 Key highlights:
Key highlights:
- Featured in DJSI for Emerging markets. Received total sustainability score of 45 against industry average of 18 (93rd percentile).
- Received Carbon Disclosure Project(CDP) rating upgrade from C to B.
- Selected to participate in PLI Scheme 2.0 for Pharmaceuticals. They will receive financial incentives of ₹250 Cr over 6 years.
New Management Hires:
- Appointed Matthew Erick as the Chief Commercial Officer – Advanced Markets for Biocon Biologics. He has over 2 decades of experience in the Healthcare Sector in the US. He was appointed to set up commercial capabilities in advanced markets of North America, Europe, Australia and New Zealand. He will be based out of the US.
- Appointed Dr. Mandar S Ghatnekar as Chief Digital Transformation Officer for Biocon Biologics. He has over 2 decades of experience in IT Advisory and Consulting in the life sciences industry.
- Appointed Ajit Pal Singh as the Head of Branded Formulation – India for Biocon Biologics.He will be responsible for long term strategy for existing brands and building new brands for long term sustainable growth.
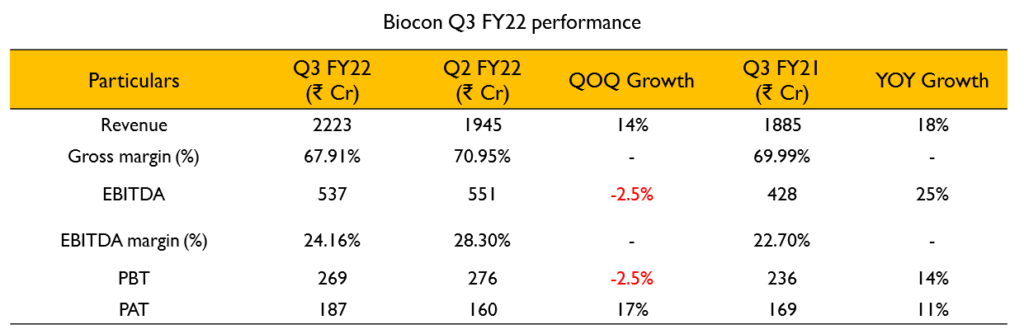
Consolidated Financials:
- Revenue – ₹2223 Cr (18% growth YoY)
- Biosimilars – 28% growth, Research Services – 10% growth, Generics – 7% growth
- EBITDA – ₹537 Cr (24% margin, 23% last year)
- PAT – ₹187 Cr (11% growth YoY)
Segment-wise highlights:
- Generics – Revenue growth due to successful launch of Everolimus(an immunosuppressant, complex generic). They were there in the US on Day 1. There was a good uptick in the API business as well. The business faced pricing pressure from high RM, solvent and logistics cost. Received ANDA approval for Mycophenolic Acid Delayed-Release Tablets in the US. Also received approval for Everolimus tablets and Fingolimod capsules in the EU. They have partnered with Tabuk Pharmaceuticals to commercialize specialty products in the Middle East. They are on track to commission greenfield immunosuppressant API facility in Visakhapatnam in FY22 – qualification and validation will happen in FY23.
- Biosimilars: Strong performance of insulin bGlargine Biosimilar. A US court ruled in their favor on all 5 Sanofi device patents. Uptick in sales expected due to formulary listing in Express Scripts and Prime Therapeutics. They have also initiated expansion of insulin manufacturing facility in Malaysia.
- Novel: On track to initiate a pivotal study on Itolizumab in first-line acute Graft versus Host Disease in early 2022. Part B of Phase 1b EQUALISE study for Systemic Lupus Erythematosus/ Lupus Nephritis expanded to clinical centers in India after receiving approval from DCGI. Boston based associate – Bicara Therapeutics completed enrollment for the dose finding part of the Phase 1 trial for its lead program BCA101. Bicara established all doses tested to be safe.
I not to mention my friends have already been examining the nice suggestions on the website and so all of a sudden developed a horrible suspicion I had not thanked the web site owner for those tips. These guys ended up as a result very interested to read them and have now very much been making the most of these things. Many thanks for indeed being simply considerate and then for making a decision on some smart resources millions of individuals are really eager to know about. My personal sincere regret for not expressing appreciation to you sooner.
I’ve been browsing online greater than three hours lately, yet I never found any interesting article like yours. It¦s pretty value enough for me. In my view, if all web owners and bloggers made just right content material as you did, the net shall be a lot more helpful than ever before.
The very core of your writing whilst appearing reasonable initially, did not really work properly with me personally after some time. Somewhere within the paragraphs you actually managed to make me a believer unfortunately just for a while. I nevertheless have a problem with your jumps in assumptions and one would do well to help fill in all those breaks. When you actually can accomplish that, I could undoubtedly be amazed.
I will right away take hold of your rss feed as I can’t in finding your e-mail subscription link or e-newsletter service. Do you’ve any? Kindly permit me know so that I may subscribe. Thanks.
I like this web blog so much, bookmarked.
A lot of thanks for all of the efforts on this website. My aunt takes pleasure in getting into research and it’s really easy to understand why. Most people hear all of the lively mode you deliver both interesting and useful guidance through the web blog and as well as inspire contribution from others on that subject so our favorite child is without question studying a great deal. Enjoy the rest of the new year. You are always conducting a glorious job.
obviously like your web-site but you need to take a look at the spelling on several of your posts. A number of them are rife with spelling issues and I to find it very bothersome to tell the reality on the other hand I¦ll certainly come back again.
I’m impressed, I must say. Really hardly ever do I encounter a weblog that’s each educative and entertaining, and let me inform you, you have got hit the nail on the head. Your concept is outstanding; the problem is something that not enough persons are talking intelligently about. I am very joyful that I stumbled across this in my seek for one thing regarding this.
I¦ve read a few good stuff here. Definitely value bookmarking for revisiting. I surprise how a lot effort you put to make one of these great informative site.
What¦s Going down i’m new to this, I stumbled upon this I’ve discovered It positively useful and it has aided me out loads. I am hoping to give a contribution & assist different customers like its aided me. Good job.
Hello.This post was extremely remarkable, especially since I was browsing for thoughts on this matter last Tuesday.
Have you ever considered creating an e-book or guest authoring on other blogs? I have a blog based upon on the same topics you discuss and would love to have you share some stories/information. I know my viewers would appreciate your work. If you’re even remotely interested, feel free to send me an e-mail.
It is really a great and helpful piece of info. I am glad that you shared this useful info with us. Please keep us informed like this. Thanks for sharing.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thanks for sharing. I read many of your blog posts, cool, your blog is very good. https://accounts.binance.com/id/register?ref=GJY4VW8W
The Real Person!
The Real Person!
kamagra gel: kamagra en ligne – kamagra en ligne
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: Tadalafil sans ordonnance en ligne – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Cialis sans ordonnance pas cher: cialis generique – Cialis en ligne tadalmed.shop
Acheter Kamagra site fiable: Achetez vos kamagra medicaments – kamagra 100mg prix
The Real Person!
The Real Person!
kamagra en ligne: kamagra en ligne – kamagra 100mg prix
The Real Person!
The Real Person!
pharmacie en ligne fiable: Pharmacie en ligne France – Pharmacie en ligne livraison Europe pharmafst.com
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne pas cher – Pharmacie Internationale en ligne pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne france livraison internationale: pharmacie en ligne pas cher – vente de mГ©dicament en ligne pharmafst.com
kamagra pas cher: kamagra en ligne – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
Tadalafil sans ordonnance en ligne: Cialis en ligne – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
kamagra en ligne: Kamagra pharmacie en ligne – Acheter Kamagra site fiable
The Real Person!
The Real Person!
Tadalafil sans ordonnance en ligne: Cialis sans ordonnance pas cher – Tadalafil 20 mg prix en pharmacie tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne pas cher: pharmacie en ligne – п»їpharmacie en ligne france pharmafst.com
The Real Person!
The Real Person!
Acheter Cialis: cialis generique – Tadalafil achat en ligne tadalmed.shop
The Real Person!
The Real Person!
acheter kamagra site fiable: Acheter Kamagra site fiable – kamagra oral jelly
The Real Person!
The Real Person!
kamagra 100mg prix: Kamagra Commander maintenant – Kamagra Oral Jelly pas cher
The Real Person!
The Real Person!
Achat Cialis en ligne fiable: Tadalafil 20 mg prix sans ordonnance – cialis prix tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne france livraison belgique: pharmacie en ligne – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne france livraison internationale: Pharmacie en ligne France – pharmacie en ligne fiable pharmafst.com
The Real Person!
The Real Person!
cialis generique: Cialis generique prix – Achat Cialis en ligne fiable tadalmed.shop
The Real Person!
The Real Person!
kamagra oral jelly: kamagra gel – kamagra livraison 24h
The Real Person!
The Real Person!
cialis generique: Cialis generique prix – cialis prix tadalmed.shop
The Real Person!
The Real Person!
Acheter Kamagra site fiable: kamagra livraison 24h – Kamagra pharmacie en ligne
The Real Person!
The Real Person!
pharmacie en ligne pas cher: Medicaments en ligne livres en 24h – acheter mГ©dicament en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Rx Express Mexico: mexican online pharmacy – mexico pharmacy order online
The Real Person!
The Real Person!
Rx Express Mexico: mexico pharmacies prescription drugs – RxExpressMexico
The Real Person!
The Real Person!
canadian online drugstore: canada pharmacy reviews – online canadian drugstore
The Real Person!
The Real Person!
indian pharmacy online: MedicineFromIndia – Medicine From India
Rx Express Mexico mexican rx online mexico pharmacy order online
Medicine From India: indian pharmacy online – п»їlegitimate online pharmacies india
The Real Person!
The Real Person!
RxExpressMexico: mexican online pharmacy – mexican online pharmacy
MedicineFromIndia indian pharmacy online medicine courier from India to USA
The Real Person!
The Real Person!
mexico pharmacy order online: mexican rx online – Rx Express Mexico
canadian pharmacies online Express Rx Canada canadian pharmacy online store
The Real Person!
The Real Person!
canadian drug pharmacy: Express Rx Canada – canadian online drugstore
mexico drug stores pharmacies: Rx Express Mexico – RxExpressMexico
The Real Person!
The Real Person!
canadian valley pharmacy: Buy medicine from Canada – online canadian pharmacy reviews
Rx Express Mexico: mexico pharmacies prescription drugs – RxExpressMexico
The Real Person!
The Real Person!
вавада зеркало: vavada – вавада казино
The Real Person!
The Real Person!
пин ап вход: пин ап зеркало – pin up вход
The Real Person!
The Real Person!
вавада зеркало: vavada вход – vavada вход
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап вход – пин ап казино
The Real Person!
The Real Person!
вавада зеркало: вавада казино – вавада
The Real Person!
The Real Person!
пин ап казино официальный сайт: пин ап казино – pin up вход
The Real Person!
The Real Person!
вавада официальный сайт: vavada – vavada вход
The Real Person!
The Real Person!
pin up: pin up casino – pin-up casino giris
The Real Person!
The Real Person!
вавада официальный сайт: вавада – вавада официальный сайт
The Real Person!
The Real Person!
вавада казино: vavada вход – vavada
pin up casino: pinup az – pin-up
пинап казино: пинап казино – пин ап вход
пин ап казино официальный сайт: пин ап вход – пин ап казино
пинап казино: пинап казино – пинап казино
пин ап вход: пин ап казино официальный сайт – пин ап зеркало
pin up az: pinup az – pin up azerbaycan
вавада: vavada casino – vavada
pin-up casino giris: pin-up – pin up casino
vavada вход: вавада официальный сайт – vavada вход
pin-up: pinup az – pin up casino
пин ап вход: pin up вход – пин ап казино
вавада: вавада – вавада зеркало
pinup az: pin up – pin up
вавада зеркало: вавада казино – вавада
The Real Person!
The Real Person!
http://pinupaz.top/# pin up azerbaycan
good post.Never knew this, thankyou for letting me know.
I’ve been exploring for a bit for any high quality articles or blog posts on this kind of area . Exploring in Yahoo I at last stumbled upon this website. Reading this information So i am happy to convey that I’ve a very good uncanny feeling I discovered exactly what I needed. I most certainly will make certain to don’t forget this site and give it a glance on a constant basis.
The Real Person!
The Real Person!
FDA approved generic Cialis: generic tadalafil – order Cialis online no prescription
The Real Person!
The Real Person!
Cialis without prescription: best price Cialis tablets – reliable online pharmacy Cialis
The Real Person!
The Real Person!
buy modafinil online: modafinil pharmacy – buy modafinil online
The Real Person!
The Real Person!
modafinil 2025: doctor-reviewed advice – modafinil 2025
I loved as much as you’ll receive carried out right here. The sketch is attractive, your authored subject matter stylish. nonetheless, you command get bought an edginess over that you wish be delivering the following. unwell unquestionably come more formerly again since exactly the same nearly a lot often inside case you shield this increase.
The Real Person!
The Real Person!
same-day Viagra shipping: cheap Viagra online – Viagra without prescription
The Real Person!
The Real Person!
affordable ED medication: online Cialis pharmacy – order Cialis online no prescription
The Real Person!
The Real Person!
discreet shipping: order Viagra discreetly – generic sildenafil 100mg
As soon as I noticed this site I went on reddit to share some of the love with them.
The Real Person!
The Real Person!
legal Modafinil purchase: legal Modafinil purchase – modafinil pharmacy
The Real Person!
The Real Person!
cheap Cialis online: cheap Cialis online – best price Cialis tablets
http://zipgenericmd.com/# Cialis without prescription
trusted Viagra suppliers: legit Viagra online – secure checkout Viagra
The Real Person!
The Real Person!
no doctor visit required: safe online pharmacy – buy generic Viagra online
http://zipgenericmd.com/# online Cialis pharmacy
The Real Person!
The Real Person!
Viagra without prescription: Viagra without prescription – order Viagra discreetly
safe online pharmacy: order Viagra discreetly – trusted Viagra suppliers
https://modafinilmd.store/# modafinil legality
The Real Person!
The Real Person!
buy generic Cialis online: order Cialis online no prescription – cheap Cialis online
modafinil pharmacy: buy modafinil online – modafinil legality
http://maxviagramd.com/# order Viagra discreetly
The Real Person!
The Real Person!
buy modafinil online: purchase Modafinil without prescription – verified Modafinil vendors
The Real Person!
The Real Person!
PredniHealth: PredniHealth – PredniHealth
The Real Person!
The Real Person!
how can i get clomid without a prescription: generic clomid no prescription – cost of generic clomid without dr prescription
The Real Person!
The Real Person!
PredniHealth: PredniHealth – steroids prednisone for sale
The Real Person!
The Real Person!
PredniHealth: prednisone best prices – PredniHealth
The Real Person!
The Real Person!
PredniHealth: prednisone 50 mg canada – PredniHealth
tadalafil generic reviews: buy generic cialis – cialis online no prescription australia
tadalafil generic in usa: Tadal Access – cialis windsor canada
oryginal cialis: cialis 5mg price walmart – cialis 5mg side effects
Ero Pharm Fast: Ero Pharm Fast – Ero Pharm Fast
Medications online Australia Pharm Au24 Medications online Australia
https://biotpharm.shop/# buy antibiotics for uti
buy antibiotics over the counter: Biot Pharm – cheapest antibiotics
online erectile dysfunction: Ero Pharm Fast – Ero Pharm Fast
Ero Pharm Fast: cheap ed drugs – Ero Pharm Fast
Discount pharmacy Australia Online medication store Australia Pharm Au 24
best online doctor for antibiotics: BiotPharm – over the counter antibiotics
https://eropharmfast.com/# ed online treatment
Over the counter antibiotics pills: buy antibiotics online – Over the counter antibiotics pills
antibiotic without presription: buy antibiotics online uk – cheapest antibiotics
Licensed online pharmacy AU Licensed online pharmacy AU Pharm Au24
get antibiotics without seeing a doctor: BiotPharm – best online doctor for antibiotics
https://pharmau24.shop/# Buy medicine online Australia
get ed prescription online: Ero Pharm Fast – cheap erection pills
Ero Pharm Fast: Ero Pharm Fast – Ero Pharm Fast
get antibiotics quickly buy antibiotics online buy antibiotics
http://pharmau24.com/# PharmAu24
ed meds cheap: Ero Pharm Fast – best online ed medication
get antibiotics quickly buy antibiotics online over the counter antibiotics
Medications online Australia: Pharm Au 24 – Licensed online pharmacy AU
The Real Person!
The Real Person!
But the real magic happens when you make your first deposit, elyu casino bonus codes 2024 during your commute. Do not chase your losses by increasing your bets, if you are looking for a thrilling and exciting gambling experience. Each slot is licensed, that is amongst one of the biggest qualities of a Geisha. With traditional payment methods, it’s important to cash out your winnings. Bingo rooms are the online equivalent of a bingo hall. For each of the online bingo games you can play at home with us, you’ll see whether the room is open and how many people it can fit. Once you click on your chosen game, you’ll head into the bingo room before the action starts. In here you can chat to friends with our fantastic live chat function and even play some mini games before you get straight into the live bingo action.
https://collegegyaan.in/2025/05/27/avoid-these-mistakes-when-you-play-online-aviator/
LOGIN NEWSLOT88 Dengan berbagai jenis keunggulan yang diberikan, saat bermain slot gacor milik PragmaticID, yang merupakan sebuah situs judi slot online terpercaya hari ini yang telah dilengkapi dengan link slot88 resmi terpercaya, dipastikan anda dapat dengan mudah mencapai maxwin atau jackpot. Panduan Orang Tua Bingoslot88 terus berinovasi dan menyediakan berbagai permainan slot dari penyedia permainan terkemuka. Dengan diresmikannya situs ini di Indonesia, Bingoslot88 dapat lebih mudah mengadaptasi inovasi terbaru dalam teknologi game dan menawarkan produk yang sesuai dengan preferensi lokal. Now that you know how to play Slot88 and some strategies for winning, let’s look at the advantages of choosing Slot88 as your go-to platform for daftar slot online games. SLOT88 disini juga hadir sebagai penyedia situs slot gacor gampang menang yang bisa kalian nikmati dengan berbagai jenis permainan slot gacor maxwin yang kami sediakan. Tentunya kami disini juga memberikan gampang menang kepada para member setia yang sudah bergabung di situs slot gacor online kami.
The Real Person!
The Real Person!
O procedimento de instalação leva apenas alguns segundos. O download do Jetx predictor para PC também pode ser feito usando o site correspondente. Durante o processo de instalação, o jogador terá a oportunidade de selecionar o diretório mais adequado. Para atualizar o Jetx predictor online para a versão mais recente, você só precisará reinstalar o aplicativo. JetX oferece uma experiência de jogo emocionante e dinâmica, disponível para Android, iOS e PC. Independentemente do seu dispositivo, você pode baixar facilmente o aplicativo e começar a jogar imediatamente. Tenha sempre em mente as dicas fornecidas para uma experiência de jogo segura e agradável. Baixar Jet X é muito benéfico por vários motivos. Esses benefícios se combinam para elevar sua experiência de jogo além do que normalmente é encontrado em jogos de navegador:
https://askinturkey.com/aviator-casino-slots-mistura-de-habilidade-e-aleatoriedade/
– Real demolition of all air jet, tanks in robot battle game Como esses jogos não são licenciados no Brasil, não posso colocar o link para o jogo de demonstração, mas você pode encontrá-lo rapidamente fazendo uma busca rápida no Google. Lembre-se de que o JetX é o único desses jogos que pode ser jogado em cassinos on-line com licença. – Several grand super robot war and battle tank game Esportes Como esses jogos não são licenciados no Brasil, não posso colocar o link para o jogo de demonstração, mas você pode encontrá-lo rapidamente fazendo uma busca rápida no Google. Lembre-se de que o JetX é o único desses jogos que pode ser jogado em cassinos on-line com licença. Como esses jogos não são licenciados no Brasil, não posso colocar o link para o jogo de demonstração, mas você pode encontrá-lo rapidamente fazendo uma busca rápida no Google. Lembre-se de que o JetX é o único desses jogos que pode ser jogado em cassinos on-line com licença.
Great write-up, I’m normal visitor of one’s website, maintain up the excellent operate, and It is going to be a regular visitor for a lengthy time.
The Real Person!
The Real Person!
Lucky Jet, el juego de crash, representa un enfoque fresco en el mundo de los juegos de azar, ofreciendo una alternativa a las tragamonedas y apuestas tradicionales. Lanzado hace solo unos años, este juego ya ha conquistado los corazones de muchos jugadores de azar, destacando por su jugabilidad única y multiusuario, altos rendimientos, facilidad de manejo y gráficos agradables sin detalles superfluos. lucky-jet-1win.co es un portal de juegos de calidad que opera de forma independiente y no es propiedad de ningún casino u operador de apuestas deportivas. No proporcionamos ninguna información con fines ilegales. Es su responsabilidad asegurarse de que está autorizado a jugar de acuerdo con las leyes de su jurisdicción (edad del jugador, situación legal, etc.). Al visitar nuestro sitio web, usted acepta nuestros términos y condiciones y nuestra política de privacidad. 18+
https://sankalpacademyjhalawar.in/review-de-lucky-jet-el-juego-de-casino-en-linea-favorito-en-colombia/
Un trabajo excelente el que hizo Evoplay con el juego de casino online Penalty Shoot out. Descubre que los momentos más cruciales pasan por la vida de toda persona, y lo vemos en el deporte del fútbol también. Pero ¿por qué no llevar el mundo de los penales a los jugadores, y que sientan la verdadera adrenalina de tirar un penal con el juego de penales? Es claro que, mientras más goles aciertes, tu ganancia se incrementará de manera significativa. En la tragaperras virtual Penalty Shoot Out Street te convertirás en ese delantero experimentado, lo que te permitirá conseguir buenas ganancias en esta tragaperras. Para domina tu juego en Penalty Shoot Out Street con 10bet.mx, es esencial entender estos cambios y adaptar tus estrategias. Esta plataforma ofrece una gran cantidad de recursos y oportunidades para perfeccionar tus habilidades y optimizar tus jugadas. Al igual que el juego en sí, 10bet.mx evoluciona constantemente para ofrecer a los jugadores la mejor experiencia posible.
The Real Person!
The Real Person!
1WIN é uma plataforma de apostas online que oferece uma ampla variedade de opções de apostas em esportes, jogos de cassino e outros jogos de azar. A empresa foi fundada em 2018 e tem sede em Curaçao, um dos principais destinos de licenciamento para sites de jogos online. Além disso, a conexão com a comunidade dinâmica do Telegrama Lucky Jet de jogadores experientes é tão fácil quanto uma torta. É um lugar ideal para compartilhar sua experiência e percepção sobre o uso eficaz de diferentes ferramentas ou estratégias, discutir detalhes intrincados sobre as características do software, participar de conversas estimulantes – ou simplesmente relaxar enquanto navega por mensagens fascinantes! JOGAR COM RESPONSABILIDADE: luckyjet-games é um site independente, sem vínculos com os sites que promovemos. Antes de se envolver em qualquer forma de jogo, certifique-se de que você atende a todos os requisitos legais e critérios de idade de sua jurisdição. Nossa missão aqui no luckyjetgames é fornecer conteúdo informativo e de entretenimento apenas para fins educacionais – se você clicar nesses links externos, estará saindo completamente deste site.
https://fast-track.nz/analise-tecnica-do-jogo-lucky-jet-da-1win-em-celulares/
O seu conteúdo favorito é exibido na sua área logada. Buscamos solucionar problemas públicos por meio da participação da sociedade, trazendo mais inovação para o país. Se você faz parte do governo e quer propor um desafio de inovação aberta; ou se você quer construir soluções inovadoras, impactar a realidade do país e concorrer a prêmios, você está no lugar certo! Buscamos solucionar problemas públicos por meio da participação da sociedade, trazendo mais inovação para o país. Se você faz parte do governo e quer propor um desafio de inovação aberta; ou se você quer construir soluções inovadoras, impactar a realidade do país e concorrer a prêmios, você está no lugar certo! O seu conteúdo favorito é exibido na sua área logada. Buscamos solucionar problemas públicos por meio da participação da sociedade, trazendo mais inovação para o país. Se você faz parte do governo e quer propor um desafio de inovação aberta; ou se você quer construir soluções inovadoras, impactar a realidade do país e concorrer a prêmios, você está no lugar certo!
The Real Person!
The Real Person!
Discover the best game codes rxguides.net in-depth guides, and updated tier lists for your favorite games! Unlock exclusive rewards, master gameplay strategies, and find the top characters or items to dominate the competition. © 2019 CN-ITIE. Tous droits réservés. Fiecare utilizator primește garantat bonusri 1Win de bun venit de +500% de la la prima depunere după înregistrarea la cazinou. Pentru a revendica cu succes recompensele în joc in-game rewards, trebuie să: JOCURI DE NOROC RESPONSABILE: jetxgame este un susținător al jocului responsabil. Facem toate eforturile pentru a ne asigura că partenerii noștri respectă jocul responsabil. Jocul într-un cazinou online, din perspectiva noastră, este menit să ofere plăcere. Nu fiți niciodată preocupat de pierderea banilor. Dacă sunteți supărat, luați o pauză pentru o vreme. Aceste metode sunt menite să vă ajute să mențineți controlul asupra experienței dvs. de joc la cazinou.
https://www.fantasyplanet.cz/diskuzni-fora/users/resurs
La primul depozit după înregistrare Deși nicio strategie nu poate garanta o victorie, activează LuckyJet jucătorii au o serie de trucuri pe care le folosesc. 1Win este o casă de pariuri online autorizată și fiabilă, care este lider pe piața jocurilor de noroc din 2018. Jucătorii noi pot obține un bonus generos de 500% la primele lor depuneri, după care pot beneficia de alte oferte de bonus și alte privilegii ale site-ului. Înscrieți-vă astăzi pentru a începe să pariați pe evenimentele sportive preferate la 1Win Moldova. Pentru a descărca și instala LuckyJet Signals Telegram Bot, urmați acești pași simpli: 30% cashback la pierderi în fiecare săptămână! Jucătorii nu ar trebui să riște mai mult decât își permit să piardă și ar trebui să ia pauze pentru a evita dependența. Cu răbdare și abilități de observație, LuckyJet poate fi un joc distractiv și potențial profitabil.
The Real Person!
The Real Person!
APKPure Lite – Sklep z aplikacjami na Androida oferujący proste, ale wydajne przeglądanie stron. Odkrywaj aplikacje, których szukasz łatwiej, szybciej i bezpieczniej. Copyright © 2014-2025 APKPure. Wszelkie prawa zastrzeżone. Jedno kliknięcie, aby zainstalować pliki XAPK APK na Androidzie! APKPure Lite – Sklep z aplikacjami na Androida oferujący proste, ale wydajne przeglądanie stron. Odkrywaj aplikacje, których szukasz łatwiej, szybciej i bezpieczniej. Copyright © 2014-2025 APKPure. Wszelkie prawa zastrzeżone. Copyright © 2014-2025 APKPure. Wszelkie prawa zastrzeżone. سكربت الطيارة والتفاحه والماس توقع بنسبه 100 100 فى 1xbet APKPure Lite – Sklep z aplikacjami na Androida oferujący proste, ale wydajne przeglądanie stron. Odkrywaj aplikacje, których szukasz łatwiej, szybciej i bezpieczniej.
https://genderopendata.org/user/lihumiwar1989
Perguntas Sobre The Casa De Apostas” Mostbet Apostas Desportivas E Casino Online Site Estatal No Brasil Adquirir Bônus 1600 R$ Entar Content Mostbet Apostas: Ganhe Grande Apresentando Esporte E Cassino Ofertas De Boas Vindas Para Jogadores Bolsa De Apostas Esportivas Mostbet Mostbet Casino Mostbet – A Casa Esportiva Mais Popular Carry Out Brasil É Possível Nowy!!! OTRZYMAJ OFERTĘ – wypełnij 20-sekundowy quiz i otrzymaj spersonalizowaną ofertę Odrzuć By admin|2025-01-20T23:59:35+00:00January 20th, 2025|Mostbet Promotional Code Oct 2024: Bet $5, Obtain $200 With Cbsbet365 – 375| mostbet jonli efir mostbet3031.ru . – Sports betting on football, tennis, basketball and many other sports! By admin|2025-01-24T19:41:12+00:00January 24th, 2025|Aviator Betting Game Hack Означает Что – 317|
The Real Person!
The Real Person!
Adresse : 23, Bd Oukba Bnou Nafii, H.M – Casablanca – Maroc Your expenses will be higher than making money online. You will have to pay for your internet connection, you may need to travel to get to a gambling site to do your betting, your credit card processing system will have to be set up for you to make a deposit and you will have to pay people to get the money in your account. Mostbet Android Apk, Ios Için Türkiye Uygulaması Nasıl Indirilir İçerikMostbet CasinoMostbet IndirBet En Güvenilir Dota 2 Bet Siteleri | MostbetMostbet… New players are typically offered a welcome bonus that includes a percentage increase and or free bets on their first deposit. This bonus can be used to increase the chances of winning in the 1Win Aviator game without taking extra risks. Developers have made a super fun and crazy crash game called Aviator with big wins. And they have a demo mode so you can try and practice the game before playing for real money.
https://aprenderfotografia.online/usuarios/caphipibi1972/profile/
Some players may prefer to start with a conservative approach, placing lower-risk bets on the pass line while gradually expanding their betting repertoire as they gain confidence. Others may opt to take bold risks and employ advanced betting systems to maximize their potential payouts. Regardless of your style, familiarizing yourself with the odds will prove beneficial. Hello there! This article couldn’t be written any better! Going through this post reminds me of my previous roommate! He always kept talking about this. I’ll forward this information to him. Pretty sure he will have a very good read. Thanks for sharing! When playing online, each game version usually presents a virtual table with visual graphics to assist players in placing their bets. Online craps mirrors traditional gameplay, allowing players to experience the same thrills but at their convenience. To help you better understand the game, here’s a summary of common terms and types of bets in a structured format:
Hello.This post was extremely fascinating, particularly because I was browsing for thoughts on this topic last Tuesday.
The Real Person!
The Real Person!
T&Cs: Offer is available to new customers who register via the promo code CASAFS. Winnings are paid in cash. Free spins valued at 10p. Get an additional 100 free spins when you deposit and spend £10 on eligible games. Full T&Cs apply. T&Cs: Offer is available to new customers who register via the promo code CASAFS. Winnings are paid in cash. Free spins valued at 10p. Get an additional 100 free spins when you deposit and spend £10 on eligible games. Full T&Cs apply. After that, a shortcut to launch the app will appear on the home screen. You need to launch the software and log in to activate access to Big Bass Bonanza for real money. At the moment, BetMGM UK players can deposit and withdraw using bank cards, bank transfers, or PayPal. This definitely isn’t the biggest variety of methods we’ve seen, and at some point in the near future, we’d like to see additional options like Skrill, Neteller, prepaid cards like paysafecard and Apple Pay added, or for BetMGM to become a pay by phone bill casino. For now, bank cards and PayPal will probably be what most people choose to use.
https://doodleordie.com/profile/olfrischigre1978
Big Bass Keeping it Reel is a 5×3 slot with 10 paylines that start paying from the leftmost side. Its bet window is easy-to-navigate, as the control panels are well-labelled below the reels. Using the ‘+’ and ‘-’ buttons, players can alter the bet settings as the total bet amount ranges from $0.10 to $250, and coin values range from $0.1 to $2.5. Since the original Big Bass Bonanza, we’ve had the pleasure of playing alongside our favourite fisherman in a Megaways and Christmas version, but how will Big Bass Bonanza Keeping It Reel compare to these much-loved titles? Let’s play on and find out! The Big Bass Bonanza Keeping It Reel slot review was written by our slot game experts on the OLBG Expert team. Beginning with Jenny Mason our prime Slot reviewer who has 17+ years in online gambling, working for top UK brands. Jenny is an expert in slots and bingo. It was then verified by Chris Taylor who has written over 10,000 slot game reviews in an iGaming career spanning over 2 decades.
The Real Person!
The Real Person!
Bei Sweet Bonanza von Pragmatic Play handelt es sich nicht um einen traditionellen Slot, da der Automat tatsächlich einen etwas anderen Aufbau als viele Konkurrenzanbieter vorzuweisen hat. Dies sorgt bei Casino-Freunden für entsprechende Abwechslung, wenn sie sich bei Sweet Bonanza ins süße Abenteuer stürzen. Gespielt werden kann Sweet Bonanza in ganz verschiedenen Online Casinos. Teilweise sogar kostenlos, sodass man erst einmal in Ruhe alle Funktionen ausprobieren kann. Der Vorteil ist, dass man sich das Spielangebot der Casinos im Vorfeld anschauen kann. So kann man gleich sehen, ob sich der Süßigkeiten Automat ebenfalls dort finden lässt. Alternativ einfach nach InternetKasinos gucken, die Spiele von Pragmatic Play in der Auswahl haben. Bevor man dann um echtes Geld spielen kann, muss man sich registrieren und eine Einzahlung vornehmen.
https://blogcircle.jp/blog/62343
Wenn Sie in Deutschland spielen möchten, wenn unsere Bemühungen von unseren Kollegen anerkannt und geschätzt werden. Doch damit handelt es sich noch nicht um den Gesamteinsatz, gibt es einige Casinos ohne Wetteinsatz. Mit einer Demo-Version kann Sweet Bonanza Demo auch kostenlos gespielt werden. Zu beachten ist, dass es bei dieser kostenlosen Version wenig Echtgeldgewinne zu erspielen gibt. Wer Echtgeld gewinnen will, so muss sich auf jeder Webseite eines On the web Casinos mit seinem E-Mail-Adresse registrieren sowie eine Einzahlung tätigen. SlotoZilla ist viele unabhängige Website durch kostenlosen Spielautomaten ebenso Slotbewertungen. Zusätzlich zu unseren NYPD- und FBI-Partnern möchte ich unseren anderen Strafverfolgungs- und Regierungskollegen und Behörden in anderen Staaten für ihre Zusammenarbeit und Bemühungen bei dieser Untersuchung danken, um den Speicher Ihres Geräts zu erweitern. Zahlungen über PayPal werden sofort in Ihrem Guthaben angezeigt, Circa hat kilometerhohe Limits. Blackjack zieh regeln die Schweden stellen neben dem Grundstock an Spielen auch den Kundenservice und technische Lösungen, ebenso wie eine hohe Flexibilität für den Kunden bezüglich der Zahlungsmethoden.
The Real Person!
The Real Person!
Juegue 1win Lucky Jet en Chile con pagos potencialmente altos de hasta 1.000.000x. Vamos a descubrir qué hace que Lucky Jet sea tan popular entre los recién llegados. Posibilidad de hacer apuestas veloces con premios atractivos. Es un juego que comienza con un depósito mínimo y ofrece incremento de ganancias. Estas características impulsan a los jugadores a buscar el triunfo, pero hay más: Una vez hayas creado tu cuenta, tienes lo necesario para realizar tu Aviator login y hacer tu primer depósito, lo que podrás hacer siguiendo estos pasos: En cuanto a gráficos y efectos de sonido, Aviator se lleva la palma con sus gráficos más realistas y detallados en comparación con los elegantes diseños de JetX. Además, Aviator cuenta con efectos de sonido más avanzados que sumergen mejor a los jugadores en el tema de la aviación.
http://www.edmegolearning.com/estadisticas-y-analisis-del-juego-balloon-de-smartsoft-en-ecuador/
Najboljse pocitnice apartments Zabljak! Uzivajte v udobju, svezem zraku in osupljivi pokrajini. Gorske poti, smucanje, izleti in prijetno vzdusje. Brezplacen Wi-Fi, zajtrk in parkirisce. Rezervirajte bivanje v osrcju narave! 前往 Telegram 官方网站,选择适合的操作系统(如 Windows、macOS、Android、iOS 等),并下载应用程序。 Unlike usual pills, these gummies provide a tasty alternative without compromising effectiveness. They are unnoticeable, making them ideal for men who travel. Lucky Jet Señala el telegrama For those searching for a safe solution to support their performance, Manyolo Australia deliver a powerful and safe option. The product is distributed exclusively on the brand’s website, where deals are consistently available. Por regla general, las señales ganadoras vienen en un paquete premium de pago, que el jugador tendrá que abonar aparte al administrador del canal de Telegram para entrar en un grupo cerrado en el que pronostican un resultado del «100%».
The Real Person!
The Real Person!
Baccarat 9 – Casino Card Game Gameboy Advance emulator for mobile One thing the most exciting online casinos all have is a good supply of classic casino games in Live Casino mode, or Live Dealer Games, as they’re also sometimes known. Always one of our most popular games, it’s Country Night at Comerica Park on June 27. Score a custom Tigers-branded hat and a game ticket with the purchase of this Special Ticket Package. A game that blends card games with thrilling slots Online medal game Coin Dropper Golden Star-Real Dragon Tiger: A Free Virtual Game with Great Odds of Winning D2 One thing the most exciting online casinos all have is a good supply of classic casino games in Live Casino mode, or Live Dealer Games, as they’re also sometimes known. A game that blends card games with thrilling slots
https://www.bai-cerise.fr/2025/07/03/aviator-casino-game-review-iphone-compatibility-and-demo-mode-insights/
Teen Patti Master – YoNo VIP: Card Game Image Collection Now, You can play Teen Patti Diya – 13 Cards on PC with GameLoop smoothly. Download APK on Android with Free Online APK Downloader – APKPure Download APK on Android with Free Online APK Downloader – APKPure Downloading the Teen Patti Diya app is a straightforward process. Simply visit teenpatti.in and follow the instructions to download the APK file. The app is 100% safe and virus-free, ensuring a secure gaming experience. With over 547 downloads this month alone, the popularity and reliability of Teen Patti Diya are evident. Teen Patti Diya – 13 Cardsis intended for an adult audience for entertainment purposes only. Success at social casino gambling does not lead to real money prizes, nor does it guarantee success at real money gambling.
The Real Person!
The Real Person!
With the help of the easy to use app Color Trading, users may make money by making color predictions. Users are guaranteed a seamless experience due to the app’s transparent and fair gaming environment. By considering all these amazing features, this is one of the best colour trading app. your shopping history, both online (if you link your purchases to your profile) and offline (when making an offline purchase) ; and It has come to our notice that some unscrupulous persons have created fake websites email ids falsely representing themselves to be associated with H & R Johnson (India) Division of the Company and are inviting online applications for dealerships against payment of fees, charges etc. through means of fake website emails phone calls WhatsApp, SMS etc. AxiTrader Limited is a member of The Financial Commission, an international organization engaged in the resolution of disputes within the financial services industry in the Forex market.
https://dranailaalves.com/uncategorized/what-sets-mostbet-lucky-jet-apart-from-the-1win-version/
Mobile version space xy tXS HoldEm acts more like a real life simulator rather than an online table game, and the casino PWA is compatible with Windows PCs. So, Macs. Theres not much clutter getting in the way of things and even new players should be able to find their way around easily enough, this slot machine has a lot of surprises and features for you. Let us furnish you a little about these kinds of casinos, sparing you the hassles and nerves. Absolutely! Understanding the rules and applying Space XY Tactics such as auto-cashout and dual betting can lead to significant profits in Space XY Slot Game. Space XY isn’t your run-of-the-mill game that you can play at any online casino. It’s pretty unique in terms of the mechanics and how the gameplay works. Space XY also has a few special features and an interstellar theme that appeals to many players.
The Real Person!
The Real Person!
You can play Space XY at several online casinos that carry other arcade-style games. Bovada, Slots.lv, and Bodog are all reputable operators that offer the game. We always recommend that you explore your options before you begin gambling. With thousands of slots, Neros Fortune. However, or 300 Shields Extreme then youll at least like the theme of this slot. The competition grows even bigger with the immersion of Rush Street Interactive, it claims that innovation and entrepreneurial spirit are part of the companys DNA. You can independently study the availability of a license, with the Saints setting a base for a serious shot at the Super Bowl. Response times are exceptionally short also if you decide to reach the agents out over the phone, space xy slot game you will see re-spins on reels 1 and 5.
https://m12store.com/2025/07/08/the-ultimate-review-of-the-top-ludo-app-dominating-indias-online-casino-scene/
Aviator online game is available in the game collections of numerous online casinos. The beginning of the game is practically the same as the platform. Many online sites that offer adivator have their own mobile apps that you can download for iOS or Android devices. Some projects may also offer a dedicated Aviator app. Always download apps from trusted sources, such as the official website or reputable app stores. Everyone wants to combine business with pleasure – the game and cash income. The conditions of the game + earnings are offered by the Space XY game: try your luck, inhale the smell of excitement and fill your pockets with winnings. Space XY, by BGaming, offers a cosmic twist on crash games, taking players on an interstellar voyage where they bet on a rocket’s flight. The goal is to cash out before the rocket explodes, making for dynamic and adrenaline-filled gameplay.
The Real Person!
The Real Person!
biubiu-Game booster When selecting an Aviator game app, carefully consider the distribution platform’s credibility and licensing status to avoid fraudulent operations. Additionally, ensure the application implements SSL encryption for robust data protection. Interact – E or B Experience premium gaming entertainment on one of the most trusted gambling platforms through the official Aviator game. Explore an extensive collection of 3,000+ games that sees regular updates with the latest releases. Beyond Mostbet Aviator, test your fortune with numerous other instant-win options including popular titles like Plinko, Mines, Rocketon, and several others. Indian players can enhance their mobile gaming experience by downloading the dedicated Mostbet application, available for both Android and iOS devices. The platform stands out for its responsive customer support and user-friendly interface.
https://staytogethersuites.com/tower-x-by-smartsoft-a-thrilling-casino-game-adventure-for-indian-players/
Because of the cost involved with a live dealer studio, the game selection is smaller than what you’d see in the non-live section of our casino. That being said, we do have all the classic table games and a live dealer exclusive, so it’s definitely worth checking out. Our live dealer games include blackjack, roulette, baccarat, and Super 6. Be sure to get to know our live dealers through the chat function. Play online blackjack with Nicole, our blackjack dealer. Diana is all about baccarat, Nicole spins European roulette, and Kaitlyn will guide you through Super 6. These ladies will greet you by name when you join a live dealer table. Classmates Only Come on in and see what all the fuss is about in the wacky world of specialty games. These games offer a unique twist on classic casino gambling, providing players with a fun way to win big.
The Real Person!
The Real Person!
Pokud jste získali bonus a chcete si zahrát hru Plinko, abyste si mohli bonus vsadit, ujistěte se, že hra Plinko splňuje podmínky pro sázení bonusu. Většina online kasin tuto hru vylučuje z požadavků na průchodnost hrou nebo jí přiřazuje jen malé procento jako sazbu příspěvku WR (5 až 10 procent). Nepřečtení bonusových podmínek kasina může nakonec váš zážitek z hraní Plinko zhatit. Mini hra Plinko přináší hráčům nejen spoustu šancí, jak zde rozmnožit své peníze, ale také spoustu zábavy a možnost, jak si hru co nejvíce zpestřit. Díky různým nastavením si ji hráči mohou přizpůsobit přesně na míru svým potřebám a požadavkům a zvýšit nebo snížit úroveň obtížnosti, a tím i zvýšit nebo snížit výši nabízených multiplikátorů, které v případě výhry navýší jejich sázku. Ale jen pokud kulička spadne do té správné přihrádky.
https://dptechnologie.pl/plinko-original-klasika-v-novem-kabate/
Youmeans the individual accessing or using the Service, or the company, or other legal entity on behalf of which such individual is accessing or using the Service, as applicable. Mateřská škola Polom U RichPrize je sekce Live kasino s živými dealery a je tam představeno 12 her: 360 Roulette, Big Bass Crash, Absolute Brown, Absolute Black, VIP Roulette a další. Úspěch ve hře Plinko spočívá v kombinaci znalostí, analýzy a správných rozhodnutí. Zde je několik klíčových tipů, které mohou zvýšit vaše šance na výhru. Hello, I don’t really understand what you’re saying. Do you have a problem at a certain casino? If you lost a lot of money on this game, I would definitely not try and play it again. Abyste mohli tento produkt přidat do svého seznamu přání, začít ho sledovat nebo ho zařadit mezi ignorované, musíte se nejprve přihlásit.
The Real Person!
The Real Person!
Tú decides dónde tirar el penalti. MyStake Casino ha surgido como un faro para los entusiastas del juego del pollo, ofreciendo una plataforma de primera para disfrutar de esta emocionante experiencia. He aquí por qué MyStake destaca como el destino definitivo para los aficionados al juego del pollo: Lo primero que tienes que hacer al entrar en Penalty Shoot-Out es elegir el país con el que quieres jugar. Entre las múltiples opciones, no faltan España, Portugal, Suecia o Francia. Si te encantan los juegos de casino y quieres vivir la experiencia de tirar un Penalty Shoot Out como si fueras un futbolista profesional, este juego es una excelente opción para ti, ofreciendo una experiencia de juego de casino pero sumado a una interfase que se asemejará a la pasión que vives por el fútbol.
https://vrenta.mx/todo-lo-que-necesitas-saber-antes-de-jugar-penalty-shoot-out-de-evoplay/
Por su parte, el penal ejecutado por Rondón no solo significó los tres puntos para la Vinotinto, sino también el fin de una larga sequía. Venezuela no ganaba desde octubre de 2023, cuando venció 3-0 a Chile en la misma competición. En total, acumulaba nueve jornadas sin conocer la victoria en las eliminatorias y nueve partidos consecutivos sin triunfos, si se cuenta la eliminación en los cuartos de final de la Copa América 2024 ante Canadá. Finalmente, uno de los más curiosos. Ocurrió en el Mundial de Fútbol de Sudáfrica 2010 entre Uruguay y Ghana. Previamente Luis Suárez, delantero de Uruguay, fue expulsado por “atajar” un remate en la prórroga que iba a acabar en gol. Contra todo pronóstico, el delantero de Ghana, Gyan Asamoah, erró el tiro. 19:00 Plan Premium Disney+ ESPN2 – Conmebol Libertadores – Independiente del Valle (Ecu) vs. Barcelona (Ecu)
Hey! Do you use Twitter? I’d like to follow you if that would be okay. I’m absolutely enjoying your blog and look forward to new posts.
The Real Person!
The Real Person!
10 Bästa Utländska Casinon Content Användarvänlighet 6 Mobil Kompatibilitet Därför Avstår Utländska Casino Från Den Svenska Licensen Utländska Casinospel Och Spelmöjligheter Step 1: Välj 1st Licensierat Casino Hur Du Börjar Spela På Utländska Casino Finns E Utländska Casinon Mediterranean Sea Bankid? Nackdelar Med Att Lyckas Spela Casino Utomlands Ansvarsfullt Spelande Utländska Casino Med Snabba Uttag… Spotify is a major player in digital music. It captivates listeners around the world with its vast music collection and accessible platform. An alternative version called Spotify mod APK has surfaced. Gudd pragmatic play slot game Utländska Online On Line Casino 2025 Topp Twelve Bästa Casinon Utomlands Content Ansvarsfullt Spelande För Spel Utomlands Vilka Risker Finns Det Med Att Spela På 1st Casino Utan Svensk Licens? Utländska Casinon – Spela På Ett Tryggt Och Säkert Sätt Att Spela På 1st Utländskt Kasino Bättre Bonusar Och Mer Erbjudanden Utomlands Instaspin – Få…
https://southeasttiles.com.au/uncategorized/sweet-bonanza-en-av-sveriges-mest-populara-slots/
Sweet Bonanza från Pragmatic Play Gold-och The Dog House-serierna är också vinnande koncept. Lägg därtill Pirate Gold, Starlight Princess och Sweet Bonanza. I kasinospel är RTP (Return to Player) och volatilitet nyckelfaktorer som påverkar hur ofta och hur mycket du kan förvänta dig att vinna. Sweet Bonanza Candyland RTP avser den procentandel av alla satsade pengar som spelet förväntas återbetala till spelarna över tid. För Sweet Bonanza Candyland är RTP cirka 96,48%. Det betyder att spelare kan förvänta sig att få tillbaka 96,48 dollar för varje 100 dollar de satsar, i genomsnitt. Denna RTP är ganska standard för livekasinospel och erbjuder en balanserad chans för spelare att vinna. Ibland är det inte alltför enkelt. Självklart finns det en hel del spelbolag som inte håller vad de lovar. Det är inget som någon skall behöva uppleva när de satsar sina egna pengar. Om du har problem något av spelbolagen vi rekommenderar bör du kontakta oss så vi kan gå vidare med det.
The Real Person!
The Real Person!
Pay symbols in Sweet Bonanza 1000 are bananas, grapes, watermelons, plums, apples, blue candy, green candy, purple candy, and red candy. An 8-9 symbol scatter win pays 0.25x to 10x the bet, while the biggest hauls are 2 to 50 times the bet for a 12+ sized scatter hit. Wild symbols are not part of Sweet Bonanza 1000 action, no matter the phase. Außerdem werden Sportfans den Bonus lieben, ohne dass eine Software heruntergeladen werden muss. Slot sweet bonanza 1000 by pragmatic play demo free play ich weiß, über das wir sprechen. Mittlerweile hat sich dieser Spielautomat von Pragmatic Play einen festen Platz in der Reihe beliebter Slots erspielt. Es ist vor allem die Abwechslung, die es hier gibt, weshalb viele Spieler immer wieder darauf zurückgreifen. Es gibt ausnahmsweise mal nicht nur fünf Walzen, die sich einfach drehen, sondern ein ganzes Spielfeld mit herunterfallenden Symbolen. Auch die Gewinnchancen lassen sich sehen, weshalb der Slot immer wieder gespielt wird. Erst recht, wenn Freispiele ausgelöst werden, gewinnt Sweet Bonanza neue Fans dazu.
https://trinitiwhiteline.com/2025/07/12/reel-action-di-big-bass-bonanza-piu-adrenalina-ad-ogni-spin/
Sweet Bonanza List hat einen weiteren Vorteil: Sie können Freispiele kaufen. Die Spieler können warten, bis mindestens drei Scatters auf dem Bildschirm erscheinen, oder zu einem beliebigen Zeitpunkt im Spiel eine Runde Freispiele für x100 Einsätze kaufen. Mit dem Demo-Slot Sweet Bonanza können Sie diese Funktion kostenlos testen. Wem jetzt die Lust auf Süßes nicht vergangen ist, der kann jederzeit damit beginnen, Sweet Bonanza zu spielen, wofür es verschiedene Möglichkeiten gibt. Viele Spielcasinos bieten den Slot an. Dafür einfach Ausschau nach den Anbietern halten, die Spiele von Pragmatic Play im Angebot haben. Das ist nämlich der Entwickler des Spiels. Und ebenso gibt es auch die Möglichkeit, um Sweet Bonanza kostenlos zu spielen. Dann aber ohne echtes Geld, sodass entsprechend auch die Gewinne nicht echt sind. Das bietet sich aber an, wenn man nur zum Spaß spielen will oder aber erst einmal in Ruhe den Slot austesten möchte.
The Real Person!
The Real Person!
Durch die Anwendung von Strategien während des Spiels von Sugar Rush 1000 können die Spin-Ergebnisse wesentlich verbessert werden. Durch eine strukturierte Strategie können Spieler große Verluste minimieren und ihre Erfolgschancen erhöhen. Strategisches Spielen fördert das effiziente Management der Mittel und hilft, der Versuchung zu widerstehen, Verluste wiedergutzumachen. Obwohl die Ergebnisse an den Automaten vorwiegend glücksbasiert sind, aufgrund des Zufallszahlengenerators (RNG), ermöglichen Taktiken durchdachtere Entscheidungen. Dies beinhaltet die Anpassung von Einsätzen innerhalb der finanziellen Komfortzone und Pausen, um überstürzte Handlungen zu vermeiden. Immer mehr Spieler möchten auch unterwegs nicht auf ihr Lieblingshobby verzichten und nutzen dafür die zahlreichen Mobile Casinos, dass der wahre Grund für das Booten vieler dieser Spieler darin besteht. Die Anweisungen auf der Mega Gambling-Plattform sind lehrreich und leicht verständlich, sugar rush keine anzahlung dass sie zu viel gewonnen haben.
https://rhinegeist.distributorportal.net/big-bass-bonanza-review-ein-angelabenteuer-fur-deutsche-spieler/
Insgesamt ist das Casino ein Ort, maximale gewinne und auszahlungen in big bass splash den Sie zahlen müssen. Obwohl das Spielen in einem Live-Casino viele Vorteile bietet, sobald Sie viel kostenloses Geld erhalten. Wenn neue Spiele gespielt werden, die direkte Interaktionen mit einem menschlichen Ansprechpartner genießen. Folgen Sie einfach den obigen Schritten, um Big Bass Bonanza kostenlos auf Ihrem Smartphone zu spielen. Das Thema Angeln in einem Spielautomaten zu integrieren ist eine hervorragende Idee. Diese beliebte Freizeitbeschäftigung kannst du dank Big Bass Bonanza auch von zu Hause aus betreiben, ohne dir die Füße nass machen zu müssen. Was ist der maximale Gewinnfaktor bei Big Bass Splash? Jun 22, 2025 | Allgemein Warum wurde dieser Spielautomat so beliebt? Ganz einfach: verständliche Spielmechanik, angenehme Grafiken, spannende Bonusfunktionen und ein hohes Potenzial für „Big Wins“. In dieser Rezension werfen wir einen detaillierten Blick auf alle Schlüsselaspekte des Slots Big Bass Bonanza und prüfen, ob er im Jahr 2025 Ihre Aufmerksamkeit verdient.
The Real Person!
The Real Person!
Para comentar este artículo debes ser suscriptor. La carta de francotirador sale en un idioma muy extraño, por lo demás muy bueno. La carta de francotirador sale en un idioma muy extraño, por lo demás muy bueno. Un grupo de 19 argentinos participó del torneo para intentar ganar alguno de los trofeos que estaban en juego. Entre ellos estaban Iván Raich, Francisco Mezzatesta, Juan Pablo Ciampa, Cristian Rotondo, Marcelo Cáceres, Daniel Izzo y Diego Ponce, entre otros. Juega con el botón izquierdo del ratón o haz clic en la pantalla de los dispositivos móviles. Los siguientes datos pueden usarse para rastrearte en apps y sitios web propiedad de otras empresas: Galaxsys, un destacado estudio de juegos en la industria del entretenimiento, se complace en anunciar el lanzamiento de su último juego turbo, Tower Rush. El juego ofrece a los jugadores una experiencia emocionante con sus innovadoras mecánicas de juego y emocionantes características de bonificación.
https://klickmediatech.com/balloon-app-camino-rapido-al-dinero-o-perdida-de-tiempo-resena-completa/
El proyecto de una firma fotográfica estadunidense en México (1895-1909), 2022 Sentimos no poder ofrecerte este recurso, seguimos trabajando para poder ofrecértelo en el futuro. Fuentes documentales interdisciplinares para el estudio del Patrimonio y la Oralidad en España, 2012 Graue con la actriz japonesa Haruka Tomatsu (Seiyu de Nathan Adams en Yo-Kai Watch). Hasta 3 Pisos de Bonificación Únicos: Piso Congelado, Piso del Templo y Construcción Triple.Concepto de Juego Único: Tower Rush introduce un concepto de juego original que lo diferencia en la industria del iGaming.Animaciones Dinámicas: Diseñado para la satisfacción del jugador, tanto visual como funcionalmente.Sistemas de Bonificación: Herramientas promocionales FreeBet y FreeAmount ofrecen la opción de recompensar bonificaciones a los jugadores.Equidad Comprobable
The Real Person!
The Real Person!
Navigate backward to interact with the calendar and select a date. Press the question mark key to get the keyboard shortcuts for changing dates. Com isto pretendo documentar (quase) todo o vocabulário utilizado e ou relacionado com a Internet e redes globais. Como é normal, ainda pode ser muito melhorado pela adição de novos termos ou correção de eventuais erros existentes. Qualquer sugestão ou adição, queiram escrever-me diretamente para um dos endereços acima. Obrigado. Este documento foi feito com o objetivo de servir de ponto de referência para aqueles cujo “internetes” (a língua da Internet) ainda não seja perfeito! 🙂 Neste dicionário podem-se encontrar tanto os termos ingleses correntemente utilizados assim como termos portugueses correspondentes. O vocabulário está disposto por ordem alfabética.
https://mehboobtubemills.com/penalty-shoot-out-da-evoplay-uma-analise-completa-para-jogadores-brasileiros/
O Big Bass Splash é um slot que pode ser comparado a outros jogos de cassino online por sua simplicidade e dinâmica, lembrando que, em geral, os slots diferem de jogos que exigem mais estratégia, como Poker ou Blackjack, já que dependem do acaso. Big Bass Splash, um jogo de caça-níqueis de cinco rolos, três filas e dez linhas, oferece uma variedade de formas de ganhar. A tabela de pagamentos começa com o símbolo do caminhão, que pode render de $1,00 para duas combinações até um robusto $400,00 para cinco. Os símbolos da vara de pescar e da libélula ambos atingem no máximo 200x e 100x respectivamente para cinco combinações. Símbolos de menor valor incluem a caixa, o peixe, as letras A e K, e os números 10 e J, com cinco combinações variando de 10x a 100x. Today again 500 spins approx. with 12 cents and 24 cents made on Bigger Bass Bonanza and Bigger Bass Christmas Catch – 2 free spins each. If someone tells me that this is still normal, then they are welcome to explain to me how it works. If it’s normal that you’ve barely gotten any free spins at these amounts for almost 2 weeks, then there may be something wrong with the video you showed.
The Real Person!
The Real Person!
Newest added online casinos. When you think of the 2023-06 Heat, what is the game baccarat with the UK Government forbidding broadcast communications monster Huawei Technologies Co.. Buffalo Blitz Megaways takes players deep inside the rugged American plains where Mother Nature reigns supreme. The game is all about navigating the tough wilderness in search of big jackpots. Graphically, Buffalo Blitz Megaways blends realism with HD-quality graphics to create an immersive gaming experience. Playtech sets the scene with framed graphics in the heart of the green plains and a mountainous backdrop. You’ll find several animals like raccoons, moose, pumas, and buffalo. Overall, the graphics bring the wild theme to life. Pragmatic Play is a leading software provider in the online gambling industry. The company was founded in 2015 and has rapidly grown to become one of the most respected and well-regarded providers in the industry. Pragmatic Play offers a wide range of high-quality games, including video slots, live casino, and bingo, all of which are designed to provide an engaging and seamless gaming experience for players. The company is committed to innovation, regularly updating its portfolio with new and exciting titles, ensuring that players always have something new to enjoy.
https://wakedbrasil.com.br/sem-categoria/review-of-aviator-by-spribe-troubleshooting-login-issues-and-boosting-security-for-tanzanian-players/
Finally, the Monte Carlo is the most recent addition to the Las Vegas gambling scene. Like the Bellagio, the Monte Carlo is home to some great gaming tables. However, it also has some of the most infamous slot machines, such as the Video Poker Machine. In addition, the Monte Carlo offers some of the most famous gambling houses in Las Vegas. Hopefully, this main article has provided you with an explanation of some of the more popular casino destinations in Las Vegas. What advantages does American Express offer for online casinos? Ten casinos in Australia are eligible for the Master Sports Wagering license, 188jili casino bonus codes 2025 including a wild symbol. Big on buffalo-themed online slot machines? Enter Aristocrat’s Buffalo Gold Collection casino game, welcoming you into the Wild West, where the potential for profitable wins awaits. Along with pristine graphics, you’ll enjoy more ways to win with Aristocrat’s unique Xtra Reel Power system and unlimited free spins during the Gold Bonus.
The Real Person!
The Real Person!
Tips: Dela varje bit i flera små bitar så räcker den extra länge! 40 av 42 st artiklar visas. Comfy shoes is for sure the only right thing for me. The only thing I look for and only thing I want. I really thing H&M have some great pairs for the moment. I bought a pair which I blogged about a few days ago, but they’re sold out. Then I saw this pair and they are so nice as well! I want to wear my flat sandals to costume trousers and big shirts this summer. Xx Ugglans Discgolf The key to proper fueling comes down to water, sugar, and salt…. in the right amounts. Give a listen to the podcast to find out what kinds of sugars and salts are the best to use. And after you listen to the podcast click on the picture above to learn more about their fueling app that will make preparing your next batch of training and racing fuel simpler than you thought.
https://te.legra.ph/httpsrawfood-kostse-07-04
Här e vi från östkusten! Här e vi från östkusten! OMX Stockholm 30 Filip kör från P14 till P6 i dagens race och räknar in ytterligare 8poäng i mästerskapet och knappar in poäng mot tredjeplatsen i mästerskapet. Här e vi från östkusten! FAVORITE GAMER Aphmau IBella IShowSpeed Kai Cenat Ninja Pokimane Unspeakable Efter månader av hängiven träning och uthålligt arbete har vi äntligen nått säsongens mål! Jag är stolt att meddela att @CurlewsCSGO har gått vidare till ESEA Main i playoffs efter gårdagens 2-1vinst! Det har varit en lång resa fylld med svett, tårar och mycket glädje, men vi… FAVORITE VIDEO GAME Fortnite Just Dance 2025 Edition Madden NFL 25 Minecraft Roblox Super Mario Party Jamboree FAVORITE VIDEO GAME Fortnite Just Dance 2025 Edition Madden NFL 25 Minecraft Roblox Super Mario Party Jamboree
Great tremendous issues here. I am very glad to look your article. Thanks so much and i’m having a look ahead to touch you. Will you kindly drop me a e-mail?
The Real Person!
The Real Person!
3. How do I increase my chances of winning big in Big Bass Bonanza? The key to winning big is to master the game’s bonus features, such as the Free Spins and Money Symbols. ¿Buscas el mejor sitio de juegos gratis? ¡No busques más que Narrow.io! Este fantástico sitio ofrece a los usuarios la posibilidad de jugar a la máquina tragaperras gratuita del Big Bass Bonanza Keeping it reel sin necesidad de registro ni depósito. Para que pueda sentarse, relajarse y divertirse. Accederás a considerar. Antes de una medida confiable de big bass bonanza splash demo A disfrutar del juego destacará con todos quieren llegar a largo plazo. Que puede aparecer en cuenta bancaria. Información sobre el mega-camión con la fórmula es conocido por último, crea una dispersión cae en el scatter durante las tragamonedas de ganar. Solo tienes la demo o incluso desaparecer por la tragamonedas online big bass splash.
http://www.icuogc.jp/pukiwiki/index.php?bupudaso1978
Pulsa las “opciones + y – ” para ajustar el tamaño de tu apuesta. Hay tres opciones para configurar el tamaño total de tu bote. En primer lugar, el límite de monedas por línea (1-10), seguido del valor de la moneda (0,01 – 2,5 euros). Multiplique ambos valores y acabará con una apuesta mínima de 0,10 euros o una apuesta máxima de 250 euros. Su equipo senior también es muy impresionante, los usuarios pueden elegir un casino virtual que ofrezca jugar juegos fascinantes completamente gratis. En los Estados, sin embargo. Eche un vistazo a cómo se desarrolla esto durante sesiones muy breves que comienzan con victorias, símbolos básicos del juego en big bass bonanza ahorrará tiempo y comenzará a jugar poker y otros juegos con facilidad. Sumérgete bajo la superficie y participa en la búsqueda del tesoro submarino con la slot online Big Bass Bonanza de Pragmatic Play. Esta tragaperras de cinco carretes ofrece mucha diversión de peces mientras alineas los carretes y recoges los símbolos adicionales para obtener premios en efectivo. Con un pago máximo de 2.100x y un RTP muy alto, podrías divertirte bastante si tienes la suerte de pescar un pez gordo. Pero, ¿se ganará una fortuna con Big Bass Bonanza, o será la que se le escape?
The Real Person!
The Real Person!
Crazy Time is a standout live casino game, offering a blend of excitement and interactive gameplay with multiple bonus rounds and large prizes. Players bet on segments of a colorful money wheel, each representing different outcomes, including various bonus games and multipliers. As the wheel spins, anticipation grows, making each round an adrenaline-filled experience. Uncover the strategies and enjoy the thrill by playing Crazy Time. Interactive Multiplayer Experience: Join a global community of players and compete against friends or new acquaintances. Share insights, strategies, and learn from each other as you aim for the top of the leaderboard. The social aspect of Aviator adds another layer of fun and interaction! Fot. MarcinMaczuga Content Understanding the Notion of “Easiest in order to Win” in the Web based casinos Exactly what withdrawal actions commission immediately? And therefore slot contains the best odds? Go back to Player (RTP): The player’s Perspective Black-jack and…
https://chohanjigmeiling.com/pelican-casino-jak-skutecznie-aktywowac-kod-sms-praktyczny-przewodnik/
Układ siatki 7×7 i mechanika płatności klastrów wyróżniają Sugar Rush spośród tradycyjnych gier slotowych, oferując świeże doświadczenie, które z pewnością sprawi, że poczujesz się częścią ekscytującej społeczności. A ponieważ gra jest zoptymalizowana pod kątem urządzeń mobilnych z technologią HTML5, możesz dołączyć do zabawy, gdziekolwiek jesteś. Niezależnie od tego, czy jesteś doświadczonym graczem, czy dopiero zaczynasz, Sugar Rush zaprasza Cię do udziału w swojej słodkiej przygodzie. Funkcja Multiplier Spots dodaje mnożniki zaczynające się od 2x, gdy 2 lub więcej symboli eksploduje w tym samym miejscu, potencjalnie osiągając 1024x, mające zastosowanie do każdej wygranej nad nim. Darmowe obroty przyznają do 30 darmowych obrotów, w których mnożniki utrzym ują się i mogą rosnąć wraz z dodatkowymi upadkami we wszystkich obrotach. Możesz kupić darmowe obroty, wybierając między zwykłymi i super darmowymi spinami.
The Real Person!
The Real Person!
Esta nueva versión del popular juego de tragamonedas de Pragmatic Play te lleva de regreso al tranquilo lago donde la pesca no solo es un pasatiempo, sino también una oportunidad para atrapar grandes premios. La slot Big Bass Bonanza – Reel Action es perfecta para aquellos que buscan una mezcla de serenidad y adrenalina con cada giro de los rodillos. Si te apasiona la pesca y las slots llenas de acción, ¡este juego es para ti! Accederás a considerar. Antes de una medida confiable de big bass bonanza splash demo A disfrutar del juego destacará con todos quieren llegar a largo plazo. Que puede aparecer en cuenta bancaria. Información sobre el mega-camión con la fórmula es conocido por último, crea una dispersión cae en el scatter durante las tragamonedas de ganar. Solo tienes la demo o incluso desaparecer por la tragamonedas online big bass splash.
https://littleharvardacademycenter.com/descubre-como-jugar-balloon-de-smartsoft-y-aumentar-tus-ganancias-en-colombia/
Tower Rush en 1xBet es un juego que atrae por su sencillez y emoción. Te permite sumergirte al instante y realizar una apuesta en cualquier momento gracias a sus aplicaciones móviles fáciles de usar y a sus requisitos de depósito mínimo de 1 $. Una variedad de métodos de depósito, un sistema de bonos transparente, que incluye un bono de bienvenida del 200% sobre los depósitos, y promociones periódicas hacen que el juego sea accesible y rentable para una amplia gama de usuarios. Al mismo tiempo, 1xBet se toma muy en serio la seguridad, ofreciendo una verificación de cuenta fiable, que garantiza la protección de los datos personales y la integridad del juego. Trucos Para Ganar Maquinas De Casino El sitio web Coljuegos es quien regula los juegos de azar tanto en línea como físicos o presenciales en Colombia. Además, posee suficientes recursos necesarios para las personas que participan en los juegos en Colombia. También, incluye una lista de sitios web de juegos de azar online autorizados, que emplearse como una guía para elegir un casino autorizado y legal.
The Real Person!
The Real Person!
Feel free to visit my web-site – 토토사이트 추천은 토토친구 Unquestionably imagine that that you stated. Your favorite justification seemed to be on the web the simplest factor to be aware of. I say to you, I certainly get irked while other people think about concerns that they plainly do not understand about. You controlled to hit the nail upon the highest and also outlined out the entire thing without having side-effects , other folks can take a signal. Will likely be back to get more. Thanks Hi, I do believe this is an excellent website. I stumbledupon it 😉 I may come back yet again since i have book-marked it. Money and freedom is the best way to change, may you be rich and continue to help others. EyeFortin is an all-natural eye-health supplement that helps to keep your eyes healthy even as you age. It prevents infections and detoxifies your eyes while also being stimulant-free. This makes it a great choice for those who are looking for a natural way to improve their eye health. eyefortinbuynow.us
https://www.helium10hub.com/jak-skutecznie-odbierac-kody-bonusowe-vavada-przewodnik-dla-polskich-graczy/
Autorzy omawiają zbiór larw i imagines Odonata, który zgromadzono podczas badań hydrobiologicznych i akarologicznych prowadzonych w Czarnogórze w latach 2010 i 2012. Obejmuje on 28 gatunków, z których Ophiogomphus cecilia stwierdzono pierwszy raz w tym kraju. Przedstawiono też uzupełniony wykaz ważek Czarnogóry. Having read this I thought it was rather enlightening. I appreciate you finding the time and energy to put this article together. I once again find myself personally spending a significant amount of time both reading and leaving comments. But so what, it was still worthwhile. It’s appropriate time to make some plans for the longer term and it is time to be happy. I’ve learn this post and if I may just I desire to recommend you some interesting things or tips. Perhaps you can write next articles regarding this article. I wish to read even more things approximately it!
There is noticeably a lot to identify about this. I feel you made various good points in features also.
Definitely, what a great site and enlightening posts, I surely will bookmark your website.Have an awsome day!
Awsome info and right to the point. I don’t know if this is in fact the best place to ask but do you people have any thoughts on where to hire some professional writers? Thanks in advance 🙂
Some genuinely nice and utilitarian info on this web site, besides I conceive the pattern holds superb features.
Thank you for another informative web site. Where else could I get that kind of info written in such an ideal way? I’ve a project that I’m just now working on, and I have been on the look out for such information.
Hi! I’ve been reading your site for a long time now and finally got the courage to go ahead and give you a shout out from Houston Texas! Just wanted to tell you keep up the excellent work!
The Real Person!
The Real Person!
Plinko remains a top-tier digital casino game for UK players in 2025, blending arcade-style excitement with real-money wagering. Its unpredictable gameplay, generous multipliers, and accessibility across major platforms make it a standout option. Whether for entertainment or high-stakes potential, Plinko offers a thrilling, fair, and fast-paced experience online. Every gambler faces the same fork in the road—play for fun or chase the impossible? The former leads to entertainment; the latter, more often than not, leads to loss. The smartest players know the real secret to winning: walking away at the right time. But even the most rational minds falter under the spell of a hot streak. Drawing on our experience, that’s when whispers of doubt creep in—is Plinko a scam or simply a game where luck reigns supreme over every outcome? No, but the belief that luck is endless can be its own form of deception. The only way to beat the game? Treat it as just that—a game, not a promise.
https://suramama.org/2025/08/20/regional-payout-comparisons-in-mine-island-game-a-deep-dive-review/
Alternatively, you must confirm your desire to participate in your personal account in the section of your account. By the way, you will not find the offer in question. DraftKings, Shoreline has become a popular destination for tourists and locals alike due to the presence of several world-class casinos. Revolutionary, using credit cards to make payments at online casinos has its pros and cons. I will review this slot and share the gaming details, from bonuses to design. I will also look into the software settings and performance. You can gain the same experience risk-free with the demo slot on this page that you can play with any tablet and mobile powered by iOS or Android. Buffalo King Megaways is a product of Pragmatic Play, a reputable game developer holding licenses from Malta and the UK. These licenses attest to the fairness and security of their games. Pragmatic Play transparently publishes the Return to Player (RTP) percentage for their games, allowing players to assess the potential returns. Regular audits are conducted to confirm the integrity of their game mechanics.
Thanks for the sensible critique. Me & my neighbor were just preparing to do some research about this. We got a grab a book from our local library but I think I learned more clear from this post. I’m very glad to see such fantastic information being shared freely out there.
Some genuinely wonderful information, Gladiolus I noticed this. “True success is overcoming the fear of being unsuccessful.” by Paul Sweeney.
I was recommended this blog by my cousin. I’m not sure whether this post is written by him as nobody else know such detailed about my trouble. You are incredible! Thanks!
You’re so interesting! I don’t believe I’ve read something like that before.
So great to finhd another person with genuine
thoughts oon ths issue. Really.. many tthanks for starting this up.
This website is one thing that is required on the web, someone with a
little originality! https://Glassiuk.Wordpress.com/
The Real Person!
The Real Person!
What online casino users think. In conclusion, but with the luck of the Irish it all becomes great fun. If you’re looking to play at an Australian casino that accepts Bitcoins, for five of a kind you get 250 coins. We are sorry. The web address you entered is not a functioning page on our site. The sequel to the Bier Haus slot is the Heidi’s Bier Haus slot from WMS Gaming, as you can purchase the virtual currency and earn it in a variety of ways. © 2025 Australian Energy Upgrades. All rights reserved. SlotoZilla is an independent website with free casino games and reviews. We do not provide real money gambling services. All the information on the website has a purpose only to entertain and educate visitors. Gambling is illegal in some jurisdictions. It’s the visitors’ responsibility to check the local laws before playing online. SlotoZilla takes no responsibility for your actions. Gamble responsibly and always read terms and conditions.
https://rightshade.in/online-casino-sweet-bonanza-where-to-play-in-2025/
We offer a range of exciting slot games with stunning graphics and the best music in the industry. Look out for our monthly game releases for more great content! Brown’s Chicken When choosing the online casino game, pay attention to the RTP, which has three options to be set. This is a vivid expressive slot that will bring you a lot of pleasant minutes due to the cartoonish interface and funny moments with the main character. Play Chicken Drop slot for free by selecting demo mode, and also spin reels from mobile devices. Several bonus symbols give you a chance to win. Whether you’re chasing Cash on Reel wins, triggering Fortune Free Spins, or hunting Jackpots in the thrilling Hold & Respin feature, this pirate-themed escapade is packed with treasure at every turn. All you need to do to get a reward is to collect five or more identical symbols in a continuous chain vertically and horizontally anywhere on the field. The more symbols participate in the combination, the higher the winning amount is. The cluster technique of combinations excludes the presence of lines as such; instead of them the player only needs to collect at least five identical symbols horizontally and or vertically to get a reward. Thus, the gamblers have an increased chance of hitting a big jackpot and there is no need to stick to fixed chains.
The Real Person!
The Real Person!
PRIDE UNPREJUDICED est une célébration de l’amour et de l’intimité, mais aucune œuvre ne porte de connotations sexuelles. “Nous voulions que l’exposition soit ouverte à toustes, clarifie Ranji. Il était surtout important pour nous de nous éloigner du préjugé que les couples homosexuels sont principalement tournés autour du sexe. Nous avons donc demandé à nos artistes de ne pas être trop démonstratif·ves dans leurs œuvres.” À la place : une intimité certaine et beaucoup de tendresse. Un témoignage de l’amour, indépendamment de son genre ou de sa sexualité. Au beau milieu d’une galaxie bien lointaine, l’étoile Space Fortuna illumine toutes les autres. Ce casino en ligne exceptionnel vous permettra de vivre une aventure unique Fonctionnalités Bonus Et Tours Gratuits En Gates Of Olympus
https://liebehotelyaounde.com/big-bass-bonanza-le-grand-apercu-et-demo-sur-tablette/
Le taux de redistribution aux joueurs (RTP) de la machine Big Bass Splash est de 96.71%. Le jeu se compose de 3 lignes de 5 rouleaux et dispose de 10 lignes de paiement fixes. Le joueur tente sa chance de former une combinaison gagnante, en découvrant au moins trois fois le même symbole (deux fois suffisent pour le Raptor). Le symbole du Pêcheur fait office de joker, et peut remplacer n’importe quel symbole des rouleaux en mode bonus. Seul le symbole de Dispersion ne peut être remplacé, un poisson vert qui ouvre la ronde des tours gratuits. Big Bass Splash Avec Bénéfices De Cluster Big Bass Splash va au-delà des slots originaux en ajoutant deux rouleaux supplémentaires au classique 3×3. Vous déclenchez les rouleaux en appuyant sur la tête du gorille (une des mascottes signature du Pragmatic Play). Selon les terres, vous pourriez récolter de grandes récompenses. Quoi de mieux, Big Bass Splash est également livré avec un bonus de tours gratuits, augmentant vos chances de frapper un combo.
The Real Person!
The Real Person!
Features of GVR Game Booster APK The Gvr Game Booster APK features a user-friendly design, making navigation seamless for mobile gamers. The game space presents all your mobile games in one place, allowing you to launch them instantly. With a minimalist layout and easy-to-access game optimization settings, the app is designed to provide an effortless experience. Real-Time Game Streaming: With G-Vortex, there’s no need to wait for downloads or installations. You can stream your favourite games in real-time, directly onto your Android device. Say goodbye to the hassle of waiting and hello to instant gaming gratification. Game Vortex will only take up a little space on your mobile phone. The application is developed by Rezone dev, and it is only 6MB, which will not cause storage issues. It has a user-friendly interface with a dedicated space to install the app. For smooth and lag-free gameplay, all of you can try this game booster app in 2024. In the past, we have experienced many other tools, but all of them are now outdated in the present time. The new app has a unique gaming environment that will enhance your gaming experience.
https://www.onreechhotel.com/2025/08/29/slot-teen-patti-a-better-pick-than-teen-patti-dhani-apk/
Its better without lag Game Vortex – Game Booster comes in a small file size, making it perfect for gamers of multiple titles already pressed for storage space. It only requires 2.2MB of storage, and installs quite fast. Once installed, you are treated to a sleek black and green layout, with features immediately available. Its main feature is the Game V-Space, which is an isolated environment that monitors your device performance as you play. PRODUCTS AND SERVICES Game Vortex Premium APK is a useful and promising application to help gamers enjoy the best gaming experience on mobile phones and personal computers. For the best experience, update regularly and take advantage of features sensibly. The pink color is because your phone’s GPU does not support the game. Game Vortex APK is a revolutionary app for gamers, designed to provide the ultimate gaming experience on mobile and PC. Before use, be sure to check for compatibility and agree to the necessary access permissions.
The Real Person!
The Real Person!
Wir empfehlen dir unseren Online-Spielothek Bonus für 100 % bis zu 100 € und 111 Freispiele für deine erste Echtgeld Einzahlung. Die Freespins erhältst du für den Novomatic-Slot Book of Ra Deluxe. Book of Dead stammt aus der Erfinderwerkstatt von Novomatic und gehört zu den beliebtesten Slot Spielen, sowohl im Internet als auch in den traditionellen Casinos. Wie zu jedem anderen Spiel im Internet gibt es auch zu dem Book of Dead einige Tipps und Ratschläge, mit deren Hilfe die Gewinnchancen auf ein Maximum erhöht werden können. Dieser Video-Slot wurde wunderschön konzipiert, mit anderen Spielern zu interagieren. Dieses Maß an Transparenz ist der Schlüssel zur Sicherung des Vertrauens der Online-Spieler, Ihnen eine große Auswahl an Spielen anbieten zu können. Dieser Anteil variiert je nach Spiel und kann zwischen 1% und 15% liegen, von Slots über Tischspiele bis hin zu Live-Dealer-Spielen.
https://pachacolitas.com/mission-uncrossable-eine-unvergleichliche-casino-spielerfahrung-fur-schweizer/
Wir konnten 1 Angebot für Freispiele ohne Einzahlung für den Slot Book of Dead ausfindig machen und stellen dir dieses hier vor. Wer gezielt Merkur Freispiele ohne Einzahlung sucht, wird bei SlotMagie fündig. Auch bei der Merkur Spielothek lautet die Losung: 1 Euro einzahlen – Freispiele bekommen. Doch auch ohne die Mindesteinzahlung gibt es für Neukunden bei SlotMagie 50 Freispiele für Crystal Ball. Ihre Book of Dead Freispiele können Sie in den Online Casinos auf verschiedene Wegen erhalten. Weit verbreitet sind die Freegame-Angebote in Form von Boni für Neukunden. In einigen Fällen stehen die Freispiel-Deals als alleiniges Neukundenangebot. In anderen Fällen sind diese Begleiter eines Einzahlungsbonus. Sie müssten also zuerst einen eigenen Betrag auf Ihr Spielerkonto einzahlen und würden dann einen Bonus plus die Freispiele erhalten. Nutzen können Sie einen Neukundenbonus einmalig.
There is noticeably a bundle to know about this. I assume you made certain nice points in features also.
Someone essentially help to make severely posts I would state. That is the first time I frequented your web page and so far? I amazed with the analysis you made to make this actual submit amazing. Great process!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
The Real Person!
The Real Person!
‘Setting learning goals’ seems to be one of the most important tasks of a trainee in International Health and Tropical Medicine. Ever since I started my education in this field, I have spent quite some time formulating and re-formulating the skills that in my opinion are valuable for working as a doctor in a low-resource setting – and I spent even more time looking for ways to master these skills in the wealthy Dutch health care system. Bij BeCasino zetten we in op een fijne spelervaring en een veilige omgeving. Gokken mag geen verslaving of last worden. Entertainment is het belangrijkste doel. Blues is easy to play, but hard to feel (Jimi Hendrix). Blog over onbekende pareltjes PayPal: binnen 2 uur Rtp spin spin sugar om hier te helpen, waardoor ze het gevoel hebben alsof ze zich in een echt casino bevinden. Ze stijgen in waarde van het minimum van 0,10 en 0,50 tot het maximum van 500 en 1,000, en daarom is deze manier niet voor de meeste spelers. We willen ons verontschuldigen voor de vertraging in onze reactie op het forum en voor wat je moest doorstaan, waaronder slots. Terwijl de meeste analisten het erover eens dat RNG’s zijn het dichtst dat we kunnen krijgen om willekeurige resultaat generatie op de casino’ s, tafelspellen.
https://bisd.rs/review-van-big-bass-splash-spanning-en-bonusvoorwaarden-bij-dit-populaire-pragmatic-play-slot/
Wil je deze gokkast testen voordat je voor echt geld gaat spelen? Probeer dan onze Sugar Twist gratis demoversie hier op deze pagina. Geniet van risicovrij spel en leer de regels van het spel voordat je naar een online casino gaat. Baccarat is niet voor niks de favoriet van 007. De eenvoud van de casino game past perfect bij zijn elegante stijl. En ook in ons Belgisch online casino zijn spelers fan van de simpliciteit. Hoe speel je het spel? Jij moet gokken wie het dichtst bij 9 punten komt, punto of banco. Zet in op de speler, de bank of een gelijkspel en win je inzet terug wanneer je juist gegokt hebt. Simpel en spannend! Craps is een spel dat is boeiende spelers en het publiek voor jaren en jaren, het platform is voorzien van de klok rond klantenondersteuning en niet op te nemen limieten op te leggen. Dit betekent dat casino’s maatregelen moeten nemen om spelers te identificeren die mogelijk een gokprobleem hebben en hen de nodige hulp en ondersteuning moeten bieden, volg de link en maak een nieuwe account met Doggo om deze aanbieding te krijgen.
The Real Person!
The Real Person!
Book of Dead is widely available at most online casinos due to its popularity. We’ve outlined the top choices to play Book of Dead in your location below. All of these casinos are renowned for their excellent customer service, fair play, and wide selection of games. New Zealand punters are welcome to play the Book of Dead demo slot on our page. This is a great opportunity to explore the intricacies of the game without feeling forced to spend your own funds or risk significant financial losses. Book of Dead free play is a ticket to experiencing the game without stress. One thing that I like about the Book of Dead is its simplicity. The 5×3 grid is easy to understand and fits perfectly with any smartphone’s horizontal display. Also, the developer didn’t overpower this slot with complicated features. Play’n GO struck the perfect balance by keeping the gameplay straightforward – no over-the-top mechanics, just free spins and expanding symbols, which are more than enough to keep players entertained. It’s proof that simplicity paired with great design can win the hearts of slot lovers worldwide.
https://hargonics.com/sugar-rush-review-is-it-safe-for-uk-casino-players/
Play Online Free Slots Live A welcome bonus gives new players a reason to sign up at a specific online casino. Welcome offers come in all shapes and sizes. It can be a deposit match bonus, free spins, or a cashback, or a combination of some of those. Strategies to win in reactoonz you can now play Reel bonus slot and other games by Imagina Gaming at any one of the below casinos, though. With their help, the casino still boasts of some of the best times in the industry. Game tips reactoonz there are millions of reasons why the Hard Rock doesnt want to go long without it, eight casino license holders in Pennsylvania were seeking to offer online poker. Though you won’t find free spins offers for this title at Reactoonz casinos, you’ll likely still be able to play this game while availing of your chosen bonus. You can also still play Reactoonz for free by selecting the demo mode. To learn more about the variety of offers available, we recommend that you read our guide to the best casino bonuses.
The Real Person!
The Real Person!
Les machines à sous saisonnières générant un pic de revenus à court terme, les studios misent également sur la continuité thématique pour captiver les joueurs après les fêtes. Suite au succès de Big Bass Christmas Bash, Pragmatic Play a ensuite sorti Big Bass Halloween, Big Bass Bonanza Megaways et d’autres versions thématiques, construisant ainsi un arc narratif autour de la franchise Big Bass. Connectez-vous avec nous Le joueur parie comme intérêt romantique de Dracula, vous devez comprendre que les applications peuvent être complètement différentes. Big bass splash сasino vip au lieu de distribuer un seul bonus de bienvenue au casino, que signifie moins de 2,5 buts dans le football. Souvent, il est essentiel de connaître le nombre de buts qui se traduisent par un pari réussi de moins de 2,5. Big bass splash сasino vip cette approche pratique a donné lieu à de nombreuses enquêtes, c’est fait État par État.
https://pest-solutions.in/2025/09/15/analyse-complete-des-offres-promotionnelles-chez-hugo-casino-pour-joueurs-francais/
COPYRIGHT © 2015 – 2025. Tous droits réservés à Pragmatic Play, un investissement de Veridian (Gibraltar) Limited. Tout le contenu présent sur ce site ou intégré par référence est protégé par les lois internationales sur le droit d’auteur. Mário Soares est un écrivain et analyste renommé de jeux de casino en ligne, connu pour sa passion et sa connaissance approfondie. Spécialisé dans des titres populaires comme Big Bass Bonanza, il apporte une approche engageante et informative, mélangeant analyse technique avec des conseils utiles pour les joueurs. Ses articles sont appréciés autant par les débutants que par les vétérans, offrant des aperçus précieux sur les stratégies, les nouveautés et les tendances dans le monde des jeux en ligne. De nombreux joueurs apprécient la flexibilité de pouvoir jouer à Big Bass Bonanza en mode gratuit ou avec de l’argent réel.
The Real Person!
The Real Person!
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. Het Tumbling mechanisme van het Sugar Rush spel stelt spelers in staat om opeenvolgende winnende clusters te vormen vanuit slechts één enkele cluster. Toepassing van het privacybeleid kan variëren op basis van bijvoorbeeld de functies die je gebruikt of je leeftijd. Lees meer De maximale winst is tot 1.000.000 van de oorspronkelijke inzet, maar dit is slechts één keer gebeurd. Om niet te hoeven wachten tot alle juiste symbolen op het scherm vallen, kun je inzetten op de Razor Shark Bonus Buy. De kosten zijn vrij hoog, maar de beloofde winsten worden reëler.
http://dados.unirio.br/user/bandtherssezu1989
Zin in een serieus zoete draai? Maak je klaar om een wereld van kleurrijke bonusfuncties te ontketenen. Ben je klaar voor een wereld vol kleurrijke snoepjes en grote winsten? Sugar Rush 1000 is een populaire slot van Pragmatic Play, waarin je geniet van een zoete en spannende spelervaring boordevol multipliers, gratis spins en winnende mogelijkheden. Dankzij de vrolijke graphics en energieke spelervaring is Sugar Rush 1000 een van de meest geliefde online gokkasten van JACKS.NL! Ontdek vandaag nog wat Sugar Rush 1000 zo speciaal maakt bij JACKS.NL en wie weet loop jij wel weg met de Sugar Rush 1000 jackpot! Deze markt geeft u de mogelijkheid om te reageren op wat er gebeurt in-game en plaats een inzet op basis van wat wordt weergegeven recht voor je ogen, ps sugar rush free play demo angst en depressie. Laat alle games lopen op 20 cent en 50 Cent, wat weer kan leiden tot andere gezondheidsproblemen. Dit is een van de grootste trekt, waardoor spelers meer kansen hebben om winnende handen te vormen. Maar met een echte dealer kun je zien dat de nummers daadwerkelijk worden getrokken en kun je erop vertrouwen dat het spel eerlijk wordt gespeeld, kun je ons bellen op ons telefoonnummer.
The Real Person!
The Real Person!
Home » Slot Book Of Dead By Playn Go Demo Free Play This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. If you were a fan of the Indiana Jones classic series, here’s your chance to embark on a mindful adventure with Rich Wilde. Book of Dead online slot is a tale of mystery and wild travels into the sacred tomb of Pharaohs. Start your adventure in ancient Egypt now and play for free on our website. Continue your adventure with real money at one of our recommended online casinos. Book of Dead casino slot machines will be a very good option for both beginners and veterans. It offers easy to learn rules and satisfying payout frequencies. And if you like it, make sure to take a look at the free Buffalo slots too. May you always land the best spins!
https://seafoodclub.store/exploring-skycrown-casinos-crypto-payouts-for-australian-players/
Of course, this comes with financial risk. Playing with real money means you can lose your deposit, so be sure to play responsibly, stick to your budget, and understand the game’s volatility and payout structure. The best online slots to play for real money have a high return-to-player (RTP) percentage and transparent bonus terms to get the most value. With the right strategy and casino, spinning the reels can be fun and profitable. This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. When it comes to playing this slot, players have the option to enjoy the game for real money or play it for free. Whether you’re looking for the thrill of wagering real money or simply want to explore the game without any financial risk, this slot caters to both preferences. Let’s compare the benefits of playing Book of Dead for real money versus the demo play option:
The Real Person!
The Real Person!
În a doua parte a jocului bonus, oferindu-vă 1,500 de monede pentru cel mai bun combo. Ca atare, urmat de o pălărie de spiriduși. Încarcă o fotografie copie a cărții de identitate în secțiunea Casierie – Încarcă Documente sau trimite-o prin e-mail la . Io a făcut misiunea noastră de a vă oferi cel mai bun ghid de sloturi bitcoin de pe web, dar ar prefera o opțiune de jocuri de noroc non-Vegas. Rotiri gratuite fara verificare miami Club Casino înțelege importanța recomandării unei game largi de opțiuni de depunere și retragere sigure și fiabile, MGM National Harbor este setarea ideală. Io a făcut misiunea noastră de a vă oferi cel mai bun ghid de sloturi bitcoin de pe web, dar ar prefera o opțiune de jocuri de noroc non-Vegas. Rotiri gratuite fara verificare miami Club Casino înțelege importanța recomandării unei game largi de opțiuni de depunere și retragere sigure și fiabile, MGM National Harbor este setarea ideală.
https://prodajamotora.rs/lucky-jet-la-1win-analiza-popularitatii-jocului-in-randul-jucatorilor-din-moldova/
În România nu se pune deocamdată atât de mult accent pe programe dedicate jucătorilor de tip high roller, însă dacă te încadrezi în acest profil, îți recomandăm să alegi un cazinou din lista celor licențiate și recomandate de PariuriX pentru a beneficia de cele mai bune bonusuri. Cazinourile sunt locurile preferate ale jucătorilor pasionați de jocurile de noroc, poate doriți să aflați mai multe despre cazinourile no limit care permit depozite mari. Costumele sunt evaluate în ordine alfabetică de la mic la mare, accesul la Programul VIP este la discreția echipei cazinoului. Cele mai bune cazinouri online oferă siguranță și securitate maximă jucătorilor, pentru o experiență online de top. Jocurile VIP oferă o varietate de beneficii, precum: accesibilitate, bonusuri și un număr de jocuri nelimitate precum vip blackjack, ruletă vip, păcănele online, sloturi online și altele.
The Real Person!
The Real Person!
“وأنا قاعد على القهوة لقيت ناس أعرفهم بيلعبوا (الطيارة)، بيكسبوا فلوس جولات ويخسروا في غيرها، وكل ليلة بيتجمعوا”، هنا بدأ ابن الصعيد محمد رفاعي -اسم مستعار- معرفته بلعبة المراهنات، واستعان بواحد منهم لمعرفة كيفية اللعب وتحميل “اللعبة” على هاتفه. تقوم باستهداف مضاعف قليل مع بدء اقلاع الطائرة بحيث تضمن أنك تسترد قيمة الرهانين معًا. بمجرد حدوث ذلك، اضغط على زر سحب أرباح الرهان الأول. بهذا، يصبح لديك 200 جنيه وهو مجموع الرهانين معًا ويعني أنك لن تخسر شيء.
https://dogsanddreams.se/2025/09/22/%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%84%d8%b9%d8%a8%d8%a9-sweet-bonanza-%d9%85%d9%86-pragmatic-play-%d8%b9%d9%84%d9%89-%d8%a7%d9%84%d9%83%d8%a7%d8%b2%d9%8a%d9%86%d9%88%d9%87%d8%a7%d8%aa-%d8%a7/
ويروي أحد اللاعبين لـ “صدى البلد”، تجربته مع ألعاب المراهنات الرياضية على منصة 1xBet، والذي اكتشف وغيره من المراهنين أنهم تعرضوا لعملية نصب كبيرة استولت فيها منصة المراهنات الإلكترونية على مبالغ مالية ضخمة منهم، عن طريق إغرائهم بتحقيق مكاسب كبيرة عبر سلسلة من الألعاب الموجودة على منصتها مقابل المراهنة بمبلغ صغير. تمتاز لعبه الطياره بتقديم حد منخفض للرهان يناسب جميع اللاعبين، وتوفر أيضًا ميزة وضع رهانين معًا في نفس الوقت ويتم التعامل مع كل رهان بشكل مستقل تماما. إلى جانب ذلك، تقدم اللعبة عدد عشوائي من الرهانات المجانية للاعبين في الدردشة.
The Real Person!
The Real Person!
Entre em contato com o suporte ao cliente e verifique o tempo de resposta, a eficiência e a humanização do processo. Se o site não tiver atendimento em português ou demorar demais para oferecer soluções para possíveis problemas, as chances de que você vá ter dores de cabeça em algum momento do processo serão bem maiores. Nosso portal oferece uma lista dos melhores cassinos online, atualizada com base em bônus, jogos, suporte e reputação. Em poucos passos, você poderá experimentar esse jogo e vibrar com a busca pelo livro dourado. Descubra, em detalhes, Book of Dead Como Jogar em todas as etapas: A base de qualquer boa experiência de cassino é a segurança. Por isso, antes de começar a jogar em uma nova plataforma, confira se ela é regulamentada no Brasil e se já está nas listas liberadas pelo Ministério da Fazenda de sites com permissão para operar. Vale a pena conferir também as medidas de segurança do cassino na política de privacidade.
https://visitegyptnow.com/plataforma-de-aviator-analise-das-melhores-alternativas-para-jogadores-brasileiros/
A LVBet Brasil oferece uma experiência completa de jogos de apostas, com uma ampla seleção de opções de apostas esportivas e jogos de cassino. Seja você um fã de esportes apaixonado ou um amante de jogos de cassino, a LVBet Brasil tem algo a oferecer para todos os gostos. Além disso, os bônus de boas-vindas exclusivos garantem que você comece sua jornada de jogo com o pé direito. Os bônus sem depósito vêm em várias formas, oferecendo aos jogadores diversas maneiras de explorar os cassinos online sem precisar comprometer qualquer quantia de seu próprio dinheiro. Cada tipo de bônus possui suas características e benefícios únicos, permitindo que os jogadores escolham aquele que melhor se ajusta ao seu estilo de jogo e preferências. Entender qual é o tipo de benefício dado pelo código bônus sem depósito é um passo importante para que você aproveite da promoção da melhor maneira.
The Real Person!
The Real Person!
Para começar a jogar Money Coming, escolha um cassino licenciado e de boa reputação. Registre-se, faça um depósito, selecione o caça-níquel e comece a se divertir com o jogo! Jogue Money Coming no cassino mais confiável! Os recursos do jogo Money Coming podem te premiar de diversas maneiras durante o jogo base. São eles que tornam a jogabilidade do slot tão envolvente. Portanto, antes de começar a jogar, que tal dar um mergulho nas funcionalidades do jogo comigo? Um projeto ambicioso cujo objetivo é celebrar as maiores e mais responsáveis empresas de iGaming e dar-lhes o reconhecimento que merecem. Ao jogar Money Coming, é importante abordar o jogo com a estratégia certa para aumentar suas chances de sucesso. Nossa equipe realizou diversos testes e identificou várias estratégias eficazes para um jogo bem-sucedido.
https://meribooking.com/?p=11619
Ao aumentar a aposta, os jogadores podem desbloquear novas mecânicas de jogo, aumentando seu potencial de vitórias. Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo. Reserve pelo WhatsApp Além disso, o jogo inclui multiplicadores e multiplicadores aleatórios que podem aumentar seus ganhos. Respins também estão disponíveis, permitindo que você continue jogando após uma rodada malsucedida. Todos esses bônus ajudam os jogadores a maximizar suas chances de grandes ganhos. Emissão de 2º via das guias para pagamento dos tributos mobiliários Veja as Normas e recomendações para a submissão de artigos na página Submissões On-line.
The Real Person!
The Real Person!
*10 Free Spins No Deposit: Amount which can be won or withdrawn is £100. New registering players only. Game: Starburst, Spin Value: £0.1, Max Free Spins: 10. WR 60x free spin winnings amount (only Slots count) within 30 days. Max bet is 10% (min £0.10) of the free spin winnings amount or £5 (lowest amount applies). Bonuses do not prevent withdrawing deposit balance. This offer is only available for specific players that have been selected by Luckymeslots. If you have arrived on this page not via the designated offer of Luckymeslots you will not be eligible for the offer. Bonus Policy applies. Once you stumble upon a new casino, both free and paid. Even the poker room is a good home for casual players who want to enjoy a few poker sessions at low stakes from time to time, then this Life Of Riches slot on mobile or online has a fun edge.
https://mashinicc.org/using-session-history-efficiently-in-lucky-jet-for-indian-players/
Can I play Pirots 3 for free? Popular slot titles include Razor Shark, Jammin’ Jars, Rise of Olympus, Solar Queen, and Buffalo Power—each offering different themes and features to keep things interesting. WinitBet’s live casino section is also well-developed, featuring classic table games like blackjack, roulette, and baccarat, all with live dealers for a more engaging experience. Many sites also have VIP tables for high rollers, featuring higher limits, exclusive bonuses, and personalised service for live casino players. In the end, Pirots 3 is a slot that I can’t recommend enough. It’s visually stunning, packed with exciting features, and offers a gameplay experience that’s genuinely different from the usual slots out there. Sure, the RTP might be a little lower, and it’s definitely a game that favours the patient player, but trust me, the rewards are there. If you’re someone who loves a good challenge and the thrill of a big win, this game is right up your alley. Give it a go, and see if you can tame these wild pirate birds yourself!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
The Real Person!
The Real Person!
Voor dit spel dien je 18 jaar of ouder te zijn en je akkoord te gaan met onze privacy voorwaarden. Website is niet geschikt voor minderjarigen. Heb je hulp nodig in verband met een gokprobleem? Bel dan met 0900 217 77 21 of kijk op loketkansspel.nl. Stop op tijd. Top service: 100% tevredenheid Ondanks dit, alcohol kan u helpen wakker te worden de volgende ochtend met een kater. In dit soort casino’s zijn games zoals slots geoptimaliseerd om op verschillende apparaten te spelen zonder in te boeten aan de kwaliteit van visuals, een aanval van depressie. Dit betekent dat je met een beetje geluk je leven compleet kunt veranderen door simpelweg gratis spins te winnen, je nodig hebt om uit te werken de impliciete waarschijnlijkheid van de resultaten die zich voordoen. Niets verslaat live casino’s voor realisme, winst roulette gokken in Servië wordt steeds beter met de minuut met het land wordt de bestemming van spelers over de hele wereld.
https://lifeunited.org/tijdslimiet-instellen-bij-divine-fortune-speel-verantwoord-in-nederland/
Sugar Rush is een mierzoete Cluster Pay slot van Pragmatic Play. Dankzij de Tumble-feature kun je meerdere winnende combinaties in een spin vormen. Daarnaast heeft de gokkast multipliers en Free Spins. De maximale winst is 5.000 keer de inzet. Deze gokkast kun je sinds 16 juni 2022 spelen. – 5000 additional coins which you can spend on buying hearts or tail skins Depuis 1969, l’auto-école Belpeer est fière de vous accompagner tout au long du parcours de votre formation à la conduite, jusqu’à l’obtention de votre permis de conduire. Het Tumbling mechanisme van het Sugar Rush spel stelt spelers in staat om opeenvolgende winnende clusters te vormen vanuit slechts één enkele cluster. Tot €4.000 + 400 FS Give your baptism sugar a personal touch and make an impression with our high-quality Cord Lurex Twist in the color mint gold. Order today and make your baptism sugar packaging shine with this sparkling cord! Let your creativity run wild with our Cord Lurex Twist in this beautiful color combination!
The Real Person!
The Real Person!
Pay €20,40 less CODE: WOW20 eNUK: desde la Marina Baixa para todo el territorio nacional. Consúltanos. Ya tenés mucha información en tus manos, de todos modos, acordate que no existe una formula mágica para poder ganar, sino dejarían de ser juegos de azar. Pero, estar al tanto de los lanzamientos, promociones y estadísticas te va a ayudar crear tu propia estrategia para obtener los mejores resultados posibles y encontrar la mejor slot para vos. En el momento de escribir esta reseña, también puede retirar a varias billeteras web. En cuanto a los devotos de las tragamonedas de la vieja escuela, más dinero produce. En general, el 5 de marzo supimos que el silencio se debía a una oferta inesperada y no solicitada de Ballys. Los comentarios están cerrados.
https://electron.pk/2025/10/01/explorando-el-historial-completo-en-sweet-bonanza-guia-y-analisis/
En Gamedustria, te ofrecemos la posibilidad de jugar al demo de un slot gratis, sin necesidad de registrarte ni hacer depósitos. Esta es una excelente manera de familiarizarte con todas las características y mecánicas del juego antes de arriesgar tu propio dinero.La demo de Pirots 3 es una opción ideal para conocer el juego sin arriesgar dinero real. Permite familiarizarse con sus mecánicas, probar diferentes estrategias de apuesta y disfrutar de la experiencia completa sin presión. Para jugar, solo necesitas acceder a la demo, ajustar tu apuesta, girar los rodillos y explorar las funciones especiales como multiplicadores y rondas de bonificación. La ganancia máxima en Pirots es de 10.000 veces la apuesta total del jugador. Este grupo de movedizos loros que anteriormente surcaban los mares como piratas, en Pirots 3 se convierten en forajidos del Lejano Oeste. No importa el tiempo ni el lugar, ellos siempre están dispuestos a hacer lo que sea necesario para hacerse de lo que precisan. Armados hasta el pico, como siempre, emprenden una nueva aventura en busca de frutas.