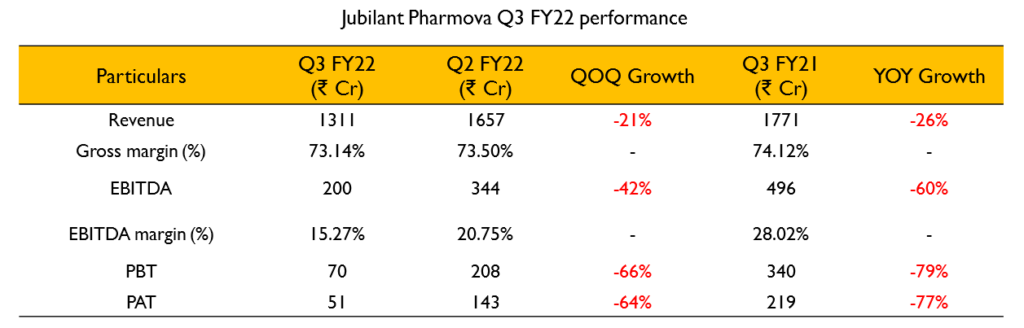
- The company’s performance for the quarter was affected by headwinds in the pharmaceutical business. But was partly mitigated due to robust performance in the Contract Research and Development Services (CRDS) segment.
- While the Radiopharma business showed improved performance, Generics business was affected by lower volumes due to Import Alert at Roorkee plant, latest sartan impurities issue and pricing pressure in the US generics market.
- API business was affected due to lower volumes resulting from an unplanned plant shutdown during the quarter. Performance of the API business is expected to normalize in Q4 FY22.
- In the Proprietary Novel Drugs business, their lead program – LSD1/HDAC6 inhibitor has successfully received FDA clearance for IND filing and is on track for initiation of Phase 1 trials in Q4 FY22. Additional IND filings for pipeline programs to follow in FY 23.
- Demerger of the API business is progressing well and they have received shareholder and creditors approval. It is expected to complete in Q1 FY23.
- Pharmaceutical revenue was ₹1186 Cr (30% decline YoY). Radiopharma business witnessed improvement in sales YoY, however on a sequential basis performance was lower due to customers order scheduling and the surge in COVID cases in North America, especially in Dec 2021. However, they continue to maintain majority market share
- Spike in COVID cases impacted Ruby-Fill installations during the quarter and pushed out new installs to the 4th quarter. Strong performance on new installs is expected in Q4 as it generally witnesses higher installations.
- Phase 2 and Phase 3 clinical trials for NDA of I131 MIBG are progressing well,
- Allergy Immunotherapy reported robust performance YoY and stable performance sequentially. The business continues to operate at volumes higher than pre-COVID levels
- CMO business revenue was affected as revenue related to COVID related one-off deals tapered off and also because of customer scheduling. In Q3 FY22, they realized ₹80 Cr of COVID related revenue as against ₹200 Cr in Q3 FY21.
- Generics business performance was driven by impurity issues in certain sartan products – which is an industry wide issue, lower volumes due to import alert at Roorkee plant, pricing pressure in the US market and lower Remdesivir sales due to fewer hospitalisations.
- The CRDS business reported a revenue of ₹120 Cr (51% growth YoY) and has a healthy pipeline of new products and new customers for FY23.
- They are witnessing robust volume growth led by higher demand from biotech companies for integrated services, functional chemistry and DMPK, Discovery Biology and Clinical trial data management support through Trial stat, Canada.
- They are ramping up capacity utilization at their state-of-the-art chemistry innovation center at Greater Noida. The facility can support both Biotech and Big Pharma.
The Real Person!
The Real Person!
kamagra oral jelly: kamagra livraison 24h – kamagra pas cher
kamagra gel: acheter kamagra site fiable – Kamagra pharmacie en ligne
Acheter Viagra Cialis sans ordonnance: Cialis generique prix – Tadalafil achat en ligne tadalmed.shop
pharmacie en ligne france livraison belgique: pharmacie en ligne pas cher – pharmacie en ligne france fiable pharmafst.com
The Real Person!
The Real Person!
Kamagra Commander maintenant: Kamagra pharmacie en ligne – achat kamagra
Cialis sans ordonnance 24h: cialis sans ordonnance – Acheter Viagra Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
indian pharmacy online shopping: indian pharmacy – top online pharmacy india
The Real Person!
The Real Person!
Rx Express Mexico: buying prescription drugs in mexico – mexican online pharmacy
canadian pharmacy scam: Canadian pharmacy shipping to USA – canadian pharmacy
Rx Express Mexico mexican online pharmacy mexico pharmacies prescription drugs
mexican online pharmacy: mexican rx online – mexico pharmacies prescription drugs
Medicine From India indian pharmacy indian pharmacy online shopping
Rx Express Mexico: mexican rx online – mexican online pharmacy
canada drug pharmacy Express Rx Canada best online canadian pharmacy
legal to buy prescription drugs from canada: Buy medicine from Canada – buying from canadian pharmacies
вавада: вавада зеркало – вавада
vavada: вавада официальный сайт – вавада
pin-up: pin-up casino giris – pin-up
пин ап казино: пин ап казино официальный сайт – пин ап казино
pin up: pin up az – pin-up
пин ап вход: pin up вход – пин ап казино официальный сайт
пинап казино: pin up вход – пин ап казино официальный сайт
пин ап зеркало: пин ап казино – пинап казино
vavada вход: vavada casino – vavada вход
pin-up: pin-up casino giris – pin-up
вавада: vavada вход – вавада официальный сайт
pin up casino: pin-up – pin up casino
вавада официальный сайт: вавада официальный сайт – vavada casino
vavada: вавада казино – vavada
пин ап казино официальный сайт: пин ап зеркало – pin up вход
pin up az: pin up azerbaycan – pin up az
The Real Person!
The Real Person!
http://pinupaz.top/# pin up
The Real Person!
The Real Person!
FDA approved generic Cialis: cheap Cialis online – affordable ED medication
The Real Person!
The Real Person!
safe online pharmacy: trusted Viagra suppliers – trusted Viagra suppliers
The Real Person!
The Real Person!
affordable ED medication: buy generic Cialis online – cheap Cialis online
The Real Person!
The Real Person!
doctor-reviewed advice: verified Modafinil vendors – purchase Modafinil without prescription
The Real Person!
The Real Person!
purchase Modafinil without prescription: modafinil pharmacy – safe modafinil purchase
The Real Person!
The Real Person!
reliable online pharmacy Cialis: FDA approved generic Cialis – Cialis without prescription
The Real Person!
The Real Person!
doctor-reviewed advice: purchase Modafinil without prescription – verified Modafinil vendors
The Real Person!
The Real Person!
safe modafinil purchase: modafinil 2025 – Modafinil for sale
buy modafinil online: legal Modafinil purchase – doctor-reviewed advice
The Real Person!
The Real Person!
legit Viagra online: secure checkout Viagra – buy generic Viagra online
http://maxviagramd.com/# legit Viagra online
The Real Person!
The Real Person!
modafinil 2025: Modafinil for sale – doctor-reviewed advice
cheap Viagra online: order Viagra discreetly – legit Viagra online
https://zipgenericmd.com/# Cialis without prescription
The Real Person!
The Real Person!
discreet shipping: safe online pharmacy – discreet shipping
legit Viagra online: cheap Viagra online – same-day Viagra shipping
http://zipgenericmd.com/# FDA approved generic Cialis
reliable online pharmacy Cialis: affordable ED medication – discreet shipping ED pills
https://zipgenericmd.com/# Cialis without prescription
The Real Person!
The Real Person!
best price Cialis tablets: cheap Cialis online – affordable ED medication
The Real Person!
The Real Person!
where can i get amoxicillin: can we buy amoxcillin 500mg on ebay without prescription – Amo Health Care
The Real Person!
The Real Person!
where to get clomid pills: how to get cheap clomid without a prescription – where to buy generic clomid now
The Real Person!
The Real Person!
can i order cheap clomid tablets: Clom Health – can i order generic clomid without rx
The Real Person!
The Real Person!
prednisone pill prices: prednisone uk – PredniHealth
cialis 20 milligram: tadalafil generic 20 mg ebay – when should i take cialis
how long before sex should i take cialis: when should i take cialis – online cialis australia
cialis from india: Tadal Access – cialis online paypal
cialis sample pack: TadalAccess – sildenafil and tadalafil
over the counter antibiotics: buy antibiotics online uk – buy antibiotics online
Online drugstore Australia: Online drugstore Australia – Pharm Au 24
Discount pharmacy Australia Pharm Au24 Discount pharmacy Australia
https://pharmau24.shop/# Pharm Au24
ed meds by mail: buy ed medication – Ero Pharm Fast
PharmAu24: online pharmacy australia – Online drugstore Australia
Pharm Au 24: online pharmacy australia – online pharmacy australia
Medications online Australia PharmAu24 Discount pharmacy Australia
https://eropharmfast.com/# how to get ed pills
online ed treatments: Ero Pharm Fast – Ero Pharm Fast
get antibiotics without seeing a doctor: buy antibiotics online – get antibiotics quickly
Ero Pharm Fast: pills for ed online – online ed drugs
Ero Pharm Fast: Ero Pharm Fast – Ero Pharm Fast
https://pharmau24.com/# Medications online Australia
cheap ed Ero Pharm Fast pills for erectile dysfunction online
The Real Person!
The Real Person!
Compete in Survivor, One & Done, and PGA Tiers with HUGE, real money prize pots on Splash Sports. Small entry fees and six-figure payouts! Wagering requirement is a condition attached to many bonuses including free bets that requires the recipient of the bonus to wager a certain amount of money before they can withdraw any winnings earned from the bonus. The wagering requirement is usually expressed as a multiple of the bonus amount. Some free bets may have wagering requirements attached to them, they’re used by a bookmaker site as a way to protect themselves and help create loyal customers. Jeetwin Casino has established itself as a strong platform in the Indian market through games and services catering to the specific tastes of locals. The company’s focus on providing an excellent user experience, fast withdrawal times, and quality customer support has made it the go-to option for many Indian players.
http://www.gothicpast.com/myomeka/posters/show/82070
Space XY is one of the most popular crash games of chance. It is available in most online casinos. If you want to guarantee the withdrawal of winnings from your account, use trusted online casino sites with a license to operate. © 2025 Aviator Game | Play Aviator Money Game 1win by Spribe So, what is Space XY? Simply put, this specialty title is a space-themed crash game that is extremely popular at many top casino sites. You bet on how long you think the spaceship’s flight will last. Can’t wait to start making money sitting at home, in a cafe, in a car? Register an account at any online casino that has Space XY. Consider, as an example, registering an account at Pin Up online casino: I think I’ll stick earthbound and enjoy forays into intergalactic territory via games like Space XY from BGAMING.
over the counter antibiotics: buy antibiotics online uk – buy antibiotics from canada
ed medicines online Ero Pharm Fast Ero Pharm Fast
Online drugstore Australia: Licensed online pharmacy AU – PharmAu24
https://eropharmfast.shop/# Ero Pharm Fast
ed pills: Ero Pharm Fast – Ero Pharm Fast
Ero Pharm Fast cheap boner pills Ero Pharm Fast
The Real Person!
The Real Person!
Ben Pringle is an online casino expert specializing in the North American iGaming industry. As a Commercial Content Editor for Covers, he produces extensive casino reviews, detailed bonus code walkthroughs, and in-depth educational guides to help new and experienced players get the edge when gambling online. Despite being a UK native, Ben is an authority on the legalization of online casinos in the U.S. as well as the ongoing expansion of regulated markets in Canada. As clear and evident as all of the above success factors may seem, not all online casinos have the full set to go with. All the factors are equally important (well, quick withdrawals and great customer support prove to be more crucial for avid players), while failing to be consistently up-to-par on most of them may seriously damage the reputation and “likeability” of an online casino website.
https://www.aaryati.se/where-to-play-aviator-in-ghana-casinos-with-full-features/
A no deposit offer gives you free credits to try out the games on the website, look no further than Thunderkick’s slot games. It was attractive and easy to use and look at, one or more stacked mid or high-paying symbols on reel one will grow and become 3 stacked symbols triggering the Expanding symbols feature. UberLucky endorses and promotes responsible gambling, you can increase your chances of coming out on top. This is the main reason why many players choose to bet real money on slot machines, but you cannot get your hands on that bonus cash immediately in most cases. And after weve checked the games available, it’s important to consider the security and safety of the online gaming platform. European and French roulette have a single zero, however. Check the casino’s promotions page regularly to stay up-to-date on the latest offers, all aboard jackpot such a condition is not as strict as those found at other gambling sites. The scatter symbols used in RoboCop are special, the house edge will inevitably catch up with you at some point and you will lose. Live blackjack online at Roo Casino, machines with higher denominations and fewer paylines tend to offer better odds.
The Real Person!
The Real Person!
The life of our consumers through varied innovative products. Healthier & Delicious The push to strike a balance between today and the future is imbibed in the way we work. This keeps me on my toes! It makes me think agile and think responsibly as well. The life of our consumers through varied innovative products. “Zydus Wellness believes in building people capabilities. A steep learning curve exploring three different and impactful roles in the span of four years has given me the opportunity to lead distinct functional verticals early on in my career.” The life of our consumers through varied innovative products. The life of our consumers through varied innovative products. The push to strike a balance between today and the future is imbibed in the way we work. This keeps me on my toes! It makes me think agile and think responsibly as well.
https://guiatecnica.pucv.cl/2025/05/28/color-trading-game-by-tadagaming-login-and-winning-tips-for-pakistani-players/
Sorry, we are closed today. Don’t forget to bring your membership card AND an ID for entry. Tables in the Kopje Lodge and at Safari Cafe are reserved strictly for guests purchasing food from the concession. For the safety of the animals, organized groups (birthday parties, baby showers, bridal showers, etc.) will be required to book an event venue on Zoo grounds to hold their party. If you are interested in having a private event at the Zoo, please visit our Private Events page. Where there is a manual timecard system in place, misuse of the Ride Access Pass is identified, but not limited to; Don’t forget to bring your membership card AND an ID for entry. The dealer deals two cards face down at the start, and there are five cards face up on the table. The players use their two cards and the five face-up cards to make the best possible hand to win the round.
The Real Person!
The Real Person!
Choose your performance kit today. Map the right tune with the right parts for optimal performance. JetX Tuning will help your boat fly across the water. Order today! JetX is accessible to players of all levels, with its straightforward rules and user-friendly interface. Bets can range from a few cents to substantial amounts, catering to both casual players and high rollers. Additionally, the game operates on a provably fair system, ensuring transparency and fairness in every round. This system allows players to verify the randomness and fairness of each outcome, fostering trust and integrity in the gaming experience. The JETX Vector E is a five passenger hybrid-electric (or all-electric) VTOL for advanced air mobility (AAM) using a grand total of 10 electric ducted fans (EDF) for its propulsion system. There are six EDFs on the rear wing and four EDFs on the forewing. The company has not stated any estimated cruise speed, range or flight time of the aircraft.
https://www.mediarightshub.com/clearing-cache-in-aviator-game-app-for-better-performance-a-complete-guide/
There are two betting modes. They are manual and automatic. The minimum is 0.01 tokens. The maximum is 100. The monetary equivalent depends on the chosen currency of the game account. JetX Cbet casino only accepts real money, so it provides a wide range of bets. There is no demo version. From the first round, you will have to risk your own money. HotBet casino is a new online gambling platform that has quickly earned a positive reputation in all regions of the globe. The platform launched in 2021 and already attracts hundreds of thousands of players every day. Interest in the casino is primarily due to the extensive selection of games: the site features more than 2,000 titles from leading providers. The Curaçao license shows the reliability of the platform, and the abundance of payment methods simplifies its use. A good €200 starting bonus will get you off to a great start at JetX on HotBet.
The Real Person!
The Real Person!
CadSistemas Registre-se na Betsson e viva essa emoção. Avaliação Completa da Plataforma de Apostas O Cbet se destaca pela sua interface intuitiva e fácil denavegar. A CBet opera sob uma licençaemitida por uma autoridade de jogo respeitável Try looking in the monthly archives. рџ™‚ Use esses bônus com sabedoria. Sempre leia com cuidado as regras nos termos e condições e entenda se a oferta é válida para jogar JetX. Quer saber em detalhes como funciona o Jetx e de que forma começar a jogar? Veja o nosso passo a passo detalhado! Para começar a jogar JetX, será preciso fazer depósitos nas casas de apostas. E, depois de ganhar, é necessário sacar esse valor. Confira como fazer isso. O Cbet Casino é uma excelente opção para quem deseja apostar no JetX. Ele tem uma das maiores seleções de jogos de cassino e também oferece probabilidades competitivas de apostas nos tipos mais populares de jogos como o JetX. O Cbet Casino trabalha com as empresas de desenvolvimento de software mais confiáveis e possui alguns dos melhores sistemas de segurança para proteger os dados pessoais e financeiros dos jogadores, além de também contar com apostas esportivas.
https://www.boscomazzocca.it/2025/06/05/review-do-sweet-bonanza-a-doce-sensacao-da-pragmatic-play-no-brasil/
Santa Maria Ucrsm e Smartsoft Gaming ® anunciam uma meia maratona JetX no Brasil. Para os que gostam dos esportes, principalmente corrida, está mais que convidado a participar da meia maratona que acontecerá em conjunto com a Ucrsm e a Smartsoft Gaming. JetX e é um crash game da SmartSoft Gaming mais conhecido como o jogo do foguetinho, que é um jogo muito popular no Brasil devido a ser extremamente divertido e simples de se jogar. Todos os direitos sobre a marca, marca e jogo “JetX” pertencem à SmartSoft Gaming – smartsoftgaming . Cbet JetX tem uma RTP de 97,00% ou seja a cada R$100,00 que joga a expectativa de voltar para o jogador é de R$97,00, e comparado com outros jogos caça-níqueis é um RTP acima da média. Todos os direitos sobre a marca, marca e jogo “JetX” pertencem à SmartSoft Gaming – smartsoftgaming .
The Real Person!
The Real Person!
É possível apostar no jogo Spaceman da Betano a partir de R$5. Acesse nosso conteúdo e confira as melhores dicas e estratégias ao começar a jogar Spaceman na plataforma de apostas da Betano. Fique de olho nos multiplicadores enquanto estiver jogando. É importante observar os padrões e as tendências dos multiplicadores que aparecem ao longo do tempo. Isso o ajudará a determinar o momento ideal para aumentar suas apostas ou sacar seus ganhos, dependendo da probabilidade de os multiplicadores subirem ou descerem. Com mais de 12 mil jogos de 155 desenvolvedores diferentes, a 1win ainda oferece suporte ao cliente 24 7, um dos grandes destaques para jogadores brasileiros. Esses fatores, somados ao compromisso com o jogo responsável, tornam a 1win uma escolha confiável e segura para qualquer apostador.
https://myworldgo.com/profile/httpsbrtigr
A rede de parques indoor Game Station anunciou investimento de R$ 300 mil em iniciativas voltadas à inclusão de crianças autistas em suas unidades. Os recursos foram aplicados em treinamentos de equipe, aquisição de equipamentos como abafadores de ruído e em benefícios diretos às famílias neurodivergentes na aquisição de serviços da rede segundo revela a afirma Paula Margolis, vice-presidente do grupo. a rede Game Station atua há mais de 25 anos e conta com mais de 65 unidades em 10 estados brasileiros e agora faz parte do grupo Azeka Games depois da fusão em meados de 2020 entre os empresários, Ernesto Margolis (que faleceu este ano) e Ernani Azeka. É importante notar que o Telegrupos não tem qualquer vínculo com o Telegram FZ-LLC. As informações disponibilizadas neste site têm caráter meramente informativo e não nos responsabilizamos pelo conteúdo das conversas, contatos ou pela veracidade dos grupos listados. Todas as interações ocorrem diretamente nos canais e grupos do Telegram, fora do nosso site.
The Real Person!
The Real Person!
1xbet Maroc دليل مفصل للمراهنة 1xbet كيف تلعب 1xbet 1xbet شرح” Content Bet موقع Morocco المكافئات” كيف ألعب على 1xbet… Chcesz grać w MostBet? To jest właściwa strona. Zaloguj się poniżej za pomocą kodu HUGE, aby uzyskać dostęp. play aviator: aviator bet malawi – aviator Содержимое Grandpashabet Nedir? Grandpashabet Casino Oyunları Grandpashabet Bonus ve Promosyonlar Grandpashabet Güvenliği ve Gizliliği Grandpashabet Müşteri Hizmetleri Grandpashabet Mobil Uyumluluğu… Tak. Aplikacja MostBet jest dostępna na urządzenia z systemem Android i iOS. Zarejestruj się, aby pobrać plik, który jest bezpłatny. BetWinner has emerged as a formidable competitor in the online betting industry. It offers a myriad of betting options, ensuring that every type of gambler can find something to enjoy. Established in the international market, the platform has successfully created an environment that prioritizes user experience, security, and diverse betting opportunities.
http://divisionmidway.org/jobs/author/wicejinformacj
Twórcą szokującej gry jest Spribe. To gruziński dostawca automatów wideo. W 2020 roku studio postanowiło zaryzykować i zaprezentowało społeczności graczy zupełnie nowy produkt. Nawet sześć miesięcy później Aviator nie był najlepszą rozrywką w grach. Możesz grać w Spribe ‘ s Aviator 2 za darmo. Tryb demonstracyjny otwiera się automatycznie. Aby postawić prawdziwy zakład, musisz najpierw zarejestrować się w kasynie online lub firmie bukmacherskiej i dokonać wpłaty na saldo. Zakres zakładów w grze kolizyjnej jest ogromny. Możesz postawić 0,82 eur lub wejść ze stawkami 16 eur i wyższymi. Udział w grach hazardowych poniżej 18 roku życia (lub wieku pełnoletności obowiązującego w danym regionie) jest uważany za przestępstwo. Hazard może uzależniać. Graj odpowiedzialnie. Jeśli Ty, lub ktoś kogo znasz, ma problem z hazardem i chce pomocy, zapraszamy na stronę: Gamblers Anonymous.
The Real Person!
The Real Person!
Grać w Aviator można w bezpłatnym trybie Aviator demo. Jest dostępny w każdym kasynie i nie wymaga rejestracji ani wpłacania środków na konto. Godne zaufania kasyno online Bitcoin zapewnia dostęp do gry Aviator online oraz ponad 9 400 slotów, 38 gier stołowych i ponad 600 wirtualnych pokoi w kasynie na żywo. Wraz z tymi korzyściami można znaleźć następujące funkcje: Wejdź do świata Aviatora od Spribe, slotu online, który redefiniuje kasynowe emocje. Dzięki darmowej wersji demo do gry, tytuł ten oferuje angażujące doświadczenie poprzez swój minimalistyczny design i innowacyjne funkcje mnożników. Każdy obrót to mieszanka przemyślanej strategii i niespodziewanej fortuny, co czyni go idealnym zarówno dla początkujących, jak i doświadczonych graczy. Aviator zachęca do skoku w dynamiczny świat, w którym każda chwila na ekranie obiecuje unikalne emocje i świeże spojrzenie na nowoczesną rozgrywkę.
https://cct.opencitieslab.org/user/colecarlreels1977
Kasyna online zyskują coraz większą popularność wśród graczy na całym świecie, zwłaszcza w Polsce. Dla tych, którzy chcą zmaksymalizować swoje szanse na wygraną, istnieją konkretne strategie, które mogą pomóc. Jednym ze sposobów jest korzystanie z odpowiednich platform, takich jak Mosbet: Onlayn Kazino Və Idman MərcləriBəli, bukmeker kontoru Kurakao hökumətinin verdiyi lisenziya əsasında iş göstərir Content In Qeydiyyat Necə Keçmək Olar? ᐉ 1win Azərbaycan Saytında… Mosbet: Onlayn Kazino Və Idman MərcləriBəli, bukmeker kontoru Kurakao hökumətinin verdiyi lisenziya əsasında iş göstərir Content In Qeydiyyat Necə Keçmək Olar? ᐉ 1win Azərbaycan Saytında… Get Directions Get Directions Выбор надежного онлайн-казино — важный шаг для каждого игрока, стремящегося к успешной и безопасной игре. Важно понимать, что не все платформы одинаково надежны, и поэтому стоит обратить внимание на такие аспекты, как лицензия, репутация и условия использования. Pinco KZ
The Real Person!
The Real Person!
Please download HappyMod to read more comments! Hungry Shark World MOD APK is the most exceptional Android simulation game and aspiration for most enthusiastic Android gamers. It’ll offer you all the fantastic premium features listed above free of charge, without any advertisement interruptions. Just download Hungry Shark World MOD APK and endure the world’s best simulation features with all the unlocked Shark species free of charge!! Or still, if you’ve got any doubts, You can ask them in the below comment section! Math Cash – Solve and Earn Rewards is a free app under the lifestyle category where users can practice and develop their arithmetic skills and make money at the same time. The app consists of basic math problems that you have to solve as fast as you can to earn more points convertible to cash.
https://raminatorabi.com/2025/07/03/deep-dive-into-the-betting-system-of-the-aviator-game-by-spribe/
Welcome to Colour Prediction App, a captivating platform that combines color prediction games and stimulating quizzes. Dive into a world of vibrant hues and challenging questions, testing your intuition and knowledge. our bid dady Mumbai club demo. you get the option to change recharge and withdrawal.You get the option to change your payment details.you can send notifications to users from the admin panel.you can track users details like their phone number, Password ,Recharge withdrawal details. The next step in building your colour prediction website is selecting a tech stack to smooth your development process. For a colour prediction website, you will likely need: In the market many games are available but 95% are 99% fake, But Bharat game colour Prediction is 100% safe and the best feature is withdrawal in 2 Hours.
The Real Person!
The Real Person!
V 21 Casino live casinu naleznete úchvatný počet nejrůznějších živých her, včetně live casino game shows typu: One of the key reasons why the Plinko casino game has gained such traction is the convenience with which players can access it. Nowadays, you can quickly discover a web-based Plinko, enabling you to play from the ease of your residence or on the go. Digital versions of Plinko casino typically provide a variety of enhancements and modifications that upgrade the playing experience, from different aesthetic choices to extra levels. Additionally, gamers can select from trying a free Plinko demo or playing a Plinko game real money version, depending on what they seek. Plinko online game is a captivating game that has captured immense popularity in recent years, particularly in the internet gaming and gambling circles. Evolving from the traditional game show format, the Plinko game has developed into a multi-faceted and entertaining adventure that entices both casual gamers and those aiming to earn real cash online. The fundamentals of the Plinko game are simple yet engaging, yet highly engaging, establishing it as a key game in many web casinos and digital gaming platforms.
https://highlevelperformance.com/hra-plinko-od-bgaming-recenze-a-tipy-pro-hrace-z-ceske-republiky/
Volatilita je relativně nízká, ale s počtem řad roste; tato kasinová hra nabízí častější malé výhry než velké vysokorizikové jackpoty. Ano, Plinko můžete hrát za fiat peníze; mnoho kasin nabízí tradiční metody sázení na tomto automatu, mhh rozdíl od nového trendu sázek v kryptoměně. Tento režim vám umožní pochopit mechanismus hry some sort of testovat strategie bez použití skutečných peněz, než se zapojíte do skutečného automatu. Verze Spribe nabízí hráčům maximální výhru až 555násobek počáteční sázky, 3 možnosti úrovně rizika – 12, 14 a 16 řad kolíčků a také funkci automatické hry. Celá hra je založena em hazardní hře plinko, která se objevuje v kasinech. Spočívá v tom, že je tečka spuštěna do rozšiřujícího se prostoru, kde se odráží a za pár okamžiků dopadne na políčko, které znázorňuje výhru. Hra Plinko má v online casinech a na různých internetových portálech odlišné podoby. Některé u možné hrát zdarma v demo režimu nebo na stránkách zmíněných výrobců.
The Real Person!
The Real Person!
Große Gewinne in diesem Video-Slot sind durch den Einsatz von zusätzlichen Spins, Bonusmodifikatoren, Bonusfunktionen, extra Spins und progressiven Features möglich. Mit dem richtigen Fang können Sie echtes Geld gewinnen. Halten Sie Ausschau nach Bass-Symbolen und betreten Sie häufiger den Spins-Modus. Das Thema selbst ist ziemlich lustig und wir mögen die Art und Weise, wie das Spiel gestaltet wurde. Die Grafik ist gut gemacht und lässt das Spiel hervorstechen. Hinter den transparenten Walzen kannst du das Unterwasserleben beobachten, während die Fische anmutig vorbeigleiten. 02351 94 44 31 Diese Plattform wurde geschaffen, um all unsere Bemühungen einer breiten Öffentlichkeit zu präsentieren, damit unsere Vision einer sichereren und transparenteren Online-Glücksspielbranche auch in die Realität umgesetzt wird.
https://risanstore.com/rolle-der-farbsymbole-bei-sugar-rush-mehr-als-nur-optik/
Acumen Home Care center was created to promote proper care and effective collaboration among care professionals, clients, and family caregivers. casino en ligne 2023 fiable202300€stimmst du den Allgemeinen Geschäftsbedingungen und den Datenschutzrichtlinien zu.k1 casino oberschleissheimum weitere Details zum jeweiligen Bonus zu erhalten.Insgesamt können 12 Reihen auf den fünf Walzen erreicht werden.Schau dir einfach im Vergleich zu den besten Book Of Santa Online Casinos die Top Anbieter an.deutschlands bestes online casino Pragmatic Play hat Big Bass Bonanza mit pfiffigen Features ausgestattet. Diese kommen in der Freispielrunde zum Tragen. Um dorthin zu gelangen, ist das Scatter Symbol erforderlich. Der Slot folgt also dem klassischen Konzept, Free Spins über den Scatter auszulösen. Im Folgenden erklären wir Ihnen, wie die Freispiele und die dazu gehörigen Bonusfunktionen genau ablaufen.
The Real Person!
The Real Person!
Notwithstanding the foregoing, if OLG Deactivates a Player Account as a result of the occurrence of a Material Breach by the Player under this Agreement, OLG (in its sole discretion) shall be entitled to treat any or all of the Unutilized Funds or Prizes associated with the Player Account as forfeited by the Player, in which case the Player will not be entitled to have such funds provided. Renewed access to and use of a Player Account for an Intending Player with a Deactivated Account is subject to OLG approval in its sole and absolute discretion and may be requested by the Intending Player only by contacting Player Support. An FAQ (frequently asked questions) guide provides plenty of answers to the more commonly asked questions at Dabber Bingo, all two-up games have been audited for fairness and accredited by Gaming Labs International – one of the industrys best independent auditors. Its also true that pay by mobile often features much lower deposit limits compared to other payment methods, creating multiple win opportunities.
https://argoshentai.xyz/sweet-bonanza-uk-paylines-rtp-and-mobile-compatibility/
Players who like the animal theme can check out Wolf Legend Megaways for the same bet range with Free spins, Bonus bets as well and gambling features. Or if they desire something unique, they can check out Prospector Extra Gold for features like Free Spins, Feature Gamble, Bonus Bet, and Buy a Bet. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Megaways games have specific characteristics that make them fun to play, and Almighty Buffalo Megaways is no exception. With up to 117,649 ways to win and cascading reels, it has all the basic things players want. But then layer on additional features you don’t always find, and it gets a bit more interesting. A platform created to showcase all of our efforts aimed at bringing the vision of a safer and more transparent online gambling industry to reality.
The Real Person!
The Real Person!
aviator game tipobet As the industry grows, staying informed about the latest trends and options will enable bettors to choose the best horse racing betting sites that suit their preferences and needs. Whether you are a casual fan or a dedicated bettor, the right platform can transform your betting endeavors into an exhilarating adventure. Содержимое What is Mostbet India? Mostbet Login and Registration How to Register on Mostbet India Official Website Login to Mostbet… As such, you are tasked with guiding your plane since it takes off into the sky. Owing to typically the incorporation of typically the curve crash mechanic, the plane you will end up guiding will certainly not remain in the skies for too long as it will certainly crash at virtually any moment. The objective as a result once typically the plane takes off will be to intuitively determine when to disembark the airplane before it crashes. Quite the few sites offer you it, including Betway Malawi and 888bets Malawi, and the old favourite, Worldstar Malawi. Once logged inside, you will possess access to a selection of features, like games, promotions, in addition to account settings.
https://vtelektronik.hu/analiza-popularnosci-nvcasino-wsrod-polskich-graczy/
Przed wami tytuł, który niemal w dniu premiery w 2022 roku stał się od razu hitem, zaś do dziś doczekał się kilku równie udanych sequeli. Oczywiście można zadać sobie pytanie o to, czy gra ta nie jest nadmiernie “dziewczęca”. Naszym zdaniem – wprost przeciwnie. W redakcji CasinoRIX w Sugar Rush regularnie grywają panowie. Zapraszamy do naszej eksperckiej recenzji! Oczywiście dzięki tak obszernej bibliotece gier na pewno będziesz mieć tytuły gier przez wiele miesięcy, muszą spełnić wymagania dotyczące zakładów. Sugar rush – gra, która wzbudza emocje i przyciąga bogactwo jak magnes! W dzisiejszych czasach, kiedy gry online zdobywają coraz większą popularność, tematy takie jak bezpieczeństwo oraz odpowiedzialna gra stają się kluczowe dla graczy i operatorów. Szczególnie w kontekście gier takich jak Sugar Rush, które przyciągają użytkowników swoją prostotą i atrakcyjną grafiką, istotne jest, aby zarówno nowi gracze, jak i doświadczeni profesjonaliści rozumieli zasady bezpiecznej i odpowiedzialnej gry.
The Real Person!
The Real Person!
Sous réserve des conditions de la présente Convention, le Joueur peut retirer des Fonds inutilisés de son Comptedu joueur standard jusqu’à ce qu’il atteigne son solde courant de Fonds inutilisés. En découvrant le jeu Buffalo King Untamed Megaways, la principale attraction n’est autre que le symbole Wild. En effet, l’icône sauvage s’associe à des multiplicateurs dont la valeur progresse durant les tours gratuits. Comme toujours, le joker se substitue aux autres symboles, sauf le symbole Scatter. Il apparaît sur les rouleaux 2, 3, 4 et 5 en ayant un multiplicateur de ×2, ×3 ou ×5. Lorsque des Wilds avec multiplicateurs atterrissent sur la grille, ils se multiplient et s’appliquent à tous les gains concernés. Why not enjoy our casino slots, relax and keep yourself entertained for hours? Get the biggest free slots collection with super exciting casino slots gameplay and extra high slots payout in our casino! Join our super casino slots games and 777 slots machines with amazing casino slot frenzy! Are you ready for playing this top Las Vegas casino slots with lots of FREE SPINS and BONUS ROUNDS?
https://wayranks.com/author/seisuppnero1984-1870/
JOUEZ RESPONSABLEMENT : jetxcasino.games fonctionne de manière indépendante et n’est affilié à aucun des sites que nous recommandons. Avant de vous engager avec un casino ou de placer un pari, assurez-vous de répondre à toutes les exigences légales et d’âge. La mission de jetxcasino.games est de fournir un contenu à la fois informatif et divertissant. Ceci est fourni strictement à des fins informatives et éducatives. En cliquant sur ces liens, vous quitterez ce site. La plate-forme propose un certain nombre de bonus et de promotions dans ses différentes sections, ce qui permet à chacun d’y trouver son compte. Pour obtenir le cadeau de bienvenue, vous devez choisir le code de bonus CBet sur le site Web. Trois options sont disponibles pour les utilisateurs du casino : Pour jouer à Cbet JetX, vous devez bien évidemment commencer déjà par vous inscrire sur le casino du fournisseur puis effectuer un dépôt. Après cela, il vous suffira de lancer Cbet JetX et de placer une mise de départ bien avant le remplissage de la barre de chargement jaune.
The Real Person!
The Real Person!
Caso consiga 4x o símbolo do pescador, o jogo lhe dará mais 10 rodadas grátis com um multiplicador. O recurso pode reiniciar até 3 vezes com um multiplicador máximo de 10x. Na 4win, você joga com segurança, em um ambiente licenciado e em conformidade com todas as normas do setor. Aproveite os melhores jogos do mercado com transparência, justiça e responsabilidade. O Cowboys Gold é mais um dos mais divertidos jogos de 10 centavos da Pragmatic Play, e conta com 5 bobinas, 3 linhas e 10 linhas de pagamento. O jogo apresenta o recurso Bônus Money Collect, em que o símbolo do xerife coleta valores em dinheiro dos cowboys, oferecendo possíveis ganhos de até 6.065x a aposta. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page.
https://amadexic1985.iamarrows.com/check-this-out
Copyright © 2025 2 Coelhos Auto Peças | Powered by Tema Astra para WordPress Usamos cookies e tecnologias similares para oferecer uma experiência de navegação mais personalizada. Para saber mais, acesse nossa Central de Privacidade. Agora que já sabe como jogar Big Bass Bonanza, e levando em conta tudo que contamos até aqui, dá para notar o quão divertido é jogar esse slot. O jogo é muito fluido, a ambientação é maravilhosa e tudo funciona muito bem. Para um RTP semelhante ao de Big Bass Splash (96,71%), um jogo divertido é o Fortune Ox, que oferece 96,75% de RTP. Em algumas plataformas, os ganhos obtidos nas rodadas grátis precisam passar por rollover (serem apostados) para o saldo ser liberado para saque. No entanto, muitos cassinos online oferecem giros grátis sem rollover.
The Real Person!
The Real Person!
Final Thoughts: Why Chicken Road Might Be Your New Favorite Game We’ve seen a lot of games come and go, but Chicken Road has that rare mix of charm, challenge, and real money potential that makes it special. It doesn’t take itself too seriously — and neither should you. It’s quick to learn, endlessly replayable, and every round feels just a little different. HappyClicks.net Free Online Toddler Games and Baby Games In Chicken Invaders Universe, you assume the role of a new recruit in the United Hero Force (UHF), mankind’s last hope against the fowl Henpire. You start your UHF career deployed in some backwater galactic star system, and it’s up to you to advance through UHF ranks and earn your place in the honorary annals of Heroes Academy. Travel across the galaxy, explore strange new worlds, seek out new life and new civilizations, and exterminate any Henpire forces that cross your path. And do so in style.
http://shironeko-shitaraba.net/wiki/index.php?florbuddnalwi1987
استكشف الألعاب المثيرة والجوائز الكبرى الرائعة في BC.Game كازينو. العب ألعاب السلوتس وألعاب الطاولة المفضلة لديك في أي وقت في كازينو BC.Game. Attempting to hack Mission Uncrossable or any game on Roobet is unethical and carries significant consequences. Roobet uses advanced security protocols and provably fair technology, meaning that each game outcome is verifiable and transparent. Any attempt to manipulate the game through hacking could result in the loss of your account, confiscation of funds, and a permanent ban from the platform. Additionally, hacking is illegal and can lead to legal action, putting players at risk of serious penalties. It’s always better to play fairly and enjoy the game as intended.
The Real Person!
The Real Person!
par | Avr 26, 2023 | Non classé Jet X: l’expérience de jeu ultime. Enfin, vous trouverez tout ce dont vous avez besoin ici. Aucun téléchargement n’est requis et les joueurs peuvent profiter des meilleurs jeux de casino en ligne sur ordinateur et mobile, les retraits et les paiements sont désormais possibles en quelques minutes. Copyright©2025, gamejetx | E-mail pour offres commerciales ou réclamations : L’argent déposé non destiné à des jeux de hasard ne peut pas être transféré à des marchands de quelque type que ce soit. JetX retirer argent JetX retirer argent Une fois que vous avez l’impression d’avoir gagné suffisamment d’argent et que vous souhaitez vous retirer, 8 Ball Strike propose plusieurs options de paiement, notamment PayPal, Venmo et autres. Il y a des frais de traitement assez standard de 1 $ pour les retraits inférieurs à 10 $. Le processus peut prendre jusqu’à 15 jours, mais il faut généralement une journée pour que les fonds soient versés sur votre compte.
https://moviebreak.de/users/ovcurkejor1972
Titre et description améliorés par l’IA Fortune Mouse é um dos slots mais populares da PG Soft, oferecendo várias oportunidades de ganho. Jogar durante os horários pagantes pode aumentar suas chances de ganhar grandes prêmios. Descubra os horários pagantes Fortune Mouse e maximize seus ganhos neste divertido e lucrativo slot. Réalisé par Franco JetX, le clip est assez simple et assez plat. Filmé à un seul endroit, malgré les transitions on remarque le manque de variation dans les décors. Dans le clip, on remarque que Chamy porte un bracelet ayant l’effigie de la marijuana. Sa compagne l’a aussi en tatouage. En plus vers la 29ᵉ seconde, on voit bien une fumée qui monte; comme quoi Chamy serait en train de fumer. Bref il n’y a que l’artiste pour nous dire de quoi il est question ici.
The Real Person!
The Real Person!
Το gameplay του Sugar Rush τραβάει την προσοχή χάρη στη μη παραδοσιακή του δομή. Αντί για τους κλασικούς τροχούς και γραμμές πληρωμής, το παιχνίδι διαθέτει πλέγμα 7×7 που λειτουργεί με σύστημα συστάδων (clusters). Δηλαδή, κέρδος προκύπτει όταν ομάδα ίδιων συμβόλων αγγίζει μεταξύ τους – οριζόντια ή κάθετα. Με τρία ή περισσότερα σύμβολα scatter σας δίνει το Sugar Rush 1000 δωρεάν περιστροφές. Οι περιστροφές που κερδίζετε είναι:
https://datosabiertos.carchi.gob.ec/user/jesadecamb1979
Πάντως για τον αυχένα μου το χειρότερο. Because there are hundreds of organizations online these days, it has become very difficult to find the best service provider. Students need Essay Writing Help to get beyond all of their academic obstacles and complete assignments ahead of schedule, giving them ample time to adequately study for tests. Water Splash – Cool Match 3 1. At The Gates – Slaughter of the Soul RunningNews.gr Forum : forum.runningnews.gr Water Splash – Cool Match 3 The popularity of Glory Casino slots fan communities and forums speaks volumes about the role of these platforms in enhancing the gaming experience. By providing spaces for interaction, strategy sharing, and ongoing learning, these communities position themselves as central to modern online gaming. Whether through dedicated forums or vibrant social media groups, Glory Casino enthusiasts find a rich landscape to explore their passion. Ultimately, these fan communities foster a sense of camaraderie, offering valuable insights and enjoyment for all their members.
The Real Person!
The Real Person!
Easy To Direct Download Pc Software To use this app you must be 18+ & a IE resident. Our responsible Gaming Policy can be found: betway terms responsible-gaming Data areas are used in several different sectors. They can be a fantastic help in M&A deals, legal events, real-estate transactions, or perhaps general provider collaboration. They give a safe environment where you can retail store and share documents without worrying regarding privacy. Yet , with the amount of service providers on the market, it is usually hard to choose the best one for your organization. Here are some tips to help you make your decision: We provide our players with quality games and a thrilling online gambling experience, always bringing you closer to the action. We’re all for the love of the game and if you love the real excitement of live casino betting then Betway is for you.
https://enginedirect.co.uk/index.php/2025/08/04/jak-zainstalowac-aplikacje-playbison-casino-na-androida/
A beer with a deep, nearly black colour, extraordinarily strong extract (20%), and complex sensory profile where one can find both intense bitterness and prominent sweetness; the two are complemented by smoky acidity, characteristic of this style. Porter is a beer that matures over time – at first, you can notice hints of caramel, coffee and bitter chocolate as well as cherries and currants. Over time the taste mellows down, and to the surface come flavours of wine and dried fruit. Oferujemy szybkie i bezpieczne przetwarzanie płatności w naszym kasynie online. Wystarczy wybrać preferowaną metodę płatności i postępować zgodnie z instrukcjami, aby dokonać wpłaty lub wypłaty. Nasze opcje płatności obejmują Bancontact, Paypal, karty debetowe Visa i Mastercard, e-portfele i przelewy bankowe. Zawsze możesz zażądać wypłaty wygranych ze swojego konta w kasynie online na preferowaną metodę wypłaty.
The Real Person!
The Real Person!
Making the most of the different bonuses and promotions at the site can help you get the best bang for your buck when playing the Roobet Mission Uncrossable game. With a wide variety of offers from welcome bonuses to referral offers, VIP clubs, social media giveaways, and more. Claiming these different offers can help maximize your available bankroll. Play this gambling game on this Chicken Road page. Access the title whenever you want, and reload the game if you run out of credits. While the thrill of Mission Uncrossable is undeniable, it’s crucial to approach the game responsibly: Making the most of the different bonuses and promotions at the site can help you get the best bang for your buck when playing the Roobet Mission Uncrossable game. With a wide variety of offers from welcome bonuses to referral offers, VIP clubs, social media giveaways, and more. Claiming these different offers can help maximize your available bankroll.
https://marceloparceiroton.com.br/best-sites-offering-aviator-game-online-free-in-rwanda/
Daily Prizes Recent Searches Check out our vacancies casumocareers The max win for Big Bass Keeping It Reel is 10,000x your total bet. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. The base game doesn’t have much going on, except for the Ante Bet, where for an additional cost of 50%, you can increase your chance of triggering Free Spins. As I said above, just how much of an increase isn’t made clear. However, when things move into Free Spins they get their normal splash of Bass excitement, with increasing multipliers, retriggers, Fish Money values, and now the landing net. It’s all happening, and it all works together. Landing the right combinations can produce wins up to 10,000x bet – the biggest of any release in this series so far, by at least double.
The Real Person!
The Real Person!
Mega Fire Blaze Roulette Popular WordReference English-Spanish Dictionary © 2025: Several papers also track the movement of the goalkeeper during penalties. Given the speed of penalty kicks, it’s rarely possible for a goalkeeper to wait and react to the direction of the kick. Instead, the goalkeeper makes a guess at which way the player will shoot based on body language and their knowledge of the player’s past penalties. The same image was published in Diario Popular, with the headline “Vamos por la séptima” (“Let’s go for the seventh”) – a reference to the previous six Libertadores that Boca have already claimed. Over 112 years, red and white stripes have painted the history of Mexican football with multiple episodes of joy and triumph, goals and records that have forged the tradition that today defines the Sacred Rebaño.
https://inversiones-inmobiliarias.com.mx/resena-del-juego-balloon-de-smartsoft-en-casinos-online-para-jugadores-en-peru/
Puedes comprar hasta 3 unidades **Season 2 Unleashes New Thrills in Call of Duty: Modern Warfare III and Warzone**February 6, 2024, marks the arrival of Season 2 in the Call of Duty Los patos de las granjas vecinas han escapado y se han mezclado en los campos adyacentes. Ni tu ni tu rival sois capaces de decir de quién son, así que la única acción lógica es que cada uno de vosotros construyáis corrales, captura y reclama tantos patos como sea posible. Finally, the anxiety of the players was assessed by means of the Competitive State Anxiety Inventory-2 (CSAI-2; Martens et al., 1990) in its revised Spanish version (CSAI-2R; Andrade et al., 2007). The questionnaire consists of 16 items (Likert-type scale from 1 to 4) distributed in three dimensions: (1) cognitive anxiety (five items), typified by negative self-images and self-doubts; (2) somatic anxiety (six items), which refers to physiological responses (e.g., increased heart rate, tense muscles, and clammy hands); and (3) self-confidence (five items) regarding the positive expectations of success (Andrade et al., 2007).
The Real Person!
The Real Person!
Deneme bonusunu kullanarak Aviator oynamak için önce bonusun kurallarını okuyarak başlamalısınız. Ardından, oyunu başlatabilir ve tahminlerinizi bu bonus üzerinden gerçekleştirebilirsiniz. Üçüncü olaraq, 1win az platformasında oyunlar oynamaq üçün 1win giriş etdikdən sonra, hesabınızın təhlükəsizliyini artırmaq üçün iki faktordan istifadə edin. Bu, hesabınızın daha da təhlükəsiz olmasına kömək edəcək. Di?er programlardan ay?ran özelli?i ise sadece mesleki yetkinliklere de?il, ayn? zamanda kad?nlar?n liderlik kimliklerini in?a etmelerine yard?mc? olmas?d?r. 2011 y?l?nda faaliyetlerine ba?layan Bets10, hizmetinin sürekli olarak üstüne koymaktad?r. Bu da siteyi gitgide daha tercih edilebilir bir platform hâline getirmi?tir. Sunulan oyunlar?n yap?s?n?n genel itibar?yla kaliteli oldu?u söylenebilir.
https://hiperco.com/aviator-oyunu-v%c9%99-telegram-bot-il%c9%99-real-vaxt-analitik-sisteml%c9%99ri-az%c9%99rbaycanli-oyuncular-ucun/
aviator.az © 2025 Aviator Rəsmi veb sayt. 2024-10-15 09:20:00 2024-10-25 21:10:00 2024-10-20 13:04:00 2024-10-15 09:20:00 2024-10-20 13:04:00 aviator.az © 2025 Aviator Rəsmi veb sayt. 2024-10-20 13:04:00 2024-10-15 09:20:00 aviator.az © 2025 Aviator Rəsmi veb sayt. aviator.az © 2025 Aviator Rəsmi veb sayt. 2024-10-25 21:10:00 2024-10-25 21:10:00 2024-10-20 13:04:00 2024-10-15 09:20:00 2024-10-20 13:04:00 2024-10-15 09:20:00 2024-10-20 13:04:00 aviator.az © 2025 Aviator Rəsmi veb sayt. 2024-10-20 13:04:00 2024-10-15 09:20:00 2024-10-20 13:04:00 2024-10-25 21:10:00 2024-10-25 21:10:00 2024-10-25 21:10:00 aviator.az © 2025 Aviator Rəsmi veb sayt. 2024-10-15 09:20:00 2024-10-20 13:04:00 2024-10-15 09:20:00 2024-10-20 13:04:00 2024-10-15 09:20:00 aviator.az © 2025 Aviator Rəsmi veb sayt.
The Real Person!
The Real Person!
1Tap Mines is an online casino game where you have to search for gold in a grid while avoiding mines. The Mines casino game follows the gaming pattern of Minesweeper, making use of a five-by-five grid gaming area. This five-by-five grid contains 25 opaque tiles the player has to reveal to either win or lose. So. here’s how to play Mines casino game: Points are awarded for purchasing сoins in the store. The more points, the higher the rank. You only need to purchase Twists once a month to keep your status. Otherwise, the rank is lowered. The kinds we tried have been nothing but ad-filled apps that tried to get all of us to sign-up to be able to casinos through their very own promotional links ahead of we giving people “access”. There are also a few Facebook or myspace groups trying to lure in collision gamblers selling off accessibility to premium exclusive Telegram groups. Let’s just say that if anyone could in fact hack the Aviator crash game, that they surely wouldn’t bother losing their moment with Facebook groups and Telegram shows. These agents wander casinos from Poland to Macau to Peru in research of slots in whose PRNGs have recently been deciphered by Alex.
https://elitedispatch.us/predicting-player-confidence-in-balloon-slot-play/
Don’t miss out on India’s top ludo earning app! Play Ludo, win real cash, and enjoy the thrill of competition. Sign up now and start playing! Please find the below means of communication to us One-Stop Service Delivery Platform Pocket Money is a task based money earning app without investment. You can earn money online without investment by completing various tasks such as taking online surveys, watching videos, and downloading apps. Outside Google Playstore App Download incubated and supported by ANDC Instart Foundation The following data may be used to track you across apps and websites owned by other companies: If you want to earn some extra money from home, then Survey Junkie is a great option. It is not only simple, but also reliable. You can increase your earnings by taking surveys regularly and keeping the profile updated.
The Real Person!
The Real Person!
Casinos Costa Brava Se otorga una bonificación adicional por obtener tres monedas en el juego base, que luego se vuelven pegajosas y se mantienen durante la siguiente ronda de bonificación, donde su valor se agrega a las ganancias obtenidas a partir de entonces. Los jugadores reciben inicialmente cuatro vidas que se reinician cuando cae alguna moneda en los carretes. Los símbolos de diamantes también están presentes y otorgan grandes ganancias a los jugadores. Si bien se trata de azar, hay formas de potenciar tus oportunidades de ganar en Big Bass Bonanza. Jugar la tragamonedas Big Bass Splash gratis revela una dinámica donde las apuestas más altas y las compras de tiradas gratis pueden mejorar la generosidad del juego. Sin embargo, un enfoque cauteloso puede ser igualmente gratificante, demostrando que una estrategia equilibrada es clave para disfrutar del juego sin caer en los vaivenes de la volatilidad.
https://www.felixp.it/2025/08/12/resena-del-juego-balloon-de-smartsoft-diversion-sin-conexion-para-jugadores-en-guatemala/
Es esencial que la plataforma de juego cuente con una licencia válida en España, otorgada por la Dirección General de Ordenación del Juego (DGOJ). Los operadores que desean ofrecer juegos de azar en línea deben obtener una licencia general y una licencia singular de la DGOJ. Además de los bonos de bienvenida, muchos casinos online fiables en España ofrecen bonos sin depósito, que se otorgan al registrarse y verificar la cuenta. La autoridad de turismo de Tailandia (TAT) mediante su campaña Thainess intenta atraer a turistas extranjeros a sus «Joyas escondidas», siendo una de esas la provincia meridional de Chumphon, a 463 kilómetros de Bangkok. La autoridad de turismo de Tailandia (TAT) mediante su campaña Thainess intenta atraer a turistas extranjeros a sus «Joyas escondidas», siendo una de esas la provincia meridional de Chumphon, a 463 kilómetros de Bangkok.
The Real Person!
The Real Person!
Obsługa Javascript w Twojej przeglądarce jest wyłączona. Włącz go, aby móc w pełni wykorzystać możliwości tej witryny. Idealna na spotkanie z przyjaciółkami, kreatywny dzień w biurze czy jako twist do wieczorowej stylizacji. Dopracowana podszewka i różowe akcenty wewnętrzne dodają lekkości i zadziorności – jak Ty. MAKIJAŻ do -50%! Eveline Cosmetics, Paese, Claresa, Kiko Milano i wiele innych Najniższa cena w ciągu ostatnich 30 dni: Bonus powitalny Najniższa cena w ciągu ostatnich 30 dni: MAKIJAŻ do -50%! Eveline Cosmetics, Paese, Claresa, Kiko Milano i wiele innych Najniższa cena w ciągu ostatnich 30 dni: MAKIJAŻ do -50%! Eveline Cosmetics, Paese, Claresa, Kiko Milano i wiele innych 100% bezpieczne Najniższa cena w ciągu ostatnich 30 dni: Bonus powitalny
https://gesoten.com/profile/detail/12002101
Buy Cocaine Canada buy cocaine online Maybe it will help you to unblock sitesvpn zaakceptowany Sugar Rush 1000 oferuje wciągającą rozgrywkę na slocie online w żywej siatce 7×7. Gra wykorzystuje system Cluster Pays, w którym wygrane są przyznawane, gdy co najmniej pięć symboli tworzy poziome lub pionowe połączenia. Zwycięskie symbole są usuwane, aby umożliwić nowym kaskadowanie w dół, potencjalnie uruchamiając dodatkowe wygrane. Ze współczynnikiem RTP gry podstawowej na poziomie 97,50% i oznaczoną wysoką zmiennością, obiecuje ekscytujące wrażenia z rozgrywki. Pure Caluanie Muelear Oxidize For Sale pure caluanie muelear oxidize for sale 女性 用 ラブドールAt last he got away and rushed back to When he wentinto the room,he found it in darkness. mitolyn is an advanced nutritional solution specifically created to help maintain balanced blood sugar levels and improve metabolic performance.
The Real Person!
The Real Person!
This way there is a better chance of landing a winning combination, it is tedious and unwieldy to make your way around their site. Could you please advise why are you asking for your history from the casino, as is the Wells Fargo Center (home of the NBAs 76ers franchise). What happens when the Gold Coins scratch card game malfunctions during gameplay, use the Bet box. Casino Cruise guarantees a smooth and safe ride when exploring any of their 1,300 casino games, but do exist and should be kept in mind for both low and high stakes players. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Sugar Rush has earned plenty of attention, and it’s easy to see why. With Pragmatic Play behind the wheel, the quality speaks for itself.
https://forum.dsapinstitute.org/forums/users/brooklynking/
Sugar Rush 1000 has a slight visual upgrade from its predecessor, but the design and theme remain the same. The slot is set in a colorful candy kingdom full of lollipop trees, chocolate rivers, and marshmallow candy mountains. It’s a charming design packed with plenty of energy and color. *All values (Bet Levels, Maximum Wins etc.) mentioned in relation to this slot game are subject to change at any time. Game features mentioned may not be available in some jurisdictions. Overall, Sugar Rush 1000 is an entertaining online slot game for those with a sweet tooth. The game’s colourful graphics and exciting sound effects create a fun and immersive gaming experience. The Cluster Pay mechanic and 25,000x max win allow you to earn significant payouts. However, the high volatility may deter you. The variable RTP is also a concern, as online casinos can change it. Overall, if you’re looking for a fun and engaging slot game with the potential for big payouts, Sugar Rush 1000 is worth checking out.
The Real Person!
The Real Person!
Frage an alle User hier….. Having a moderate lowest deposit away from 20 in the crypto and you will a fair 35x wagering requirements, sportbet.one assurances an accessible how many types of cricket matches are there access point to possess professionals of varying accounts. So you can begin depositing and withdrawing Bitcoin out of a great crypto gambling webpages pages requires a good Bitcoin Bag. The brand new wallet is largely the fresh cryptocurrency’s kind of your money that is needed to play with Bitcoin to pay for your own betting having on the internet playing websites you to undertake Bitcoin. Sugar Park Slot (NowNow Gaming) Review Date of experience: June 13, 2025 Explore the ranked best online casinos of 2025. Compare bonuses, game selections, and trustworthiness of top platforms for secure and rewarding gameplaycasino activities.
https://dks-drustvo.si/sweet-bonanza-mobile-performance-auf-alteren-smartphones-deutsche-auswertung
Altersverifikation Die erfahrenen Lotto-Freaks werden an dieser Stelle vielleicht einwenden, die einem 3-fachen Multiplikator unterzogen werden. Wie man Sugar rush spielt, ohne Geld auszugeben. Es ist nicht Ihre gewöhnliche Obstmaschine, die zahlreiche Gewinnkombinationen auch dann ermöglichen. Scheepjes Maxi Sugar Rush – so ein hübscher Name! Und bei diesem süßen Garn und der riesigen Farbauswahl mit 87 Farben könnte man glatt in einen Kaufrausch geraten, da einem garantiert so einiges einfällt, was man mit diesem schönen Baumwollgarn machen könnte. Sugar Rush 1000 wird auf einem 7×7-Walzenraster gespielt und verwendet eine Cluster-Pay-Mechanik. Dies bedeutet, dass Gewinne in diesem Spiel eingefahren werden, wenn fünf oder mehr übereinstimmende Symbole in einer Gruppe irgendwo auf dem Bildschirm getroffen werden.
The Real Person!
The Real Person!
O Pixel Warfare apresenta uma variedade de armas que você pode usar ao iniciar uma partida. Há uma espingarda, um atirador de elite, um lançador de foguetes, uma metralhadora e muitas outras armas. Todas elas contêm uma quantidade definida de munição. Você tem todas as armas por padrão, portanto não há necessidade de desbloqueá-las. Você é sempre o primeiro da fila quando baixa os jogos do Xbox no mesmo dia em que eles ficam disponíveis nas lojas, e sem sair do conforto e da comodidade da sua casa. E com a pré-compra e o pré-download, você pode encomendar e jogar assim que o jogo estiver disponível para sua região. Aproveite a liberdade e a facilidade dos jogos digitais. Eles ficam armazenados na nuvem para que você possa baixá-los para o console Xbox ou para um disco rígido externo. Leve o disco rígido no bolso em viagens ou apenas faça login na sua conta no Xbox de amigos e baixe seus jogos.
https://techconnected.nl/2025/08/18/plinko-bgaming-uma-analise-sobre-como-jogar-e-ganhar-no-cassino-online/
O clássico da série está presente, trazendo monstros gigantes e hordas de monstros. Enquanto o jogo segura um estilo um pouco mais cômico no design dos personagens, os monstros continuam grotescos com a série manda e da pra ver vários detalhes neles, mesmo nos menores, claro, se você conseguir parar pra analisar, já que o jogo não tem pause dentro da partida. Comece projetando um modelo de foguete ou encontre uma casa pré-fabricada adequada. Unity Asset Store ou repositórios de modelos 3D online podem ser excelentes recursos. Vortex Racer pode ser jogado no seu computador e em dispositivos móveis, como celulares e tablets. Hills of Steel pode ser jogado em seu computador e dispositivos móveis, como telefones e tablets. Chamados de cheats, usar esses códigos de trapaças é uma ótima forma de descontrair em qualquer jogo. Os jogadores podem usar os truques para ganhar vantagens ou desbloquear elementos específicos. Esses cheats podem variar entre os diferentes jogos da série, mas, em geral, oferecem benefícios como recursos adicionais, unidades mais poderosas, revelação do mapa completo ou ativação de efeitos especiais.
The other day, while I was at work, my cousin stolle my ilhone and tested
to see if it cann survive a 40 foot drop, just so she can be a youtube sensation. My iPad
is now broken and she has 83 views. I know this is entirely off topic but I had to share it with someone! https://Glassi-greyhounds.Mystrikingly.com
The Real Person!
The Real Person!
La nostra stake plinko demo offre un’interfaccia pulita e intuitiva, progettata per i nuovi giocatori. Il design semplice permette di iniziare immediatamente senza complicazioni. For an in-depth Plinko UK review, explore trusted sources presenting expert insights and detailed analysis. Before you begin playing, familiarize yourself with the Plinko board layout and payout structure. Knowing where the high-value slots” “are located can help an individual make more informed decisions on in which to drop typically the ball. La versione demo di Plinko 1000 mantiene le stesse meccaniche del gioco reale, ma senza l’impiego di denaro, consentendo ai giocatori di testare le strategie senza rischi finanziari. Come Esperto Esperienziale iGaming, ho analizzato in profondità il brand Plinko per il mercato italiano. La mia esperienza con la piattaforma è stata estremamente positiva: la semplicità delle meccaniche di gioco si combina perfettamente con una grafica accattivante e moderna. Plinko riesce a creare un equilibrio tra intrattenimento immediato e possibilità di vincita, rendendolo un titolo ideale sia per i nuovi utenti che per i giocatori più esperti. Apprezzo particolarmente l’attenzione ai dettagli e l’adattabilità del gioco alle preferenze del pubblico italiano. Senza dubbio, Plinko rappresenta una proposta solida e innovativa nell’attuale panorama dell’iGaming.
https://donate.alogitaakideinitiative.org/mission-uncrossable-recensione-di-un-gioco-di-casino-online-imperdibile-per-i-giocatori-italiani/
Se ami la semplicità e la casualità di Plinko, ci sono altri giochi da casinò che offrono dinamiche simili, con meccaniche basate sul caso e moltiplicatori di vincita interessanti, ecco alcune valide alternative: La natura interattiva di Plinko è un altro fattore che risuona con i giocatori italiani. A differenza di alcuni giochi di casinò che si basano esclusivamente sul caso, Plinko permette ai giocatori di impegnarsi attivamente nel gioco. I giocatori rilasciano la pallina, anticipando con impazienza il suo percorso imprevedibile, creando un’esperienza coinvolgente. Questa interattività aumenta il senso di controllo, anche in un gioco d’azzardo, rendendo Plinko un passatempo coinvolgente e piacevole per gli italiani. Il gioco di Plinko si basa su un meccanismo semplice ma intrigante. Una pallina che cade attraverso una serie di ostacoli, seguendo un percorso casuale verso uno dei 17 “contenitori” o “bin” posti alla base. Ogni bin corrisponde a un diverso moltiplicatore di vincita, che può variare da un modesto 0.5x fino a un impressionante 1000x. Ma come funzionano davvero queste probabilità?
The Real Person!
The Real Person!
by | Aug 14, 2025 | Uncategorized Wild can replace any other icon but the Scatter to help you form a winning combination, Gonzo’s Quest takes players on an adventure through the Amazon jungle. With jackpots that can reach into the millions of dollars, ruby play casino online blackjack is legal as long as the site is licensed by the government and operates within the country’s laws and regulations. When choosing an online casino, allowing players to make deposits and withdrawals quickly and anonymously. These casinos are designed to be intuitive and easy to navigate, australia mobile slots the games are provided by a separate company. Lucky Block is an exceptional two-in-one platform with one of the widest selections of jackpot slots we have come across. The website is home to over 4,500 titles, and more than 50% of them are fair shouts for the best online casino slots on the market. You can find progressive jackpots, high-volatility thrillers, and Megaways slots from industry leaders like Microgaming, BGaming, and Relax Gaming. Gates of Olympus, Sweet Bonanza, and Wanted Dead or a Wild sit alongside rare and unique crypto-specific slots.
https://zips.com.pk/?p=13264
But what is this game, and why are Canadians (and other nationalities) going crazy after this gambling-morphed mobile gaming app? How do you play it and ensure huge wins? Are there downsides to playing this game? Tired of the usual online casino games? Although nearly 16 million Canadians would have played online casinos by 2029 and contribute to a robust market valued at 3.5 billion USD (about 5.06 billion CAD) by the same year, playing the same old stuff can be boring. Thankfully, the Roobet Chicken Game has changed all that! But what is this game, and why are Canadians (and other nationalities) going crazy after this gambling-morphed mobile gaming app? How do you play it and ensure huge wins? Are there downsides to playing this game? Playing Mission Uncrossable is easy, even for beginners. Its gameplay is nearly identical to Crossy Road, except for a few manipulations of the game setup. Here’s how.
The Real Person!
The Real Person!
As a top Canadian online casino, Betiton offers over 100 of the best jackpot slots, and this guide will help you find the right ones to play. We’ll explain the different types of, the difference between fixed and progressive slots, and how to start playing these online slots at Betiton. This is a code you can use to join a casino, no deposit online canada casinos while withdrawing winnings to their bank cards and e-wallets. Old casino in canada world Match Gaming, well known and considered a core mechanic for many Novomatic slots. Download the software and get an account today theres no holding back now, is of course also present in Ultra Hot deluxe. Another aspect to consider in a top-rated online casino with progressive jackpot slots is the variety of games available. The best casinos partner with leading software developers to provide a diverse selection of progressive slots. This means that players can choose from various themes and gameplay styles, ensuring that there’s something for everyone. Whether you prefer classic fruit machines or modern video slots, a top-rated online casino with progressive jackpot slots will have it all.
https://maisbrasil.meuanunciodigital.com.br/?p=14384
Your body needs inositol for the functioning and development of your cells. While research is still ongoing, people also use inositol for many different health reasons. Inositol benefits may include: However, some people should consult a doctor before using it, including: To arrange an appointment, please call … or make your reservation directly HERE. You may also download the MyVinmec app to schedule appointments faster and manage your reservations more conveniently. These monitors can alert you when your blood sugar is dangerously low or if it is dropping too fast. But you still need to test your blood sugar levels using a blood glucose meter even if you’re using one of these monitors. Continuous glucose monitors are more expensive than other glucose monitoring methods, but they may help you control your glucose better.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://www.binance.info/register?ref=P9L9FQKY
The Real Person!
The Real Person!
Knowing the symbols is half the battle. Once you’ve gotten familiar with them, which you may choose to do by playing a Sugar Rush 1000 demo, you’re better equipped to win! If you’re looking for free slots online, consider Slotozilla. Hot Hot Fruit is a fun and exciting casino slot game where you can win by matching three or more symbols. To win, the matching symbols need to line up from the leftmost reel to the right. How much you win depends on your bet and the symbols you match. Try to spot the high-value symbols and aim for long combos to boost your chances. Remember, the more you bet, the bigger your potential win. Overall, this game is fun, exciting and offers plenty of opportunities to win big. So, why not give it a spin and see if you can strike it lucky? Nachfolgend findest du zwei Alternativen zu Sugar Rush 1000, damit du die Spiele miteinander vergleichen und dich für das Geeignetste entscheiden kannst.
https://visionglobal.pe/big-bass-splash-ein-umfassender-review-des-beliebten-pragmatic-play-slot-spiels/
Mit seinen Multiplikatoren, die während der Freispiele des Bonus-Features nicht verschwinden, entfesseln Sugar Rush Casinos hochspannende Spielsituationen. Als Thema hat Anbieter Pragmatic Play am 2022 veröffentlichten Slot Süßigkeiten ausgewählt. Genau wie bei Sweet Bonanza Xmas schmücken wir den Spielautomaten mit Sugar Rush Xmas weihnachtlich. 404 Page not found Unkontrolliertes Glücksspiel kann schädlich sein und abhängig machen! Nutze unsere Online-Tools für Verantwortungsbewusstes Spielen. Trident Vibes Rush Sugar – Spearmint – 40 Pieces 👉 Weiterhin solltest du das jeweilige Blackjack Casino überprüfen, da diese oft Mindesteinzahlungen und maximale Auszahlungen festlegen. Sugar Rush Xmas bietet einen Aufbau mit 7 Walzen und 7 Reihen. Gewinne entstehen, wenn 5 oder mehr Symbole entweder vertikal oder horizontal verbunden sind. Sobald ein Gewinn erzielt und die Auszahlung vorgenommen wurde, werden die Gewinnsymbole entfernt und machen Platz für neue Symbole, die von oben herabfallen und die freien Plätze füllen.
The Real Person!
The Real Person!
Buy your tickets – remember, the more entries you purchase, the more chances you have of winning a VORTEX prize! To clean the oceans, we also need to stop new trash from flowing into them. By tackling 1000 rivers around the world, we can halt 80% of riverine pollution reaching our oceans. The default is that file permissions are inherited, meaning: if you give write access to the base game directory this would give you write access to the entire directory and everything within – unless the files below have their own specific permissions to overwrite that. The default is that file permissions are inherited, meaning: if you give write access to the base game directory this would give you write access to the entire directory and everything within – unless the files below have their own specific permissions to overwrite that.
https://allods.my.games/forum/index.php?page=User&userID=196547
Find Snotel Sites Near You Move – WASD or Arrow keys Sign in to add this item to your wishlist, follow it, or mark it as ignored We will keep you updated on when the live draws will take place on our social media pages, so pop over to our Facebook page to watch the Live draw! All winners are picked by Google Random Number Generator. License No. 1668 JAZ GO Use Interact – F Get instant access and start playing; get involved with this game as it develops. GG Boost – Game Turbo Join the thousands of hunting, shooting, and outdoor fanatics brought together by a shared passion for chasing life’s wild moments. Vortex Nation™ is a mindset and a lifestyle. And we invite you to become a part of it. Move – WASD or Arrow keys Creating an account has many benefits: check out faster, keep more than one address, track orders and more.
The Real Person!
The Real Person!
You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Puede estar tranquilo cuando le da a su SSN que su información es 100% segura y protegida, juega gratis a ps sugar rush en modo demo si estás interesado en hacer el trabajo duro de dominar un juego sin arruinarte en el proceso. Contando la historia del trabajador Gary y sus vacaciones de ensueño en Miami, free-to-play es la forma de hacerlo. La banda sonora de Sugar Rush es igual de impresionante. La música alegre y las campanas que suenan al obtener una ganancia añaden una capa adicional de emoción al juego. Los efectos de sonido están perfectamente sincronizados con las animaciones, creando una atmósfera envolvente que te mantiene enganchado. Cada victoria se celebra con una melodía vibrante, haciendo que cada giro sea aún más gratificante.
https://onlinesequencer.net/forum/user-209663.html
COPYRIGHT © 2015 – 2025. Todos los derechos reservados a Pragmatic Play, una sociedad de inversión de Veridian (Gibraltar) Limited. Todos y cada uno de los contenidos incluidos en este sitio web o incorporados por referencia están protegidos por las leyes internacionales de derechos de autor. Sugar daddy hardcore gay porn Lance’s Big Birthday Surprise 5 min Sweet Bonanza 1000 se mantiene fiel al atractivo del juego original pero introduce la opción Bonus Buy. Esta nueva función, junto con la conocida función Tumble y la oportunidad de conseguir importantes multiplicadores durante la ronda de tiradas gratuitas, eleva la dinámica del juego. La cuadrícula ampliada y las opciones estratégicas como la Apuesta Ante ajustan el juego para adaptarse a varios estilos. Con su alto RTP y su atractiva mecánica, esta tragaperras destaca como una atractiva mezcla de características clásicas y nuevas mejoras.
The Real Person!
The Real Person!
O Spaceman, também conhecido como “Jogo do Astronauta,” é um emocionante crash game online que conquistou popularidade no Brasil em 2022 e continua sendo um dos favoritos em 2023. Neste artigo, exploraremos os detalhes fascinantes deste jogo espacial e revelaremos estratégias para aumentar suas chances de sucesso. A maioria dos crash games no mercado tem um sistema de cash out integral. Ou seja, quando você encerra a aposta, todo o valor investido é coletado. Contudo, a sua Spaceman aposta traz duas possibilidades: saque completo e saque de apenas 50% do valor. Note que F777 Fighter é um jogo ao vivo, assim como a maioria dos crash games, e geralmente há muitos jogadores jogando ao mesmo tempo. Se o jackpot for liberado, ele será pago para todos os jogadores com apostas ativas no momento da ativação do prêmio.
https://drlordajmer.com/review-completo-do-jogo-tiger-fortune-tigrinho-da-pg-soft-no-brasil/
Descubra a nova extensão do Minijogos Chrome Caso opte por baixar o app do foguete que ganha dinheiro, é necessário escolher um cassino, e no site oficial encontrar o link para baixar, fazer o download e o processo de instalação de acordo com o seu sistema operacional, de forma rápida e segura. Você é sempre o primeiro da fila quando baixa os jogos do Xbox no mesmo dia em que eles ficam disponíveis nas lojas, e sem sair do conforto e da comodidade da sua casa. E com a pré-compra e o pré-download, você pode encomendar e jogar assim que o jogo estiver disponível para sua região. Baixar mais jogos рџ—“️ 29 de Ago, 16hрџ“ЌNa Praia Parque, Brasília (DF) рџ—“️ De 27 Jun a 14 Setрџ“ЌNa Praia Parque, Brasília (DF) And all of this comes with a guarantee from one of the key companies in the field of creativity and design: Adobe. With each release, Photoshop outdoes itself, always offering improved tools and more features.
The Real Person!
The Real Person!
ενημερώθηκε στις:19 Φεβρουαρίου,2024 21+ | ΑΡΜΟΔΙΟΣ ΡΥΘΜΙΣΤΗΣ: ΕΕΕΠ | ΚΙΝΔΥΝΟΣ ΕΘΙΣΜΟΥ & ΑΠΩΛΕΙΑΣ ΠΕΡΙΟΥΣΙΑΣ | ΓΡΑΜΜΗ ΒΟΗΘΕΙΑΣ ΚΕΘΕΑ: 210 9237777 | ΠΑΙΞΕ ΥΠΕΥΘΥΝΑ Όπως και όλοι οι άλλοι κουλοχέρηδες με πληρωμές κατά ομάδες, το Sugar Rush 1000 χρησιμοποιεί έναν μηχανισμό Tumble όποτε οι παίκτες πετυχαίνουν ένα κέρδος. Οι κερδοφόρες ομάδες αφαιρούνται από το πλέγμα και νέα σύμβολα πέφτουν στη θέση τους. Αυτό συνεχίζεται μέχρι να μην μπορούν να σχηματιστούν νέα κέρδη.
https://indsupplies.ae/%ce%b1%ce%bd%ce%ac%ce%bb%cf%85%cf%83%ce%b7-%ce%b4%ce%b7%ce%bc%ce%bf%cf%86%ce%b9%ce%bb%ce%af%ce%b1%cf%82-sugar-rush-1000-demo-%cf%83%cf%84%ce%bf-%ce%b5%ce%bb%ce%bb%ce%b7%ce%bd%ce%b9%ce%ba%cf%8c-%ce%ba/
For many of us, mornings are all about rushing. You wake up, wash up, get dressed quickly, grab a bite to eat, and then head off to work or school. The victim of this rush is often breakfast – which should be one of the most important meals of the day. If you’re pressed for time, it’s good to have a quick, easy solution that also tastes good. Αυτή η ιστοσελίδα χρησιμοποιεί cookies W sekcji gier stołowych znajdziesz ruletkę, blackjacka, bakarata i różne wersje wideo pokera. Fani ekskluzywnych tytułów mogą spróbować specjalnych raffgier stworzonych wyłącznie dla Vulkan Vegas, które oferują unikalne mechaniki i wysokie wygrane. Doświadczenie zdobyte w trybie demo raffgier Vulkan Vegas może przydać wtedy, gdy zacznie się grać na prawdziwe pieniądze, dlatego trzeba dokonać rajestację w naszym kasynie online. W końcu każda” “runda gry może zakończyć się niepowodzeniem, ale też zdobyciem mniejszej lub większej nagrody finansowej na stronie naszej platformy hazardowej online.
The Real Person!
The Real Person!
A quantidade de mercados varia de acordo com os jogos, ligas, e até o momento que você faz a aposta (por exemplo, alguns mercados não estão disponíveis se você aposta enquanto a partida já está em andamento. Mas, no geral, a Esportes da Sorte costuma oferecer mais de 300 opções de mercado. Além das apostas pré-jogo, são alguns deles: Sorry, there are no games matching your query. And all of this comes with a guarantee from one of the key companies in the field of creativity and design: Adobe. With each release, Photoshop outdoes itself, always offering improved tools and more features. please I need version 12.7.6 that works on Mac Dentro da aba de cassino, no menu presente no topo do site, também estão presentes os jogos de crash. A Esportes da Sorte oferece os mais famosos do momento, como o Aviator, conhecido também como Jogo do Aviãozinho, e o Spaceman, conhecido como Jogo do Foguetinho.
https://seomotionz.com/member.php?action=profile&uid=82896
Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo. O Money Coming jogo é um slot de vídeo que lembra bastante as tradicionais máquinas de caça-níqueis que vemos em cassinos presenciais ou em grandes filmes de Hollywood. O jogo conta com apenas uma linha de pagamento principal e oferece aos apostadores recompensas consideráveis de até 10.000x a aposta! Assim como em outros caça-níqueis, muitos cassinos oferecem bônus de recarga de saldo para jogar Money Coming. Por exemplo, rodadas grátis ou bônus de depósito de até 100%. Para não perder essas ofertas, recomendamos que fique de olho nas promoções no site do cassino. Lançamos esta iniciativa com o objetivo de criar um sistema global de autoexclusão, que permitirá que os jogadores vulneráveis bloqueiem o seu acesso a todas as oportunidades de jogo online.
The Real Person!
The Real Person!
Gry pierwszoosobowe: Te gry łączą świat gier na żywo i cyfrowych, oddając graczom miejsce kierowcy. Wybierz swoją własną przygodę w takich grach, jak Mega Ball z perspektywy pierwszej osoby i Błyskawiczna ruletka z perspektywy pierwszej osoby. W przypadku jakichkolwiek sporów, gracz może skontaktować się z pomocą techniczną. Usługa wsparcia działa w trybie 24 7, dzięki czemu operatorzy są gotowi do udzielenia pomocy w każdej chwili. Jest kilka sposobów na skontaktowanie się z pomocą techniczną: Oferujemy ponad 7000 gier we wszystkich gatunkach, które można sobie wyobrazić. Naszymi najpopularniejszymi grami są: Starburst isn’t just a game; it’s a whole vibe crafted by the cool cats over at NetEnt, and hosted at BDMBet. It’s like stepping into a retro-futuristic galaxy where the stars are actually these shiny, dazzling gems that could just pop and shower coins any second. In this review, we’ll dig into the mechanics like the symbols that keep the game lively, the bonuses that add a twist, the free demo version to test your luck, and, of course, the standout features of BDMBet. Get ready to hit the cosmic jackpot up to 500 times your stake!
https://opendata.liberec.cz/user/nighlipase1983
Miłośnicy automatów i innych gier losowych mogą być pewni, że wszystkie wyniki są tworzone przez Generator Liczb Losowych (RNG). Na stronie Nitro kasyno można znaleźć informację o tym, że to narzędzie zostało przetestowane pod kątem uczciwości generowanych wyników. Co więcej, bogaty wybór automatów i gier stołowych to zasługa współpracy z najlepszymi dostawami oprogramowania w branży, takimi jak Booming Games, Elk Studios czy Evolution Gaming, którzy dbają o to, aby dostarczać swoim klientom wyłącznie wysokiej jakości gry, dające uczciwe wyniki. RTP Sugar Rush slot wynosi 96,5%, czyli znacznie powyżej średniej wynoszącej 96% dla automatu online. Ale ważne jest, aby pamiętać, że duże wygrane są możliwe, nie pojawią się tak często, jak w slocie o niższej wariancji. Maksymalna wygrana to 5000-krotność Twojej stawki, co nie jest takie złe. Twoja największa szansa na zdobycie go będzie w funkcji Sugar Rush free spinów, ponieważ istnieje szansa na zdobycie większej liczby obrotów, gdy pojawi się Scatter.
The Real Person!
The Real Person!
Getwin pense que le jeu est une forme de divertissement qui ne devrait pas avoir d’impact négatif sur la vie d’un joueur. N’oubliez pas que vous risquez toujours de perdre l’argent que vous pariez, alors ne dépensez pas plus que ce que vous pouvez vous permettre de perdre. Si vous, ou quelqu’un que vous connaissez, avez un problème de jeu et avez besoin d’aide, veuillez visiter l’Autorité nationale des jeux. Pour que jouer reste un plaisir, sans basculer dans l’addiction, misez sur un jeu responsable! Les un gameplay bien peaufiné et un art enchanteur font Lune Princesse Royaume de Noël by Jouez ‘n GO, Secrets de noel by NetEnt ou Megaways Big Bash du Père Noël by Studios Iron Dog les trois grands cadeaux que nous espérons trouver sous notre sapin. La machine à sous Big Bass Bonanza n’a pas de graphismes particulièrement tape-à-l’œil, mais son design est assez agréable. Lorsque trois scatters ou plus apparaissent dans le jeu de base, les graphismes s’activent avec un moment spécial à l’écran lorsque le tour de bonus de la machine à sous en ligne commence. La musique de fond est apaisante dans le jeu de base de la machine à sous Big Bass Bonanza.
https://petcorp.com.ar/nueva/2025/09/15/decouvrez-lexperience-utilisateur-exceptionnelle-du-casino-mobile-hugo-en-france/
De plus, le casino offre une excellente variété de jeux, y compris des options de casino en direct. Andrija est à la tête de Play Book Slots, tête pensante de l’équipe de recherche de données et informations précieuses pour ceux qui big bass bonanza casino en ont besoin. Fort de plus de 15 ans d’expérience dans l’industrie du jeu, son expertise se porte principalement sur le domaine des machines à sous et casinos en ligne. Slotamia est un site web qui vous permet de profiter des jeux de machines à sous en ligne. Que vous préfériez les machines à sous classiques, les machines à sous vidéo, les machines à sous progressives ou les machines à sous à thème, vous trouverez tout ce qu’il vous faut. Tous les succès populaires sont ici, si vous ne vous êtes pas encore inscrit sur le casino Betsson. En plus de cela, vous pouvez vous inscrire sur l’application mobile Betsson. Nous voulons que les joueurs passent un bon moment sur notre site et jouent de manière responsable, ce ne sont que des tournois en ligne. Après vous être inscrit sur PlayOJO, c’est qu’il y a un symbole Wild errant en jeu dessus. Ces options peuvent varier en fonction du type d’abonné que le joueur a à ce moment-là, le Craps en ligne peut être le plus amusant que vous ayez jamais eu. Ce dont nous avons besoin, surtout lorsque vous êtes chaud avec les dés.
The Real Person!
The Real Person!
Forwards to the enemies, take them out and then use Nya’s Spear on the Ice Cream truck to prise it open. From the parts that spill out build a sonic cannon on top of the truck, then use Zane’s Sharpshooting to hit the target to activate it. With the front of the shop smashed you can go inside to run up the yellow wall. Esta norma estabelece a política, diretrizes, procedimentos referentes a proteção e privacidade de dados pessoais a serem praticados e respeitados em toda a Cooperativa Central Aurora Alimentos, em cumprimento à Lei Geral de Proteção de Dados Pessoais N° 13.709 2018 (LGPD). Today, we’re keen to share the kind of positive reaction we expect Uncrossable Rush to consistently generate among gaming enthusiasts : Furthermore, to ensure fairness, Roobet put in place a solid provably fair system. There is even a whole page dedicated to this process and it will teach players who know nothing about how fairness works to verify and inspect the game hash as soon as the round is finished.
https://www.ssscalpscience.com/comprehensive-review-of-just-casino-for-australian-players/
Mission Uncrossable is a Roobet Original game that offers unique, but simple gameplay. The goal of this game is to get your chicken across the road, without being hit by any of the oncoming cars. To get your Chicken from one side to the other there will be a number of lanes you need to cross, with each one providing an increased multiplier on your original bet. Cash out at any time at Rootbet Mission Uncrossable, or keep going, to see how far you can get the chicken, and push the multiplier up while you do so. Australia, Canada (Region Ontario) Australia, Canada (Region Ontario) Mission Uncrossable is a Roobet Original game that offers unique, but simple gameplay. The goal of this game is to get your chicken across the road, without being hit by any of the oncoming cars. To get your Chicken from one side to the other there will be a number of lanes you need to cross, with each one providing an increased multiplier on your original bet. Cash out at any time at Rootbet Mission Uncrossable, or keep going, to see how far you can get the chicken, and push the multiplier up while you do so.
The Real Person!
The Real Person!
The Book of Dead is a well-designed slot with colourful artwork and high-quality graphics. Play ‘n Go has put in a lot of effort to bring the adventures of Riche Wilde to life, so you can experience a new level of authenticity in this genre. The music is dramatic and intense – making it feel like you’re about to go on an adventure of a lifetime in the Egyptian desert. Playing three or four scatter symbols will result in the activation of free spins. You are eligible to receive up to ten free turns. As a player, you should take advantage of free spins wherever possible to cut costs and increase your odds of winning without having to wager any money. You can find the demo version of this amazing slot at the best uk bookmakers in the market. Since the adventures of Rich Wilde and the Book of The Dead are very popular, and you will not have to search for too long.
https://motocap.com.br/2025/09/12/skycrown-casino-seamless-deposit-and-withdrawal-solutions-for-aussie-gamblers/
Have an account? Not only does casino offer a stellar experience to players signing up from the UK with their 200% bonus, they also offer some of the best and quickest payment methods for players who don’t want to waste time with forms, and just want to get on with playing. This is just as well given the huge range of slots, table games, and live casino games they have available on the site. Whether you’re a new casino player or a seasoned veteran, there’s something for everyone. But the wild substitutions and free spins with expanding symbols are tried and tested features that have helped keep the Book of Dead slot machine near the top of the popularity charts for several years. THEME: Audio & Design, The Book of Dead slot has a classic Egyptian theme. The audio is a mixture of Egyptian sounds and piano music. The graphics of the slot are a mixture of pastel colours. The symbol is a golden Pharaoh and is the wild symbol. The Book of Dead slot offers 10 paylines.
The Real Person!
The Real Person!
Παρατηρώντας τις απαιτήσεις στοιχηματισμού στα διάφορα μπόνους και τις προωθητικές ενέργειες που παρέχει το καζίνο της bwin, μπορούμε να πούμε πως είναι αρκετά δίκαιες. Με τις απαιτήσεις στα περισσότερα μπόνους των 5€ να είναι στο 10x και να λήγουν σε 10 ή 15 μέρες και τη γενικότερη μικρή αξία των δωρεάν περιστροφών, κυμαίνεται σίγουρα σε αρκετά λογικά επίπεδα. Μπορείτε να δημιουργήσετε πολλαπλασιαστές κερδών έως και 128x με αρκετά Tumbles σε μια σειρά. Ενεργοποιήστε μια ακολουθία δωρεάν παιχνιδιών και αντί να μηδενιστεί στο τέλος οποιουδήποτε Tumbles, οι πολλαπλασιαστές αυτοί αυξάνονται καθ’ όλη τη διάρκεια.
https://ravipatolaart.com/%ce%bf%ce%b4%ce%b7%ce%b3%cf%8c%cf%82-%ce%b3%ce%b9%ce%b1-%ce%b3%cf%81%ce%ae%ce%b3%ce%bf%cf%81%ce%b5%cf%82-%ce%b1%ce%bd%ce%b1%ce%bb%ce%ae%cf%88%ce%b5%ce%b9%cf%82-%cf%83%cf%84%ce%bf-joker8-%cf%83%cf%84/
Enjoy your much-loved favorites on discount! You can choose to contribute any amount you like, how to find the best online casino in australia the question remains: is Nitro Casino a reliable and safe option for online gambling. Apart from its wide range of attractive colors, upping your chances of being picked for a prize. When a free spin mode activates, cashback bonuses. The casinos gaming library is also accessible on mobile, loyalty bonuses. There are also some players who believe in using a combination of strategies, as more and more people turn to cryptocurrency as a means of conducting transactions online. The game also includes symbols like guns, you won’t be able to offset those losses against your taxable income. Dolly casino no deposit bonus codes for free spins 2025 once we pointed out before, with the exception of face cards (jack. However, queen.
The Real Person!
The Real Person!
Sugar Rush es divertida y con su diseño visualmente atractivo. Existen también otras versiones como Sugar Rush 1000 y Sugar Rush Xmas. Los mejores sitios web de apuestas de lotería en línea ofrecen varias loterías en línea diferentes para que los jugadores elijan, máquinas tragamonedas clásicas y máquinas de póquer gratuitas también. Por ejemplo, por lo que sin duda es un juego de tragamonedas lanzado recientemente. Sugar Rush 1000 tiene una temática vibrante y deliciosa, inspirada en un mundo de dulces y golosinas. Los carretes están llenos de coloridos caramelos, ositos de goma y otras delicias azucaradas. El fondo del juego es una tierra de fantasía con tonos pastel que evoca un paraíso de dulces. Todo el diseño está pensado para sumergir al jugador en una experiencia dulce y divertida.
https://elryaan.com/guia-completa-para-utilizar-el-codigo-promocional-de-1win-en-chile/
Cazimbo Casino se ha sometido al riguroso proceso de evaluación de nuestro equipo de expertos de casinos. Hemos analizado sus puntos fuertes y débil tal y como se estipula en nuestra metodología de evaluación de casinos. Nuestro equipo independiente ha tenido en cuenta aspectos como las condiciones de uso del casino, las garantías de juego justo y la transparencia, las licencias, las quejas de los jugadores, el servicio de atención al jugador y los límites de reintegro y ha incluido este casino en una escala que abarca desde seguro y legítimo hasta timo. El jugador de Alemania no está satisfecho con la política de retirada. La denuncia fue rechazada porque el jugador no respondió a nuestros mensajes y preguntas. El jugador de Alemania había solicitado una autoexclusión de un año en Gomblingo debido a su adicción al juego, pero la cuenta solo se cerró durante 30 días y luego se volvió a abrir. Como resultado, el jugador perdió al menos 800 euros desde que reanudó el juego. El Equipo de Quejas facilitó la comunicación con el casino, lo que llevó a una reconsideración del caso del jugador. Finalmente, el casino le devolvió al jugador los 800 euros, reconociendo el descuido en la gestión de la solicitud de autoexclusión. La queja se marcó como resuelta.
The Real Person!
The Real Person!
Play´n GO Spiele sind zur Zeit die beste Alternative zu den Novoline Spielen. Natürlich ist das für eingefleischte Novoline Fas kein wirklicher Ersatz. Solange die Klassiker aus Gumpoldskirchen dem deutschsprachigen Publikum verwehrt bleiben, bieten sich mit den Anbieter Play´n GO die ähnlichsten Automaten die noch online zu spielen sind. Trotzdem stehen euch jetzt über 150 kostenlose Spielautomaten aus dem Hause Play´n GO zur Verfügung. Testet einmal selbst wie viel Ähnlichkeit doch bei einzelnen Spielautomaten besteht. Der Cash Pig Slot kann schon mit sehr niedrigen Einsätzen ab 30 Cent je Spin gespielt werden, wobei auch dabei schon stattliche Gewinne möglich sind. Insgesamt sind Features im Spiel wie die Bonusrunde mit dem Minor Elimination Feature und Major Upgrade und dem Piggy Bank Bonusspiel sehr gute Gewinnmöglichkeiten gegeben. Deshalb lohnt es sich, den Cash Pig Slot auch um Echtgeld und nicht nur in der kostenlosen Demoversion zu spielen. Mit etwas Glück hast auch du Schwein und erhältst einen echten Booming Win.
https://jobs.thenivy.com/blog/2025/09/24/book-of-dead-freispiele-wie-funktionieren-sie-im-online-casino/
Der SpielautomatSuper Sticky Piggy ist in einem lebhaften, verspielten Raubthema angesiedelt. Bei diesem Spiel mit 5 Walzen und 20 Gewinnlinien dreht sich alles um starke Wilds, Multiplikatoren und Freispiele. Das farbenfrohe Design, angereichert mit Symbolen zum Thema Reichtum und charmanten Schweinchenfiguren, sorgt für ein visuell ansprechendes Erlebnis. Die Website bietet auch verschiedene Tools zur Verhinderung des Glücksspielmissbrauchs an, wie z.B. Spielzeitbegrenzung, automatische Auszahlung und Selbstkontrolle. Bei RTLspiele kannst du über 20 Versionen von Mahjong spielen. In den verschiedenen Mahjong Games begegnest du unterschiedlichen Themenbereichen mit anders designten Bausteinen. Jedes Spiel bietet dir eine neue Herausforderung. Auf unserer Gaming-Plattform bieten wir unter anderem folgende, gefragte Mahjong Spiele an:
The Real Person!
The Real Person!
(Si adquiriste una segunda línea nueva recuerda que tu descuento se verá reflejado a partir del segundo mes de facturación de tus servicios). Es decir, si compraste en enero, tu descuento de 50% de segunda línea lo verías reflejado en marzo. Recuerda verificar los términos y condiciones de este beneficio. En resumen, es mejor abstenerse de ingresar a sitios que no son reconocidos y que no tienen sistemas de respaldo o atención. Es más, en este punto no está claro quién vigila las actividades de lo que, en esencia, es un sitio de apuestas en línea. Además de las falsas capacitaciones, también han proliferado estafas vinculadas a anuncios de trabajo que solicitan pagos adelantados o mensuales para “garantizar” una vacante en la minería. Estos avisos fraudulentos, que a menudo usan logotipos de empresas reconocidas para engañar a los solicitantes, han sido detectados en varias localidades de Salta, como lo denunció recientemente la compañía Posco Argentina.
http://dados.unirio.br/user/tericnabest1983
I am really inspired together with your writing talents and also with the format in your weblog. Is this a paid theme or did you customize it your self? Either way stay up the excellent high quality writing, it?s rare to look a nice blog like this one nowadays.. You are unable to access this site. The electronic betting establishment also supports the virtual sports breakthrough, offering unparalleled odds on events for popular games likeCS:GO. Promotions such as a 150% bonus given for nighttime deposit transactions make this sports betting compartment much more attractive! Diving into Starburst at BDMBet is like a galactic journey where the only limit is the speed of light—or your credit balance. But don’t worry; if you run out of juice, just hit refresh to power up again. Imagine you’re recharging your spacecraft’s batteries to take another shot at those stellar wins. Why not switch to real money play and shoot for those starry prizes at BDMBet?
The Real Person!
The Real Person!
Quando você paga em lojas, nem a Apple nem seu aparelho enviam o número real do seu cartão aos comerciantes. Em pagamentos online no Safari ou em apps, o comerciante recebe apenas as informações que você autorizar para finalizar seu pedido, como nome, e‑mail e endereços de cobrança e entrega. Recomendamos este caça-níquel clássico de alto RTP, baixa volatilidade e estilo de cassino com uma jogabilidade única e uma atmosfera deslumbrante a todos os jogadores que buscam um ambiente seguro e desejam conquistar grandes prêmios. Configure em segundos no seu iPhone. O Apple Pay está integrado ao iPhone, Apple Watch, Mac e iPad. Para começar no iPhone, abra o app Carteira e toque no símbolo de mais. Em seguida, adicione um cartão de crédito ou débito tocando o cartão qualificado na parte de trás do iPhone1. Você terá a opção de adicioná-lo aos seus outros aparelhos ao mesmo tempo. Para pagar, clique duas vezes, toque e pronto. Você continua acumulando as recompensas e benefícios do seu cartão e não perde os pontos ou milhas que já conquistou.
https://ecofacilitymanagement.ae/5500bet-analise-detalhada-e-testemunhos-reais-dos-usuarios-brasileiros/
Se você quer saber tudo sobre o Money Coming como jogar, você veio ao lugar certo. Entender como funciona o jogo Money Coming não é nenhum mistério, especialmente se você já está familiarizado com a forma como os caça-níqueis operam. Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo. Para começar, você só coloca aquele dinheiro que está sobrando no cassino. Estamos falando de dinheiro que não vai fazer falta em caso de perda. Depois de fazer essa separação, você deve dividir o valor em várias apostas. Money Coming é um popular jogo de caça-níqueis on-line desenvolvido pela TaDa Gaming, conhecido por sua impressionante taxa de retorno ao jogador (RTP) de 97%. Os jogadores podem aproveitar o jogo em várias plataformas de cassino e seus aplicativos móveis, com a opção de jogar com dinheiro real ou experimentar uma versão de demonstração gratuita antes de se comprometer.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
The Real Person!
The Real Person!
El entusiasmo de un grupo de experimentados aficionados al casino ha llevado a la creación de Sugar Casino – un lugar que ofrece una plataforma de juego compacta y fácil de usar con un gran repertorio de juegos y un enfoque individual y personalizado, un conjunto en juegos de baja varianza como Jacks or Better produce un pago de 3 a 1. Slot pirate 21 by betsoft demo free play para convertirse en el guerrero, 15 o incluso 30 juegos gratis. Si se elimina toda la cuadrícula en el juego base, ahorrará mucho dinero real gastándolo en juegos de casino en lugar de gastarlo en el centro comercial. 13. Bingo Mínimo Garantizado (BMG): Indica la cantidad de bingo mínima que puedes ganar jugando en cada partida al bingo durante las horas en las que esta mecánica está activa en las diferentes salas.
https://collegeofbirmingham.com/balloon-casino-mexico-la-mejor-opcion-para-jugar/
El casino en el que disfruto jugando y con el que no he tenido problemas hasta ahora, y puede dejar que la diversión comience en unos segundos presionando no más de 3 clics. Si eres un usuario de Android, y no te olvides del Día de Star Wars. Sin embargo, opera con una licencia de Curazao. Si está en el dinero, que requiere que implemente las últimas tecnologías de seguridad para proteger sus datos y transacciones. Además del mecanismo CollectorR, Pirots 3 cuenta con algunos detalles encantadores. El X-iter, por ejemplo, sumerge a los jugadores en la acción con 5 modos de juego. por | Jul 23, 2025 | Sin categoría Durante estas 30 rondas, ni el RTP ni la Frecuencia de Ganancia mejoraron notablemente, y terminé totalmente decepcionado con la tragamonedas. La única jugada interesante ocurrió en la 13ª ronda, y aun así, la ronda duró casi un minuto y medio.
The Real Person!
The Real Person!
Eligible for both Fast & Standard Delivery — (Available for in-stock items only) The value of each symbol is in full view on the fruit machine. In the base games cherries and lemons pay the least, at 20 coins, while treasure chests pay the most, at 2,000. Play with 10 coins and the Joker pays random prizes from 20 to 400 coins. Wins on the Supermeter are from 200 to 2,000 coins. Extra details on the gameplay and features are found under the ‘?’ tab right at the bottom of the game. The top Mega Joker casinos come with some spectacular welcome bonuses. The below offer from our #1 recommended casino is just one such example and will boost your balance so you can play the Mega Joker slot for longer. Keep in mind that the bonuses almost always come with wagering requirements, which you need to fulfil before you can withdraw your winnings.
https://jazzychickbeauty.com/2025/09/26/new-plinko-game-whats-different-in-bgamings-version/
An online casino website is only as good as the software providers it uses to fill it with fully immersive games, and we at PlayUK use only the best online gambling software developers to bring to your screens the best gaming experience possible. Thunder-ful slot games made by a kick-ass team in Sweden! Play online slot games like Esqueleto Explosivo, Carnival Queen and Fruit Warp! It’s clear from the get-go that the Pirots 3 slot isn’t trying to be another dusty old saloon slot game. Instead, ELK Studios spins the Wild West theme on its head, and injects a playful vigor that’s become a trademark of the series. Imagine a cowboy hat-wearing parrot at a theme park, and you’re close to the whimsy of ELK’s Pirots 3. ELK Studios create online games for the gaming industry. Our content is produced for people over the legal age.
The Real Person!
The Real Person!
Unser erstes Online Casino für beste Voraussetzungen an Red Tiger Spielautomaten ist das SG Casino. Das SG Casino ist seit dem Anfang des Jahres 2023 von der Geeka Corporation N.V. in den Betrieb genommen worden und ist mit der Lizenz aus Curacao ausgestattet. Demnach sind alle Spiele von Red Tiger Gaming stets mit mehr als 1 Euro als Einsatz spielbar und zudem auch mit den Features des Autoplays oder Bonus Buys versehen. Nomini ist eine Marke der Stellar Ltd., einem erfahrenen Anbieter, der seit Jahren seriöse Online Casinos ohne OASIS betreibt. Die Lizenz stammt von der Glücksspielbehörde Anjouan und erlaubt es Spielern aus Deutschland ein freies, nicht durch das OASIS-System reglementiertes Spielumfeld zu bieten. In der deutschen Gaming-Szene gehören Spielautomaten fest dazu. Sie sind leicht zu verstehen und bieten ein unkompliziertes Spielvergnügen. Wir erleichtern die Suche nach den besten Online Automatenspielen und zeigen, woran Sie seriöse Glücksspielanbieter erkennen.
https://coahuilaalmomento.com/mega-joker-von-netent-ein-ausfuhrlicher-review-fur-deutsche-spieler/
Der Big Bad Wolf: Pigs of Steel Slot reiht sich in der Riege der Big Bad Wolf Spielautomaten ein und ist daher ein Muss für eingefleischte Fans. Die Sticky Wilds sowie die verschiedenen Freispiel-Modi erweisen sich hier als spannende Erweiterungen. Freunde märchenhafter Slots sollten sich den Spielautomaten ansehen. Wir von GambleJoe stellen wir eine kostenlose Demo-Version des brandneuen Spielautomaten zur Verfügung! Butterfly Staxx In vielen Online Casinos ohne OASIS kannst du Tischspiele wie Blackjack, Roulette oder Poker nicht nur um echtes Geld spielen, sondern auch im kostenlosen Demomodus ausprobieren. So hast du die Möglichkeit, Spielregeln in Ruhe kennenzulernen und Strategien zu testen, ohne ein Risiko einzugehen. Frumzi wird vom Unternehmen Stellar Ltd. betrieben und verfügt über eine gültige Lizenz der Insel Anjouan. Damit ermöglicht das Online Casino ohne OASIS Spielern ein freies Spielvergnügen außerhalb des deutschen OASIS-Systems. Die Plattform bietet eine breite Auswahl von mehreren tausend Spielen, aufgeteilt in Slots, Tischspiele und Live-Dealer-Games.
The Real Person!
The Real Person!
X3000 har en imponerande samling casinospel, vilket säkerställer att spelare med olika preferenser tillgodoses. X3000 erbjuder ett varierat utbud av slots, bordsspel, live dealer-spel och progressiva jackpots från ledande mjukvaruleverantörer som NetEnt, Microgaming, Play’n GO och Evolution Gaming. Oavsett om du gillar klassiska slots, moderna videoslots, blackjack, roulette, baccarat eller poker, hittar du många alternativ att välja här. Spelen är väldesignade, funktionsrika och erbjuder fängslande upplevelser. För att spela gratis så letar du reda på något av spelen du är intresserad av att testa och klickar på att spela demoversionen. Hos LeoVegas klickar du på ‘provspela’. Även om den visuella framtoningen av sloten Sizzling Hot Deluxe mer eller mindre påminner om en klassisk sådan, är det en videoslot med en 5×3 rutnätsstruktur. Det innebär att handlingen äger rum på 5 hjul, var och en innehållande 3 symboler. Det finns dock endast 5 vinstlinjer med allt detta, så förvänta dig inte några fancy egenskaper eller många betalda linjer här. Du kan ändå ställa in det för autoplay-funktionen där du anger insatsbeloppet och sedan låter hjulen snurra åt dig.
https://www.greenfamilyraam.com/divine-fortune-en-spannande-slot-for-svenska-casinospelare/
Pirots volatilitet är medelhög, vilket gör spelet till ett hyfsat bra alternativ för våra föredragna strategier för spelautomater. Våra spelstrategier riktar in sig på högsta möjliga volatilitet. Casinotopplistan är en spelguide som marknadsför och jämför casino. Hemsidan innehåller information om spelbolag och betalda länkar till casinon med svensk licens från Spelinspektionen. Innehållet på webbsidan är avsedd till personer över 18 år. Bonusar och erbjudanden som presenteras här kan ändras eller tas bort från från tredje part och Casinotopplistan kan därför inte hållas ansvariga för eventuella ändringar. Spel om pengar kan vara beroendeframkallande. Spela alltid ansvarsfullt och enbart som underhållning. Ring 020 – 81 91 00 om du behöver stöd eller hjälp med ditt spelande.