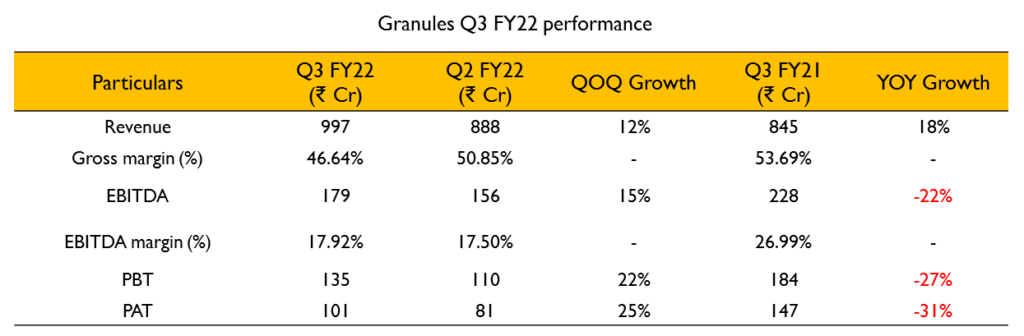
- Revenue for the quarter stood at ₹996.8 Cr (18% growth YoY). EBITDA for the quarter was at ₹173 Cr (17.9% decline YoY). EBITDA margins were at 17.4% compared to 25.1% in Q3 FY21.
- They faced challenges during the quarter such as RM price increases, unstable supply from China and logistics issues.Their volumes were down compared to last year but they have been able to pass on a significant amount of price increases to their customers.
- The overall gross margin recorded is lower due to change in segment mix in the total revenue. Share of Finished dosage has come down from 57% in Q2 to 46% in Q3 due to higher inventory build-up at USA and year end.
- During the quarter they filed two ANDAs, two Canadian Dossiers, one US DMF, one CEP and received three ANDA approvals.
- They are going to be backward integrating deep into the value chain. They plan to start from basic chemicals that are commercially available in India.
- R&D expenditure for the quarter was at ₹35 Cr. The YTD R&D spend has been ₹109 Cr.
- Capex spend for the quarter was at ₹95 Cr. Total capex done in the 9 months was at ₹327 Cr.
- The margins on Paracetamol have shrunk. Although they were able to pass on some price increases, the RM prices continued to increase. So the sale value is high but the margins are low.
- They bought some batches of PAP from one Indian manufacturer but that had some quality issues. They were hoping another manufacturer’s plant would start but it hasn’t. So PAP availability is still an issue. However, the biggest plant in China which had shutdown is opening up again in a couple of months.
- The revenues from non-core molecules is expected to increase significantly over the next few years as they are planning the R&D in such a way that they can capture the dominant market share in those molecules.
- They are focusing on green chemistries and sustainable technologies and are trying to be a carbon neutral company in the next few years.
- In the non-core molecules, they are not being too aggressive right now as most players in the industry are defending their market share which will lead to unsustainable prices. They are only bidding on contracts where the prices and margins are acceptable to them.
- For the new molecules going forward, they will be starting from KSMs itself so the need for outside suppliers will be reduced. For the existing molecules, they are working on backward integration as far as possible in a green way. This could take about 2 years to yield results.
- Capacity utilization for Paracetamol is 60%. But the price of Paracetamol is so high currently that the revenues from this 60% is equivalent to running the plant at 100% in the past.
- They had a JV in China for manufacturing Ibuprofen API but they have exited it. It helped them secure supply when there were Ibuprofen shortages but currently there is a surplus of Ibuprofen capacity. BASF is the greenest and cheapest producer with the best technology and they can make Ibuprofen much cheaper in the US than anyone else in the world. So there are going to be tough times ahead for Ibuprofen manufacturers. But formulation manufacturers are well protected.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? https://accounts.binance.com/en/register?ref=JHQQKNKN
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://www.binance.info/sk/register-person?ref=OMM3XK51
I’m very happy to find this page. I want to to thank you for your time for this particularly
wonderful read!! I definitely loved every little bit oof iit and I have you
saved to fav to see new stuff in ypur site. https://Glassi-Freespins.Blogspot.com/2025/08/how-to-claim-glassi-casino-free-spins.html
Aw, this was a very nice post. Taking the time and actual effort
to make a great article… but what can I say…
I procrastinate a lot and never mannage to get nearly anything done. https://lr-mediconsult.de/Unternehmen/tonebet-casino/
I am curious to find out what blog platform you’re utilizing?
I’m having some small security issues with my latest blog and I
would like to find something more risk-free.
Do you have any solutions? https://Usnationalgoalkeeper.wordpress.com/