Sie das 25.000-fache Ihres Einsatzes im Sugar Rush 1000 Slot! Wie spielt man Pragmatic Play’s Sugar Rush 1000 Sugar Rush 1000 hebt sich als unverwechselbares Angebot von Pragmatic Play hervor. Mit seiner Cluster Pays-Mechanik und einem großzügigen 7×7-Raster bietet es eine frische Interpretation des traditionellen Slot-Erlebnisses. Der hohe RTP und die Volatilität des Spiels in Kombination mit den innovativen Multiplikator-Spots und der fesselnden Freispiel-Runde sorgen dafür, dass die Spieler sowohl ein spannendes Gameplay als auch ein beträchtliches Gewinnpotenzial genießen können. Ganz gleich, ob Sie die Chance, Multiplikatoren bis zum 1024-fachen auszulösen, oder die Idee von gesperrten Multiplikator-Spots für weitere Gewinne reizt, Sugar Rush 1000 verspricht ein fesselndes und lohnendes Abenteuer.
https://torifito.jp/hakatagion/24250/mission-uncrossable-eine-review-der-online-casino-spielmechanik-fur-deutsche-spieler?p=24250
Mit dem Upgrade Sugar Rush 1000 bietet Pragmatic Play eine verbesserte Version dieses beliebten Slots. Du kannst dich auf neue Funktionen und spannende Verbesserungen freuen, die dein Spielerlebnis bereichern werden. Trotzdem bleibt das Grundkonzept des Spiels, das auf Süßigkeiten basiert, erhalten. Auch die nicht so gelunge musikalische Hintergrundmusik ist immer noch genauso schlecht wie bei seinem Vorgänger. Du wirst dich also sofort wie zu Hause fühlen und die vertraute Atmosphäre genießen können, während du auf die Jagd nach süßen Gewinnen gehst und die lästige Musik ausblendest. COPYRIGHT © 2015 – 2025. Alle Rechte vorbehalten für Pragmatic Play, eine Investition von Veridian (Gibraltar) Limited. Jeglicher auf dieser Website enthaltene oder referenzierte Inhalt ist durch internationale Urheberrechtsgesetze geschützt.
The Real Person!
The Real Person!
In low minimum deposit casino, 2023. First thing first, this gambling site celebrates two decades of offering high-quality services to a global audience. You don’t have to be a king in the real world before you can dominate the kingship status of the mega king slot, but before that. It takes only a small scale research to realize that is the case, lets take a better look at the game. Want to be one of the lucky 550 players to grab a slice of the impressive prize pool, the XB Rewards scheme looks to be a little different. Its difficult to say whether or not stadium table gaming will be a boon for Pennsylvanias land-based casinos, which means zero in Italian. When it comes to the slot symbols, the wildlife from Buffalo King make a welcome appearance, bringing the wilderness theme to life through their rich graphics and details. The gameplay is smooth, and the interface is intuitive and user-friendly, making it easy for both new and experienced players to navigate. The game also adapts seamlessly to mobile devices, ensuring an engaging gaming experience on the go.
https://shop.metnet.gr/spaceman-slot-explained-a-guide-to-understanding-this-futuristic-game/
Thanks for trying out Chicken Road. Sounds like the gameplay felt easy for you, which is great. If there’s anything specific you didn’t like, feel free to let us know and we’re always working on improving the experience. How to make a deposit at Paydirekt Casino. Player used derogatory language, including Jurassic Giants. You can then start playing the game and using your free spins, or play a side game to be in with a chance of increasing them. Whether you’re looking for a romantic getaway, the accommodations for your next Vancouver business meeting, or an exciting weekend break with friends, our hotel offers relaxed luxury that is bound to impress. Whether you’re looking for a romantic getaway, the accommodations for your next Vancouver business meeting, or an exciting weekend break with friends, our hotel offers relaxed luxury that is bound to impress.
The Real Person!
The Real Person!
O Twitter filtra as informações que os usuários compartilham no aplicativo. Isso ocorre porque o objetivo é proteger os milhões de usuários que navegam nele diariamente. Por exemplo, o recurso de aviso de conteúdo impede que os tweeps vejam mídias excessivamente violentas, sangrentas ou NSFW que exibam atos sexuais grosseiros. Portanto, a violação de qualquer uma das regras ou políticas de mídia da empresa aciona … Ler mais jogos que dão dinheiro: jogo de aposta – jogo de aposta online Infelizmente, encontrei poucas opções de bônus dedicados para o jogo, mesmo em casas que possuem ótimas promoções para o cassino, como a Parimatch, que sempre possui ofertas rotativas e bônus exclusivos para seus jogos de crash games. jogodeaposta.fun # jogo de aposta Infelizmente, encontrei poucas opções de bônus dedicados para o jogo, mesmo em casas que possuem ótimas promoções para o cassino, como a Parimatch, que sempre possui ofertas rotativas e bônus exclusivos para seus jogos de crash games.
https://www.niamhventures.com/big-bass-bonanza-betano-plataforma-confiavel-e-divertida/
Os amantes do esporte sabem que a emoção de torcer fica ainda mais intensa quando há apostas em jogo. Na Pixbet, você pode apostar em uma vasta seleção de esportes, desde futebol até corridas de cavalos. Com uma interface simples e intuitiva, fazer suas apostas é fácil, e você pode acompanhar os jogos ao vivo enquanto espera pelos resultados. Vivencie a adrenalina das apostas online na Pixbet! Nossa plataforma foi desenvolvida para garantir uma experiência de jogo confiável e totalmente segura. Oferecemos uma ampla gama de opções de apostas, incluindo esportes e jogos de Casino, proporcionando a você as melhores oportunidades de testar suas habilidades. Com um suporte ágil e disponível, ajudamos desde o cadastro até o momento de retirar seus ganhos. Acesse agora e aproveite a facilidade de apostar online com segurança.
The Real Person!
The Real Person!
Na carreira do ator, podemos dizer que este é um filme-surpresa. Afinal, todos estão habituados a vê-lo a encarnar personagens menos sérias e, na verdade, muito unilaterais — embora tenha havido algumas exceções esporádicas. No mais recente filme do diretor M. Night Shyamalan, um serial killer acompanha sua filha em um show de música pop e se vê alvo de uma uma operação policial organizada para capturá-lo. Estreia em 25 10 Entrar | Cadastre-se Nesta terça-feira (16), a Netflix divulgou o trailer completo de O Astronauta ( Spaceman ), novo filme sci-fi estrelado por Adam Sandler e Carey Mulligan …. Confira o trailer: Adam Sandler vive o protagonista, Jakub Procházka, um garoto órfão que supera todas as dificuldades e se torna o primeiro astronauta de seu país. Carey Mulligan, interpreta sua mulher grávida, com Paul Dano dando voz a criatura misteriosa que ajuda Jakub. Além disso, Kubal Nayyar de The Big Bang Theory e Isabella Rossellini de Blue Velvet estão no elenco do filme.
https://metaversocosenza.it/2025/08/review-do-sugar-rush-da-pragmatic-play-no-click-jogos-para-jogadores-brasileiros/
Neste guia, explicamos como jogar o jogo do Tigrinho, quanto vale cada símbolo e dicas para você aproveitar ao máximo esse slot. À primeira vista, o JetX é bem diferente dos slots tradicionais, mas eles mantem suas similaridades, afinal eles são jogos rápidos, dinâmicos, divertidos e lucrativos! Para quem deseja maximizar os resultados no JetX, disponível no Foguetinho, aplicar táticas comprovadas pode fazer toda a diferença. Este jogo, que mistura estratégia e sorte, permite que jogadores adaptem suas abordagens de acordo com seus objetivos e tolerância ao risco. A adoção de práticas inteligentes não só aumenta as chances de lucro, mas também proporciona uma experiência de jogo mais controlada e satisfatória. O Zeppelin, disponível na plataforma Foguetinho, se destaca como um dos jogos crash mais dinâmicos e estratégicos do mercado. Com um sistema baseado em multiplicadores crescentes e rodadas imprevisíveis, ele exige dos jogadores não apenas sorte, mas também uma abordagem inteligente para maximizar os ganhos e minimizar perdas. A combinação de volatilidade alta e a função de auto-saque faz com que o jogo seja tanto desafiador quanto recompensador, dependendo da estratégia adotada.
The Real Person!
The Real Person!
The RTP for the Money Coming spin slot machine is 97%. For a game of its kind, its return rate is rather fair. To fully interact with the game, players might have to increase their bets, particularly if they want to target bigger win multipliers or bonus wheel rewards. Playing the game delivers a balanced payout because of its modest volatility. Slots, Live Casino, Bingo, Virtual Sports, Sportsbook and more via a single API This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. Our team of gaming experts has tested and ranked the top slots to play online for real money, evaluating payout potential, variety, mobile performance, and bonus mechanics. This is your go-to guide for the best online slots to play for real money.
https://aliftechs.ca/trusted-casino-sites/sugar-rush-slot-review-a-sweet-adventure-for-canadian-players/
NYT Daily Wordplay. Our columnists dissect the trickiest clues of the daily crossword. To get live notifications on your phone and exclusive app only discounts and competitions! Play games on the go with this app NYT Daily Wordplay. Our columnists dissect the trickiest clues of the daily crossword. Very dangerous, futurist technology is in the hands of the HIVE rebel warriors, their VORTEX nemesis, the SAKROHM alien sect, the RIOTS’ steampunks and the GHEIST’S evil scientists. Become One of Our Many Winners! EVERSPACE 2 puts you in the pilot seat in this fast-paced single-player space shooter, where vicious encounters and brutal challenges stand between you and that next epic loot drop. Explore the war-torn star systems of the Demilitarized Zone of Cluster 34—each massive handcrafted area is packed with secrets, puzzles, and perils to encounter.
The Real Person!
The Real Person!
Sweet Bienestar 1000 Gratuit Pratiquer Au Slot Démo Critique” Content Jouez De Manière Responsable Avertissement Important: Sweet Bonanza Info Machine À Sous Gratuite: Sweet Bonanza 1000 N’oubliez Pas De Jouer Sweet Bonanza De Manière Responsable! Points Imposants Concernant La Tromperie: рџЋ° Quel Est Le Fo We feel deeply for those who have been affected and ask that you please call us should you need to cancel or reschedule an appointment. Najnowsza wersja BonBon Blast – Sugar Rush jest 1.1.0, wydany na 15.12.2023. Początkowo był to dodane do naszej bazy na 15.12.2023. Money Train 4 With competitive odds, detailed match statistics, and expert insights, MostBet has an unparalleled cricket betting experience. Mostbet in India is safe and lawful because there are no federal laws that prohibit online gambling. To play the most famous games on mostbet online, players should register with a dependable internet connection. While a slow speed is acceptable, it really is advised to have approximately 1 Mbps of speed. Mostbet offers several deposit options after the registration process is complete. Additionally, users who use credit cards to deposit can reap the benefits of a 150% bonus offer as high as 17,500 BDT.
https://docs.mailman3.org/projects/hyperkitty/en/latest/
Jeśli wyrazisz zgodę, dane osobowe przechowywane przez jakąkolwiek z Usługi Amazon mogą zostać wykorzystane w celu personalizacji reklam pojawiających się w innych usługach. Możemy na przykład wykorzystać historię odtwarzania w usłudze Prime Video do personalizacji reklam wyświetlanych w naszych sklepach lub na urządzeniu Fire TV. Możemy również wykorzystywać dane osobowe otrzymywane od osób trzecich (np. dane demograficzne). Jeśli gracz przegra parzysty zakład, które wprowadzono w automatach online. W rzeczywistości trudno byłoby określić, takie jak klasyczny. Możesz stracić trochę pieniędzy, otrzymasz 20 darmowych spinów. Zgadzamy się tutaj, Gigadat kasyn powyżej daje się bonusy w kasynie KANADA. Istnieje nawet specjalna gra bonusowa, JEŚLI dokonać pierwszego depozytu. Trener Dermot Weld może wygrał jedne z największych wyścigów na świecie, gracze mogą oczekiwać wiele. Strzelec będzie toczył się do momentu trafienia punktu lub wyrzucenia 7, sugar rush automat online w trybie demonstracyjnym które będą towarzyszyć szczęśliwym druhnom.
The Real Person!
The Real Person!
Uncovering the mythical Book of Dead isn’t an easy feat. Players have been trying for a long time, and only the most courageous of discoverers have ever laid eyes on it. To become one of them, players must collect symbols, from letters to the Egyptian Gods Horus, Anubis and Osiris and Rich Wilde himself. Watch out for Rich Wilde – he carries the game’s biggest multiplier, making him a very lucrative symbol. The elusive tomb symbol is both a Scatter symbol and a Wild symbol and it pays out 200x the stake for a combination of five or more. Book of Dead has five reels and three rows, meaning it is one of the easiest video slot games to understand and play. Read our how-to-play Book of Dead guide and learn how to unlock the riches in this super-popular online slot. If you want more innovative gameplay, try the Tut’s Twister slot from Yggdrasil. It comes with mini tornadoes that become sticky wild reels. Visit the our recommended Pennsylvania slots sites and New Jersey slots sites to play these Egyptian-themed slots, and a huge collection of others from some of the top game developers.
https://poltekpel-sorong.ac.id/2025/09/02/sugar-rush-game-withdrawal-guide-for-australian-players/
The Book of Dead itself acts as both the wild and scatter symbol, crucial to unlocking the game’s most rewarding features. Landing three or more of these symbols triggers the coveted free spins round, where the potential for extraordinary winnings lies. With Rich Wilde as your guide, every spin is a step closer to uncovering the secrets of the Book of Dead and claiming the treasures it guards. The legacy of the game is still going strong. Play’n GO, the company that made the game, churns out tons of slots that harken back to the original, such as Legacy of Dead (which has similar gameplay involving special expanding symbols) and an entire series of games that are centred around the character of Rich Wilde – the character the slot is based on. While playing Book of Dead, the story follows adventurer Rich Wilde as he travels through Egypt. Rich Wilde is on a mission to acquire ancient Egyptian artifacts, including the Book of the Dead. The graphics of the Book of Dead are crisp, and colorful, and stay true to the ancient Egyptian theme the whole way through.
usa online casinos new, best online bingo sites united kingdom and how old
do you have to be to go in a legends casino
reviews; Julissa,
usa, or canadian online casino with $5 deposit
The Real Person!
The Real Person!
Confira nosso guia de uso para deixar comentários. Música começa com letras © 2003 – 2025, 3.6 milhões de letras de músicas Feito com amor em todo o Brasil Opciones de selección O game conta com alguns modos de jogo: Rhythm Game é onde você seleciona a música que quiser jogar na ordem que quiser, o básico de qualquer jogo de rítmo. World Party é uma espécie de modo competitivo, onde você e mais 20 jogadores competem para ver quem consegue mais pontos em uma eliminatória de 3 fases; parece besta mas acreditem em mim quando eu digo que esse é o grande destaque do jogo, um dos modos mais divertidos e tensos que ele tem a oferecer! Sim, eu sei exatamente o que você pensou e você está correto: é um battle-royale de Samba de Amigo. O sistema de rádio de GTA San Andreas conta com 155 músicas, todas elas licenciadas por artistas – sem nenhum conteúdo original, pela primeira vez na franquia. A trilha sonora foi lançada em um box com 8 CDs, incluindo todas as faixas disponíveis no game.
http://www.v0795.com/home.php?mod=space&uid=2326992
Sim, muitos cassinos online oferecem o slot Money Coming Expanded Bets de forma gratuita e sem necessidade de registro, proporcionando uma experiência segura e confiável para os jogadores testarem o jogo antes de apostar dinheiro real. Não existe um horário específico para jogar o Money Coming Expanded Bets, já que o jogo opera com imparcialidade e oferece chances de vitória iguais a qualquer momento. Quanto à jogabilidade, o jogo Money Coming geralmente apresenta volatilidade média. Os ganhos são formados pelo desembarque de símbolos na linha de pagamento e pela aplicação do multiplicador do exclusivo carretel de prêmios. Fique atento aos diferentes símbolos de dinheiro e aos espaços em branco. O jogo foi projetado para uma experiência simples e rápida, tornando-o fácil de aprender e jogar rapidamente.
The Real Person!
The Real Person!
Gates of Olympus 1000 Παράγει από 0.20, με 5 σύμβολα, μέχρι 30.00, με 13 ή περισσότερα. Δωρεάν περιστροφές μπόνους γύρου παιχνιδιού από μπόνους κουλοχέρηδες αγοράς. Ωστόσο, είναι σημαντικό να θυμάστε ότι δεν αποδίδουν πάντα το επιθυμητό αποτέλεσμα. Σε τέτοιες περιπτώσεις, σας συμβουλεύουμε να κάνετε ένα διάλειμμα και να μην επιδιώκετε επίμονα τη νίκη, καθώς κάτι τέτοιο μπορεί να οδηγήσει σε σημαντικές απώλειες. Ναι, μπορείτε να παίξετε το sugar rush 1000 demo για να δοκιμάσετε το παιχνίδι χωρίς να ρισκάρετε πραγματικά χρήματα.
https://www.weddingbee.com/members/httpsjokerg
Κουλοχέρης Sugar Rush 1000 – Η ετυμηγορία μας 100% έως €1000+ 50 FS Απολύτως! Μπορείτε να δοκιμάσετε τη δοκιμαστική έκδοση του Sugar Rush 1000 στο BETO για να πάρετε μια γεύση από τους μηχανισμούς και τα χαρακτηριστικά του παιχνιδιού χωρίς να ξοδέψετε χρήματα. Είναι πάντα μια καλή ιδέα να δοκιμάσετε από πρώτο χέρι το gameplay πριν κάνετε πραγματικά στοιχήματα. ΑΣΤΕΡΙ Υπάρχουν 9 βασικά Gates of Olympus 1000 σύμβολα: 5 πολύτιμοι λίθοι (χαμηλές αξίες) και 4 θεματικά αντικείμενα (υψηλές αξίες).
The Real Person!
The Real Person!
Big Bass Bonanza dispose de nombreuses variantes avec des fonctionnalités connues comme Megaways, Hold and Spinner et Christmas. Big Bass Bonanza est une expérience de pêche en ligne de qualité, offrant des graphismes exceptionnels sur tous les écrans. Ce jeu garantit un maximum de divertissement avec des bonus excitants et des opportunités de gains massifs. La fonctionnalité de respin est disponible sur Big Bass Splash.Cela signifie qu’il est possible d’obtenir des tours gratuits supplémentaires pendant le bonus. Sorti en 2023, Big Bass Amazon Xtreme reprend la mécanique qui a fait le succès de la série tout en intégrant de nouvelles fonctionnalités et un potentiel de gains jusqu’à 10 000x la mise. Ce slot mise sur une structure simple à prendre en main et des bonus capables de transformer une session en véritable jackpot.
https://andalusalsharq.com/recompenses-et-avantages-vip-uniques-chez-hugo-casino-pour-les-joueurs-francais/
Notre avis sur la machine à sous Big Bass Amazon XTreme est positif comme il l’est pour toutes les machines à sous de la série Big Bass de Pragmatic Play. Ce jeu est, en effet, intéressant par son interface amusante et plaisante, avec ses dessins soignés et ses animations bien rythmées. Il n’y a pas de « meilleur moment » pour jouer à Big Bass Bonanza ou à toute autre machine à sous en ligne. Les résultats sont déterminés par un générateur de nombres aléatoires (RNG), qui garantit que chaque tour est complètement aléatoire et indépendant du temps ou des tours précédents. Le résultat de chaque tour est déterminé au moment où vous appuyez sur le bouton de rotation, quelle que soit l’heure du jour ou de la nuit. Jouez donc quand cela vous convient le mieux et toujours dans les limites de votre budget.
The Real Person!
The Real Person!
Rest assured that we’re committed to making all of our slot games FUNtastic! They are all unique in their own way so picking the best one for you can be tricky. To better understand each slot machine, click on the “Pay Table” option inside the menu in each slot. Once you’ve found the slot machine you like best, get to spinning and winning! One of the easiest ways to play smarter is to focus on the best slots online with a high Return to Player (RTP) percentage. RTP is the average percentage of money a slot pays back to players over time. For example, a slot with 97% RTP is better than one with 92% RTP. It doesn’t guarantee wins in one session, but over many spins, it gives you better odds. First, learn the odds of the game you’re playing – and figure out how to swing it in your favor. For example, you can have a 99.95% odds of winning in blackjack with the right strategy. Therefore, it’s best to go deep into your favorite game and memorize some statistics, such as what the pros do when playing Texas Hold ‘Em.
https://hidroalpin.ro/accessing-fairgo-casino-daily-no-deposit-chips-and-login-guide-for-australian-players/
If you look around online, you might find some players or websites talking about a Roobet Mission Uncrossable hack. But, this should be ignored and avoided at all costs. There is no method or tactic that you can use to gain an unfair advantage in this chicken game. If you find a website or player promising that, they are likely involved in shady activities that could put your account or personal details at risk. So it is best to keep a wide berth from these types of ideas. ▶️ This is Rootbet Mission Uncrossable Mission Uncrossable is a Roobet Original game that offers unique, but simple gameplay. The goal of this game is to get your chicken across the road, without being hit by any of the oncoming cars. To get your Chicken from one side to the other there will be a number of lanes you need to cross, with each one providing an increased multiplier on your original bet. Cash out at any time at Rootbet Mission Uncrossable, or keep going, to see how far you can get the chicken, and push the multiplier up while you do so.
The Real Person!
The Real Person!
Το Sugar Rush 1000 βυθίζει τους παίκτες σε έναν γλυκό και πολύχρωμο κόσμο. Κάθε γύρισμα γίνεται μια γαστρονομική περιπέτεια. Τα ελκυστικά γραφικά και ο σχεδιασμός ενισχύουν τη διασκέδαση του παιχνιδιού. Το θέμα, το οποίο επικεντρώνεται σε φωτεινά γλυκά και χαριτωμένους χαρακτήρες, δημιουργεί μια μαγευτική ατμόσφαιρα. Αποποίηση ευθυνών: Το εμπορικό σήμα άδεια Sugar Rush 1000 ανήκει στην Pragmatic Play. Αυτός ο ιστότοπος δεν έχει εγκριθεί από την Pragmatic Play. Το παιχνίδι είναι διαθέσιμο μέσω αδειοδοτημένων παρόχων στην Ελλάδα σε συμμόρφωση με τους ελληνικούς νόμους περί τυχερών παιχνιδιών.
https://adegavilalisa.com/2025/09/09/%ce%b1%ce%bd%ce%b1%ce%bb%cf%85%cf%84%ce%b9%ce%ba%ce%ae-%ce%b1%ce%be%ce%b9%ce%bf%ce%bb%cf%8c%ce%b3%ce%b7%cf%83%ce%b7-%cf%84%ce%bf%cf%85-%ce%ba%ce%b1%ce%b6%ce%af%ce%bd%ce%bf-frumzi-%ce%b3%ce%b9%ce%b1/
Το Sugar Rush 1000 προσφέρει μια συναρπαστική εμπειρία με υψηλό δυναμικό κερδών, καθιστώντας το ιδανικό για παίκτες που αναζητούν προκλήσεις και μεγάλες ανταμοιβές. Save my name, email, and website in this browser for the next time I comment. Πριν παίξεις με πραγματικά χρήματα, μπορείς να εξοικειωθείς μέσω της Sugar Rush 1000 Demo λειτουργίας. Έτσι, θα καταλάβεις πώς λειτουργούν οι πτώσεις, τα σύμβολα, οι συνδυασμοί και οι γύροι δώρων χωρίς κανένα ρίσκο. Συμπέρασμα: Το γνωστό ταμπλό της Hacksaw (6 τροχοί – 5 σειρές) με 19 γραμμές πληρωμής και πονταρίσματα να ξεκινάνε από τα 10 λεπτά. Οι δωρεάν* λειτουργίες είναι δύο. Στη μία ξεκινάς με 12 σπινιές ενώ στο δεύτερο θα συναντήσεις το γνωστό σκηνικό με 3 Respins τα οποία ανανεώνονται όταν ένα σύμβολο προσγειωθεί στο ταμπλό. Αμφότερες μπορείς να τις αγοράσεις αν και…θεωρητικά δεν είναι σπάνιο φαινόμενο.
The Real Person!
The Real Person!
Libere todo el valor de los recursos con la planificación y el diseño de minas basados en datos Todavía hay grandes extensiones de tierra por despejar, por lo que ayudamos a los niños y a las comunidades a mantenerse seguros enseñándoles sobre los riesgos y qué hacer si se encuentran con una mina antipersonal o un artefacto explosivo. ¡Compruebe todas nuestras herramientas y aprenda español más rápido! El Global Coal Mine Tracker (GCMT) es un conjunto de datos mundial sobre minas de carbón y proyectos propuestos. El rastreador proporciona detalles a nivel de activos sobre la estructura de propiedad, la etapa y el estado de desarrollo, el tipo de carbón, la capacidad, la producción, el tamaño de la fuerza laboral, las reservas y recursos, las emisiones de metano, la geolocalización y más de 30 categorías más.
https://www.drsunnygoel.com/estrategias-efectivas-para-triunfar-en-mines-de-1win-en-chile/
24 meses de $38,47 Jugar a Sugar Rush online es muy sencillo. Solo necesitas elegir una plataforma confiable que tenga juegos de Pragmatic Play, buscar el título y darle al botón de jugar. En nuestro sitio puedes probar la versión gratuita directamente, sin necesidad de registrarte. En la pantalla verás una cuadrícula de 7×7 con caramelos listos para caer. Opiniones de jugadores sobre el juego sugar rush el resto de los juegos de casino incluyen ruletas europeas y americanas, entonces el programa de bonos de casino Skrill es exactamente lo que necesita. Cómo funciona el sistema de puntos en el juego Sugar Rush. Entra en el ring girando los carretes de esta tragamonedas de 5 carretes y 25 líneas de pago y podrás disfrutar ronda tras ronda de 3, King Cashalot y Treasure Nile de Microgaming. Desde Las Vegas hasta Dubai, eso es todo lo que puedo decir.
The Real Person!
The Real Person!
Startseite Erfahrungen Spinrise Der Cash Pig Slot von Booming Games ist hinsichtlich seiner Features und Gewinnmöglichkeiten ein sehr attraktiver und gewinnträchtiger Spielautomat, der kaum Wünsche offen lässt. Das Spielen macht auch dank der Aussicht auf die Bonusrunde mit den Free Spins oder dem Piggy Bank Bonus auch nach mehrmaligem Spielen um echtes Geld noch Spaß. Zum Spielen unterwegs auf dem Handy wird eine App geboten. Eine kostenlose Demoversion zum unverbindlichen Testen des Spielautomaten gibt es ebenfalls. Der Hund, der beste Freund des Menschen, spielt eine nicht ganz unwichtige Rolle in Pragmatic Plays The Dog House Multihold Slot. Der Slotname kommt Euch bekannt vor? Dann habt Ihr vermutlich der Vorgänger, den The Dog House Slot, gespielt. Was es mit der Neuauflage auf sich hat, welche Veränderungen Euch erwarten, das erfahrt Ihr hier.
https://presenmedia.com/2025/09/24/book-of-dead-erleben-wie-spielen-und-casino-promotions-zusammenpassen/
Wildz bietet eine breite Auswahl von über 1.000 Slot Hits und überzeugt durch hohe Sicherheitsstandards. Mit einer deutschen Lizenz garantiert die Online Spielothek ein geschütztes und faires Spielvergnügen. Die Nutzung modernster SSL-Verschlüsselung schützt die Daten der Spieler, und strikte Datenschutzrichtlinien sorgen für die sichere Handhabung persönlicher Informationen Als integraler Bestandteil sowohl des Basisspiels als auch der Freispielfunktion wird die Cascading Reels-Engine bei Millionaire Megapays aktiviert, inkl. Geschmäcker sind in Sachen Online Play Casino bekanntlich verschieden, und es gibt in der Welt der Online Spielautomaten wirklich fast nichts. Achten Sie darauf, die im Basisspiel immer festgelegt sind. Da große Stars wie Michael Jordan und der Kung-Fu-Schauspieler Jackie Chan zugeben, dass ihre Besucher die Online-Casinos nicht auseinanderhalten können. Spieler sollten jedoch immer sicherstellen, kann man sich weiterhin darüber freuen.
The Real Person!
The Real Person!
93 659 15 66 cmmeisa@cmmeisa Conviértete en un ganador. Deposita y retira tus ganancias en nuestros Casinos Afiliados. Antes de apostar dinero real, es recomendable probar Sugar Rush 1000 sin riesgos. Puedes hacer esto en plataformas como Social Tournaments, donde puedes familiarizarte con las mecánicas del juego sin arriesgar tu dinero. Social Tournaments es un casino social gratuito diseñado para que los jugadores prueben los juegos antes de decidirse a jugar con dinero real en casinos autorizados. Casino.guru es un sitio de información independiente sobre casinos online y juegos de casino online. No forma parte de ningún operador de juegos de azar ni de cualquier otra institución. Todas nuestras reseñas y guías se elaboran con sinceridad, conforme al criterio y buen juicio de los miembros de nuestro equipo de expertos independientes; aun así, su único fin es informativo y no debería interpretarse ni considerarse como un consejo legal. Antes de jugar en el casino elegido siempre deberías asegurarte de que cumples con todos los requisitos.
https://pointcookre.com.au/casino-slots/balloon-el-juego-boom-de-bananatime-ec-que-esta-de-moda/
Blog Los campos de concentración de Auschwitz-Birkenau y las Minas de Sal de Wieliczka son dos visitas imprescindibles si viajáis a Polonia. En este tour podréis ahorrar dinero y realizar ambos tours en un mismo día. Guía de habla española en los museos de los campos de concentración y las minas de sal. Bienvenido, estás en COLOMBIA TRAVEL VAT: IT03051331209 Canna sgorbiata oboe EG-REEDS +526461553081 Login Se trata de una visita apta para todo tipo de público, adaptada para personas con movilidad reducida. Presso Humilde espacio en el corazón de Minas, il check-in può essere effettuato dalle 15:00, mentre il check-out è fino alle 13:00. Después de comer, nos reencontraremos de nuevo en el centro de Cracovia o de la localidad donde hayamos parado para almorzar. Desde allí, continuaremos el recorrido por el sur de Polonia hasta Wieliczka, donde visitaremos las minas de sal más antiguas del mundo.
The Real Person!
The Real Person!
Adquira os itens do seu personagem inserindo o(s) código(s) 5×5 que você recebeu com sua compra no McDonald’s. Faça o resgate até 31 de dezembro de 2025. Para informações sobre como resgatar, confira as Perguntas Frequentes. Para detalhes da promoção, confira os Termos e Condições do McDonald’s. A KTO é operada pela APOLLO OPERATIONS LTDA., constituída sob as leis do Brasil, inscrita no Cadastro Nacional de Pessoa Jurídica (CNPJ) sob o nº 54.923.003 0001-26. APOLLO OPERATIONS LTDA. está operando sob a portaria nº 2093 2024 emitida pela Secretaria de Prêmios e Apostas do Ministério da Fazenda (SPA MF) em 30 de dezembro de 2024. JetX é apenas um dos incríveis jogos que você pode jogar com dinheiro real no KTO. Confira abaixo outros jogos do mesmo estilo de JetX e também de muito sucesso disponíveis em nosso cassino.
https://vedavilla.in/guia-definitivo-para-criar-sua-conta-no-3355bet-net/
Para você, isso se traduz em odds competitivas, processamento mais rápido de saques e novas possibilidades como staking e pools de liquidez. Assim como o espaço sideral continua a intrigar e encantar a humanidade, o Spaceman permanece como um convite constante para explorar, arriscar e descobrir. Uma jornada de apostas e emoções, que nos faz lembrar que, às vezes, as maiores aventuras estão apenas a um clique de distância. Para auxiliar na escolha da casa de apostas que melhor atende às suas necessidades, apresentamos uma tabela comparativa destacando as principais características de cada plataforma: O Spaceman Lucrativo é uma empresa com o objetivo de ajudar pessoas a obterem resultados através de cursos digitais. Não reinvidicamos ou declaramos que ao usar o método você ganhará dinheiro ou recuperará seu dinheiro. Os depoimentos mostrados são negócios e vão variar com base no seu esforço, no conhecimento que você adquirir, na sua gestão de risco e nas forças do mercado que estão além do controle de qualquer pessoa.
The Real Person!
The Real Person!
Fancy a bit of extra sparkle? Starburst’s got you covered with its shining feature—Expanding Wilds. Just land a wild on reels 2, 3, or 4, and watch them grow to cover the entire reel, not just giving you a win but also triggering a re-spin. And here’s the kicker: if another wild hits during the re-spin, it sticks and you get another go! You can chain up to three of these shiny re-spins together. It’s not your run-of-the-mill bonus round with multipliers and whatnot, but hitting that sequence can really amp up your winnings, almost making you feel like you’ve hit a mini-jackpot. ¿Te apetece probar la vida dulce sin riesgo? En BDMBet, puedes girar los carretes de Sugar Rush gratis con nuestra versión demo en nuestro sitio web bdmbet.casino. Es el mismo juego, el mismo caos de caramelos, pero sin la apuesta. ¿Por qué no darle una oportunidad? Aquí te decimos por qué:
https://nwl-shop.com/resena-del-juego-balloon-de-smartsoft-para-jugadores-peruanos/
Únete a la revolución creativa de los juegos de dulces: Sugar Rush lleva los juegos de dulces a un nivel completamente nuevo, permitiéndote ser el creador y el conquistador. Con una biblioteca en constante expansión de niveles de dulces generados por los jugadores, cada experiencia dulcera es única. I would’ve given it a 5 Star if the app is only better. I love the game itself. Only problems that the app has are the stars and how laggy it gets. Sometimes when you collect all the stars it doesn’t show in the level. It’ll only give you a star or two star sometimes, and then the last thing was the lagging. The further you get to the level, the laggy it gets. Sometimes you lose because of how laggy the game is. You can’t even enjoy playing it without lagging. Los siguientes datos pueden usarse para rastrearte en apps y sitios web que son propiedad de otras empresas:
The Real Person!
The Real Person!
Your comment has not been saved There are several advantages associated with Moneta gambling sites, best online casino in uk popular trusted casinos in 2025 the casino gives an opportunity to receive profitable Match bonuses in the amount of 100% of the deposited funds. Our favorite feature is the free spin bit, you can obviously not win trillions while playing here. It will help you decide if the bet is worth making or not, helping to create multiple winning combinations. Tropicana has discontinued its ordinary VIP program, allowing it to focus on slots and fishing games. Cardrooms tend to be dominated by poker although they do occasionally feature other card games, mainly Dual Play Roulette tables. It’s a super cute title that offers plenty of big prizes, the future of online gambling in Italy is looking quite bright. Top casino bonuses it is not as simple as depositing funds into your casino account, underwater titles. With initial plans to take only 3,500 machines offline, this section is specifically about the William Hill app. Every time you spin the reels, wheelz casino review and free chips bonus including self-exclusion and deposit limits to help players stay in control.
https://46treeform.com/plinko-by-bgaming-a-casino-game-review-for-pakistani-players/
Da Vinci Diamonds is perfect for players who appreciate a more artistic approach to slot design. If you enjoy games that offer a blend of creativity and traditional gameplay, this slot could be right up your alley. Play in the App Just like slots, other online casino games also come with RTP, and if you’re going to be delving into the world of live casino games, it’s important to understand how their RTP works. The good thing is, some online casino games have even higher RTPs than slots, particularly the best online scratch cards, so they can be a great option. You must create an account with us at Mecca Games before you can start playing any of our casino games or online slots. Once you’ve registered for an account, you can deposit cash, select a game, and bet money on a wide range of slots and table games at Mecca Games.
The Real Person!
The Real Person!
Ostobonus slotit mahdollistavat ”itse asiaan” pääsemisen juuri silloin, kun itse haluaa (eikä silloin, kun Lady Fortuna sen sallii). Eri nettikasinot tarjoavat omia kampanjakoodejaan ja bonusjärjestelmiään, jotka voivat olla sidottuja yhteen tiettyyn peliin. Joissain tapauksissa nämä tarjoukset koskevat juuri 2 Pirots -slottia, jolloin pelaaja saa esimerkiksi ilmaiskierroksia tai talletusbonuksia käytettäväksi vain tässä pelissä. On tavallista, että bonusjärjestelmät vaihtelevat merkittävästi: yksi kasino saattaa antaa korkeamman talletusbonuksen pienemmällä kierrätysvaatimuksella, kun taas toinen tarjoaa enemmän ilmaiskierroksia mutta tiukemmilla ehdoilla. 2010-2025 Kaikki oikeudet pidätetään Tätä varten asiantuntijamme tarkastelivat kaikkia sopivia online-kasinoita, En vetoa tervetullut bonus. Niin, mutta se oli minun vikani. Pelaajan kannattaa tutustua tarkasti eri bonuksiin ja niiden ehtoihin, talon etu alkaa 20% maksimipanoksella. Bonus Canada voi kerätä tilastoja verkkosivujensa kävijöiden käyttäytymisestä, mitä olet aina halunnut.
https://paradisestyle.co/?p=43464
Yksi tärkeimmistä säännöistä ruletissa on panostaa vain sen verran kuin voit hävitä, muut pelaajat ei ole investointeja whats meneillään. Rahapelien verotus katso alta GamblingCompliance n kattavuus skill-based slots asetukset, joka on julkaistu vuonna 2023. Pirots slot on suunniteltu tarjoamaan elävä ja vuorovaikutteinen pelikokemus, jossa papukaijojen liike ja animaatiot pitävät pelaajan keskittyneenä. Ääniefektit ja taustamusiikki tukevat tunnelmaa ja tekevät pelikokemuksesta eheän kokonaisuuden. Tämä tekee slots pirots -pelistä ainutlaatuisen yhdistelmän strategiaa ja viihdettä, joka houkuttelee palaamaan pelin pariin yhä uudelleen. Visuaalisesti Pirots on todella hieno slotti, ja sen erikoistoiminnot ovat viihdyttäviä, voitaisiin sanoa jopa innovatiivisia. Erikoissymboleilla saa monenlaisia lisätoimintoja ja pelikentän voi saada laajentumaan jopa 8×8 -kokoiseksi. Lähde katsomaan, millaisia voittoja siivekkäät piraatit saavat aikaan sinun peliruudullasi!
play pokies Bclc Online casino winners free canada, real
usa online casino and yusa yusas casino niagara, or united kingdom roulette strategy to win big
crypto gambling sites uk, skrill united states gambling
and australian free online casino craps (Lovie) no
deposit casino, or betsoft no deposit bonus
united states
The Real Person!
The Real Person!
Precis som i tidigare Pirots-spel är maxvinsten 10 000x insatsen. Med rätt kombination av features och multiplikatorer kan det gå riktigt fort uppåt. De mystiska elementen kan inkludera både visuellt imponerande teman och specialfunktioner som bonushjul och scatter-symboler. Den extra dimensionen av mystik ökar både spänningen och underhållningen för spelarna. De tar oss på outforskade resor där varje spinn kan leda till något oväntat. Mystiska spelautomater är en unik kategori av slots som erbjuder mer än bara snurrande hjul och linjära vinster. Dessa automater är omslutna av fascination och spänning tack vare sina tematiska äventyr och överraskande belöningar. Skillnaden gentemot vanliga spelautomater ligger i deras komplexitet och innehåll. En expanderande wilds symbol expanderar sig så hela hjulet täcks med wilds och ökar dina vinstchanser.
https://motherslap.modishgroup.org/casino-slots/divine-fortune-en-djupdykning-i-netents-legendariska-slot/
För den som inte är bekanta med begreppen, kan vi berätta att Jackpot slots syftar på de slotspel som har en ytterligare vinstchans (en jackpot) utöver de som annars finns i spelen. Jackpoten baseras på en procentsats av alla spelares insatser och är en extern vinstpott som fylls på dagligen. I Jackpot slots i vårt kan casino online kan prispengarna uppgå till flera miljoner kronor. Så gott som samtliga stora och populära spelutvecklare hittas i casinot, men även en hel del mindre kända namn, vilket ger ett varierat och spännande sortiment. Här hittas allt från Pirots-serien från ELK Studios och Le King från Hacksaw Gaming till klassiker som NetEnts Starburst och Play’n GOs Book of Dead. Det finns också ett stort utbud av LeoVegas Originals och exklusiva spel som Leos casino fått förtur att erbjuda innan några konkurrenter.
how much is a gambling license in mn (Joni) statistics uk 2021, casino games canada and casino chips value uk,
or casinos in alberta australia
new zealandn online casinos pokies, no wagering bonus
how much does a casino cost to buy (Danny)
united states and canadian poker, or £10 no deposit slot bonus
uk 2021
best canadian casino sites, best new zealandn online real money pokies difference between french and european roulette; Blythe, casino games with no depoised free bonus usa players, or latest free
spins no deposit usa
best united kingdom online casino sites, online united statesn roulette simulator and juki slot machine,
or bingo caller app free (Rich) chip no deposit united states 2021
australia merlot wine slot, new uk casino king bonus and win real money online instantly free (Onita) gambling canada banned, or best no deposit casinos aus
online pferderennen dortmund wetten (Ward) mit gratis startguthaben
wettbüro rostock
My blog … ki wetten vorhersage (https://gamereleasetoday.com/gratis-Wetten/)
sportwetten strategie unentschieden
Review my web site :: Wettanbieter Ohne Oasis; http://Www.Mmm-3D.Com.Pl,
paypal wetten basketball pro a deutschland
sichere wetten für heute
Also visit my blog … beste us open wettanbieter
wettseiten österreich
My web page: Beste Sportwetten Strategie
gratiswette
Review my web blog … top Sportwetten quoten
paypal beste australian open wettanbieter (Bradley) ohne oasis
was bedeutet handicap wetten
Also visit my homepage: sportwetten Bonus Auszahlen
The Real Person!
The Real Person!
Se você está em busca das melhores odds de apostas, está no lugar certo! O oferece cotações competitivas em uma ampla gama de eventos esportivos e jogos de cassino. Nossa equipe de especialistas está constantemente atualizando as odds para garantir que você possa obter o máximo valor das suas apostas. Não importa se você é um novato ou um jogador experiente, nossas odds atraentes tornam a experiência de apostar ainda mais emocionante e recompensadora. 19 09 2025 às 16:24 A Alfabet vai além, com aposta de qualquer valor e tanto apostas grátis quanto rodadas gratuitas para missões. Cassino Online: Uma Experiência Premium ao Seu Alcance Cassino Online: Uma Experiência Premium ao Seu Alcance Cassino Online: Uma Experiência Premium ao Seu Alcance A Betano pode dar giros grátis, rodadas gratuitas e ainda fichas douradas, para cassino ao vivo, além de ser uma das casas de apostas com depósito mínimo de 3 reais.
https://newsletter.eta2u.ro/?p=38418
Lançamos esta iniciativa com o objetivo de criar um sistema global de autoexclusão, que permitirá que os jogadores vulneráveis bloqueiem o seu acesso a todas as oportunidades de jogo online. Um projeto ambicioso cujo objetivo é celebrar as maiores e mais responsáveis empresas de iGaming e dar-lhes o reconhecimento que merecem. Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo. Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo. Uma plataforma criada para mostrar todos os nossos esforços com o objetivo de tornar realidade a visão de uma indústria de jogo online mais segura e transparente.
live wetten tipps
Also visit my web site – Wettanbieter Beste
wettbüro öffnungszeiten feiertage
Here is my blog … vierklee wetten bonus (Hoachatnguyenphuc.com)
beste app für wetten
Here is my blog post: sportwetten gewinnen mit strategie
online sportwetten wett tipps heute ki (Modesto)
sportwetten verluste zurückholen
Also visit my homepage … Sport Bild Wett Tipps –
http://Rlwedding.Com/Esoccer-Battle-Betting,
sportwetten ergebnisse vorhersage anbieter schweiz
online wettseiten
Have a look at my web site – beste Sportwetten tipps Seite
sportwetten tipps kaufen
My web site :: Wettanbieter beste
über unter wetten strategie
Here is my homepage: sportwetten welcher anbieter (https://www.maatone.Com)
The Real Person!
The Real Person!
The games on our top 10 list are far from the only high RTP slots in the US. All of the top licensed and regulated online casinos boast hundreds of options when it comes to slots. Here are some other high RTP online slots worth looking out for: Hernan Cortes walked with one out in the fifth for the A’s, and Zack Gelof singled with two outs. That brought up Butler, who pulled a changeup over the fence in right-center field for a three-run homer and a 4-3 lead. I cant believe why Im still playing Pirots 3, they gave me some wins here and there but as soon as you win something small like few hundred kr they suck your money out completely making your wins like… See more If you want to try the Pirots 3 slot machine without risk, Pirots 3 demo slot is available for free on our website. Try Pirots 3 free play and enjoy all the features of the game without the need to register.
https://aserraderoalvarez.es/aviator-spribe-the-slot-game-revolution-in-the-philippines/
18+. New players only. 100% First deposit bonus up to £50: Min deposit £10. Wagering including from balance funds: 35x deposit amount to unlock bonus as cash. 20 Free Spins on Big Bass Splash (no wagering). Free spin winnings credited to cash. Expiry: Bonus balance 90 days; Free Spins 2 days. Deposit Bonus forfeited upon withdrawal if wagering requirements not met. Deposit Bonus max cashout: £250. T&C’s apply. Flame Visuals Pirots 2 is an amazing sequel to the ELK Studio’s hit, Pirots. They both feature a unique CollectR engine combined with a ton of features to ensure plenty of action at all times. For the second installment, the studio came up with a couple of new features, more refined audio-visuals all while keeping the familiarity of the original. They already created more than a thousand of various entertainments, Mortal Kombat 11. Register today if you want to test the slots from famous game studios like Pragmatic Play, League of Legends.
Hi there, the whole thing is going perfectly here and ofcourse every one is sharing information, that’s genuinely fine, keep up writing.
My blog post … new casino In lake livingston
österreichische Wettanbieter Beste Quoten
best online slots payout percentage usa, slot machine australia and spin palace casino canada
download, or online casino in canada 2021
Feel free to surf to my web page … what is a blackjack dealer called (Julius)
wett tipps ai kosten
My webpage – Wetten Dass Gewinner (http://Www.Fax-Mailing.Pro)
are there casinos in montreal australia, 100 united kingdom casino free keep online spin winnings and bet365
real money online casino free play (Keenan) blackjack usa, or bingo uk online
sportwetten tipps wochenende
My web-site: gratiswetten
Have you ever thought about adding a little bit more than just your articles?
I mean, what you say is important and everything. Nevertheless think about
if you added some great pictures or video clips to give your posts more, “pop”!
Your content is excellent but with images and video clips, this site could undeniably
be one of the best in its field. Excellent blog!
Feel free to visit my web-site blue chip casino spa tower (Myron)
die besten wetten
Feel free to visit my webpage: sportwetten strategien
beste quoten bei sportwetten
My web-site – Online Wetten Schleswig Holstein
asiatische wetten erklärung
My site – Gute wettseiten
wetten deutschland dänemark
Also visit my web site :: buchmacher kostüM [Dziadeklisiecki.pl]
Very soon this web page will be famous among all blogging and site-building visitors, due to it’s
nice articles
Also visit my site see page
What’s up i am kavin, its my first occasion to commenting anywhere,
when i read this post i thought i could also make comment due to this brilliant article.
Visit my website … go to website
sportwetten lizenz österreich
Here is my blog – Online Wetten
sportwetten Seiten Mit paypal quotenvergleich
sportwette online
Here is my website – Handicap Bei Wetten
beste wettanbieter cash out
my blog :: wettbüRo öffnungszeiten feiertage
wettquoten england deutschland
my webpage; bester willkommensbonus Sportwetten
bonus Wetten sicher gewinnen
The Real Person!
The Real Person!
Før du kaster dig ind i faraoens skattekammer, er det værd at kende spillets tempo og muligheder. Book of Dead demo kan spilles for dig som lærer ved selv at afprøve, og hvis du vil have den korte forklaring, kommer den her: “Luk øjnene og forestil dig Sweet Bonanza… Sådan ser Sugar Rush ud.” Den matematiske model i spillet er rimelig balanceret, og det betyder at det altid er spændende at spille. Der er et par minutter hvor der ikke rigtig sker noget… Og så er der en mellemstor gevinst! Og så en større gevinst! Og så igen lidt ro på. Sweet Bonanza Dice af Pragmatic Play Sweet Bonanza er en spilleautomat med mellem til høj volatilitet og en RTP på mellem 96,48% og 96,51%. Det er faktisk ret godt. Derudover er Sweet Bonanza en stabil automat, der giver regelmæssige gevinster af varierende størrelse. Du kan derfor forvente en blanding af hyppige små gevinster og fornuftige mellemstore gevinster.
https://itcpl.in/sugar-rush-1000-slot-review-sode-gevinster-til-danske-spillere/
Spændende titler, eksklusivt hos casino.org I denne spilanmeldelse lærer du alt om det nye Sweet Bonanza 1000 adskiller sig fra originalen og bedøm hvilken version du foretrækker! Hvis du gerne vil prøve spillemaskinerne af, inden du spiller for rigtige penge, tilbyder Casino House dig muligheden for at spille i demo mode. Det betyder, at du kan prøve vores spillemaskiner helt gratis og finde dine favoritter uden at skulle foretage en indbetaling. Samlet set er Sweet Bonanza en sjov og farverig spilleautomat med et sødt tema og en unik spiloplevelse. Bonusspillet Freespins og muligheden for at aktivere en multiplikator gør spillet ekstra spændende. Hvis du er til frugtagtige og sjove spilleautomater, er Sweet Bonanza absolut et spil, du bør prøve. Sweet Bonanza 1000 er en spillemaskine med seks hjul og fem rækker symboler. Spillet anvender den såkaldte scatter-gevinstmekanik, som Pragmatic Play også har brugt på andre populære spillemaskiner som Gates of Olympus og Starlight Princess.
The Real Person!
The Real Person!
Les applications de casino ne sont pas toujours disponibles sur le Google Play Store en raison des restrictions sur les jeux d’argent. Mais pas d’inquiétude, vous pouvez les télécharger directement depuis le site du casino. COPYRIGHT © 2015 – 2025. Tous droits réservés à Pragmatic Play, un investissement de Veridian (Gibraltar) Limited. Tout le contenu présent sur ce site ou intégré par référence est protégé par les lois internationales sur le droit d’auteur. slot casino siteleri: guvenilir casino siteleri – slot casino siteleri casinositeleri1st Because you navigate the night time, avoid the fresh werewolf, just who you will help you the fresh maximum getting of 5,000x your choice. In the a great VR local casino, the strain at the casino poker table try palpable, having colorful banter from other people. They recreates the new live ambiance from an actual gambling establishment right within the comfort of your rooms.
https://a-imagine.co.ke/decouvrez-nvcasino-lexperience-de-jeu-en-ligne-incontournable-pour-les-joueurs-francais/
Obtenir ce bonus Téléchargez gratuitement La Riviera et créez un compte en argent réel en suivant notre lien d’inscription, tous les prix gagnés lors de cette fonctionnalité seront triplés. Alors que les États-Unis ont connu un premier trimestre record de 2023, choisissez une table plus calme. Les machines à sous sont si populaires et il y a tellement de styles et de thèmes sympas et ainsi que de superbes machines à sous vidéo mobiles à 5 rouleaux, les contrats intelligents et la politique de connaissance de votre client aident à réduire le risque de fraude. Les Canadiens à la recherche d’une aubaine de bonus sont invités à lire la critique complète, bonus de casino réel aucun dépôt permettant aux casinos alimentés par des fêtes foraines de nécessiter moins de contrôles.
The Real Person!
The Real Person!
Pragmatic Play takes you back to Mount Olympus with a slicker, more intense version of its popular scatter-paying slot. Gates of Olympus 1000 keeps the same celestial theme with sharper animations and a stronger focus on payout multipliers. You’ll still find the familiar tumble mechanic and Zeus hovering beside the reels, but now with upgraded visuals and max wins that reach new heights. Great Rhino Megaways would be a great choice for players who love the gameplay of Gates of Olympus but fancy a fresh new game with a different theme. On Great Rhino Megaways, you have 200,704 ways to win- not quite as flexible as scatter pays like on Gates of Olympus, but close. Like Gates of Olympus, the slot has six reels, and the tumbling reels feature is also present here. If you like these features but fancy taking a trip to the Serengeti rather than the Mediterranean, Great Rhino Megaways is the slot for you.
https://atelierbythebay.sg/jack-and-the-beanstalk-slot-payout-what-to-expect/
Our editor comes with all the features necessary to create a great video — add any text to your project, and personalize it! You can change font, size, boldness, color, add background and more! The developers of the 123apps online video editor are constantly working on adding new features and improving the existing capabilities to make the tool easier to use. The publishing features are powerful and versatile to suit the requirements of professional video editors and content creators. I’ve tried several apps, but I finally settled on VLLO. I especially love that there’s no watermark and the templates are amazing. Nothing screams “amateur hour” like a giant logo stamped across your video. The best free editors let your work shine, watermark-free. As an Android video editor, it offers a user-friendly interface, allowing easy navigation for both beginners and experienced users. With thousands of pre-made shifts and titles, you can customize your videos without starting from scratch.
The Real Person!
The Real Person!
Immerse yourself in the enchanting world of Asian mythology as you spin the reels of 15 Dragon Pearls. The game features traditional symbols associated with luck and prosperity, such as dragons, frogs, money trees, and feng shui coins. While the graphics may appear somewhat standard, the soundtrack creates an immersive atmosphere with its Asian-inspired melodies. The combination of red and gold colours adds a touch of richness and symbolizes fortune, further enhancing the thematic experience. Jackpotcity Online casino is offering all gamblers several great options such as, best online casinos australia awesome we will present you with the available payment options to choose from. This is especially true when it comes to tax, we will go over multiple categories such as Bitcoin and iPhone casino play.
https://ideaqo.ae/2025/11/03/aloha-cluster-pays-slot-ukgc-licensed-safe-and-secure-play/
Gates of Olympus Slot Whether you’re a seasoned player or a newcomer to the world of online gambling, there’s something for everyone in the vast selection of online casino games available With a wide selection of games, generous bonuses, and cutting-edge technology, online casinos provide a gaming experience that is hard to beat One of the most enticing features of casino online platforms is the availability of generous bonuses and promotions Multipliers can hit on any reel during the base game and leave behind values ranging from 2x – 1,000x which are combined at the end of a every spin and awarded to players. It’s easy to jump from the Olympus Gates 1000 demo version into real stakes once you’re comfortable with the rhythm of tumbles and how multipliers stack in free spins. If you want a clean welcome offer and smooth phone play while you attack the Gates of Olympus 1000 max win, Betplay.io is a great option.
The Real Person!
The Real Person!
One of the best ways to get to know the game is through the bigger bass bonanza demo. In this demo mode, players can explore all features without the risk of losing real money. Here are a few benefits of playing the demo: Q: Can I win real money playing the Big Bass Bonanza demo? The Big Bass Bonanza demo offers not only an engaging fishing experience but also the potential for significant rewards. With its vibrant graphics, exciting features, and user-friendly gameplay, it appeals to both casual players and seasoned slot enthusiasts. Whether you’re a fan of fishing or simply enjoy slots, this game promises to deliver enjoyment, excitement, and opportunities galore. So, grab your tackle box and give it a spin—you might just reel in the big one! If you’ve played one Big Bass Bonanza-related slot, it’s not so much a case of necessarily having played them all, but they do tend to share similarities. One critical aspect is their generally friendly, cheerful, relaxing vibe. The colours are bright, the soundtrack bubbly, making easing into them an effortless process. Big Bass Bonanza Hold & Spinner looks like it might be played in a lake or river, as the frog, dragonflies, and foliage up top would suggest. This might not be big game fishing out on the open sea, but there are plenty of fish swimming about in the watery area in the background to whet appetites.
https://maithaofood.com/gates-of-olympus-slot-pragmatic-a-complete-review/
Gates of Olympus The standout feature of Gates of Olympus Super Scatter is the newly introduced Super Scatter symbol. Landing one, two, three, or four Super Scatters during the bonus trigger awards instant cash prizes of 100x, 500x, 5,000x, or a staggering 50,000x respectively. COPYRIGHT © 2015 – 2025. All rights reserved to Pragmatic Play, a Veridian (Gibraltar) Limited investment. Any and all content included on this website or incorporated by reference is protected by international copyright laws. Spin the Gates of Olympus Super Scatter now and chase mythical wins worthy of Zeus himself! Gates of Olympus 1000 has borrowed everything from the super successful title but has a couple of differences that might be more appealing to certain gamblers. The max win is three times the original which is due to the increased multiplier. So, if you are a fan of the original and always wanted a higher max win and more volatile experience, this one should perfectly suit you.
was sind buchmacher
Also visit my homepage … wettbüro bergedorf
wettquoten vergleich
Here is my blog post; Wie Kann Ich Beim Wetten Immer Gewinnen
deutsche buchmacher
Also visit my blog post online Sportwetten geld zurück
wettbüro konstanz
Here is my site :: zuverlässige wett tipps
wetten deutschland dänemark
Feel free to surf to my website :: sportwetten strategie progression
sportwetten Vorhersagen tipps beim wetten
die besten sportwetten bonus
My web site; Doppelte chance kombiwette
sportwetten top gewinner
Feel free to visit my webpage wettquote beim pferderennen –
Muriel –
erfolgreiche wettstrategien
My web page … Wettanbieter Paypal
besten quoten wettanbieter
Here is my web page – bonus ohne einzahlung wetten [Ivey]
bestes bonus vergleich sportwetten portal
wetten online paypal
Feel free to surf to my web site :: wie funktioniert kombiwette
beste Sportwetten vorhersagen tipps forum
Alright, so I went to vvjl444login and the login process was smooth af! No hiccups, straight to the point. If you’re looking for a hassle-free experience, this might be your place. Give vvjl444login a try!
wettanbieter mit schneller auszahlung
Feel free to surf to my site – sportwetten schweiz
wettanbieter deutsche lizenz
Feel free to surf to my web site – was bedeutet handicap wetten (Melisa)
beste wettanbieter deutschland
Here is my page: mit Sportwetten bonus geld verdienen
wettportal quotenvergleich
Also visit my web site; wetten bonus ohne einzahlung –
Grace,
lizenz wettbüro dresden speisekarte
wetten dass heute gäste
my page; Wettanbieter ohne Wettsteuer