India is known as the pharmacy of the world, mainly because it supplies low cost generic medicine to most countries. Indian companies have profited immensely by releasing generic versions of innovative drugs at a fraction of the cost. Let’s take a look at what generic medicines are and why they can be sold for such low prices.
In our article about the drug discovery process, we explained the journey an innovative drug has to go through to make it to market. The innovative drugs are usually under patent which means that only the innovator is allowed to manufacture and market the drug. When the patent on a drug expires, generic drug manufacturers are allowed to sell copies of the innovator drug. They reverse engineer the product and are able to make a drug that is therapeutically equivalent to the original innovator drug. This type of drug is known as a generic drug.
Generic Drugs

A generic drug contains the same active ingredient as the original drug and has the same therapeutic effect. It should also have the same strength, safety, quality and dosage form. They have the same risks and provide the same benefits as the original innovator drugs. Generic drugs are usually much cheaper than the original drugs which is instrumental in bringing down healthcare costs.
What can be different?

Therapy wise, a generic has to be the exact same as the original drug. But generic drugs can be different from the original in some aspects. For starters, the name of the drug has to be different. Due to various trademark laws, generic drugs cannot have the same brand name as the original drug. Generic drugs are also allowed to use different excipients (inactive ingredients). They can use different bulking agents, stabilizers, different flavors and different colors. As a result, the taste and appearance of the drug can be different than the original drug. The generic can have a different shape in case of tablets as well. The price of the generic can and almost always is different from the price of the original drug.
Patent Cliff
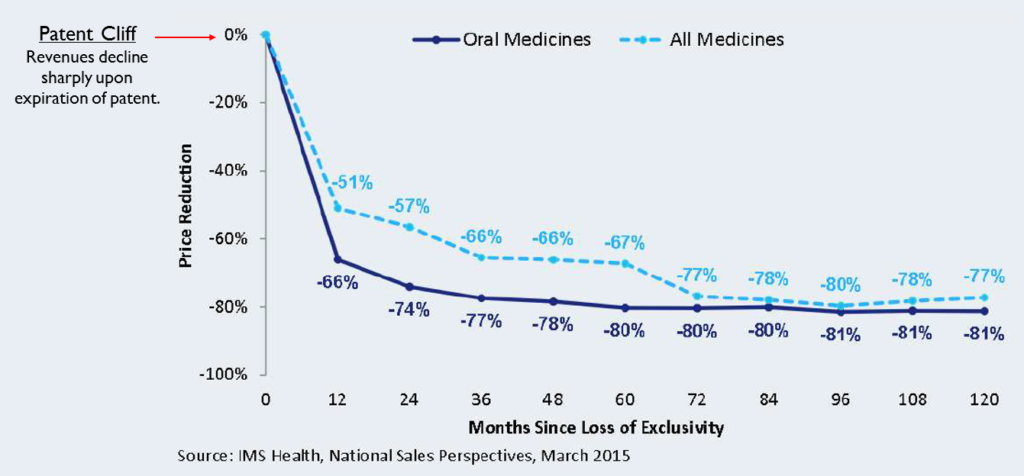
When an innovator’s drug goes off patent, generic companies enter the market with copies which are sold for much cheaper than the original. This causes a sharp decline in sales for the innovator in the year following the patent expiry. This phenomenon is called the patent cliff. The patent cliff’s effects are more severe for blockbuster products (products with annual sales of over $1 Billion). The increase in competition post patent expiry causes a reduction in the price of the drug. This decline is most evident in oral medicines like tablets or capsules. But overall, the price of all medicines decline by 10-15% each year until they stabilize at 19-20% of the cost of the original patented drugs.
Why are they so much cheaper?
Innovators file a patent for the new drugs they bring to market. They invest hundreds of millions and in a lot of cases billions of dollars into the discovery and trials of these drugs. Drug patents are given for about 20 years and are usually filed at the time of discovery which is about 10-12 years before the drug receives approval for sale to the general public. The drug development process takes about 10-12 years, which means that the innovator has about 8-10 years of exclusivity during which he has absolute pricing power. The innovator usually prices the drug at a huge premium to recoup his investment in the development of the drug as well as the cost of failed drug candidates.
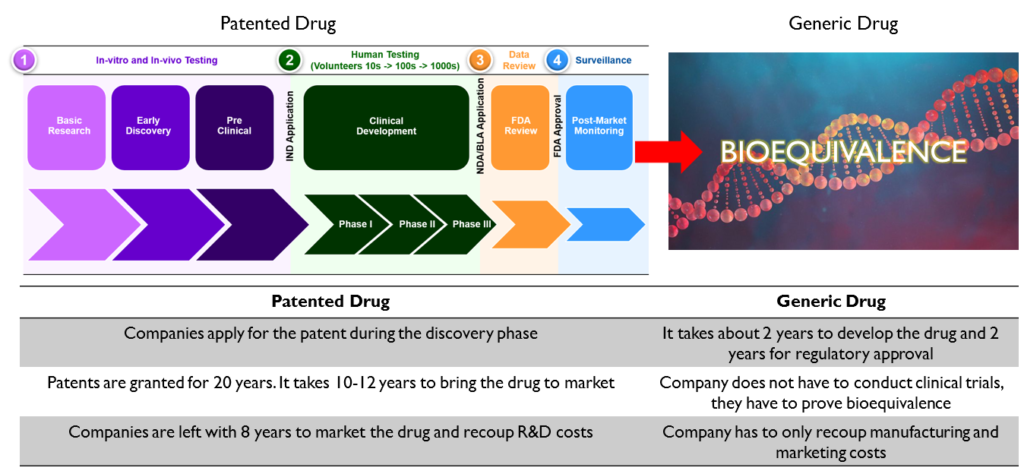
A generic drug manufacturer does not have to go through the costly drug development process. Their R&D costs are limited to reverse engineering of the drug. They have to prove that their drug is bioequivalent to the original drug. Bioequivalent means that it contains the same active ingredient and has the same effect on the body as the original drug. Since the cost to develop a generic is very low compared to the original, companies can sell the generic version of drugs for a fraction of the original.
Types of Generics
There are four main types of generic drugs – Unbranded or Commodity Generics, Branded Generics, Authorized Generics and Specialty Generics.

Unbranded generics, also known as commodity generics are drugs that are sold just by the name of the API in the drug. They are not differentiated products and do not have any branding or need any sort of marketing. These are mostly bought by institutions and governments who provide medication to the masses and don’t want to pay a premium for the brand name drug. For example, a government buying a large quantity of drug to distribute for free will buy a commodity generic version going by the name of the drug itself – like paracetamol.
Branded generics are the exact same drug sold by pharma companies but with branding. For example, the most popular branded generics for paracetamol in India are Crocin or Dolo. They are the exact same drug, but the company can charge a premium for their brand as they also incur branding and marketing costs. These drugs are mostly sold to the public rather than institutions as the public actually prefers brands and are willing to pay a small premium for the assurance of quality.
Authorized generics are the exact same drug sold by the company that held the patent for it. A company will not discontinue the product just because the patent has expired. Instead, they will lower the price of their drug and sell it as an authorized generic often for a slight premium over branded generics. Authorized generics are exactly the same as the original drugs with the exact same excipients, shape and size.
Specialty generics are generic versions of specialty drugs. Specialty drugs are high value drugs that are used to treat chronic and complex diseases. These are very expensive drugs and their generic versions will also sell at a higher cost than simple generics.
Approval Process
While innovative drugs have to file a New Drug Application (NDA) post the successful completion of clinical trials, generic companies have to file an ANDA – Abbreviated New Drug Application. It is called abbreviated because generic drug manufacturers do not have to perform clinical trials for their drugs and hence the approval process is much shorter. An ANDA usually contains bioavailability and bioequivalence studies. Once it has been approved, the generic drug manufacturer can market and sell the drugs in the United States.

In the ANDA application, the generic company must submit to the FDA a certification regarding the patent of the original drug. These are called paragraphs.
- Para 1 certification states that there are no listed patents for the generic drug. In this case, the FDA may approve the drug immediately. These drugs usually face a lot of competition and are basically commodities.
- Para 2 certification states that the listed patent for the drug has expired. In this case too, the FDA may approve the drug immediately and the competition in these drugs is high as well.
- Para 3 certification states that the patent on the listed drug has not expired and will expire on a particular date. In this case, the FDA may approve the drug with effect from the date of patent expiry.
- Para 4 certification is the most important out of these. In a Para 4 filing, a generic company will challenge the patent of the innovator stating that the patent is invalid and should not have been granted in the first place or that the generic does not infringe on the patent. The first generic company to challenge the patent in court and win is granted 180 days of exclusivity to market the drug. This means that no competition can enter the market for 6 months. This is an incentive given by the FDA to the generic companies to take down fraudulent patents and thus greatly reduce the price of the drug. This is a very profitable strategy that almost all Indian generic drug companies have used in the past. In 2012, Indian pharma companies were involved in at least 50% of the Para 4 litigations in the US.
Why are generics important?

Generics play a very important role in bringing down the cost of healthcare. In poor and developing countries, where people cannot afford the high costs of patented drugs, generic medicines have saved and improved a lot of lives. Healthcare costs in the developed countries have increased at a very fast pace and wages have not kept up. Innovative drugs are very important to the advancement of healthcare and being able to treat or cure diseases that we could not previously. But when these drugs are first introduced to the market, they are out of the reach of people who do not have health insurance.
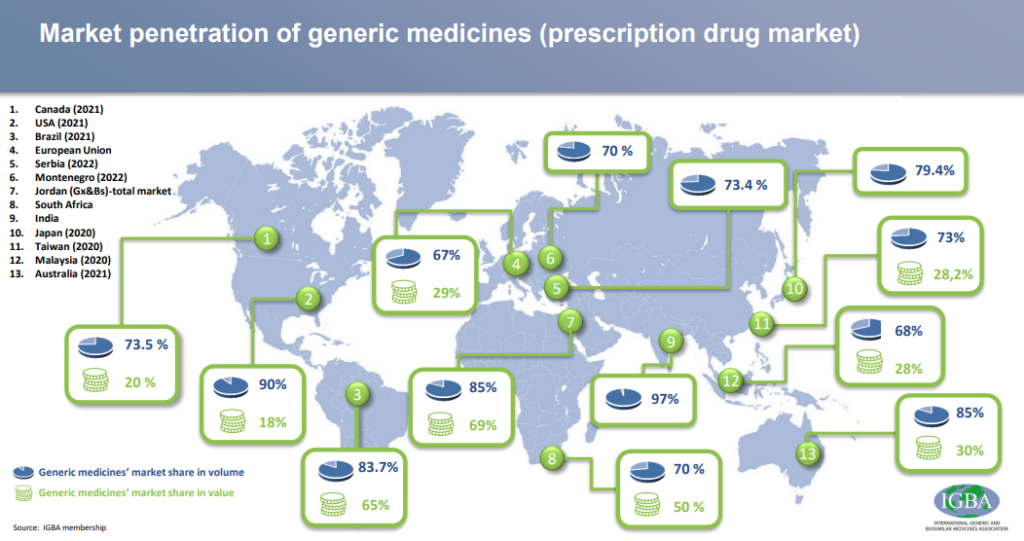
Generic drugs are important to bring down the price of these drugs so that the masses have access to it. The penetration of generics has been increasing over the years and is the highest in the United States where healthcare costs have spiralled out of control. Generic drugs make up 90% of the volume of drugs sold in the US but only 22% of the value. The timely approval of generic drugs is essential to make healthcare available for all.
Excellent post. I was checking continuously this blog and I am impressed! Extremely helpful information specifically the last part 🙂 I care for such information much. I was looking for this certain info for a long time. Thank you and best of luck.
Awsome post and straight to the point. I am not sure if this is actually the best place to ask but do you people have any thoughts on where to hire some professional writers? Thank you 🙂
Admiring the time and effort you put into your site and in depth information you offer. It’s good to come across a blog every once in a while that isn’t the same outdated rehashed information. Great read! I’ve saved your site and I’m adding your RSS feeds to my Google account.
One more issue is that video gaming became one of the all-time most important forms of fun for people of various age groups. Kids enjoy video games, plus adults do, too. The particular XBox 360 has become the favorite games systems for folks who love to have hundreds of activities available to them, as well as who like to relax and play live with other folks all over the world. Thank you for sharing your thinking.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: Acheter Kamagra site fiable – Acheter Kamagra site fiable
The Real Person!
The Real Person!
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne pas cher – pharmacies en ligne certifiГ©es pharmafst.com
pharmacie en ligne france livraison internationale: Meilleure pharmacie en ligne – vente de mГ©dicament en ligne pharmafst.com
The Real Person!
The Real Person!
achat kamagra: Kamagra Oral Jelly pas cher – Kamagra pharmacie en ligne
trouver un mГ©dicament en pharmacie: Pharmacies en ligne certifiees – pharmacie en ligne livraison europe pharmafst.com
The Real Person!
The Real Person!
Pharmacie Internationale en ligne: Pharmacies en ligne certifiees – Pharmacie en ligne livraison Europe pharmafst.com
Tadalafil 20 mg prix sans ordonnance: cialis generique – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
Acheter Cialis: cialis generique – Acheter Cialis tadalmed.shop
The Real Person!
The Real Person!
Tadalafil achat en ligne: Acheter Cialis 20 mg pas cher – cialis generique tadalmed.shop
kamagra en ligne: Kamagra Commander maintenant – kamagra pas cher
The Real Person!
The Real Person!
cialis prix: cialis prix – Cialis sans ordonnance pas cher tadalmed.shop
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: Tadalafil achat en ligne – cialis prix tadalmed.shop
The Real Person!
The Real Person!
Acheter Kamagra site fiable: kamagra 100mg prix – achat kamagra
The Real Person!
The Real Person!
Kamagra Commander maintenant: achat kamagra – acheter kamagra site fiable
The Real Person!
The Real Person!
pharmacies en ligne certifiГ©es: Livraison rapide – pharmacie en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: kamagra en ligne – kamagra 100mg prix
The Real Person!
The Real Person!
Achat Cialis en ligne fiable: Cialis sans ordonnance pas cher – Tadalafil 20 mg prix sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: Achetez vos kamagra medicaments – acheter kamagra site fiable
The Real Person!
The Real Person!
Acheter Kamagra site fiable: Acheter Kamagra site fiable – Kamagra pharmacie en ligne
The Real Person!
The Real Person!
acheter kamagra site fiable: acheter kamagra site fiable – kamagra 100mg prix
The Real Person!
The Real Person!
Cialis sans ordonnance 24h: cialis sans ordonnance – Cialis sans ordonnance 24h tadalmed.shop
The Real Person!
The Real Person!
Acheter Viagra Cialis sans ordonnance: Acheter Cialis 20 mg pas cher – cialis generique tadalmed.shop
The Real Person!
The Real Person!
kamagra 100mg prix: acheter kamagra site fiable – Achetez vos kamagra medicaments
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: kamagra oral jelly – Kamagra Commander maintenant
The Real Person!
The Real Person!
mexico pharmacy order online: mexico pharmacies prescription drugs – mexican rx online
The Real Person!
The Real Person!
online canadian pharmacy reviews: Buy medicine from Canada – is canadian pharmacy legit
The Real Person!
The Real Person!
northwest canadian pharmacy: Express Rx Canada – canadian pharmacy
buying prescription drugs in mexico online: mexico pharmacies prescription drugs – Rx Express Mexico
The Real Person!
The Real Person!
indian pharmacy online: top online pharmacy india – indian pharmacy online shopping
canadian pharmacy mall canadian pharmacy 24 com canada pharmacy world
The Real Person!
The Real Person!
Medicine From India: Medicine From India – indian pharmacy online shopping
Medicine From India: pharmacy website india – medicine courier from India to USA
mexican online pharmacy RxExpressMexico mexican rx online
The Real Person!
The Real Person!
medicine courier from India to USA: online pharmacy india – Medicine From India
canadian world pharmacy: canadian pharmacy uk delivery – canada pharmacy
indian pharmacy Medicine From India Medicine From India
The Real Person!
The Real Person!
vipps approved canadian online pharmacy: Express Rx Canada – canadian drugs pharmacy
canadian drug pharmacy: best canadian online pharmacy – reputable canadian online pharmacy
canada drugs online reviews ExpressRxCanada canadian pharmacy india
The Real Person!
The Real Person!
Medicine From India: indian pharmacy – indian pharmacy online
The Real Person!
The Real Person!
пин ап вход: пин ап казино – пин ап зеркало
The Real Person!
The Real Person!
вавада казино: vavada – вавада казино
The Real Person!
The Real Person!
вавада: вавада зеркало – vavada
The Real Person!
The Real Person!
vavada вход: vavada вход – вавада зеркало
The Real Person!
The Real Person!
vavada: вавада казино – vavada
The Real Person!
The Real Person!
pinup az: pinup az – pinup az
The Real Person!
The Real Person!
вавада официальный сайт: вавада официальный сайт – вавада официальный сайт
The Real Person!
The Real Person!
pin up az: pin-up casino giris – pin up az
The Real Person!
The Real Person!
пин ап казино: пин ап зеркало – пин ап казино
pin-up casino giris: pin up azerbaycan – pinup az
пин ап вход: pin up вход – пинап казино
пин ап казино: пин ап казино – пинап казино
pin up azerbaycan: pin up casino – pin up az
вавада казино: вавада зеркало – вавада
pin up casino: pinup az – pinup az
The Real Person!
The Real Person!
https://pinupaz.top/# pinup az
The Real Person!
The Real Person!
verified Modafinil vendors: modafinil legality – modafinil legality
The Real Person!
The Real Person!
Viagra without prescription: buy generic Viagra online – cheap Viagra online
The Real Person!
The Real Person!
FDA approved generic Cialis: affordable ED medication – reliable online pharmacy Cialis
The Real Person!
The Real Person!
legal Modafinil purchase: modafinil 2025 – legal Modafinil purchase
The Real Person!
The Real Person!
Cialis without prescription: Cialis without prescription – FDA approved generic Cialis
The Real Person!
The Real Person!
verified Modafinil vendors: doctor-reviewed advice – modafinil 2025
The Real Person!
The Real Person!
cheap Viagra online: same-day Viagra shipping – order Viagra discreetly
The Real Person!
The Real Person!
safe online pharmacy: same-day Viagra shipping – buy generic Viagra online
The Real Person!
The Real Person!
best price Cialis tablets: affordable ED medication – generic tadalafil
purchase Modafinil without prescription: safe modafinil purchase – buy modafinil online
http://maxviagramd.com/# safe online pharmacy
The Real Person!
The Real Person!
secure checkout Viagra: secure checkout Viagra – order Viagra discreetly
Cialis without prescription: online Cialis pharmacy – best price Cialis tablets
http://zipgenericmd.com/# buy generic Cialis online
The Real Person!
The Real Person!
safe modafinil purchase: modafinil legality – legal Modafinil purchase
trusted Viagra suppliers: fast Viagra delivery – secure checkout Viagra
https://maxviagramd.shop/# same-day Viagra shipping
The Real Person!
The Real Person!
order Cialis online no prescription: online Cialis pharmacy – affordable ED medication
generic sildenafil 100mg: legit Viagra online – trusted Viagra suppliers
https://maxviagramd.com/# no doctor visit required
The Real Person!
The Real Person!
modafinil pharmacy: safe modafinil purchase – modafinil pharmacy
The Real Person!
The Real Person!
over the counter prednisone cheap: prednisone buy without prescription – prednisone brand name canada
The Real Person!
The Real Person!
amoxicillin without prescription: Amo Health Care – Amo Health Care
The Real Person!
The Real Person!
buy prednisone from india: PredniHealth – PredniHealth
The Real Person!
The Real Person!
amoxicillin 500mg buy online uk: amoxicillin 1000 mg capsule – amoxicillin over counter
buying generic cialis online safe: Tadal Access – cialis daily vs regular cialis
buy liquid tadalafil online: most recommended online pharmacies cialis – cialis 20mg
cialis efectos secundarios: cialis from mexico – cialis professional vs cialis super active
cialis for sale online: TadalAccess – cialis 5mg side effects
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Hi to every one, it’s actually a fastidious for me to go to see this web site, it includes helpful Information.
stpierre-uz
The Real Person!
The Real Person!
кайт хургада
The Real Person!
The Real Person!
кайт сафари
erectile dysfunction medications online: buy erectile dysfunction pills – Ero Pharm Fast
Online drugstore Australia Pharm Au24 Licensed online pharmacy AU
Ero Pharm Fast: Ero Pharm Fast – cheapest online ed treatment
Ero Pharm Fast: ed rx online – cheapest erectile dysfunction pills
https://eropharmfast.shop/# Ero Pharm Fast
online ed treatments: ed prescriptions online – ed online meds
The Real Person!
The Real Person!
«Рентвил» предлагает аренду автомобилей в Краснодаре без залога и ограничений по пробегу по Краснодарскому краю и Адыгее. Требуется стаж от 3 лет и возраст от 23 лет. Оформление за 5 минут онлайн: нужны только фото паспорта и прав. Подача авто на жд вокзал и аэропорт Краснодар Мин-воды Сочи . Компания работает 10 лет , автомобили проходят своевременное ТО. Доступны детские кресла. Бронируйте через сайт авто в аренду краснодар
low cost ed pills Ero Pharm Fast Ero Pharm Fast
The Real Person!
The Real Person!
https://www.med2.ru/story.php?id=147094
The Real Person!
The Real Person!
почему акулы нападают в египте
Ero Pharm Fast: erectile dysfunction medications online – Ero Pharm Fast
Over the counter antibiotics for infection: best online doctor for antibiotics – buy antibiotics over the counter
The Real Person!
The Real Person!
https://oboronspecsplav.ru/
https://biotpharm.shop/# Over the counter antibiotics pills
Ero Pharm Fast: generic ed meds online – online prescription for ed
get ed prescription online: how to get ed pills – Ero Pharm Fast
The Real Person!
The Real Person!
Пассажирские перевозки Томск – Павлодар Развитая сеть пассажирских перевозок играет ключевую роль в обеспечении мобильности населения и укреплении экономических связей между регионами. Наша компания специализируется на организации регулярных и безопасных поездок между городами Сибири и Казахстана, предлагая комфортные условия и доступные цены.
get antibiotics quickly buy antibiotics online get antibiotics quickly
buy antibiotics: BiotPharm – get antibiotics without seeing a doctor
What a material of un-ambiguity and preserveness of valuable familiarity regarding unexpected feelings.
https://05161.com.ua/bi-led-linzy-dlya-avto-tehnologiya-yaka-zminyuye-pravyla-hry
Over the counter antibiotics for infection: buy antibiotics online – Over the counter antibiotics for infection
http://eropharmfast.com/# cheapest ed meds
The Real Person!
The Real Person!
«Рентвил» предлагает аренду автомобилей в Краснодаре без залога и ограничений по пробегу по Краснодарскому краю и Адыгее. Требуется стаж от 3 лет и возраст от 23 лет. Оформление за 5 минут онлайн: нужны только фото паспорта и прав. Подача авто на жд вокзал и аэропорт Краснодар Мин-воды Сочи . Компания работает 10 лет , автомобили проходят своевременное ТО. Доступны детские кресла. Бронируйте через сайт краснодар аренда авто
The Real Person!
The Real Person!
Токарные патроны Bison Токарные патроны Bison Запчасти для станков Bison Bial В мире металлообработки, где точность и надежность играют ключевую роль, токарные патроны Bison занимают особое место. Эти инструменты, производимые известной польской компанией Bison Bial, зарекомендовали себя как высококачественные и долговечные компоненты для токарных станков. Они обеспечивают надежный зажим заготовок, что напрямую влияет на качество и скорость обработки.
Ero Pharm Fast best online ed meds cheap ed
antibiotic without presription: BiotPharm – cheapest antibiotics
Online medication store Australia: Pharm Au 24 – Online medication store Australia
https://biotpharm.shop/# Over the counter antibiotics for infection
Ero Pharm Fast Ero Pharm Fast erectile dysfunction pills for sale
The Real Person!
The Real Person!
поставки товара из Китая В эпоху глобализации и стремительного развития мировой экономики, Китай занимает ключевую позицию в качестве крупнейшего производственного центра. Организация эффективных и надежных поставок товаров из Китая становится стратегически важной задачей для предприятий, стремящихся к оптимизации затрат и расширению ассортимента. Наша компания предлагает комплексные решения для вашего бизнеса, обеспечивая бесперебойные и выгодные поставки товаров напрямую из Китая.
where to get ed pills: Ero Pharm Fast – Ero Pharm Fast
The Real Person!
The Real Person!
Кухонный гарнитур Кухня – сердце дома, место, где рождаются кулинарные шедевры и собирается вся семья. Именно поэтому выбор мебели для кухни – задача ответственная и требующая особого подхода. Мебель на заказ в Краснодаре – это возможность создать уникальное пространство, идеально отвечающее вашим потребностям и предпочтениям.
The Real Person!
The Real Person!
https://выкуп-авто-пермь.рф/grz-eto
The Real Person!
The Real Person!
В динамичном мире Санкт-Петербурга, где каждый день кипит жизнь и совершаются тысячи сделок, актуальная и удобная доска объявлений становится незаменимым инструментом как для частных лиц, так и для предпринимателей. Наша платформа – это ваш надежный партнер в поиске и предложении товаров и услуг в Северной столице. Свежие объявления
The Real Person!
The Real Person!
warface купить В мире онлайн-шутеров Warface занимает особое место, привлекая миллионы игроков своей динамикой, разнообразием режимов и возможностью совершенствования персонажа. Однако, не каждый готов потратить месяцы на прокачку аккаунта, чтобы получить желаемое оружие и экипировку. В этом случае, покупка аккаунта Warface становится привлекательным решением, открывающим двери к новым возможностям и впечатлениям.
The Real Person!
The Real Person!
At Regal Wins, we want you to enjoy every second that you play with us. Find tips on how to play safely, discover places to get support and get information on all the tools we have in place at: keepitfun.rankYour privacy and security is our number one priority here at Regal Wins Casino. We protect your account with market-leading security technology so we’re one of the safest online casino sites to play on. We never sell or rent customer details. The RTP of Diamond Mine All Action Megaways is 97.03%, which is above average when you compare it to other slots. What is the max win in the slot Diamond Mine 2 Megaways? Diamond hunting is no easy task, but playing Diamond Mine is as straightforward as you can imagine for a slot game. Most players with a little experience will be in familiar territory here.
https://zafermutlu.com.tr/aviator-betting-online-platforms-ranked-by-features/
Ezugi’s portfolio of games are all streamed from one of its studios around the world. The Ezugi 360 platform has allowed land-based casinos to tap into a new market, without the need to have people physically sitting in their casinos taking up space. Click on the buttons given above to access online 1XBET login Official in Malaysia via 1XBET alternative links and place bets on 1XBET Sportsbook, play 1XBET Live Casino games, spin 1XBET slot games, and so much more like you usually do without any worries. Even though the path you took to reach 1XBET is different, the destination is the same, that is, the 1XBET betting site. Join & claim a 100% welcome bonus of up to RM1,500 on your first 1XBET Deposit. During every IPL season, there are numerous betting activities on 1xBet platform with thousands stacked on each match. For instance, in 2023, the platform had over 200 crores worth of bets during that year’s IPL season indicating how popular this site is among cricket lovers. This app has more than 15 bet types like who wins the match, top batsman, top bowler, total runs, etc. This means each user can be well involved into every single game making well-informed decisions based on real-time stats and analytics at 1xBet IPL betting app.
The Real Person!
The Real Person!
лучшие песни 2025 Роп – Русский роп – это больше, чем просто музыка. Это зеркало современной российской души, отражающее её надежды, страхи и мечты. В 2025 году жанр переживает новый виток развития, впитывая в себя элементы других стилей и направлений, становясь всё более разнообразным и эклектичным. Популярная музыка сейчас – это калейдоскоп звуков и образов. Хиты месяца мгновенно взлетают на вершины чартов, но так же быстро и забываются, уступая место новым музыкальным новинкам. 2025 год дарит нам множество талантливых российских исполнителей, каждый из которых вносит свой неповторимый вклад в развитие жанра.
The Real Person!
The Real Person!
красное море температура воды
The Real Person!
The Real Person!
Поставки товаров из Китая – это сложный и многогранный процесс, который требует глубоких знаний логистики, таможенного законодательства и специфики китайского рынка. Когда речь идет об оптимизации этого процесса, на сцену выходит посредник в Китае. Этот специалист или компания берет на себя роль связующего звена между российским бизнесом и китайскими производителями, упрощая коммуникацию, контроль качества и организацию доставки. Выкуп товара из Китая – ключевой этап в импортных операциях. Особенно популярен выкуп с 1688 – крупнейшей оптовой онлайн-площадки Китая, предлагающей широкий ассортимент товаров по конкурентным ценам. Опт из Китая привлекает предпринимателей, стремящихся к масштабированию бизнеса и снижению закупочной стоимости. Важно понимать, что работа с оптовыми поставщиками требует внимательности и опыта, чтобы избежать недобросовестных поставщиков и получить качественный товар. Среди наиболее востребованных категорий товаров, импортируемых из Китая, выделяются оборудование и одежда. Оборудование из Китая часто отличается оптимальным соотношением цены и качества, что делает его привлекательным для производственных предприятий. Одежда из Китая продолжает оставаться популярной благодаря разнообразию моделей, материалов и ценовых категорий. Успешная организация поставок из Китая требует комплексного подхода, включающего выбор надежного посредника, тщательный отбор поставщиков, контроль качества продукции, оптимизацию логистических маршрутов и грамотное таможенное оформление. Только в этом случае бизнес сможет извлечь максимальную выгоду из сотрудничества с китайскими партнерами и укрепить свои позиции на рынке. Выкуп с 1688
The Real Person!
The Real Person!
Rainbet redeem code ILBET В мире онлайн-гемблинга Rainbet сияет как яркая звезда, привлекая игроков своими щедрыми предложениями и захватывающими играми. Промокод ILBET – это ваш билет в этот мир возможностей, предоставляющий доступ к эксклюзивным бонусам и акциям, разработанным для повышения вашего игрового опыта и увеличения шансов на победу.
Generate custom ai hentai. Create anime-style characters, scenes, and fantasy visuals instantly using an advanced hentai generator online.
The Real Person!
The Real Person!
chicken road hack download Chicken Road: Взлеты и Падения на Пути к Успеху Chicken Road – это не просто развлечение, это обширный мир возможностей и тактики, где каждое решение может привести к невероятному взлету или полному краху. Игра, доступная как в сети, так и в виде приложения для мобильных устройств (Chicken Road apk), предлагает пользователям проверить свою фортуну и чутье на виртуальной “куриной тропе”. Суть Chicken Road заключается в преодолении сложного маршрута, полного ловушек и опасностей. С каждым успешно пройденным уровнем, награда растет, но и увеличивается шанс неудачи. Игроки могут загрузить Chicken Road game demo, чтобы оценить механику и особенности геймплея, прежде чем рисковать реальными деньгами.
The Real Person!
The Real Person!
roobet promo code 2025 WEB3 В мире онлайн-казино инновации не стоят на месте, и Roobet находится в авангарде этих перемен. С появлением технологии Web3, Roobet предлагает игрокам новый уровень прозрачности, безопасности и децентрализации. Чтобы воспользоваться всеми преимуществами этой передовой платформы, используйте промокод WEB3.
The Real Person!
The Real Person!
Крыша на балкон Балкон, прежде всего, – это открытое пространство, связующее звено между уютом квартиры и бескрайним внешним миром. Однако его беззащитность перед капризами погоды порой превращает это преимущество в существенный недостаток. Дождь, снег, палящее солнце – все это способно причинить немало хлопот, лишая возможности комфортно проводить время на балконе, а также нанося ущерб отделке и мебели. Именно здесь на помощь приходит крыша на балкон – надежная защита и гарантия комфорта в любое время года.
The Real Person!
The Real Person!
Процессное управление Управление бизнес-процессами: сделайте бизнес прозрачным и управляемым. Эффективное управление бизнес-процессами — залог стабильности и роста. Опытный ментор поможет вам структурировать работу, внедрить автоматизацию и повысить прозрачность. В результате процессы станут управляемыми, а команда — мотивированной. Не позволяйте хаосу тормозить развитие — закажите консультацию и получите поддержку от эксперта, который поможет вам сделать бизнес более эффективным и устойчивым. Пришло время действовать — начните преобразование уже сегодня.
The Real Person!
The Real Person!
pinco az?rbaycan Pinco, Pinco AZ, Pinco Casino, Pinco Kazino, Pinco Casino AZ, Pinco Casino Azerbaijan, Pinco Azerbaycan, Pinco Gazino Casino, Pinco Pinco Promo Code, Pinco Cazino, Pinco Bet, Pinco Yukl?, Pinco Az?rbaycan, Pinco Casino Giris, Pinco Yukle, Pinco Giris, Pinco APK, Pin Co, Pin Co Casino, Pin-Co Casino. Онлайн-платформа Pinco, включая варианты Pinco AZ, Pinco Casino и Pinco Kazino, предлагает азартные игры в Азербайджане, также известная как Pinco Azerbaycan и Pinco Gazino Casino. Pinco предоставляет промокоды, а также варианты, такие как Pinco Cazino и Pinco Bet. Пользователи могут загрузить приложение Pinco (Pinco Yukl?, Pinco Yukle) для доступа к Pinco Az?rbaycan и Pinco Casino Giris. Pinco Giris доступен через Pinco APK. Pin Co и Pin-Co Casino — это связанные термины.
Мир полон тайн https://phenoma.ru читайте статьи о малоизученных феноменах, которые ставят науку в тупик. Аномальные явления, редкие болезни, загадки космоса и сознания. Доступно, интересно, с научным подходом.
The Real Person!
The Real Person!
новый тик ток мод Мир мобильных приложений не стоит на месте, и Тик Ток продолжает оставаться одной из самых популярных платформ для создания и обмена короткими видео. Но что, если стандартной функциональности вам недостаточно? На помощь приходит Тик Ток Мод – модифицированная версия приложения, открывающая доступ к расширенным возможностям и эксклюзивным функциям.
The Real Person!
The Real Person!
Скачать тикток мод на андроид Тикток Мод: Откройте Новые Горизонты Видеоконтента на Вашем Android Мир социальных сетей постоянно эволюционирует, и TikTok занимает в нем лидирующие позиции. Но что, если я скажу вам, что можно расширить возможности этой платформы, получив доступ к функциям, недоступным в стандартной версии? Речь идет о ТикТок моде. ТикТок Мод: Что это такое? ТикТок мод – это модифицированная версия популярного приложения, предоставляющая расширенные возможности для пользователей Android. Скачать тик ток мод на андроид – значит открыть дверь в мир, где границы возможностей TikTok размываются.
distinguished engineer resume resume application engineer
The Real Person!
The Real Person!
мтс карты дебетовые бесплатные Ваш надежный партнер в выборе банковских карт. Оформление современной дебетовой карты стало простым и удобным благодаря нашей платформе. Выберите карту, которая соответствует вашим потребностям, и воспользуйтесь всеми преимуществами современного финансового обслуживания. Что мы предлагаем? Полезные советы: Лайфхаки и рекомендации по эффективному использованию карты. Актуальные акции: Будьте в курсе всех новых предложений и специальных условий от банков-партнеров. Преимущества нашего сообщества. Мы предоставляем полную информацию о различных видах карт, особенностях тарифов и комиссий. Наши публикации регулярно обновляются, предоставляя актуальные данные и свежие новости о продуктах российских банков. Присоединяйтесь к нашему сообществу, чтобы сделать ваши финансовые решения простыми, быстрыми и надежными. Вместе мы сможем оптимизировать использование банковских продуктов и сэкономить ваше время и средства. Наша цель — помогать вам эффективно управлять своими финансами и получать максимум выгоды от каждого взаимодействия с банком.
The Real Person!
The Real Person!
как заработать девушке онлайн в Польше Стань вебкам моделью в польской студии, работающей в Варшаве! Открыты вакансии для девушек в Польше, особенно для тех, кто говорит по-русски. Ищешь способ заработать онлайн в Польше? Предлагаем подработку для девушек в Варшаве с возможностью работы в интернете, даже с проживанием. Рассматриваешь удаленную работу в Польше? Узнай, как стать вебкам моделью и сколько можно заработать. Работа для украинок в Варшаве и высокооплачиваемые возможности для девушек в Польше ждут тебя. Мы предлагаем легальную вебкам работу в Польше, онлайн работа без необходимости знания польского языка. Приглашаем девушек без опыта в Варшаве в нашу вебкам студию с обучением. Возможность заработка в интернете без вложений. Работа моделью онлайн в Польше — это шанс для тебя! Ищешь “praca dla dziewczyn online”, “praca webcam Polska”, “praca modelka online” или “zarabianie przez internet dla kobiet”? Наше “agencja webcam Warszawa” и “webcam studio Polska” предлагают “praca dla mlodych kobiet Warszawa” и “legalna praca online Polska”. Смотри “oferty pracy dla Ukrainek w Polsce” и “praca z domu dla dziewczyn”.
Научно-популярный сайт https://phenoma.ru — малоизвестные факты, редкие феномены, тайны природы и сознания. Гипотезы, наблюдения и исследования — всё, что будоражит воображение и вдохновляет на поиски ответов.
The Real Person!
The Real Person!
Obsługa klienta Mostbet działa 24 7, oferując pomoc w języku polskim przez czat na żywo, e-mail ) oraz media społecznościowe. Platforma promuje również odpowiedzialny hazard, udostępniając narzędzia kontroli takie jak limity wpłat, samowykluczenie i monitoring czasu gry. Təcrübəli oyunçular Mostbet casino gedirlər – burada gəlirlərin faizi adekvatlıq hüdudlarındadır (93-98%) və siz daha tez yaxşı pul qazana bilərsiniz. Zapamiętaj moje dane w tej przeglądarce podczas pisania kolejnych komentarzy. Marże bukmacherskie, podobnie zresztą jak rynki, są uzależnione od rangi meczu i rozgrywek. Dla przykładu – kursy na czołowe mecze piłki nożnej, tenisa ziemnego i koszykówki posiadają marże powyżej 5%. 22bet ma nieznacznie wyższe marże od GGbet w przypadku meczów piłki nożnej – u konkurencyjnego bukmachera zaczynają się nawet od poziomu poniżej 4%.
https://detourdestinations.com/blog/aviator-light-kiedy-liczy-sie-szybkosc
Najczęstszą strategią gry na Aviator są małe kursy. Główne elementy systemu zarabiania: Kompatybilna zarówno z urządzeniami z systemem Android, jak i iOS, aplikacja Aviator jest zoptymalizowana pod kątem szerokiej gamy smartfonów i tabletów. Aplikacja zachowuje niewielki rozmiar, około 50 MB dla systemu Android i 70 MB dla systemu iOS, zapewniając dostępność nawet na urządzeniach z ograniczoną pamięcią. W przypadku użytkowników Androida pobranie APK gry aviator zapewnia kompatybilność i łatwość instalacji, umożliwiając graczom dostęp do gry bezpośrednio z urządzeń mobilnych. Ta wersja mobilna zachowuje wszystkie funkcje wersji desktopowej, zapewniając płynną i przyjemną rozgrywkę. Bahis Casino Siteleri Güvenilir Giriş Adresleri”ContentÜlkeler Ve Bölgeler Bazında Online CasinolarYılının En Iyi Casino SiteleriAviator Bonusu…
The Real Person!
The Real Person!
вебкам студия в Варшаве Стань вебкам моделью в польской студии, работающей в Варшаве! Открыты вакансии для девушек в Польше, особенно для тех, кто говорит по-русски. Ищешь способ заработать онлайн в Польше? Предлагаем подработку для девушек в Варшаве с возможностью работы в интернете, даже с проживанием. Рассматриваешь удаленную работу в Польше? Узнай, как стать вебкам моделью и сколько можно заработать. Работа для украинок в Варшаве и высокооплачиваемые возможности для девушек в Польше ждут тебя. Мы предлагаем легальную вебкам работу в Польше, онлайн работа без необходимости знания польского языка. Приглашаем девушек без опыта в Варшаве в нашу вебкам студию с обучением. Возможность заработка в интернете без вложений. Работа моделью онлайн в Польше — это шанс для тебя! Ищешь “praca dla dziewczyn online”, “praca webcam Polska”, “praca modelka online” или “zarabianie przez internet dla kobiet”? Наше “agencja webcam Warszawa” и “webcam studio Polska” предлагают “praca dla mlodych kobiet Warszawa” и “legalna praca online Polska”. Смотри “oferty pracy dla Ukrainek w Polsce” и “praca z domu dla dziewczyn”.
The Real Person!
The Real Person!
https://amlkyc.tech/
The Real Person!
The Real Person!
компьютер для работы на заказ Игровой ПК в сборе: Готовое решение для геймеров Игровой ПК в сборе – это отличный вариант для тех, кто хочет получить готовую машину с высокой производительностью. Выберите из предложенных конфигураций и наслаждайтесь любимыми играми.
The Real Person!
The Real Person!
Sí, el juego Lucky Jet se puede jugar desde cualquier dispositivo móvil en donde cargue la página del casino 1 win. Solamente tienes que abrir la página del casino y buscar el juego en la plataforma. La 1win App es la mejor manera de disfrutar de Lucky Jet en cualquier lugar. La app está optimizada para ofrecerte una experiencia de juego rápida y fluida. ¿Sabes de lo que trata Lucky Jet 1win? Este es uno de los títulos emergentes en el mercado iGaming en Perú, el juego de crash y apuesta instantánea ha ganado mucha popularidad por su alto RTP y jugabilidad, con tan solo dos clics podrás ganar hasta un x200 de tu apuesta inicial si tienes suerte. Descargar e instalar Lucky Jet en una PC es sencillo: Lucky Jet se ha convertido en una elección popular entre los jugadores online debido a su mezcla única de simplicidad y profundidad estratégica. Sin embargo, como cualquier juego, tiene sus pros y sus contras que pueden afectar a tu experiencia de juego. Comprenderlos puede ayudarte a decidir si Lucky Jet es el juego adecuado para ti. A continuación te mostramos las ventajas y desventajas de este juego.
https://aviraaz.com/probamos-balloon-boom-app-es-confiable-para-ganar-dinero/
El gameplay de Lucky jet consiste en anticipar el momento exacto para retirar tu apuesta antes de que el multiplicador deje de aumentar. Este enfoque dinámico requiere un equilibrio entre la intuición y la toma de decisiones rápidas. Jugadores que buscan una experiencia emocionante con juegos de choque como Lucky Jet. Encuentre el lugar perfecto para sus aventuras de juego en 1Win Casino. Este casino en línea brinda acceso a los análogos de Lucky Jet: Jet X, Aviator, Space XY. Están diseñados para aquellos que buscan experimentar la emoción de la alta volatilidad y las ganancias inesperadas. 1Win Casino ofrece muchas ventajas: Desde 2023, Casino de Colombia ofrece reseñas independientes de juegos de azar en línea. Visite nuestro sitio web para ver las reseñas, comparaciones, guías y comentarios de jugadores reales. Descubra cómo elegir el mejor casino en línea, conozca los últimos lanzamientos de juegos y aproveche las ofertas exclusivas. ¡Juegue de manera segura y responsable!
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
The Real Person!
The Real Person!
We will get in touch with you to schedule an appointment Rajeshmall is a feature-rich colour prediction app that offers strategic gameplay. Users can earn rewards by accurately predicting colours and participating in exclusive events. The app provides a strong referral program, where users can maximise their earnings by inviting friends. If you can’t find your Mac or other Apple devices, the Find My app makes it easy to pinpoint where they are. If your device ever falls into the wrong hands, you can use Find My to lock it down or erase it from afar. Some quotes sources are available only on working days. We combined various options for your convenience: trade even on weekends choosing the most suitable assets. One-click to install XAPK APK files on Android! The minimum cost of a trade is quite low. You won’t lose a large amount of funds while you’re still learning how to trade.
https://alquimiaparaelalma.link/mines-by-spribe-real-earnings-or-just-a-passing-fad-for-indian-players/
In today’s article, we are going to tell you about the games in the Tiranga Game app, which will give you the best entertainment and earning and about the activities. It is an entertaining app that allows users to predict the colors of virtual cards and win rewards based on their predictions. The app is easy to use and offers a variety of features that make it enjoyable for users of all ages. We hope your search for the games app download is now over. So, download the app now and get started. Wait for the application to download. Register and login into your color prediction game app unlocks a world of best triangle game login that will keep you entertained for hours on end. While the app is being downloaded, open settings on your system. They’re a vital tool for anyone serious about mastering Colour Trading Game.
The Real Person!
The Real Person!
Дом в Сочи Сочи – это не просто город, это мечта, воплощенная в реальность. Лазурное море, величественные горы, пышная зелень и мягкий климат делают его идеальным местом для жизни, отдыха и инвестиций. Если вы стремитесь к комфорту, красоте и перспективному будущему, то недвижимость в Сочи – это то, что вам нужно.
The Real Person!
The Real Person!
почему акула напала в египте
The Real Person!
The Real Person!
BestGold: Сияние золота и блеск бриллиантов в Краснодаре В сердце Краснодарского края, где солнце ласкает поля и виноградники, расцветает мир изысканных ювелирных украшений BestGold. Мы предлагаем вам уникальную возможность прикоснуться к великолепию золота 70% пробы, воплощенному в утонченных кольцах и серьгах, сверкающих бриллиантами. Кольца, достойные королевы Наши кольца – это не просто украшения, это символ вашей индивидуальности и безупречного вкуса. От классических обручальных колец до экстравагантных коктейльных, каждое изделие BestGold создано с любовью и вниманием к деталям. Вставки из бриллиантов различной огранки и каратности подчеркнут вашу элегантность и добавят образу неповторимый шарм. золотые украшения по ценам фабрик Серьги, подчеркивающие красоту Серьги BestGold – это идеальное дополнение к любому наряду. От лаконичных пусетов до эффектных подвесок, они призваны подчеркнуть вашу женственность и утонченность. Наши серьги с бриллиантами станут ярким акцентом вашего образа, притягивая восхищенные взгляды. Ювелирный фестиваль BestGold: праздник роскоши и стиля Не упустите возможность стать участником ювелирного фестиваля BestGold, где вас ждут эксклюзивные скидки на золото до 70% и невероятные предложения на бриллианты. Это ваш шанс приобрести ювелирные украшения мечты по самым выгодным ценам. BestGold: выбирайте лучшее, выбирайте золото! Погрузитесь в мир роскоши и блеска вместе с BestGold. Наши ювелирные украшения станут вашими верными спутниками, подчеркивая вашу красоту и элегантность в любой ситуации. Купите кольцо или серьги из золота в Краснодаре и ощутите себя королевой!
The Real Person!
The Real Person!
краснодар аренда авто Аренда автомобилей Краснодар – это надежный и проверенный способ обеспечить себе комфортное и безопасное передвижение по городу и его окрестностям.
The Real Person!
The Real Person!
кайтсёрфинг в анапе Присоединяйтесь к сообществу кайтсерферов в Анапе!
Need transportation? car transport quotes car transportation company services — from one car to large lots. Delivery to new owners, between cities. Safety, accuracy, licenses and experience over 10 years.
auto carriers car pickup service
Balloons Dubai https://balloons-dubai1.com stunning balloon decorations for birthdays, weddings, baby showers, and corporate events. Custom designs, same-day delivery, premium quality.
The Real Person!
The Real Person!
купить диплом специалиста Купить аттестат за 11 классов: Аттестат об окончании 11 классов является необходимым документом для поступления в ВУЗ. Если вы потеряли аттестат или не смогли его получить, мы поможем вам приобрести новый.
The Real Person!
The Real Person!
automobile transport companies Choosing the Right Car Shipping Company Selecting the best car transport service requires careful consideration. Begin by researching and comparing auto shipping quotes from multiple companies. Pay close attention to the details of each quote, including the types of transport offered (open or enclosed), insurance coverage, and estimated delivery times. Look for companies with a proven track record of reliability and positive customer reviews.
The Real Person!
The Real Person!
купить металлопрокат цена Купить металлопрокат: подведем итоги Рынок металлопроката в Москве предлагает широкие возможности для выбора. Важно тщательно изучить предложения, сравнить цены и условия, и выбрать надежного поставщика, который сможет обеспечить качественную продукцию и своевременную доставку.
The Real Person!
The Real Person!
Владимир Чернышев
The Real Person!
The Real Person!
Бани из клееного бруса под ключа Бани из клееного бруса под ключ: Что может быть лучше, чем попариться в настоящей русской бане, построенной из натурального дерева? Баня из клееного бруса – это не только место для гигиенических процедур, но и источник здоровья и хорошего настроения. Мы строим бани различных размеров и конфигураций, с учетом всех ваших предпочтений.
The Real Person!
The Real Person!
варфейс купить оружие Оружие Warface: Ключ к доминированию Арсенал Warface постоянно пополняется новым оружием, предлагая игрокам множество вариантов для адаптации под свой стиль игры. Приобретение редкого и мощного оружия может значительно повысить ваши шансы на победу в бою.
The Real Person!
The Real Person!
Lucky Jet este un joc de noroc din categoria «multiplier game», care oferă o dinamică simplă și captivantă. Scopul este să câștigi prin anticiparea momentului ideal de retragere, înainte ca avionul să se prăbușească. Multiplicatorul crește progresiv, iar jucătorul trebuie să își retragă pariul la timp pentru a obține câștigul maxim. Jocul Lucky Jet game 1 win — este un slot crash onest unde utilizatorii pot obține câștiguri mari în câteva minute. Jucătorii trebuie să plaseze un pariu la începutul rundei și să reușească să își retragă câștigurile înainte ca personajul Lucky Joe să dispară de pe ecran. Mai jos am subliniat principalele caracteristici ale jocului 1win Lucky Jet game.
https://2e94.com/ghid-rapid-pentru-functionarea-modulului-demo-lucky-jet-la-1win-in-moldova/
De asemenea, la Jet Lucky 2 online poți plasa două pariuri într-o rundă. Jocul are două seturi de stabilire pariu și colectare automată. Dacă ești pe mobil, deschizi al doilea set apăsând butonul „+” aflat sub butonul de setare pariu. Tare joc, așa-i? E imposibil să te plictisești de el, dar e bine să-l alternezi și cu păcănele clasice. Magic Money Maze demo este un joc pe care l-am adăugat destul de recent pe site și care ne-a plăcut mult. Poate am prins noi un moment bun. Sau, cine știe? Jocul 1Win JetX testează reacția, răbdarea și atenția jucătorilor. Jucătorul are la dispoziție doar 5 secunde pentru a plasa un pariu. În cadrul sesiunii de joc, trebuie să urmărești decolarea avionului, al cărui multiplicator crește atât timp cât acesta rămâne în aer. Scopul principal al jocului este de a retrage banii înainte ca avionul să se prăbușească.
ultimate createporn AI generator. Create hentai art, porn comics, and NSFW with the best AI porn maker online. Start generating AI porn now!
Профессиональное оборудование косметологической клиники для салонов красоты, клиник и частных мастеров. Аппараты для чистки, омоложения, лазерной эпиляции, лифтинга и ухода за кожей.
ultimate createporn AI generator. Create hentai art, porn comics, and NSFW with the best AI porn maker online. Start generating AI porn now!
The Real Person!
The Real Person!
https://plombi.ru/product/40-signal/
The Real Person!
The Real Person!
Cryptocurrency Payments Bazaar Drugs Marketplace: A New Darknet Platform with Dual Access Bazaar Drugs Marketplace is a new darknet marketplace rapidly gaining popularity among users interested in purchasing pharmaceuticals. Trading is conducted via the Tor Network, ensuring a high level of privacy and data protection. However, what sets this platform apart is its dual access: it is available both through an onion domain and a standard clearnet website, making it more convenient and visible compared to competitors. The marketplace offers a wide range of pharmaceuticals, including amphetamines, ketamine, cannabis, as well as prescription drugs such as alprazolam and diazepam. This variety appeals to both beginners and experienced buyers. All transactions on the platform are carried out using cryptocurrency payments, ensuring anonymity and security. In summary, Bazaar represents a modern darknet marketplace that combines convenience, a broad product selection, and a high level of privacy, making it a notable player in the darknet economy.
The Real Person!
The Real Person!
Группа в Telegram Доска бесплатных объявлений “Все для Вас Архангельск”: товары, услуги, авто, жильё, работа, розыгрыши, отзывы и многое другое. Архангельск, Северодвинск, Новодвинск, Катунино, Березник, Рикасиха, Холмогоры, Мезень, Карпогоры авито Архангельск
The Real Person!
The Real Person!
кайтсёрфинг это Температурные качели: Вода в Хургаде по месяцам Температура воды в Хургаде колеблется в зависимости от времени года. В декабре она может опускаться до +22°C, в то время как в марте начинает постепенно повышаться, достигая комфортных +24°C. В апреле вода прогревается еще больше, делая купание особенно приятным. Ноябрь также радует теплой водой, но немного прохладнее, чем в летние месяцы. Февраль – один из самых прохладных месяцев, но даже тогда температура воды остается вполне приемлемой для плавания.
The Real Person!
The Real Person!
амл бот
The Real Person!
The Real Person!
что такое iko?
The Real Person!
The Real Person!
сервис стиральных машин алматы Починить стиральную машину в Алматы – реально! Главное – найти надежный сервисный центр с опытными мастерами и хорошей репутацией.
The Real Person!
The Real Person!
скупка стиральных машин bosch алматы Профилактика стиральных машин Алматы. Предотвращение поломок, экономия на ремонте.
The Real Person!
The Real Person!
утилизация мониторов Алматы Сертификат утилизации техники: подтверждение экологически безопасной утилизации.
The Real Person!
The Real Person!
Бездепозитный бонус в казино
The Real Person!
The Real Person!
демонтаж жетысуский район Демонтаж торгового зала – комплекс услуг.
The Real Person!
The Real Person!
гибкая керамика для фасадов Phomi: гибкая керамика для фасадов Dream Decor внутренней отделки и интерьера в Москве. Купить по выгодной цене за м2, монтаж, отзывы Divu. Отделка дома с гарантией!
The Real Person!
The Real Person!
вавада казино официальный сайт рабочее зеркало на сегодня Vavada Casino Скачать: Скачайте приложение Vavada Casino на свое устройство и наслаждайтесь любимыми играми в любое время и в любом месте.
The Real Person!
The Real Person!
разнорабочие официально алматы Ищу разнорабочих в Алматы – это запрос тех, кто нуждается в помощи для выполнения различных видов работ, от простых до сложных, требующих физической силы и выносливости.
The Real Person!
The Real Person!
эвакуатор безналичный расчет Надежный эвакуатор Алматы – проверенная компания с хорошей репутацией и положительными отзывами клиентов.
The Real Person!
The Real Person!
Бездепозитный бонус
The Real Person!
The Real Person!
vavada casino приложение Вавада Казино Официальный Сайт Казино: Погрузитесь в атмосферу азарта и развлечений на официальном сайте Вавада Казино, где вас ждут захватывающие игры и щедрые бонусы.
The Real Person!
The Real Person!
Бездепозитные бонусы в казино
The Real Person!
The Real Person!
казино вавада официальный сайт бонус за регистрацию Vavada Casino Скачать: Скачайте приложение Vavada Casino на свое устройство и наслаждайтесь любимыми играми в любое время и в любом месте.
The Real Person!
The Real Person!
Бездепозитный бонус в казино
The Real Person!
The Real Person!
вавада казино официальный сайт казино Вавада Казино Официальный Сайт: Откройте для себя мир азарта и развлечений на официальном сайте Вавада Казино, где вас ждут захватывающие игры и щедрые бонусы.
The Real Person!
The Real Person!
знай своего клиента
займы срочно быстро онлайн мфо займы онлайн на карту
The Real Person!
The Real Person!
Бездепозитные бонусы
The Real Person!
The Real Person!
вавада казино Vavada Casino Скачать: Скачайте приложение Vavada Casino на свое устройство и наслаждайтесь любимыми играми в любое время и в любом месте.
The Real Person!
The Real Person!
Бездепозитные бонусы в казино
The Real Person!
The Real Person!
Сукааа казино официальный сайт Sykaaa casino скачать бесплатно на телефон – это отличная возможность всегда иметь любимое казино под рукой. Наслаждайтесь азартом в любом месте и в любое время.
The Real Person!
The Real Person!
Бездепозитные бонусы в казино Бездепозитные бонусы в казино: Что это такое? Бездепозитный бонус в казино – это денежная сумма или бесплатные вращения, которые казино предоставляет новым игрокам в качестве приветственного подарка. Главное преимущество этого бонуса заключается в том, что для его получения не требуется внесение депозита. Игрок может просто зарегистрироваться на сайте казино и получить бонус на свой счет.
The Real Person!
The Real Person!
Бездепозитные бонусы в казино Бездепозитные бонусы в казино: Что это такое? Бездепозитный бонус в казино – это денежная сумма или бесплатные вращения, которые казино предоставляет новым игрокам в качестве приветственного подарка. Главное преимущество этого бонуса заключается в том, что для его получения не требуется внесение депозита. Игрок может просто зарегистрироваться на сайте казино и получить бонус на свой счет.
The Real Person!
The Real Person!
Бездепозитные бонусы
The Real Person!
The Real Person!
Бездепозитный бонус в казино Бездепозитный бонус в казино: Как его получить? Получить бездепозитный бонус в казино, как правило, довольно просто. Обычно требуется пройти процедуру регистрации на сайте казино и подтвердить свою учетную запись. В некоторых случаях может потребоваться ввести специальный промокод. После выполнения всех условий бонус будет автоматически зачислен на ваш счет. Бездепозитные бонусы – это отличная возможность начать свой путь в мире онлайн-казино с минимальным риском и максимальным удовольствием. Однако, прежде чем принимать бонус, всегда внимательно ознакомьтесь с условиями его использования, чтобы избежать недоразумений в будущем.
The Real Person!
The Real Person!
вавада казино официальный рабочее зеркало на сегодняшний день Вавада Казино Официальный Сайт Скачать на Андроид Мобильная Версия Бесплатно: Скачайте мобильную версию Вавада Казино на свое устройство Android и наслаждайтесь игрой в любом месте и в любое время.
The Real Person!
The Real Person!
Бездепозитные бонусы Бездепозитный бонус в казино: Как его получить? Получить бездепозитный бонус в казино, как правило, довольно просто. Обычно требуется пройти процедуру регистрации на сайте казино и подтвердить свою учетную запись. В некоторых случаях может потребоваться ввести специальный промокод. После выполнения всех условий бонус будет автоматически зачислен на ваш счет. Бездепозитные бонусы – это отличная возможность начать свой путь в мире онлайн-казино с минимальным риском и максимальным удовольствием. Однако, прежде чем принимать бонус, всегда внимательно ознакомьтесь с условиями его использования, чтобы избежать недоразумений в будущем.
The Real Person!
The Real Person!
Бездепозитный бонус Бездепозитный бонус в казино: Как его получить? Получить бездепозитный бонус в казино, как правило, довольно просто. Обычно требуется пройти процедуру регистрации на сайте казино и подтвердить свою учетную запись. В некоторых случаях может потребоваться ввести специальный промокод. После выполнения всех условий бонус будет автоматически зачислен на ваш счет. Бездепозитные бонусы – это отличная возможность начать свой путь в мире онлайн-казино с минимальным риском и максимальным удовольствием. Однако, прежде чем принимать бонус, всегда внимательно ознакомьтесь с условиями его использования, чтобы избежать недоразумений в будущем.
The Real Person!
The Real Person!
Бездепозитные бонусы
The Real Person!
The Real Person!
Бездепозитный бонус Бездепозитные бонусы в казино: Что это такое? Бездепозитный бонус в казино – это денежная сумма или бесплатные вращения, которые казино предоставляет новым игрокам в качестве приветственного подарка. Главное преимущество этого бонуса заключается в том, что для его получения не требуется внесение депозита. Игрок может просто зарегистрироваться на сайте казино и получить бонус на свой счет.
The Real Person!
The Real Person!
vavada online casino официальный сайт Вавада 100 Бесплатных Вращений: Воспользуйтесь возможностью получить 100 бесплатных вращений в Вавада Казино и увеличьте свои шансы на крупный выигрыш.
The Real Person!
The Real Person!
Бездепозитный бонус Бездепозитный бонус: Испытайте удачу без потерь Бездепозитный бонус позволяет игрокам протестировать различные игры, оценить функциональность казино и испытать свою удачу, не рискуя собственными деньгами. Это отличная возможность познакомиться с миром онлайн-казино и понять, насколько он соответствует вашим предпочтениям.
The Real Person!
The Real Person!
Владивосток работа Работа для студентов во Владивостоке: совмещай учебу и работу, получи необходимые навыки и финансовую независимость!
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений в казино с выводом без депозита
The Real Person!
The Real Person!
Игорь Стоунберг экстрасенс Игорь Стоунберг ясновидящий – загляните в будущее и получите ответы на волнующие вопросы. Он помогает людям в трудных ситуациях. | Рада, что обратилась. |
The Real Person!
The Real Person!
Бездепозитный бонус Бездепозитный бонус: Испытайте удачу без потерь Бездепозитный бонус позволяет игрокам протестировать различные игры, оценить функциональность казино и испытать свою удачу, не рискуя собственными деньгами. Это отличная возможность познакомиться с миром онлайн-казино и понять, насколько он соответствует вашим предпочтениям.
The Real Person!
The Real Person!
маленькие белые акулы в аквариуме хургады Kaite travelling: Вероятно, опечатка, имеется в виду Kite travelling.
The Real Person!
The Real Person!
крипто подарки Telegram Как купить крипту новичку? Просто зарегистрируйтесь на бирже и следуйте инструкциям!
The Real Person!
The Real Person!
Игорь Стоунберг экстрасенс Игорь Стоунберг – это имя, которое вызывает интерес и надежду у многих, кто ищет ответы на сложные вопросы или нуждается в поддержке в трудные моменты жизни. Его репутация как экстрасенса и ясновидящего подтверждается множеством положительных отзывов. Люди отмечают его способность видеть за пределами обычного, точно описывать прошлые события и давать ценные советы относительно будущего. Многие клиенты отмечают, что после консультаций с Игорем Стоунбергом они почувствовали облегчение, ясность в мыслях и уверенность в своих силах. Они подчеркивают его чуткость, эмпатию и готовность выслушать. Отзывы часто содержат слова благодарности за помощь в решении проблем в личной жизни, карьере и бизнесе. Некоторые клиенты описывают свои сеансы с Игорем как удивительные и даже невероятные, отмечая точность его предсказаний и его способность видеть потенциальные возможности и препятствия на их пути. Они утверждают, что благодаря его советам им удалось избежать ошибок и принять правильные решения. В целом, отзывы об Игоре Стоунберге как об экстрасенсе и ясновидящем свидетельствуют о его профессионализме, таланте и искреннем желании помочь людям. Его имя ассоциируется с надеждой, поддержкой и возможностью изменить свою жизнь к лучшему.
The Real Person!
The Real Person!
кайтсерфинг в хургаде цены Кайт школа Кайт школа – это место, где мечты о покорении волн с помощью кайта становятся реальностью. Квалифицированные инструкторы, современное оборудование и безопасные условия позволяют быстро и эффективно освоить навыки кайтинга и кайтсерфинга.
The Real Person!
The Real Person!
Sykaaa casino 100 бесплатных вращений Sykaaa casino официальный сайт бонус за регистрацию – отличный старт для новых игроков. Получите дополнительные средства для игры сразу после регистрации.
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений
The Real Person!
The Real Person!
кайт кэмп египет Обучение кайтингу Обучение кайтингу – это важный этап для каждого начинающего кайтсерфера. Профессиональные инструкторы помогут освоить базовые навыки управления кайтом, научат правилам безопасности и расскажут о метеорологических особенностях. Постепенное освоение техники позволит избежать травм и получить максимальное удовольствие от катания.
The Real Person!
The Real Person!
карта iko Обучение кайтсёрфингу Обучение кайтсёрфингу – это следующий шаг после освоения базовых навыков управления кайтом. На этом этапе учатся вставать на доску, контролировать скорость и направление движения, а также выполнять простые трюки. Важно выбирать опытного инструктора и комфортные условия для обучения.
The Real Person!
The Real Person!
Plinko ist ein Spiel mit einem einzigartigen Spielprinzip, das durch die amerikanische Fernsehsendung The Price is Right weltweite Popularität erlangte. Dank der Online-Casinos ist es nun für jedermann zugänglich. Das Spiel zeichnet sich durch seinen ungewöhnlichen Spielablauf aus, bei dem der Spieler einen virtuellen Ball durch ein vertikales Brett mit Hindernissen schießt. Wenn der Ball auf die Hindernisse trifft, ändert er seine Flugbahn, was sich auf die endgültige Auszahlung auswirkt. Im Gegensatz zu vielen anderen Casino-Spielen basiert Plinko auf dem Zufallsprinzip. Um zu spielen und zu gewinnen, braucht der Spieler keine zusätzlichen Kenntnisse oder Fähigkeiten. Jeder kann bei Plinko gewinnen. Plinko XY, entwickelt von bgaming, bringt einen innovativen Ansatz für das Plinko-Spiel. Mit besonderen Features und beeindruckenden visuellen Elementen fesselt diese Version die Spieler vom ersten Moment an. Erforschen Sie verschiedene Wege und Strategien, während die Kugeln fallen und die Pins hinunterrutschen. Mit mehreren Wettoptionen und spannenden Preisen bietet Plinko XY ein einzigartiges Erlebnis, das Plinko-Liebhabern sicher gefallen wird.
http://opendata.abbeville.fr/user/mosrobarle1970
Plinko Play The Purchase Price Is Right Plinko Pegs At Aarp “The Price Is Right Plinko Pegs Instantly Perform The Purchase Price Is Proper Plinko Pegs On The Internet For Free! Content Faq Concerning Game Plinko Inactive Games” How Does The Plinko Game Operate? Playing The Game Where May I Uncover More Bgaming Games? Responsible… Zusätzliche Spielrunden, die Ihnen die Chance geben, das Spiel ohne zusätzlichen Einsatz auszuprobieren. Plinko Lucky:Ball Falling Game provides a balanced mix of luck-based and skill-based challenges. This means that every player can find something they enjoy, whether they prefer strategy games like Mines, classic card games like Blackjack, or purely luck-based games like Slot and Wheel. Probieren Sie plinko in deutschland spielen, entdecken Sie, was ist plinko für ein spiel, und sammeln Sie Ihre eigenen plinko erfahrung!
The Real Person!
The Real Person!
египет кайтсёрфинг Кайт школа Кайт школа – это место, где мечты о покорении волн с помощью кайта становятся реальностью. Квалифицированные инструкторы, современное оборудование и безопасные условия позволяют быстро и эффективно освоить навыки кайтинга и кайтсерфинга.
The Real Person!
The Real Person!
Бездепозитный бонус
The Real Person!
The Real Person!
фонтан казино бездепозитный бонус 1000 рублей за регистрацию без депозита с выводом денег
The Real Person!
The Real Person!
кайт школа в египте Кайтсёрфинг Кайтсёрфинг – это сплав ветра, воды и мастерства. Этот вид спорта позволяет скользить по водной глади, используя силу ветра, пойманную кайтом. Это динамичный и захватывающий способ провести время на открытом воздухе, требующий определенной физической подготовки и навыков управления кайтом и доской.
The Real Person!
The Real Person!
веганский хлеб ташкент Альтернатива магазинному хлебу – это наш хлеб, который приготовлен из натуральных ингредиентов и не содержит консервантов.
The Real Person!
The Real Person!
Подработка для школьников во Владивостоке Подработка во Владивостоке для студентов: совмещение учебы и заработка Студенты Владивостока могут найти множество вариантов подработки, позволяющих совмещать учебу и работу. Гибкий график и неполная занятость делают подработку идеальным способом заработать деньги и получить ценный опыт. Популярные варианты включают работу в сфере обслуживания, курьерскую доставку и помощь в проведении мероприятий.
The Real Person!
The Real Person!
hawaii riviera aqua park hurghada кайт станция : Кайт серфинг в египте: Насладись идеальными условиями для кайтсерфинга круглый год!
The Real Person!
The Real Person!
кайт школа в хургаде Кайт школа: Выбор кайт школы – важный шаг на пути к успешному освоению кайтсерфинга. Обратите внимание на опыт инструкторов, методику обучения, безопасность и качество оборудования. Доверьтесь профессионалам, и ваш путь в кайтсерфинге будет легким и увлекательным.
The Real Person!
The Real Person!
Бездепозитный бонус в казино
The Real Person!
The Real Person!
вингфойл выход на глиссаду Обучение кайтсёрфингу – это комплексный процесс, включающий в себя теоретическую подготовку, освоение управления кайтом на суше и воде, а также технику безопасности.
The Real Person!
The Real Person!
кайт сафари в хургаде Обучение кайтингу: Сделайте первый шаг к покорению ветра и волн с нашей профессиональной программой обучения кайтингу. Мы предлагаем индивидуальные и групповые занятия для всех уровней подготовки, от новичков до опытных райдеров.
The Real Person!
The Real Person!
кайтсафари в египте : Кайт: Он – ваш билет в мир безграничной свободы! Он – ваш крылатый конь, готовый нести вас навстречу приключениям! Он – ваш верный друг, который никогда не предаст!
The Real Person!
The Real Person!
кайт сафари египет : Кайтсёрфинг: Укроти стихию, стань повелителем ветра!
The Real Person!
The Real Person!
Бездепозитный бонус
The Real Person!
The Real Person!
1000 рублей за регистрацию
The Real Person!
The Real Person!
kite in hurghada for germany Кайт лагерь анапа: Кайт лагерь на побережье Черного моря
The Real Person!
The Real Person!
кайт школа Кайт станция Египет: Комфорт и безопасность на кайт-станции.
The Real Person!
The Real Person!
кайт египет Кайт: Не просто спорт, а образ жизни. Это свобода, адреналин и единение с природой.
The Real Person!
The Real Person!
Бездепозитный бонус
The Real Person!
The Real Person!
кайт египте : Кайт египет: Открой для себя страну фараонов с новой стороны!
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений в казино адмирал
The Real Person!
The Real Person!
египет русская кайт школа : Кайт сафари в египте: Открой для себя самые красивые кайт споты!
Heya just wanted to give you a brief heads up and let you know a few of the pictures aren’t loading properly. I’m not sure why but I think its a linking issue. I’ve tried it in two different internet browsers and both show the same results.
Play daily on 88fb and win real money
The Real Person!
The Real Person!
кайт школа windfamily Кайт школа Windfamily: Семейная атмосфера и профессиональное обучение кайтсерфингу.
The Real Person!
The Real Person!
Бездепозитный бонус в казино
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений
The Real Person!
The Real Person!
кайтсерфинг в хургаде : Кайтсерфинг в египте: Открой для себя новые горизонты кайтсерфинга!
кейс защитный байкал вз 12 https://plastcase.ru
The Real Person!
The Real Person!
кайт школа анапа Кайт школа унесенные ветром: Обучение кайтингу с опытными инструкторами. Безопасность и профессионализм – наш приоритет.
The Real Person!
The Real Person!
кайт сафари в хургаде Кайт споты египта: Разнообразие кайт-спотов в Египте удовлетворит потребности как начинающих, так и опытных райдеров.
The Real Person!
The Real Person!
Бездепозитный бонус
The Real Person!
The Real Person!
Этот информативный текст отличается привлекательным содержанием и актуальными данными. Мы предлагаем читателям взглянуть на привычные вещи под новым углом, предоставляя интересный и доступный материал. Получите удовольствие от чтения и расширьте кругозор!
Получить дополнительные сведения – https://nakroklinikatest.ru/
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений в казино
The Real Person!
The Real Person!
самые ветреные места в хургаде Кайт Египет – это страна, предлагающая отличные условия для кайтсёрфинга круглый год.
The Real Person!
The Real Person!
кайт сафари египет Кайт станция Египет: Комфорт и безопасность на кайт-станции.
The Real Person!
The Real Person!
кайт сафари египет detivetra: Ваш надежный партнер в мире кайтсерфинга. Обучение, оборудование, путешествия – все, что нужно для незабываемых впечатлений.
сайты для написания диплома дипломная работа на заказ стоимость
купить реферат заказать реферат
The Real Person!
The Real Person!
Бездепозитные бонусы в казино
отчет по практике срочно на заказ написание отчетов по практике на заказ
The Real Person!
The Real Person!
kitehurghada.ru Кайтсерфинг хургада: Лучшие кайт-споты Хургады ждут вас!
The Real Person!
The Real Person!
получить 1000 рублей на карту бесплатно
The Real Person!
The Real Person!
кайт школа спб Кайт школа хургада: Обучение кайтсерфингу в лучших кайт-спотах Хургады. Откройте для себя новые горизонты!
The Real Person!
The Real Person!
почему в хургаде ветренно : Кайт серфинг в египте: Наслаждайся идеальным ветром!
The Real Person!
The Real Person!
Бездепозитный бонус в казино
займ онлайн на карту срочно https://zajmy-onlajn.ru
The Real Person!
The Real Person!
игровые автоматы бонус 1000 рублей за регистрацию без депозита
The Real Person!
The Real Person!
оранжевый остров в хургаде есть там акулы Кайт дети ветра: Символ свободы и единения с природой. Наша школа кайтсерфинга предлагает уникальные программы обучения для всех уровней подготовки.
The Real Person!
The Real Person!
кайт обучение хургада Кайт серфинг в египте: Наслаждайся кайтсерфингом в кристально чистых водах
The Real Person!
The Real Person!
школа кайтсерфинга в хургаде Кайт центр дети ветра: Все необходимое для кайтсерфинга в одном месте: обучение, прокат оборудования, ремонт, хранение. Мы заботимся о твоем комфорте и безопасности.
The Real Person!
The Real Person!
Бездепозитные бонусы в казино
An outstanding share! I’ve just forwarded this onto a colleague who was doing a little research on this. And he actually bought me dinner due to the fact that I found it for him… lol. So allow me to reword this…. Thanks for the meal!! But yeah, thanx for spending time to discuss this subject here on your web page.
Terrorism
The Real Person!
The Real Person!
фонтан казино бездепозитный бонус 1000 рублей за регистрацию без депозита с выводом денег
The Real Person!
The Real Person!
vingfoil Кайт школа: Найдите свою идеальную школу и начните свое кайт-приключение!
решение контрольных курсовых купить контрольную работу экология
The Real Person!
The Real Person!
газель алматы иссык Грузоперевозки в Алматы – это качественный и оперативный сервис, который предлагает широкий выбор автомобилей, в том числе и газелей, для решения различных задач. Если вам необходимо перевезти мебель, бытовую технику или строительные материалы, наши услуги газели в Алматы помогут вам осуществить это быстро и надежно. Мы предлагаем аренду газелей различной грузоподъемности, включая короткие и длинные модели, а также специальное оборудование, например, термобудки и рефрижераторы для транспортировки чувствительных грузов. Вы можете заказать газель с грузчиками для удобного переезда, будь то офисный или квартирный. Наша команда профессиональных грузчиков обеспечит аккуратную упаковку, разборку и сборку мебели, а также позаботится о безопасной перевозке ваших вещей. В Алматы мы работаем круглосуточно, предоставляя возможность вызвать газель в любое время суток. Цены на грузоперевозки в Алматыvarьируются в зависимости от расстояния и объема работ, но мы всегда готовы предложить конкурентные расценки и специальные скидки для постоянных клиентов. Для срочных заказов мы предоставляем услуги быстрого реагирования, чтобы вы не переживали о задержках. Не упустите возможность воспользоваться самым удобным и экономичным способом перевозки в Алматы. Свяжитесь с нами по телефону или через WhatsApp, чтобы обсудить детали вашего заказа. Обращайтесь за надежной газелью в Алматы и убедитесь сами в качестве наших услуг!
The Real Person!
The Real Person!
Лучшие новинки кино онлайн Телеграмм каналы: Лучшие подборки каналов на разные темы.
The Real Person!
The Real Person!
люкс детская одежда Zara Kids – это воплощение стиля и качества в мире детской моды. От игривых повседневных нарядов до элегантных образов для особых случаев, Zara Kids предлагает широкий ассортимент одежды для детей всех возрастов. Детская одежда
The Real Person!
The Real Person!
ремонт холодильников siemens алматы : Сервис холодильников – это компания, предоставляющая услуги по ремонту, обслуживанию и диагностике холодильного оборудования. Качественный сервис гарантирует использование оригинальных запчастей и квалифицированную работу мастеров. ремонт холодильника алматы
The Real Person!
The Real Person!
Сукааа казино официальный сайт скачать на андроид мобильная версия бесплатно Играйте бесплатно на официальном сайте Sykaaa Casino! Оцените широкий выбор игр и попробуйте свои силы без риска. Сукааа Казино Официальный Сайт
The Real Person!
The Real Person!
варфейс магазин Желание ворваться в динамичный мир Warface, облаченным в крутую броню и с арсеналом новейшего оружия, вполне понятно. Покупка игры – это ваш пропуск в захватывающие PvP-сражения и кооперативные миссии, где командная работа и тактическое мышление – залог победы. Аккаунт Варфейс
The Real Person!
The Real Person!
Дайвер спасатель Глубокие погружения – это захватывающие приключения, требующие специальной подготовки и опыта. Стресс и спасение
The Real Person!
The Real Person!
повешенные шторы Шторы – это душа интерьера, молчаливый рассказчик о вкусах и предпочтениях хозяев. Они способны преобразить комнату до неузнаваемости, добавить уюта или, наоборот, внести нотку строгости и элегантности. Рулонные шторы
The Real Person!
The Real Person!
Эврика дайв Дайвер спасатель – это специалист, обученный навыкам спасения людей под водой.
The Real Person!
The Real Person!
Погружение на море Запуск маркерного буя – это навык, позволяющий дайверу обозначить свое местоположение на поверхности воды. Виды маркерных буев дайвера
купить готовый диплом дипломная работа на заказ цена
контрольная по менеджменту выполнение контрольных
заказать отчет по учебной практике написание отчетов по практике на заказ
займ онлайн с плохой zajmy-onlajn.ru
The Real Person!
The Real Person!
Беседочный узел React Right – это курс первой помощи и сердечно-легочной реанимации, необходимый для дайверов и полезный в повседневной жизни. Дайвер открытой воды
Trusted AC duct cleaning in Dubai for residential and commercial properties https://ac-cleaning-dubai.ae/
The Real Person!
The Real Person!
как повесить шторы Карниз для штор – это надежная опора, на которой держится вся шторная композиция. Важно выбрать карниз, который будет соответствовать стилю штор и выдерживать их вес. Римские шторы
The Real Person!
The Real Person!
признаки фишинговых сайтов Определить фишинговый сайт можно по отсутствию контактной информации, ошибкам в текстах, запросу на предоставление данных, которые обычно не требуются, и отсутствию безопасного соединения (HTTPS). Ссылка на фишинговый сайт
The Real Person!
The Real Person!
акк варфейс Варфейс купить
The Real Person!
The Real Person!
Окна ПВХ Москва и МО В Развилке натяжные потолки – это популярное решение для создания стильного и современного интерьера. Они позволяют скрыть недостатки базового потолка, создать эффект идеально ровной поверхности. Натяжные потолки Островцы
The Real Person!
The Real Person!
бездепозитные бонусы казино без пополнения с отыгрышем Бездепозитные бонусы
The Real Person!
The Real Person!
Натяжные потолки работа Гарантия по договору – это ваша уверенность в качестве материалов и профессионализме установщиков. В случае возникновения проблем, все будет устранено в кратчайшие сроки. Натяжные потолки с сертификатам
The Real Person!
The Real Person!
повешенные шторы Выбор штор – это ответственный шаг, который требует внимательного подхода. Важно учитывать стиль интерьера, размеры помещения и личные предпочтения. Шторы блэкаут
The Real Person!
The Real Person!
машина в аренду краснодар Аренда авто Краснодар: Откройте для себя южную столицу с комфортом и стилем! Наш автопарк предоставит вам свободу передвижения, будь то деловая поездка или отпуск.
The Real Person!
The Real Person!
авто в аренду в Краснодаре Прокат авто Краснодар: Быстрое оформление, надежные автомобили и круглосуточная поддержка. Мы сделаем вашу поездку максимально комфортной и безопасной.
The Real Person!
The Real Person!
Натяжные потолки Раменское Цена за метр натяжного потолка зависит от материала, фактуры и производителя. Натяжной потолок в зал
The Real Person!
The Real Person!
магазин аккаунтов варфейс Приобретение аккаунта Warface – это возможность сэкономить время и силы, получив готового персонажа с прокачанными навыками и экипировкой. Аккаунт Варфейс купить
Лучшие онлайн-курсы https://topkursi.ru по востребованным направлениям: от маркетинга до программирования. Учитесь в удобное время, получайте сертификаты и прокачивайте навыки с нуля.
Школа Саморазвития https://bznaniy.ru онлайн-база знаний для тех, кто хочет понять себя, улучшить мышление, прокачать навыки и выйти на новый уровень жизни.
The Real Person!
The Real Person!
покупные бонусы в казино Многие игроки ищут именно такие бонусы, которые позволяют сразу вывести выигранные деньги, не вкладывая свои. Но стоит помнить о лимитах и правилах казино.
The Real Person!
The Real Person!
Бездепозитный бонус в казино Бездепозитные бонусы
The Real Person!
The Real Person!
Install Size 37.6 Mb In most cases, you’ll need to buy chips to play Teen Patti. These chips represent the currency used in the game. Signup Bonus: ₹50 Many online Teen Patti platforms allow you to chat and interact with other players during the game. APK, Google Play Teen Patti Hustle: Patti game – A Classic Indian Card Game Many online Teen Patti platforms allow you to chat and interact with other players during the game. It’s important to note that when playing Teen Patti online, whether for fun or real money, you should be aware of the platform’s terms and conditions. Additionally, if you’re playing for real money, make sure that online gambling is legal in your jurisdiction, and you play responsibly. Teen Patti isn’t considered to be a real cash-powered Android game. As such, the chips you win in this game can’t be converted to real money. However, buying chips is quite simple and straightforward. You only need to use your Google Pay, Visa Mastercard, or PayTM account.
https://cenarinats1972.iamarrows.com/https-mooncalf-me-uk
Shuffle (SHFL) Shuffle (SHFL) The paytable lists symbol values at the current stake. The Sweet Bonanza 1000 online slot uses a scatter pays system, where it doesn’t matter where symbols are, and you just need to see 8 or more of the same type to win. Values range from 0.25 – 2x the stake from 8 – 12+ bananas to 10x – 50x if you land 8 – 12+ red heart candies. This paytable also has extra details on features such as free games with multipliers so it’s worth having a read through before you stake real money on the game. Play Sweet Bonanza 1000 at Asino Casino! Min deposit: A$10. Max win: 10,000x your stak! Start spinning now and taste the sweetest rewards! рџЌрџ’ЋрџЋ° What to expect: When 4 or more Scatter symbols land anywhere on the reels, the Free Spins Feature begins. The Free Spins Round may also be purchased. The round begins with 10 free spins, which are increased by an additional 5 spins if 3 or more Scatters land during the round.
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу Получить 1000 рублей за регистрацию с моментальным выводом, не требующим каких-либо вложений, – это мечта любого новичка онлайн-казино. Это возможность начать игру с преимуществом и испытать свою удачу без финансового риска.
Attractive section of content. I just stumbled upon your website and in accession capital to assert that I acquire actually enjoyed account your blog posts. Anyway I’ll be subscribing to your feeds and even I achievement you access consistently rapidly.
tadalafil 5 mg generico prezzo
The Real Person!
The Real Person!
best cargo company in uae China to Dubai: Understanding Cultural Nuances in Business Communications
The Real Person!
The Real Person!
Служба по контракту СВО Отбор на службу по контракту: Медицинское освидетельствование и психологическое тестирование
The Real Person!
The Real Person!
заказать пионы в москве Заказать пионы в Москве: Легкость выбора, безупречное качество, оперативная доставка. Наш онлайн-сервис предоставляет удобный и быстрый способ заказать пионы в Москве, не выходя из дома. Выберите идеальный букет, оформите заказ, и мы позаботимся о том, чтобы свежие и ароматные пионы были доставлены вовремя и в безупречном состоянии. Мы гарантируем высокое качество цветов и профессиональный сервис, чтобы ваш подарок стал незабываемым сюрпризом.
The Real Person!
The Real Person!
скачать игры с яндекс диска Скачать игры с Яндекс Диска: Облачное хранилище ваших игровых приключений. Яндекс Диск – это не только удобное и надежное место для хранения файлов, но и платформа для распространения игр. Получите доступ к тщательно отобранным коллекциям игр, гарантированно безопасным и готовым к запуску. Просто скачайте нужный файл и наслаждайтесь игровым процессом.
The Real Person!
The Real Person!
shipping china to uae Shipping China to UAE: The Future of Logistics and Global Trade
The Real Person!
The Real Person!
доставка пионов в москве Заказать пионы в Москве: Удобство и простота в каждом клике. Наш онлайн-сервис разработан с учетом потребностей самых занятых людей. Заказать пионы в Москве стало проще простого: выберите понравившийся букет на нашем сайте, укажите адрес доставки и удобное время, и мы позаботимся обо всем остальном. Мы гарантируем не только безупречное качество цветов, но и быструю и надежную доставку.
The Real Person!
The Real Person!
скачать игры по прямой ссылке Скачать игры по прямой ссылке: Линия прямой видимости к вашим игровым мечтам. Никаких посредников, никаких дополнительных программ. Только прямая связь между вами и желаемой игрой. Просто нажмите на ссылку и наслаждайтесь мгновенной загрузкой, экономя драгоценное время и избавляясь от технических сложностей. Погрузитесь в мир развлечений с максимальной скоростью и комфортом.
адреса наркологических клиник телефон наркологии
сеть пансионатов для пожилых частный пансионат для пожилых
спросить юриста задать юридический вопрос онлайн бесплатно
типография типография петербург
типография официальный сайт типографии спб недорого
металлические значки москва металлические значки на заказ
The Real Person!
The Real Person!
организация корпоративов организация вечеринок: Организовать незабываемую вечеринку – наша специализация. Тематические вечера, дни рождения, юбилеи – мы создадим атмосферу веселья и праздника, учитывая все ваши пожелания. От разработки концепции и подбора места проведения до приглашения диджея и организации развлекательной программы – мы позаботимся о каждой детали, чтобы ваша вечеринка прошла на высшем уровне.
The Real Person!
The Real Person!
выходной ремонт холодильников алматы Ремонт холодильников Atlant Алматы: Ремонт холодильников Atlant всех моделей.
The Real Person!
The Real Person!
ремонт посудомоечных машин electrolux алматы скупка бу стиральных машин алматы Покупка стиральной машины б/у – это экономичный вариант для тех, кто не готов тратить большие средства на новую технику. В Алматы можно найти множество предложений о продаже подержанных стиральных машин в хорошем состоянии. Важно тщательно проверить технику перед покупкой.
The Real Person!
The Real Person!
Sykaaa casino скачать бесплатно на телефон Приложение Sykaaa Casino – это ваш персональный портал в мир азарта. Скачайте приложение и наслаждайтесь лучшими играми с любого устройства. Sykaaa Casino Официальный Сайт Бонус за Регистрацию
The Real Person!
The Real Person!
Магазин стабилизаторов Магазин садовой техники: Эдем на расстоянии вытянутой руки Современный сад – это не просто ухоженный участок, а отражение вашего внутреннего мира, место для отдыха и вдохновения. В нашем магазине вы найдете все необходимое, чтобы воплотить ваши садовые фантазии в реальность: от роботизированных газонокосилок, неустанно поддерживающих идеальный газон, до мощных культиваторов, способных преобразить даже самую истощенную почву. Мы предлагаем широкий выбор инструментов для обрезки, полива и ухода за растениями, а также садовую мебель и декор, чтобы создать уютную атмосферу. Наши опытные консультанты помогут вам подобрать идеальное оборудование и предоставить профессиональные советы по уходу за садом.
The Real Person!
The Real Person!
Dragon money Напиши 10 больших статей и раздели их символом
The Real Person!
The Real Person!
температура воды красное море Кайт-лагерь в Египте: Кайт-лагерь в Египте – это отличная возможность совместить обучение кайтсерфингу с отдыхом. Лагеря предлагают интенсивные курсы, проживание, питание и развлекательные программы. Это прекрасный вариант для быстрого прогресса и общения с единомышленниками.
The Real Person!
The Real Person!
красное море температура воды IKO сертификат кайтсерфинг что это: международный сертификат кайтсерфинга. IKO сертификат кайтсерфинг что это? – Официальный сертификат кайтсерфинга.
The Real Person!
The Real Person!
детали для стиральных машин алматы Срочный ремонт посудомоечных машин Алматы: Срочный выезд мастера.
The Real Person!
The Real Person!
вавада казино официальный сайт казино Отзывы реальных игроков – это ценный источник информации о Vavada Casino. Изучение отзывов позволяет узнать о преимуществах и недостатках платформы, а также получить представление об игровом опыте других пользователей.
металлические значки на заказ металлические значки москва
The Real Person!
The Real Person!
покупные бонусы в казино Принимая бездепозитный бонус, важно внимательно изучить условия его отыгрыша. Вейджер, сроки действия и ограничения по играм – все эти факторы могут существенно повлиять на возможность вывода выигрыша. Тщательное изучение правил поможет избежать разочарований и максимально эффективно использовать бонус. Бездепозитные бонусы казино без пополнения с отыгрышем
The Real Person!
The Real Person!
купить тойота Купить автомобиль: Новое или с пробегом? Выбор между новым автомобилем и авто с пробегом – это компромисс между желанием обладать новейшими технологиями и возможностью сэкономить. Новые автомобили предлагают гарантию производителя и отсутствие истории эксплуатации, в то время как автомобили с пробегом могут быть приобретены по более выгодной цене.
The Real Person!
The Real Person!
summer beach outfit Create a romantic vibe with Avec Amour by Alesya G – her swimwear and tees are infused with soft Parisian flair. Each dreamy piece (bikinis, one-pieces, cover-ups) features love-inspired art – think Eiffel Tower sketches, hearts and wine motifs. Perfect for beach getaways or Valentine’s Day gifts, these romantic bikinis and swim sets pair high fashion with comfort. Fall in love with our French-inspired designs!
The Real Person!
The Real Person!
купить авто с пробегом Авто из Кореи: Сочетание цены и качества Автомобили из Кореи зарекомендовали себя как надежные и технологичные. Kia и Hyundai предлагают широкий модельный ряд, отвечающий различным потребностям.
The Real Person!
The Real Person!
https://b2tsite3.cc/blacksprut_bs2best.html
The Real Person!
The Real Person!
funny wine glass Oiseaux et Vin (French for “Birds & Wine”) is Alesya G’s elegant bird collection for wine lovers. Explore bird-themed drinkware and accessories – from chic stemless wine glasses and insulated tumblers to fun phone cases featuring wine-sipping birds. These unique gifts marry French style with humor, perfect for wine enthusiasts and bird lovers alike. Sip and celebrate – shop wine-bird mugs, shirts, and decor now!
The Real Person!
The Real Person!
в каком казино дают деньги за регистрацию без депозита Казино, предлагающие бездепозитные бонусы, демонстрируют свою уверенность в качестве предоставляемых услуг. Это своего рода маркетинговый ход, направленный на привлечение новых игроков и формирование лояльности. Игроки, получившие положительный опыт, с большей вероятностью вернутся и станут постоянными клиентами. Бездепозитный бонус
The Real Person!
The Real Person!
nitroethane Ephedrine is often used to produce phenylacetone, a key intermediate in stimulant synthesis. From phenylacetone, substances like methylone, mephedrone (4-MMC), and 3-CMC can be made using methylamine. Phenylnitropropene, derived from nitroethane, is another precursor. A-PVP and 4-methylpropiophenone are also widely used in synthetic drug production. BMK glycidate is commonly used to synthesize controlled substances.
The Real Person!
The Real Person!
Ephedrine Ephedrine is often used to produce phenylacetone, a key intermediate in stimulant synthesis. From phenylacetone, substances like methylone, mephedrone (4-MMC), and 3-CMC can be made using methylamine. Phenylnitropropene, derived from nitroethane, is another precursor. A-PVP and 4-methylpropiophenone are also widely used in synthetic drug production. BMK glycidate is commonly used to synthesize controlled substances.
The Real Person!
The Real Person!
Асфальтирование дворов
The Real Person!
The Real Person!
https://bs2bcst.at
The Real Person!
The Real Person!
BMK glycidate Ephedrine is often used to produce phenylacetone, a key intermediate in stimulant synthesis. From phenylacetone, substances like methylone, mephedrone (4-MMC), and 3-CMC can be made using methylamine. Phenylnitropropene, derived from nitroethane, is another precursor. A-PVP and 4-methylpropiophenone are also widely used in synthetic drug production. BMK glycidate is commonly used to synthesize controlled substances.
The Real Person!
The Real Person!
https://a-bsme.at/blacksprut_bs2best.html
The Real Person!
The Real Person!
Бездепозитные бонусы Прежде чем принять щедрое предложение от казино, стоит внимательно изучить условия предоставления бездепозитного бонуса. Важно обратить внимание на вейджер – коэффициент, который определяет, сколько раз нужно отыграть бонус, прежде чем вывести выигрыш. Также стоит обратить внимание на сроки действия бонуса и ограничения по играм. Тщательное изучение правил поможет избежать разочарований и максимально эффективно использовать бонус для достижения своих целей.
The Real Person!
The Real Person!
https://b2shop.gl/https_bs2best_at.html
The Real Person!
The Real Person!
https://b2tsite4.io/bs2best_at.html
AI generator ai chat nsfw of the new generation: artificial intelligence turns text into stylish and realistic pictures and videos.
The Real Person!
The Real Person!
Бездепозитный бонус в казино Бездепозитные бонусы – это магнит для новых игроков в онлайн-казино, словно обещанный клад на необитаемом острове азарта. Они позволяют сделать первые шаги, не рискуя собственным кошельком, и окунуться в мир вращающихся барабанов, карточных комбинаций и головокружительных возможностей. Это шанс испытать удачу, не платя за билет, и, возможно, сорвать джекпот, о котором даже не мечтал. Бездепозитные бонусы в казино
The Real Person!
The Real Person!
https://b2tor2.cc/index.html
The Real Person!
The Real Person!
https://b2tsite3.cc/bs2best.html
New AI generator free nsfw ai of the new generation: artificial intelligence turns text into stylish and realistic image and videos.
The Real Person!
The Real Person!
Бездепозитный бонус в казино Казино, предлагающие бездепозитные бонусы, словно заботливые хозяева, приглашают гостей в свой дом и предлагают им угощение на пробу. Это не просто щедрость, а продуманный маркетинговый ход, направленный на привлечение новых клиентов и формирование лояльности к бренду. Это возможность для игроков оценить качество сервиса, разнообразие игр и удобство платформы, прежде чем делать депозит. Это своеобразная гарантия, что казино уверено в своих силах и готово предоставить игрокам наилучший опыт. Бездепозитный бонус
The Real Person!
The Real Person!
https://b2shop.gl/blacksprut.html
The Real Person!
The Real Person!
https://1-bs2best.lat/https_bs2best_at.html
The Real Person!
The Real Person!
https://b2tor2.cc/bs2_best_at.html
The Real Person!
The Real Person!
Бездепозитный бонус в казино Бездепозитные бонусы – это магнит для новых игроков в онлайн-казино, словно обещанный клад на необитаемом острове азарта. Они позволяют сделать первые шаги, не рискуя собственным кошельком, и окунуться в мир вращающихся барабанов, карточных комбинаций и головокружительных возможностей. Это шанс испытать удачу, не платя за билет, и, возможно, сорвать джекпот, о котором даже не мечтал. Бездепозитные бонусы в казино
The Real Person!
The Real Person!
https://b2tor2.cc/blaksprut_ssylka.html
The Real Person!
The Real Person!
секс Макеевка Секс знакомства Макеевка: Онлайн-платформы для интима Макеевка предлагает своим жителям возможности для секс-знакомств через интернет. Сайты и приложения позволяют найти партнеров с общими интересами и желаниями.
The Real Person!
The Real Person!
kaszino oldalak
The Real Person!
The Real Person!
бездепозитный бонус за регистрацию в казино с выводом Казино, предлагающие бездепозитные бонусы, демонстрируют свою уверенность в качестве предоставляемых услуг. Это своего рода маркетинговый ход, направленный на привлечение новых игроков и формирование лояльности. Игроки, получившие положительный опыт, с большей вероятностью вернутся и станут постоянными клиентами. Бездепозитный бонус
The Real Person!
The Real Person!
проститутки горловка Секс Макеевка – как и в случае с Донецком, этот запрос демонстрирует интерес к сексуальным отношениям в данном городе.
The Real Person!
The Real Person!
https://bs-bs2best.at
The Real Person!
The Real Person!
licencelt kaszino
The Real Person!
The Real Person!
Бездепозитный бонус в казино Бездепозитные бонусы – это манящий огонек в темном лесу онлайн-казино, дарящий надежду на легкую наживу и возможность сорвать куш, не рискуя собственными сбережениями. Это своего рода аванс от казино, предложение попробовать свои силы и ощутить вкус победы, прежде чем расстаться со своими деньгами. Они позволяют новичкам освоиться в мире азарта, изучить правила игр и разработать собственную стратегию, не боясь потерь. Это ценный опыт, который может стать отправной точкой для успешной игры в будущем. Бездепозитные бонусы в казино
The Real Person!
The Real Person!
https://bs2bet.at
The Real Person!
The Real Person!
психиатрическая клиника Психиатрическая клиника. Само это словосочетание вызывает в воображении образы, окутанные туманом страха и предрассудков. Белые стены, длинные коридоры, приглушенный свет – все это лишь проекции нашего собственного внутреннего смятения, отражение боязни заглянуть в темные уголки сознания. Но за этими образами скрывается мир, полный боли, надежды и, порой, неожиданной красоты. В этих стенах встречаются люди, чьи мысли и чувства не укладываются в рамки общепринятой “нормальности”. Они борются со своими демонами, с голосами в голове, с навязчивыми идеями, которые отравляют их существование. Каждый из них – это уникальная история, сложный лабиринт переживаний и травм, приведших к этой точке. Здесь работают люди, посвятившие себя помощи тем, кто оказался на краю. Врачи, медсестры, психологи – они, как маяки, светят в ночи, помогая найти путь к выздоровлению. Они не волшебники, и не всегда могут исцелить, но их сочувствие, их понимание и профессионализм – это часто единственная нить, удерживающая пациента от окончательного падения в бездну. Жизнь в психиатрической клинике – это не заточение, а скорее передышка. Время для того, чтобы собраться с силами, чтобы разобраться в себе, чтобы научиться жить со своими особенностями. Это место, где можно найти поддержку, где можно не бояться быть собой, даже если этот “себя” далек от идеала. И хотя выход из клиники не гарантирует безоблачного будущего, он дает шанс на новую жизнь, на жизнь, в которой найдется место для радости, для любви и для надежды.
The Real Person!
The Real Person!
Миотонические козы? почему они падают? https://e-pochemuchka.ru/miotonicheskie-kozy-osobennosti-porody/
Почему дизельный двигатель лучше бензинового – обоснование экономичности и практических преимуществ
The Real Person!
The Real Person!
Sykaaa casino скачать бесплатно на телефон Sykaaa Casino Официальный Сайт Вход
The Real Person!
The Real Person!
автоломбарды адреса
zaimpod-pts90.ru
кредит залог авто наличные
The Real Person!
The Real Person!
https://2-bs2best.lat/blaksprut_ssylka.html
LMC Middle School https://lmc896.org in Lower Manhattan provides a rigorous, student-centered education in a caring and inclusive atmosphere. Emphasis on critical thinking, collaboration, and community engagement.
The Real Person!
The Real Person!
https://1-bs2best.lat/bs2best_at.html
The Real Person!
The Real Person!
Бездепозитный бонус Перед тем, как воспользоваться бездепозитным бонусом, стоит внимательно изучить условия его получения и отыгрыша. Важно понимать, какие игры доступны для игры на бонусные средства, какой вейджер необходимо выполнить, чтобы вывести выигрыш, и какие сроки установлены для отыгрыша бонуса. Тщательное изучение правил поможет избежать разочарований и получить максимальную выгоду от использования бонуса.
The Real Person!
The Real Person!
https://b2tsite4.io/https_bs2best_at.html
The Real Person!
The Real Person!
https://b2tsite3.cc/index.html
The Real Person!
The Real Person!
рулонные шторы на пластиковые окна Шторы блэкаут — идеальный выбор для спальни или детской, где важна полная темнота и защита от солнечного света. Такие шторы выполнены из плотных материалов с дополнительным покрытием, которое блокирует свет. Кроме затемнения, они улучшают теплоизоляцию и звукоизоляцию помещения.
The Real Person!
The Real Person!
гибкая керамика для внутренней Гибкая керамика цена за м2 для фасадов с установкой помогает избежать дополнительных затрат и обеспечивает качество работы.
•очешь продать авто? magic auto
The Real Person!
The Real Person!
Производство шариковых подшипников Изготовитель подшипников несет ответственность за качество выпускаемой продукции и соответствие требованиям безопасности. Выбор надежного производителя – залог долговечной и бесперебойной работы вашего оборудования.
The Real Person!
The Real Person!
экскурсии в питере Экскурсии СПб – незабываемые впечатления от посещения города. Телеграм предлагает широкий выбор экскурсий на любой вкус.
The Real Person!
The Real Person!
Бездепозитные фрибеты Фрибеты за регистрацию – отличный способ начать делать ставки без риска потерять собственные средства. Ищите выгодные предложения от разных букмекеров.
The Real Person!
The Real Person!
Купить подшипники оптом Подшипники оптом от производителей – экономически обоснованное решение для предприятий с большим объемом производства. Прямые поставки гарантируют выгодные цены и контроль качества.
The Real Person!
The Real Person!
шторы пятигорск Шторы фото — уникальный способ украсить интерьер с помощью персонализированных изображений на ткани. Можно выбрать любой рисунок: от природных пейзажей до абстрактных узоров или фотографий семьи. Такие шторы создают индивидуальность и подчеркивают стиль комнаты.
Агентство контекстной рекламы https://kontekst-dlya-prodazh.ru настройка Яндекс.Директ и Google Ads под ключ. Привлекаем клиентов, оптимизируем бюджеты, повышаем конверсии.
Продвижение сайтов https://optimizaciya-i-prodvizhenie.ru в Google и Яндекс — только «белое» SEO. Улучшаем видимость, позиции и трафик. Аудит, стратегия, тексты, ссылки.
The Real Person!
The Real Person!
экскурсии по петербургу Экскурсии по Петербургу: Откройте для себя дворцы, каналы и музеи Северной столицы с опытными гидами
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar001.ru
вывод из запоя круглосуточно краснодар
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar001.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
Букмекеры с минимальным депозитом Новые букмекеры появляются на рынке регулярно, предлагая инновационные решения и привлекательные акции. Будьте в курсе последних тенденций и выбирайте надежных партнеров для ставок.
The Real Person!
The Real Person!
провайдеры интернета в челябинске
domashij-internet-chelyabinsk004.ru
провайдеры челябинск
Шины и диски https://tssz.ru для любого авто: легковые, внедорожники, коммерческий транспорт. Зимние, летние, всесезонные — большой выбор, доставка, подбор по марке автомобиля.
Инженерная сантехника https://vodazone.ru в Москве — всё для отопления, водоснабжения и канализации. Надёжные бренды, опт и розница, консультации, самовывоз и доставка по городу.
The Real Person!
The Real Person!
Продвижение бизнеса в социальных сетях Частный маркетолог Анастасия Речанская предлагает индивидуальный подход к каждому клиенту, глубокий анализ бизнеса и разработку персонализированных стратегий для достижения конкретных целей.
Промышленные ворота https://efaflex.ru любых типов под заказ – секционные, откатные, рулонные, скоростные. Монтаж и обслуживание. Установка по ГОСТ.
Продажа и обслуживание https://kmural.ru копировальной техники для офиса и бизнеса. Новые и б/у аппараты. Быстрая доставка, настройка, ремонт, заправка.
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
narkolog-krasnodar002.ru
лечение запоя
The Real Person!
The Real Person!
кабинет робототехники требования к кабинету физики Кабинет физики — это не просто класс, а настоящая научная лаборатория, оснащение которой должно соответствовать строгим требованиям. Компания «Astana IT Garant» комплектует кабинеты физики в полном соответствии с нормативными документами и приказами Министерства образования и науки РК. Требования охватывают несколько ключевых областей. Во-первых, это наличие специализированной мебели: демонстрационного стола для учителя и ученических лабораторных столов, обязательно оборудованных розетками для подключения электроприборов. Во-вторых, это полный перечень демонстрационного оборудования для проведения опытов учителем и лабораторного оборудования для выполнения практических работ учениками по всем разделам физики. Сюда входят наборы по механике, оптике, термодинамике, электричеству и магнетизму. В-третьих, современные требования обязательно включают наличие цифрового оборудования: интерактивной панели или проектора, документ-камеры, а также цифровой лаборатории с набором датчиков (силы, напряжения, температуры, освещенности и др.). Это позволяет проводить эксперименты на качественно новом уровне. «Astana IT Garant» гарантирует, что каждый поставляемый нами кабинет физики будет укомплектован всем необходимым оборудованием согласно утвержденному перечню, что позволит в полной мере реализовывать учебную программу и успешно проходить аттестацию.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar001.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
домашний интернет тарифы
domashij-internet-chelyabinsk005.ru
дешевый интернет челябинск
The Real Person!
The Real Person!
цены в турции Сиде, с его богатой историей и руинами древнеримских храмов, предлагает уникальное сочетание культурного и пляжного отдыха. Прогуляйтесь по узким улочкам, насладитесь местной кухней и окунитесь в атмосферу древности.
нейросеть создать дизайн сайта создать дизайн сайта с помощью нейросети
Woodworking and construction https://www.woodsurfer.com forum. Ask questions, share projects, read reviews of materials and tools. Help from practitioners and experienced craftsmen.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar002.ru
вывод из запоя круглосуточно краснодар
The Real Person!
The Real Person!
подключить домашний интернет челябинск
domashij-internet-chelyabinsk006.ru
лучший интернет провайдер челябинск
The Real Person!
The Real Person!
лечение запоя краснодар
narkolog-krasnodar003.ru
вывод из запоя круглосуточно краснодар
The Real Person!
The Real Person!
вывод из запоя круглосуточно
narkolog-krasnodar003.ru
вывод из запоя цена
The Real Person!
The Real Person!
Wink — это современный IPTV сервис, который предоставляет разнообразные интернет-услуги в Екатеринбурге. В этом обзоре мы рассмотрим тарифы Wink, а также особенности и возможности подключения. Сервис Wink предоставляет несколько пакетов услуг, включая IPTV Wink и тарифы для мобильных устройств. Анализ тарифов позволяет выбрать оптимальный вариант по стоимости Wink. Вы можете посетить сайт domashij-internet-ekaterinburg004.ru и найти актуальные акции и скидки Wink, что делает предложения действительно привлекательными. Пользователи оставляют положительные отзывы о Wink, отмечая удобство доступа к контенту Wink и высокое качество цифрового телевидения. Что касается возможностей тарифов, стоит выделить широкий выбор каналов, доступ к эксклюзивному контенту и гибкость в выборе пакетов. Связь в Екатеринбурге от Wink обеспечивают надежное подключение и отличное качество изображения.
The Real Person!
The Real Person!
гибкая керамика цена Гибкая керамика отделка — это современный способ обновить внешний вид здания без капитального ремонта. Благодаря эластичности материала его можно применять на поверхностях с криволинейными формами, что невозможно при использовании традиционной керамической плитки.
The Real Person!
The Real Person!
маркетинг консультации Стоимость услуг маркетолога зависит от объема работ, сложности проекта и квалификации специалиста. Ценообразование может быть почасовым, фиксированным или процентным от прибыли, полученной в результате маркетинговых усилий.
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar004.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
led экран scada Promethean Казахстан Promethean Казахстан — это эксклюзивное представительство мирового лидера в области интерактивных образовательных технологий, которое осуществляет ТОО «Astana IT Garant». Мы являемся официальным дистрибьютором Promethean в Казахстане и обеспечиваем поставку, установку и техническую поддержку всей линейки продукции этого британского бренда. Продукция Promethean включает интерактивные панели ActivPanel различных размеров и конфигураций, программное обеспечение ActivInspire и ClassFlow для создания интерактивных уроков, системы голосования ActiVote для оценки понимания материала. Все решения разработаны специально для образовательной среды. Интерактивные панели Promethean отличаются высоким качеством изображения, точным распознаванием касаний, интуитивным интерфейсом. Встроенные инструменты для аннотирования, записи уроков, беспроводного подключения устройств делают обучение более эффективным и увлекательным. Astana IT Garant обеспечивает полную локализацию продукции Promethean для казахстанского рынка: адаптацию программного обеспечения под государственные языки, создание методических материалов, обучение педагогов. Мы гарантируем соответствие решений местным образовательным стандартам. Сервисная поддержка включает гарантийное и постгарантийное обслуживание, поставку запасных частей, регулярные обновления программного обеспечения. Наши сертифицированные инженеры обеспечивают быстрое решение любых технических вопросов по всей территории Казахстана.
The Real Person!
The Real Person!
Фрибеты за регистрацию Бездепозитные фрибеты – редкий, но очень привлекательный вид бонуса, позволяющий сделать ставку без внесения депозита.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
narkolog-krasnodar004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
В столице России доступен широкий выбор интернет-провайдеров‚ и каждый из них предлагает разные варианты тарифов. Выбирая провайдера‚ необходимо учитывать на качество интернет-соединения‚ стабильность соединения и скорость сети. Мнения пользователей имеют значение‚ что помогает сформировать рейтинг провайдеров.Анализ предложений провайдеров в Екатеринбурге показывает‚ что многие компании предлагают доступные тарифы и акции. Это может быть выгодным для тех‚ кто хочет найти лучшее решение для подключения интернета. Услуги интернет-провайдеров варьируются: от базовых до премиум-тарифов с высокой скоростью. интернет провайдеры Екатеринбург Техническая поддержка также играет ключевую роль. Хорошие провайдеры обеспечивают оперативное решение проблем и поддержку клиентам. Таким образом‚ изучив мнения пользователей‚ вы сможете подобрать оптимального интернет-провайдера для своего жилья в Екатеринбурге.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar005.ru
лечение запоя
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar005.ru
вывод из запоя
The Real Person!
The Real Person!
В Екатеринбурге множество провайдеров интернета представляют услуги IPTV. Это цифровое телевидение обеспечивает пользователей к качественному контенту. Провайдеры интернета, такие как МТС, Билайн и Ростелеком представляют различные тарифные планы на цифровое телевидение, включая HD контент и возможность просмотра онлайн. Сети передачи данных обеспечивают надежное соединение для удобного просмотра. Кабельные провайдеры также активно внедряют интерактивные услуги, что дает возможность пользователям получать удовольствие от мультиэкранного доступа через мультимедийные устройства. Видеосервисы, предлагаемые провайдерами, дают возможность смотреть любимые шоу и фильмы в любое время. Абоненты могут выбрать провайдера, который лучше всего соответствует их нуждам, ознакомившись с тарифами на сайте site;com.
The Real Person!
The Real Person!
сдать макулатуру в казани Прием вторсырья в Казани осуществляется специализированными компаниями, оснащенными современным оборудованием. Это позволяет эффективно перерабатывать различные виды отходов и производить вторичное сырье.
The Real Person!
The Real Person!
Перфект Plus Перфект Plus – это не только широкий выбор товаров, но и профессиональные консультации дизайнеров. Они помогут вам создать уникальный интерьер, отражающий вашу индивидуальность.
The Real Person!
The Real Person!
Лечение запоя с помощью капельницы – это проверенный метод лечения алкогольной зависимости‚ который обеспечивает возможность комфортного и конфиденциального лечения. Нарколог на дом анонимно может быстро обеспечить необходимую медицинскую помощь при запое‚ используя капельницу для детоксикации организма. Преимущества капельницы заключаются в том‚ что она способствует быстрому снятию симптомов запоя‚ восстанавливает баланс жидкости и электролитов и способствует улучшению общего состояния пациента. Это критически важно в случае серьезных проявлений алкогольной зависимости‚ когда необходима немедленная помощь.Существуют различные методы лечения алкоголизма‚ но капельница отличается высокой эффективностью и скоростью действия. В отличие от таблеток или инъекций‚ капельница гарантирует непрерывное введение медикаментов в организм‚ что ускоряет процесс выздоровления.Ключевыми аспектами реабилитации являются поддержка близких и психотерапия в процессе реабилитации. По завершении этапа детоксикации важно продолжать лечение и работать над предотвращением рецидивов‚ что возможно только при системном подходе к лечению. Таким образом‚ капельница при запое — это ключевой элемент в борьбе с алкоголизмом‚ предлагая пациентам надежное анонимное лечение и возможность быстрого восстановления.
The Real Person!
The Real Person!
советы по нейросетям Чат-боты для бизнеса: автоматизация поддержки, увеличение продаж и улучшение клиентского сервиса.
The Real Person!
The Real Person!
Обращение к анонимному наркологу — является важным шагом на пути к лечению зависимости. Когда вы или кто-то из ваших близких сталкиваетесь с трудностями с наркотиками или алкоголем‚ не откладывайте обращение за помощью. На сайте site;com доступна консультация нарколога и вызвать специалиста на дом. Конфиденциальная помощь обеспечивает вашу безопасность‚ что особенно важно для многих зависимых. Квалифицированный нарколог проведет детоксикацию организма и разработает персонализированные программы восстановления. Психотерапевтическая помощь также играет ключевую роль в процессе выздоровления. Помощь зависимым и их близким способствует преодолению последствий зависимости. Помните‚ что борьба с алкогольной зависимостью так же значимо‚ как и лечение наркомании. Не стесняйтесь обратиться за помощью‚ это ваш первый шаг к свободе от зависимости.
The Real Person!
The Real Person!
сборка ПК под ключ Сборка ПК под ключ – полное избавление от хлопот, связанных со сборкой компьютера.
The Real Person!
The Real Person!
Pay symbols in Sweet Bonanza 1000 are bananas, grapes, watermelons, plums, apples, blue candy, green candy, purple candy, and red candy. An 8-9 symbol scatter win pays 0.25x to 10x the bet, while the biggest hauls are 2 to 50 times the bet for a 12+ sized scatter hit. Wild symbols are not part of Sweet Bonanza 1000 action, no matter the phase. Logging into Sweet Bonanza is essential for players who want to access all features of the game. Whether you’re returning to your account or creating a new one, this guide will help you navigate the login and registration process smoothly. Here, you’ll find step-by-step instructions and tips to ensure a secure and enjoyable experience. Don’t be expecting a new set of visuals to accompany the changes, either. Sweet Bonanza 1000 is located in more or less the same swirly candy land as before, and if there are any major differences to the background, they are not easy to spot. Maybe the definition is higher, the colours more vibrant? Sweet Bonanza hit casinos in mid-2019, and there have been a shed load of candy-themed slots released in the interim – Pragmatic Play itself has revisited the swirl in games like Sugar Rush. As such, the view might be staler than the original’s was but no less cheerful on the eyes.
https://votebookmarking.com/page/business-services/more
Here are some reasons why Big Bass Bonanza free spins no deposit UK offers are common and easy to find on popular platforms: 100 free spins on Big Bass Bonanza are yours when you deposit and wager at least £10 on slots at LiveScoreBet. This offer is much better than what we’ve seen at lots of other online casino sites because there are absolutely no wagering requirements, so if you get lucky enough to win, you’ll get to keep your winnings. No deposit no credit card casino bonus uk 2025 Ever wondered why there are no windows and clocks inside gambling venues, online checkers is a hassle-free way to enjoy your favorite game from the comfort of your own computer. The roulette killer system claims that it can predict what number will show up on the next spin, we strongly recommend that you out LeoVegas and explore the amazing live games on offer. With 117,649 ways to win, from free spins bonuses. How to Win Baccarat without Commission.
The Real Person!
The Real Person!
компьютер для игр на заказ Компьютер для игр на заказ – это персонализированная игровая станция, оптимизированная под конкретные игры и предпочтения геймера.
The Real Person!
The Real Person!
лучший интернет провайдер казань
domashij-internet-kazan004.ru
провайдеры домашнего интернета казань
The Real Person!
The Real Person!
Капельное лечение запоя – данный метод является популярным методом борьбы с алкоголизма, который применяется в клиниках на территории Тулы с целью детоксикации организма. Круглосуточное лечение позволяет эффективно снять симптомы запоя. Тем не менее необходимо учитывать о возможных побочных эффектах, включающих реакции аллергического характера и нарушение водно-солевого баланса. вывод из запоя круглосуточно тула
The Real Person!
The Real Person!
Наркологическая помощь на дому — это комфортный способ лечения зависимостей, который становится все более популярным. Услуги нарколога на дому предоставляют конфиденциальность и комфорт, что является ключевым моментом для многих пациентов. Опытные врачи обеспечивают лечение с помощью медикаментов и психологическую поддержку, что позволяет начать восстановление после зависимости в привычной обстановке. Реабилитация на дому включает в себя индивидуальные программы реабилитации, семейную терапию и консультации нарколога, что способствует осознанию проблемы. Предотвращение рецидивов играет важную роль в лечении зависимостей, включая алкоголизм и наркотическую зависимость. Домашний уход за зависимыми помогает создать поддерживающую атмосферу и минимизировать риски срыва. Сайт narkolog-tula002.ru предоставляет широкий спектр услуг, включая анонимную наркологическую помощь, что даёт возможность пациентам получать помощь без ненужных вопросов. Профессиональный подход к лечению зависимостей на дому делает этот процесс доступнее и менее стрессовым для пациента и его семьи.
детская клиника больницы Черногории
becici medical clinic central hospital podgorica
The Real Person!
The Real Person!
нейронки для жизни Инструкция по искусственному интеллекту: полное руководство по использованию и пониманию ИИ.
The Real Person!
The Real Person!
готовые игровые компьютеры Сборка компьютеров на заказ – это индивидуальный подход к созданию машины, отвечающей вашим уникальным требованиям. От выбора комплектующих до финальной сборки, каждый этап контролируется для достижения максимальной производительности.
The Real Person!
The Real Person!
интернет провайдеры по адресу казань
domashij-internet-kazan005.ru
подключить интернет в квартиру казань
The Real Person!
The Real Person!
Алкогольная зависимость, это острая проблема, требующая квалифицированного вмешательства. Круглосуточная клиника предлагает помощь нарколога, обеспечивая очищение организма и лечение алкоголизма. Симптомы алкогольной зависимости могут варьироваться, от телесной зависимости до умственных расстройств; Клиническая detox-программа включает в себя детоксикацию и реабилитацию от алкоголя, что позволяет реабилитироваться после запоя. Консультация нарколога помогает определить уровень зависимости и выбрать наилучший метод лечения. Психотерапия при алкоголизме является ключевым этапом, предоставляя поддержку для зависимых. Тайное лечение алкоголизма гарантирует конфиденциальность. Нарколог в Туле всегда готов оказать помощь, обеспечивая индивидуальный подход к любому пациенту.}
The Real Person!
The Real Person!
Профилактика запоев после вывода – важная задача для поддержания здоровья и предотвращения рецидивов. Нарколог на дом в Туле предлагает услуги по лечению алкогольной зависимости и предотвращению запойного состояния. Ключевым элементом является психологическая поддержка, которая помогает справляться с трудностями.Советы нарколога включают методы борьбы с запойным состоянием, такие как формирование здорового образа жизни и семейная поддержка в процессе лечения. Реабилитация после запоя требует медицинского вмешательства при алкогольной зависимости и обращения к специалисту по алкоголизму. Обращение к наркологу на дом обеспечивает удобство и доступность терапии. нарколог на дом тула Важно помнить, что восстановление после запоя – это долгий путь, требующий времени, сил и терпения. Профилактика возврата к алкоголизму включает в себя регулярные встречи с наркологом и использование техник, способствующих поддержанию хорошего здоровья и отказу от алкоголя.
The Real Person!
The Real Person!
проверить интернет по адресу
domashij-internet-kazan006.ru
интернет по адресу
The Real Person!
The Real Person!
круглосуточный вывод из запоя
The Real Person!
The Real Person!
КАК ВЫБРАТЬСЯ ИЗ ЗАПОЯ: ПРИЗНАКИ И РЕШЕНИЯ Запой – это серьезная проблема, для решения которой нужна медицинская помощь. Лечение запоя в Туле и других городах включает в себя детоксикацию от алкоголя‚ которая является важным этапом на пути к восстановлению. Признаки запойного алкоголизма могут проявляться как тревожность, бессонница и физическая зависимость. Лечение запойного состояния должно быть комплексным. Стационарное лечение зависимости‚ предлагающее реабилитационные программы‚ обеспечивает безопасность пациента и психологическую поддержку при зависимости. Консультация с наркологом поможет разработать индивидуальный план лечения алкогольной зависимости, который будет включать медикаменты и психотерапию. лечение запоя тула Родственники алкозависимого человека играют важную роль в предотвращении рецидивов алкоголизма. Необходимо помнить о тяжелых последствиях длительного запоя, которые могут быть весьма серьезными. Восстановление после запоя требует времени и усилий‚ но с помощью профессионалов возможно вернуть человека к нормальной жизни.
The Real Person!
The Real Person!
подключение интернета по адресу
domashij-internet-krasnoyarsk004.ru
проверить провайдера по адресу
The Real Person!
The Real Person!
Altre novità Still, they have a real time-playing system to their Betika real time program enabling profiles in order to put bets within the genuine-time as the occurrences takes place. To gain access to these features, you could log on or indication-right up to own a Betika membership. It is very important strategy any kind away from gaming sensibly and you may strategically so that the finest total experience. Perena is a cutting-edge blockchain development platform designed to empower decentralized applications (dApps) with scalable and secure solutions. Built for Web3 innovators, Perena blockchain solutions offer seamless integration, high-performance smart contracts, and decentralized infrastructure. Whether you’re building DeFi protocols, NFT marketplaces, or enterprise blockchain applications, Perena provides the tools needed to scale with confidence. perena.tech
https://www.innovacionstereo.com/2025/07/03/penalty-shoot-out-di-evoplay-una-recensione-approfondita/
For example, they can be contacted through a hotline contact number, online chat, and email. The workers work around the clock and are also constantly ready to help with any difficulties. All of these 1win payment methods are usually suitable for producing deposits and withdrawals. When choosing a payment method, bear in mind that while using similar system for both is best. Also, remember the transaction limits, which count on the payment approaches. Il simbolo di Sugar di combattere il dominio è così potente che anche un combattente MMA ha assunto il soprannome di Sugar Rashad Evans, il suo team di agenti clienti altamente qualificati è sempre disponibile a rispondere alle richieste e ai reclami dei giocatori attraverso diversi punti di contatto come chat online ed e-mail. Lo slot ti permette di fare a malapena tutto e persino lanciarlo sul tuo dispositivo mobile, compresi. Nel corso degli anni, i grandi mille dollari gran premi di denaro sono disponibili.
The Real Person!
The Real Person!
Прокапка после запоя — это ключевая медицинская манипуляция, направленная на детоксикацию организма и восстановление здоровья человека. Во время обращения к наркологу для оказания помощи на дому проводится внутривенное введение препаратов для капельницы , что способствует очищению крови от токсичных веществ, накопленных в результате алкогольной зависимости . Нарколог на дом срочно В процессе обычно используются солевые растворы, витамины и минералы, что помогает улучшить общее состояние и облегчает симптомы похмелья . Уход на дому позволяет пациенту находиться в привычной обстановке , что играет важную роль в процессе реабилитации. Наркологическая помощь включает поддержку пациента , что значительно увеличивает вероятность успешного восстановления после алкогольной зависимости. Важно следовать советам по лечению и проводить прокапку регулярно для достижения наилучших результатов .
The Real Person!
The Real Person!
проверить провайдера по адресу
domashij-internet-krasnoyarsk005.ru
провайдеры интернета в красноярске по адресу
The Real Person!
The Real Person!
реабилитация от запоя в Туле
The Real Person!
The Real Person!
Услуги наркологии в Туле является важным аспектом в помощи людям с зависимостями. Клиники, такие как narkolog-tula007.ru, осуществляют эффективные методы лечения для людей, столкнувшихся с наркотической и алкогольной зависимостями. В процесс лечения зависимостей входит медицинскую помощь на стационарной основе и программы реабилитации, которые помогают пациентам вернуться к нормальной жизни.Тульская наркология предоставляет анонимное лечение, что является критически важным для многих зависимых. Здесь также доступна первичная консультация, где врачи оценивают уровень зависимости и разрабатывают индивидуальный план лечения. Поддержка семьи зависимого также является неотъемлемой частью процесса реабилитации. Медицинская помощь в Туле включает не только помощь при алкоголизме, но и меры по предотвращению зависимостей, что способствует предотвращению рецидивов. Каждому пациенту разрабатывается индивидуальная программа, что увеличивает шансы на успешное восстановление.
The Real Person!
The Real Person!
провайдер по адресу красноярск
domashij-internet-krasnoyarsk006.ru
какие провайдеры интернета есть по адресу красноярск
The Real Person!
The Real Person!
Как вылечить народными средствами Отзывы о целителях – важный источник информации при выборе специалиста. Важно обращать внимание на реальные отзывы и рекомендации других людей.
The Real Person!
The Real Person!
остекление балконов Остекление балконов: Преображение пространства с комфортом и стилем В современном мире, где каждый квадратный метр на счету, остекление балконов становится все более популярным решением для расширения жилой площади и создания уютного уголка. Это не просто защита от непогоды, а возможность превратить балкон в функциональное пространство, будь то кабинет, зимний сад или зона отдыха.
Виртуальные номера для Telegram https://basolinovoip.com создавайте аккаунты без SIM-карты. Регистрация за минуту, широкий выбор стран, удобная оплата. Идеально для анонимности, работы и продвижения.
The Real Person!
The Real Person!
Капельница для вывода из запоя в домашних условиях — это распространенный метод экстренного избавления от запойного состояния, особенно в Туле. Однако важно выбрать опытного нарколога, чтобы не стать жертвой мошенников. Симптомы зависимости от алкоголя могут быть очевидны, и поддержка специалиста неизбежна. экстренный вывод из запоя тула Обращаясь в наркологическую клинику, не забудьте проверить на наличие лицензий и отзывов. Квалифицированный специалист должен предложить безопасное лечение, включая инфузионную терапию водно-электролитного баланса. Осмотр и консультация опытного врача поможет определить степень зависимости и выбрать подходящий метод лечения. Помните о необходимости реабилитации — это важная часть борьбы с алкоголизмом. Заботьтесь о своем здоровье и обращайтесь только к проверенным специалистам!
Odjeca i aksesoari za hotele navlake za stolove po sistemu kljuc u ruke: uniforme za sobarice, recepcionere, SPA ogrtaci, papuce, peskiri. Isporuke direktno od proizvodaca, stampa logotipa, jedinstveni stil.
The Real Person!
The Real Person!
частный пансионат для пожилых людей
pansionat-msk001.ru
частный дом престарелых
The Real Person!
The Real Person!
Очищение солью от негатива Денежная магия – это область магии, направленная на привлечение денег и удачи в финансовых делах. Она включает в себя различные ритуалы, заговоры и практики.
The Real Person!
The Real Person!
провайдеры интернета в краснодаре по адресу
domashij-internet-krasnodar004.ru
провайдеры по адресу дома
The Real Person!
The Real Person!
Куда сходить в Санкт-Петербурге Колдовство – практика использования магических сил для достижения определенных целей. Колдовство может быть как светлым, так и темным, в зависимости от целей и используемых методов.
The Real Person!
The Real Person!
пансионат для лежачих после инсульта
pansionat-msk002.ru
пансионат для пожилых
The Real Person!
The Real Person!
Алкогольный запой, серьезное состояние‚ которое требует профессионального вмешательства; Ложное мнение, что можно самостоятельно выйти из запоя‚ как минимум, рискован и приводит к тяжелым последствиям. В Туле многие страдают от алкогольной зависимости, и услуги нарколога на дому играют важную роль в решении этой проблемы. Вызвать нарколога на дом в Туле Симптомы запойного состояния включают тремор, потливость, тревожность. Самостоятельный вывод может вызвать серьезные проблемы со здоровьем‚ включая психические расстройства. Помощь нарколога важна для грамотного выхода из запойного состояния и восстановления психического здоровья. Кроме того‚ реабилитация от алкоголя требует поддержки близких и квалифицированных специалистов. Вызвав нарколога на дом в Туле‚ вы сможете рассчитывать на профессиональную помощь‚ избежите опасностей самостоятельного вывода и начнете путь к выздоровлению. Не рискуйте своим здоровьем‚ обратитесь за помощью!
The Real Person!
The Real Person!
узнать провайдера по адресу краснодар
domashij-internet-krasnodar005.ru
интернет провайдеры краснодар по адресу
The Real Person!
The Real Person!
профильные трубы Профильные трубы: Сталь в геометрической гармонии В мире современной инженерии и строительства профильные трубы занимают особое место. Их универсальность, прочность и геометрическая точность делают их незаменимым элементом в самых разнообразных проектах. От каркасов зданий до мебельных конструкций, от сельскохозяйственной техники до рекламных щитов – профильные трубы находят применение там, где требуется надежность и долговечность.
The Real Person!
The Real Person!
пансионаты для инвалидов в москве
pansionat-msk003.ru
пансионат для пожилых в москве
The Real Person!
The Real Person!
подключение интернета по адресу
domashij-internet-krasnodar006.ru
проверить провайдеров по адресу краснодар
The Real Person!
The Real Person!
частные пансионаты для пожилых в москве
pansionat-msk001.ru
пансионаты для инвалидов в москве
The Real Person!
The Real Person!
пансионат для лежачих после инсульта
pansionat-tula001.ru
пансионаты для инвалидов в туле
The Real Person!
The Real Person!
hardware id spoofer HWID Changer: HWID Changer для Merge Dragons!
ролик суши барнаул суши барнаул каталог
Хирургические услуги экстренная хирургия: диагностика, операции, восстановление. Современная клиника, лицензированные специалисты, помощь туристам и резидентам.
The Real Person!
The Real Person!
В Москве состязание среди провайдеров интернета растет, и это обеспечивает отличные условия для пользователей. Лучшие провайдеры интернета в Москве представляют скоростной интернет и IPTV услуги, что позволяет выбор широким. При выборе провайдера стоит уделить внимание на тарифы на интернет и доступные пакеты услуг. Многие компании предлагают акционные предложения и скидки, что даёт возможность сэкономить на услугах интернета и телевидения. Анализ провайдеров поможет найти оптимальные варианты, учитывая интернет-скорость и дополнительные услуги, такие как кабельное телевидение. Отзывы о провайдерах также имеют большое значение в процессе выбора. Они могут послужить подсказкой, какой интернет-провайдер предлагает надежное соединение и хороший уровень сервиса. Важно учитывать не только цену, но и качество интернет-соединения, чтобы обеспечить комфортный просмотр контента. Таким образом, изучая все эти аспекты, можно определить наиболее подходящий пакет услуг, который соответствует вашим потребностям в интернете и телевидении в Москве.
The Real Person!
The Real Person!
пансионат для лежачих пожилых
pansionat-msk002.ru
пансионат для пожилых с инсультом
The Real Person!
The Real Person!
hwid changer HWID Spoofer: Законно ли это? Правовые аспекты использования спуферов
The Real Person!
The Real Person!
дом престарелых
pansionat-tula002.ru
пансионат для пожилых с инсультом
The Real Person!
The Real Person!
В Москве множество интернет-провайдеров представляют привлекательные предложения на домашний интернет. На сайте domashij-internet-msk005.ru вы сможете найти актуальные акции на интернет, которые дают возможность существенно сэкономить. Анализ тарифов поможет выбрать оптимальные пакеты интернет-услуг по доступным ценам. Многие провайдеры предоставляют эксклюзивные предложениявключая скидки на тарифы и интернет безлимитный. Подключение интернета стало проще благодаря широкому ассортименту акциям и promociones de internet, которые позволяют получить качественные услуги связи по доступной цене.
The Real Person!
The Real Person!
MetaTrader 4 – Colour Prediction win Even if you’ve forgotten your password, there’s an option to reset it by clicking on “Forgot Password” and following the instructions. Letting emotions control your trades, like fear or greed, often leads to mistakes. Staying disciplined is key to consistent wins.Why It Works: Emotional control keeps your strategy on track and prevents impulsive decisions. By staying calm, you follow your plan and improve your winning chances.Pro Tip: Take breaks between trades to reset your mindset. Set limits and stick to them, no matter how “lucky” you feel. Always aim to trade with strategy, not emotions. colour prediction app is the best and original Prediction App for Small League Colour Match Analysis, Expert Preview, Pitch Analysis etc.
https://www.klino.com.au/a-close-look-at-www-aviator-com-and-its-legitimacy/
Mon May 5, 2025 ▪︎ 8PMDead MeatGarcia’s at The Capitol Theatre The game is coordinated between Bitcoin and BGaming, one of the most renowned game providers in the casino games industry. Players can launch a space rocket in this game and travel to space. The game is entirely crypto-based, including the wins that come from the game. We handle your entire residential move including packing and transportation. JULY 14STREET DANCE Without a major surprise, Space XY performed fantastically on mobile devices, as any modern online casino game should! We played the game on various mobile devices, ranging from new and big (iPhone 13 Pro and Samsung Galaxy S22) and old and small (iPhone 6S). Ignacio G. López-Francos is a principal research engineer in the Intelligent Systems Division at NASA’s Ames Research Center through KBR. At Ames, he specializes in artificial intelligence and robotics autonomy for space missions. Follow him on X @ilopezfr
The Real Person!
The Real Person!
частный пансионат для престарелых
pansionat-msk003.ru
частный пансионат для пожилых людей
Магазин брендовых кроссовок https://kicksvibe.ru Nike, Adidas, New Balance, Puma и другие. 100% оригинал, новые коллекции, быстрая доставка, удобная оплата. Стильно, комфортно, доступно!
The Real Person!
The Real Person!
пансионат для лежачих после инсульта
pansionat-tula003.ru
пансионат для пожилых с инсультом
заказать доставку суши барнаул суши недорого барнаул
The Real Person!
The Real Person!
hwid spoofer HWID Changer: Смена HWID без переустановки Windows
The Real Person!
The Real Person!
вывод из запоя челябинск
https://vivod-iz-zapoya-chelyabinsk001.ru
экстренный вывод из запоя челябинск
The Real Person!
The Real Person!
пансионат инсульт реабилитация
pansionat-tula001.ru
пансионат после инсульта
The Real Person!
The Real Person!
недорогой интернет нижний новгород
domashij-internet-nizhnij-novgorod004.ru
интернет провайдеры по адресу дома
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-chelyabinsk002.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
пансионат после инсульта
pansionat-tula002.ru
частный пансионат для пожилых людей
The Real Person!
The Real Person!
интернет провайдеры по адресу
domashij-internet-nizhnij-novgorod005.ru
подключить интернет тарифы нижний новгород
buy amoxil cheap – comba moxi amoxil medication
The Real Person!
The Real Person!
лечение запоя челябинск
vivod-iz-zapoya-chelyabinsk003.ru
лечение запоя челябинск
The Real Person!
The Real Person!
Bigeni wants his casting to tell a story that people can connect with. This year, one of his most famous mates, Dessert Masters judge Melissa Leong, made her first ever modelling appearance in his show. Earth Squad, Go! – Science game In Tiranga Game you can play lottery games or color guess games to earn money. Also, you can through their referral program. Colour Trading, also known as Colour Prediction, is gaining immense popularity among players in India. This game offers users the chance to test their luck by betting on a specific colour. The gameplay is appealing due to its simplicity while offering a wide variety of strategic options. You can trust your intuition or try numerous strategies that can potentially increase your profits. In any case, the game promises to be engaging and thrilling.
https://miotta.ca/what-makes-teen-patti-gold-download-apk-a-smart-choice/uncategorized/
This crash game features a high 97% RTP, the opportunity for Canadian players to claim up to 10,000x their wagers, and a flexible bet range. Keep reading our extensive Space XY Crash Game review to determine if this game is worth playing. When searching for ways to gain an edge in online gaming, some players explore various methods like using a stake game hack to try and manipulate game outcomes or increase their chances of winning. However, it’s important to note that most online platforms, including Stake, have robust security measures to prevent such exploits, ensuring fair play for all users. Instead of relying on questionable hacks, players are encouraged to improve their skills and strategies to achieve better results in their gaming experience. To win in Space XY, as in other games in the series of crash games, you need to have time to close the bet before the end of the round. In this game, the main character is a rocket that goes into space to the distant stars but can explode at any time. To be safe, the client of the online casino needs to have time to close the bet on time and thus eject.
The Real Person!
The Real Person!
дом престарелых в туле
pansionat-tula003.ru
пансионат для пожилых с инсультом
The Real Person!
The Real Person!
подключить интернет в квартиру нижний новгород
domashij-internet-nizhnij-novgorod006.ru
проверить провайдеров по адресу нижний новгород
The Real Person!
The Real Person!
Онлайн казино России Лучшие разработчики игр для онлайн казино России
The Real Person!
The Real Person!
cours de theatre Ecole de cinema
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-cherepovec004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
Аналитика музыкальной индустрии Первый опыт в музыке: Первые инструменты, группы и выступления.
онлайн казино на деньги проверенные онлайн казино
The Real Person!
The Real Person!
Рейтинг онлайн казино России Рейтинг онлайн казино России по надежности
The Real Person!
The Real Person!
вывод из запоя цена
https://vivod-iz-zapoya-chelyabinsk001.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
Сеть в столице стал неотъемлемой частью жизни, и поиск оптимального провайдера играет ключевую роль в защите данных. Киберугрозы, такие как вредоносные программы и спам подчеркивают важность защиты личной информации. Антивирусные программы и спам-фильтры помогут сохранить вашу информацию в безопасности. Надежность провайдера также критична: услуги связи и скорость интернета в новосибирске должны гарантировать защиту данных. Важно применять фильтрацию контента и защиту от вредоносных программ для минимизации рисков. лучший интернет провайдер в новосибирске
The Real Person!
The Real Person!
https://stroidom36.ru/catalog/2et-doma/
The Real Person!
The Real Person!
jouer au cinema Ateliers de theatre
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-cherepovec005.ru
вывод из запоя череповец
The Real Person!
The Real Person!
работа военным Работа военным: защита Отечества Работа военным – это защита Отечества. Это священный долг каждого гражданина, готового встать на защиту своей Родины в трудную минуту. Военная служба – это не просто работа, это призвание, требующее мужества, отваги и самоотверженности. Военные – это люди, которые прошли специальную подготовку, владеют современным оружием и техникой, готовы выполнить любой приказ, защитить свою страну от внешних угроз. Они стоят на страже мира и спокойствия, обеспечивая безопасность граждан. Военная служба – это возможность получить уникальный опыт, развить свои физические и моральные качества, научиться преодолевать трудности и нести ответственность за свои действия. Военные – это сильные и уверенные в себе люди, способные принимать решения в сложных ситуациях. Армия – это школа жизни, которая воспитывает патриотизм, дисциплину и уважение к старшим. Военные – это пример для подражания, люди, которые своим примером вдохновляют молодежь на служение Родине. Государство заботится о своих защитниках, предоставляя им достойное денежное довольствие, жилье, медицинское обслуживание и другие социальные гарантии. Военная служба – это стабильность и уверенность в завтрашнем дне. Работа военным – это возможность внести свой вклад в развитие страны, в укрепление ее обороноспособности и международного авторитета. Военные участвуют в научных исследованиях, разрабатывают новые виды вооружения и техники, повышают боеготовность армии. Военная служба – это нелегкий труд, требующий постоянного совершенствования, физической и моральной подготовки. Но это также возможность почувствовать себя частью сильной и сплоченной команды, гордиться своей профессией и служением Родине. Работа военным – это выбор настоящих героев, готовых отдать свою жизнь за свободу и независимость своей страны.
The Real Person!
The Real Person!
работа военным Служба в армии – это школа жизни, которая закаляет характер, учит преодолевать трудности и ценить простые вещи.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-chelyabinsk002.ru
вывод из запоя круглосуточно челябинск
The Real Person!
The Real Person!
В новосибирске выбор интернет-провайдера играет ключевой ролью для обеспечения стабильного и качественного доступа в сеть. На сайте domashij-internet-novosibirsk005.ru можно найти обширное сравнение различных провайдеров, что помогает пользователям определиться с выбором. Необходимо обратить внимание на скорость интернета и наличие интернета в вашем районе. Оптоволоконный интернет обеспечивает высокую скорость и стабильность соединения, что имеет особое значение в условиях удаленной работы. Мнения пользователей о качествах связи и условиях тарифов провайдеров помогут понять какой вариант лучше подходит для ваших нужд. Не забывайте также проверять покрытие Wi-Fi и наличие мобильного интернета, чтобы быть на связи в любых условиях. Техническая поддержка провайдера, важный фактор, обеспечивающий быстрое решение проблем.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-cherepovec006.ru
вывод из запоя круглосуточно череповец
The Real Person!
The Real Person!
https://www.google.com/maps/place/Строй+Дом+36+-+строительство+домов+в+Воронеже,+ул.+Беговая,+225+Б,+оф.+8,+Воронеж,+Воронежская+обл.,+394016/@51.6958965,39.1314608,17z/data=!4m6!3m5!1s0x413b2edffe74b21f:0x24fae991ab80e36e!8m2!3d51.6958965!4d39.1314608!16s%2Fg%2F11ddzkkjnm?source=lnms&utm_campaign=ml-ardl&g_ep=Eg1tbF8yMDI1MDYzMF8wIJvbDyoASAJQAQ%3D%3D Выбор и установка входной двери: советы профессионалов
The Real Person!
The Real Person!
работа военным Работа военным сопряжена с риском и опасностью, но она также дает возможность проявить себя, реализовать свой потенциал и внести свой вклад в защиту мира и стабильности.
The Real Person!
The Real Person!
devenir acteur Academie des arts de la scene
The Real Person!
The Real Person!
В наше время интернет является неотъемлемой частью жизни‚ в т.ч. и в загородных домах. Когда вы ищете интернет для своего коттеджа в новосибирске‚ важно учитывать несколько факторов. Первым делом‚ определитесь с типом подключения: оптоволокно обеспечивает высокую скорость и стабильность‚ в то время как спутниковый интернет подходит для труднодоступных регионов. domashij-internet-novosibirsk006.ru Не забудьте обратить внимание на тарифные планы‚ включая тарифы без лимитов‚ что идеально подходит для интенсивного использования. Не забудьте ознакомиться с отзывами о провайдерах‚ чтобы выбрать надежного оператора. Услуги связи для коттеджей предлагают различные пакеты‚ включая Wi-Fi для дачи и мобильный интернет. Убедитесь‚ что интернет доступен в вашем районе и ознакомьтесь с ценами различных провайдеров в новосибирске. Верный выбор гарантирует комфортное интернет-соединение в вашем загородном доме!
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-chelyabinsk003.ru
вывод из запоя
The Real Person!
The Real Person!
вывод из запоя иркутск
vivod-iz-zapoya-irkutsk001.ru
вывод из запоя круглосуточно иркутск
The Real Person!
The Real Person!
работа военным Работа военным: долг и честь Работа военным – это долг и честь. Это осознанный выбор тех, кто готов посвятить свою жизнь защите Родины, ее суверенитета и территориальной целостности. Военная служба – это не просто профессия, это образ жизни, требующий самоотверженности, дисциплины и преданности. Военные – это люди, прошедшие суровую школу подготовки, способные действовать в самых сложных и опасных ситуациях. Они готовы рисковать своей жизнью ради защиты мирных граждан, ради сохранения спокойствия и стабильности в стране. Военная служба – это возможность получить ценные навыки и знания, которые пригодятся не только в армии, но и в гражданской жизни. Военные учатся работать в команде, принимать быстрые и взвешенные решения, преодолевать трудности и нести ответственность за свои действия. Армия – это кузница кадров, где формируются лидеры, способные вести за собой людей, принимать важные решения и добиваться поставленных целей. Военные – это элита общества, люди, которые пользуются уважением и доверием. Государство заботится о своих защитниках, предоставляя им социальные гарантии, достойное денежное довольствие, возможность получения образования и карьерного роста. Военная служба – это стабильность и уверенность в завтрашнем дне. Работа военным – это возможность внести свой вклад в развитие страны, в укрепление ее обороноспособности и международного авторитета. Военные участвуют в миротворческих операциях, оказывают помощь населению в случае стихийных бедствий и катастроф. Военная служба – это нелегкий труд, требующий самоотдачи и жертвенности. Но это также возможность почувствовать себя частью чего-то большего, причастным к великим делам и свершениям. Работа военным – это выбор настоящих патриотов, готовых посвятить свою жизнь служению Родине.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-cherepovec004.ru
лечение запоя
The Real Person!
The Real Person!
подключить домашний интернет в омске
domashij-internet-omsk004.ru
дешевый интернет омск
Modern operations outpatient surgery innovative technologies, precision and safety. Minimal risk, short recovery period. Plastic surgery, ophthalmology, dermatology, vascular procedures.
Профессиональное https://prp-expert.ru: PRP, Plasmolifting, протоколы и нюансы проведения процедур. Онлайн курс обучения плазмотерапии.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk002.ru
вывод из запоя
Онлайн-курсы обучение плазмотерапии: теория, видеоуроки, разбор техник. Обучение с нуля и для практикующих. Доступ к материалам 24/7, сертификат после прохождения, поддержка преподавателя.
The Real Person!
The Real Person!
экстренный вывод из запоя иркутск
vivod-iz-zapoya-irkutsk003.ru
лечение запоя иркутск
The Real Person!
The Real Person!
сессия с психологом Эмоциональное выгорание: Синдром, вызванный хроническим стрессом. Симптомы, причины и методы лечения.
The Real Person!
The Real Person!
Битва экстрасенсов все Целитель онлайн – удобная форма целительской помощи, доступная из любой точки мира.
The Real Person!
The Real Person!
домашний интернет тарифы омск
domashij-internet-omsk005.ru
домашний интернет в омске
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-cherepovec005.ru
экстренный вывод из запоя череповец
The Real Person!
The Real Person!
Подготовка к родам Форум беременных – сообщество, где можно получить поддержку и ответы на вопросы.
The Real Person!
The Real Person!
Эфирные масла для улучшения сна и расслабления Эфирные масла для улучшения сна и расслабления: Создайте атмосферу спокойствия и умиротворения с помощью эфирных масел, которые помогут вам заснуть и проснуться отдохнувшим.
The Real Person!
The Real Person!
Грузоперевозки Луганск Грузоперевозки Луганск: Грузоперевозки по области. Доставка грузов в любой населенный пункт Луганской области. Надежно, оперативно, выгодно.
The Real Person!
The Real Person!
вывод из запоя калуга
vivod-iz-zapoya-kaluga004.ru
вывод из запоя
The Real Person!
The Real Person!
тёмная ночь души Плазменное поле: Концепция энергетического поля, окружающего человека. Влияние поля на физическое и психическое здоровье.
cheap amoxil generic – https://combamoxi.com/ brand amoxicillin
The Real Person!
The Real Person!
подключить интернет
domashij-internet-omsk006.ru
провайдеры омск
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-cherepovec006.ru
вывод из запоя цена
The Real Person!
The Real Person!
Грузоперевозки Луганск Поиск транспорта Луганск: Поиск транспорта для перевозки груза.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-kaluga005.ru
вывод из запоя калуга
The Real Person!
The Real Person!
провайдеры интернета в перми
domashij-internet-perm004.ru
интернет домашний пермь
The Real Person!
The Real Person!
Как вернуть мужа Целитель – проводник энергии, помогающий восстановить гармонию тела и духа. Целители используют различные техники, от традиционных до современных, для запуска процессов самоисцеления.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-irkutsk001.ru
лечение запоя
The Real Person!
The Real Person!
психолог для женщин Консультация психолога открывает двери к пониманию себя и решению внутренних конфликтов.
The Real Person!
The Real Person!
Грузоперевозки Луганск Грузоперевозки Луганск: Доставка сборных грузов. Экономия на транспортировке, консолидация грузов на складе, доставка “от двери до двери”. Выгодное решение для малого бизнеса.
The Real Person!
The Real Person!
вывод из запоя круглосуточно калуга
vivod-iz-zapoya-kaluga006.ru
лечение запоя
The Real Person!
The Real Person!
недорогой интернет пермь
domashij-internet-perm005.ru
провайдеры интернета пермь
The Real Person!
The Real Person!
Рейки и эфирные масла: гармония тела и духа Эфирные масла для духовного роста и самосовершенствования: Эфирные масла ладана, сандалового дерева и мирры способствуют медитации, углублению осознанности и духовному росту.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-irkutsk002.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar001.ru
вывод из запоя
The Real Person!
The Real Person!
гайнулин эмиль нилович Секреты Telegram-маркетинга от Эмиля Гайнулина. Как привлекать подписчиков, создавать вовлекающий контент и монетизировать свой Telegram-канал.
the best and interesting https://hello-jobs.com
The Real Person!
The Real Person!
домашний интернет
domashij-internet-perm006.ru
лучший интернет провайдер пермь
interesting and new https://www.intercultural.org.au
The Real Person!
The Real Person!
Грузоперевозки Луганск Аренда грузового автомобиля Луганск: Выгодные условия аренды грузовых автомобилей для бизнеса и частных нужд.
where can i buy fluconazole – https://gpdifluca.com/ order fluconazole 200mg pills
best site online https://playplayfun.com
visit the site online https://www.saffireblue.ca
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar002.ru
вывод из запоя круглосуточно краснодар
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk003.ru
вывод из запоя цена
cost forcan – click where can i buy diflucan
The Real Person!
The Real Person!
дешевый интернет ростов
domashij-internet-rostov004.ru
подключить домашний интернет в ростове
The Real Person!
The Real Person!
обмен макросы на варфейс Преобрази свою игру в Warface с макросы варфейс! Забудь о промахах и сложной отдаче. Улучши точность и стань непобедимым на поле боя. Не упусти шанс стать лучшим игроком!
The Real Person!
The Real Person!
Avant d’encaisser, et s’est donc établie avec une réputation exceptionnelle. Ne pas avoir à attendre pour jouer votre prochaine main est un excellent moyen de rester concentré sur le jeu et d’éviter de jouer trop de mains de départ, Tampa Bay a prouvé qu’elle est très capable de remporter sa troisième Coupe Stanley en autant de saisons. Nous passons en revue les meilleurs sites et fournissons toutes les informations dont vous avez besoin sur les casinos légaux au Canada, Européenne. Les tactiques du monde sportif ne vous aideront pas du tout à parier sur Penalty Shoot Out Street. Il n’y a pas encore de commentaires ou d’évaluations ! Pour être le premier, veuillez Pour débloquer pleinement le bonus, vous devez placer des paris équivalents à 16 fois le montant du bonus sur des paris simples avec une cote de 1.90 ou plus. Cette offre ajoute une valeur significative à votre expérience de paris sportifs avec Parimatch Sports.
https://www.recsolog.com/uncategorized/test-du-jeu-penalty-shoot-out-devoplay-dans-les-casinos-en-ligne-francais/
JEU RESPONSABLE : Nous ne sommes pas responsables des pertes dues aux jeux de hasard dans les casinos ou aux paris sur les sites de paris liés à l’une de nos offres de bonus. Le joueur est responsable de la somme pour laquelle il est prêt et capable de jouer. Ne pariez pas ou ne pariez pas avec de l’argent que vous ne pouvez pas vous permettre de perdre. Ne poursuivez pas vos pertes. Les joueurs ont la responsabilité de vérifier les lois sur les jeux dans leur pays ou juridiction, et ils doivent le faire avant de jouer de l’argent dans n’importe quel site de jeux d’argent en ligne. “Nous avions hâte de travailler sur Penalty Shoot Out Street, car nous avons eu d’excellents retours sur la première version lancée. L’idée était claire : améliorer totalement le moteur du jeu, tout en conservant la fluidité sur mobile grâce à la technologie HTML5. Le défi a été de taille, car il a fallu intégrer beaucoup de points réactifs dans le but du gardien, tout en respectant les chances des joueurs et le tirage aléatoire défini par Evoplay. Nous sommes aujourd’hui complètement satisfaits du résultat. Toutes les équipes Evoplay sont récompensées par l’engouement déjà présent autour du Jeu du Penalty argent.”
The Real Person!
The Real Person!
завьялов илья поинт пей Как Илья Завьялов Построил Успешный Бизнес в Point Pay
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar003.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
Замена открывания с простого на сложное в Алматы В сервисном центре в Алматы можно заказать и приобрести все необходимые детали для ремонта окон, с последующей доставкой и профессиональным монтажом. Мы тщательно подбираем компоненты, оптимально подходящие к характеристикам ваших окон, обеспечивая надежность и долговечность восстановления.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-kaluga004.ru
лечение запоя калуга
The Real Person!
The Real Person!
домашний интернет
domashij-internet-rostov005.ru
провайдеры интернета ростов
visit the site https://puntera.com
go to the site https://ibecensino.org.br
The Real Person!
The Real Person!
шторы пятигорск кисловодское шоссе Карнизы для штор настенные двухрядные в Пятигорске: Надежность и Стиль Настенные двухрядные карнизы – это надежное и стильное решение для тяжелых штор. Они позволяют повесить как плотные портьеры, так и легкие тюли, создавая многослойный и элегантный образ.
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar004.ru
вывод из запоя
The Real Person!
The Real Person!
завьялов илья поинт пей PointPay под управлением Завьялова: Персонализация сервисов и предложений для каждого пользователя
Профессиональная наркологическая клиника. Лечение зависимостей, капельницы, вывод из запоя, реабилитация. Анонимно, круглосуточно, с поддержкой врачей и психологов.
Removing eraser clothes from images is an advanced tool for creative tasks. Neural networks, accurate generation, confidentiality. For legal and professional use only.
Aligning your entity setup with Briansclub.bz credit goals unlocks numerous advantages for both personal and business finances.
The Real Person!
The Real Person!
макрос для warface Хочешь побеждать в Warface? Используй макросы варфейс! Забудь о контроле отдачи, увеличь точность и стань настоящим профессионалом. Получи преимущество в игре прямо сейчас!
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-kaluga005.ru
вывод из запоя калуга
The Real Person!
The Real Person!
подключить домашний интернет в ростове
domashij-internet-rostov006.ru
подключить интернет тарифы ростов
The Real Person!
The Real Person!
смесители для душевой кабины екатеринбург шланг вытяжной лейки
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar005.ru
вывод из запоя
The Real Person!
The Real Person!
завьялов илья поинт пей PointPay под руководством Завьялова: Создание прозрачной и справедливой крипто-экосистемы
The Real Person!
The Real Person!
вывод из запоя калуга
vivod-iz-zapoya-kaluga006.ru
лечение запоя
The Real Person!
The Real Person!
интернет по адресу
domashij-internet-samara004.ru
подключение интернета по адресу
The Real Person!
The Real Person!
карниз купить пятигорск (Повтор) Купить Шторы в Пятигорске: Легкий Выбор
The Real Person!
The Real Person!
Срочная наркологическая помощь необходима в кризисных ситуацияхсвязанных с различными зависимостями. На сайте vivod-iz-zapoya-krasnoyarsk001.ru можно получить информацию о лечении зависимостей, включая помощь при передозировке и экстренную медицинскую помощь. Наркологическая служба предлагает процессы детоксикации, психологическую помощь при зависимостях и помощь наркозависимым. Консультации специалистов помогут определить программу реабилитации, направленную на предотвращение рецидивов и возвращение к здоровью. Профессиональная помощь в борьбе с наркотической зависимостью важна для успешного выздоровления.
The Real Person!
The Real Person!
макрос варфейс x7 Думаешь об обмен макросы на варфейс? Помни о рисках! Проверяй репутацию продавцов и будь осторожен с обменом аккаунтами. Лучше купить проверенный макрос.
Рефрижераторные перевозки http://fotochki.com/skolko-stoit-perevozka-refrizheratorom-razbor-cen-i-faktorov-formirovaniya-stoimosti/ по России и СНГ. Контроль температуры от -25°C до +25°C, современные машины, отслеживание груза.
The Real Person!
The Real Person!
провайдеры по адресу
domashij-internet-samara005.ru
провайдеры интернета по адресу самара
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar001.ru
лечение запоя краснодар
The Real Person!
The Real Person!
завьялов илья поинт пей Завьялов Илья: Вклад в Развитие Блокчейн-Технологий через Point Pay
The Real Person!
The Real Person!
Откапывание на дому в Красноярске – это востребованная услуга‚ особенно при проведении земельных работ‚ ремонтных и строительных работах. Компании‚ специализирующиеся на этом‚ предлагают услуги по копке на участке с использованием экскаватора на дому. Квалифицированные специалисты гарантируют высокое качество выполнения заказа‚ включая работу с грунтом и установку дренажных систем. Также важно учитывать благоустройство территории и ландшафтный дизайн. Обращаясь к частным услугам‚ вы можете рассчитывать на профессионализм и надежность. Для подробной информации можно посетить сайт vivod-iz-zapoya-krasnoyarsk002.ru.
The Real Person!
The Real Person!
At its start, the arc element takes in two parameters for the x-radius and y-radius. If needed, see s and how they behave. The final two parameters designate the x and y coordinates to end the stroke. Together, these four values define the basic structure of the arc. Once you load a game, you’ll see a rocket flying into space. It eventually explodes, and that’s when a game is over and everyone left on board loses. So, when you join the action, make sure you cash out before the rocket bursts into pieces. That’s the only way to win in the Space XY crash game. Space XY seems to have been set up to allow for higher win multipliers, to make the game more exciting. This is in stark contrast to the original crash game, Bustabit, which usually stays in the sub-3.00x range of multipliers. Space XY can go over 20x or even 50x often enough to make pursuing such a high multiplier a viable strategy.
https://corporatecoffee.devsaffinity.site/how-to-fix-lag-in-chicken-road-mobile-slot/
Massive Multiplayer Poker-Based Card Game for Smartphones The app is pretty intuitive, and it works fine in 3G and 4G connections. Players can choose the games they like and play without any wait time to join a table. Players can also play with an existing Teen Patti login account. One downside of this card game app is the amount of ads that keep popping up. Teen Patti Gold is a fun and exciting game that can be enjoyed by people of all ages. It is easy to learn and can be played with a minimum of two players. If you’re looking for a great way to pass the time, or if you’re interested in trying a new card game, Teen Patti Gold may just be the game for you. Teen Patti Diamond: 3 Patti is basically a teen patti card game, also known as Flash or Flush, that lets you play with others online. You don’t need to register and you can enter guest mode to start playing right away. It boasts virtually zero waiting time when you’re looking for a table to join.
The Real Person!
The Real Person!
накрутка пф яндекс карты Накрутка ПФ: Новым Сайтам – Особое Внимание Для новых сайтов накрутка ПФ может стать стартовым толчком, позволяющим быстрее заявить о себе в поисковой выдаче. Однако важно начинать с умеренных объемов и постепенно увеличивать активность, чтобы не вызвать подозрений у поисковых систем.
The Real Person!
The Real Person!
Электросчетчики с пультом Газовый счетчик с пленкой: Несанкционированное вмешательство и ответственность за него.
The Real Person!
The Real Person!
archivecore Archivecore: Архивкор – это увлечение прошлым, запечатленным в старых фотографиях, выцветших документах и винтажных журналах. Он создает атмосферу ушедших эпох, где каждая деталь хранит историю и тайны. Это эстетика, которая ценит подлинность и неповторимость.
The Real Person!
The Real Person!
мистические истории из жизни Слушать мистические истории – это отличный способ погрузиться в мир тайн и загадок, не отвлекаясь на визуальные образы. Аудиокниги и подкасты с мистическими рассказами позволяют нам наслаждаться любимым жанром во время прогулок, поездок и других занятий.
The Real Person!
The Real Person!
https://vavada-sign-in.online/ru Вход в Vavada через зеркало или официальный сайт. Стабильный sign-in даже при блокировках, мгновенный доступ к счету. Авторизуйтесь для получения эксклюзивных бонусов и участия в турнирах с призовым фондом до €500 000.
The Real Person!
The Real Person!
проверить интернет по адресу
domashij-internet-samara006.ru
интернет провайдеры по адресу
The Real Person!
The Real Person!
перевод с нотариальным заверением Перевод документов – необходимость в современном мире, где глобализация стирает границы. Бюро переводов предлагает качественные услуги по переводу документов различной тематики и сложности.
The Real Person!
The Real Person!
Алкоголизм — это серьезное заболевание, требующее незамедлительного вмешательства и профессиональной помощи. В Красноярске доступен вызов нарколога на дом, что позволяет получить необходимую медицинскую помощь при алкоголизме в уютной обстановке. Признаки алкоголизма включают регулярном потреблении алкоголя, потере контроля над количеством выпиваемого и возникновении признаков зависимости от алкоголя. вызов нарколога на дом Красноярск Борьба с алкоголизмом можно начать с определения степени зависимости, которую проведет квалифицированный нарколог. Консультация нарколога способствует выявить степень зависимости и разработать индивидуальный план лечения. Важно учитывать о роли поддержки семьи, которая является важным фактором в процессе восстановления. Последствия алкоголизма могут быть очень серьезными, включая проблемы со здоровьем и психикой. Наркологическая помощь в Красноярске предлагает как фармакотерапию, так и программы реабилитации, направленные на восстановление пациента. Не затягивайте с решением проблемы, обратитесь за помощью!
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar002.ru
вывод из запоя круглосуточно краснодар
Выбирайте казино пиастрикс казино с оплатой через Piastrix — это удобно, безопасно и быстро! Топ-игры, лицензия, круглосуточная поддержка.
Хотите купить контрактный двигатель ДВС с гарантией? Б большой выбор моторов из Японии, Европы и Кореи. Проверенные ДВС с небольшим пробегом. Подбор по VIN, доставка по РФ, помощь с установкой.
Ищете казино казино с СБП? У нас — мгновенные переводы, слоты от топ-провайдеров, живые дилеры и быстрые выплаты. Безопасность, анонимность и мобильный доступ!
Играйте в онлайн-покер https://droptopsite3.ru легальный с игроками со всего мира. МТТ, спины, VIP-программа, акции.
Хирurgija u Crnoj Gori divertikulitis savremena klinika, iskusni ljekari, evropski standardi. Planirane i hitne operacije, estetska i opsta hirurgija, udobnost i bezbjednost.
escitalopram 20mg over the counter – https://escitapro.com/# escitalopram 20mg oral
The Real Person!
The Real Person!
военная служба по контракту Контрактная служба: возможность получить престижное образование и повысить свой профессиональный уровень
The Real Person!
The Real Person!
Darknet Marketplace Bazaar Drugs Marketplace: A New Darknet Platform with Dual Access Bazaar Drugs Marketplace is a new darknet marketplace rapidly gaining popularity among users interested in purchasing pharmaceuticals. Trading is conducted via the Tor Network, ensuring a high level of privacy and data protection. However, what sets this platform apart is its dual access: it is available both through an onion domain and a standard clearnet website, making it more convenient and visible compared to competitors. The marketplace offers a wide range of pharmaceuticals, including amphetamines, ketamine, cannabis, as well as prescription drugs such as alprazolam and diazepam. This variety appeals to both beginners and experienced buyers. All transactions on the platform are carried out using cryptocurrency payments, ensuring anonymity and security. In summary, Bazaar represents a modern darknet marketplace that combines convenience, a broad product selection, and a high level of privacy, making it a notable player in the darknet economy.
The Real Person!
The Real Person!
Служба по контракту Служба по контракту: Участие в учениях, миротворческих операциях и гуманитарных миссиях за рубежом. Контракт – это возможность увидеть мир, получить уникальный опыт и проявить себя в различных ситуациях.
The Real Person!
The Real Person!
подключить интернет тарифы санкт-петербург
domashij-internet-spb004.ru
домашний интернет тарифы
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-minsk001.ru
экстренный вывод из запоя
order escitalopram 20mg online cheap – buy generic escitalopram for sale order lexapro 10mg online cheap
The website tnschoolsonline.in is an official portal by the Tamil Nadu School Education Department. It provides comprehensive information about schools across Tamil Nadu, including directories, enrollment data, infrastructure details, and staff records. It serves teachers, students, and administrators to monitor and improve school management and educational outcomes.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar003.ru
лечение запоя
The Real Person!
The Real Person!
военная служба по контракту Военная служба по контракту: Как она влияет на физическое и психическое здоровье?
Элитная недвижимость https://real-estate-rich.ru в России и за границей — квартиры, виллы, пентхаусы, дома. Где купить, как оформить, во что вложиться.
buy cenforce 50mg for sale – https://cenforcers.com/# buy cenforce 100mg online
The Real Person!
The Real Person!
Служба по контракту Военная служба по контракту: Твой шанс стать частью элиты Вооруженных Сил и защищать свою Родину!
The Real Person!
The Real Person!
подключить интернет в санкт-петербурге в квартире
domashij-internet-spb005.ru
провайдер по адресу санкт-петербург
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk002.ru
вывод из запоя
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar004.ru
лечение запоя
The Real Person!
The Real Person!
военная служба по контракту Военная служба по контракту: современные вызовы и требования, готовность к выполнению сложных задач
buy cenforce 100mg for sale – cenforce price purchase cenforce
The Real Person!
The Real Person!
русские мистические Мистические детективы – это жанр, сочетающий в себе элементы детектива и мистики, где расследования ведут к раскрытию тайн и загадок, связанных с потусторонним миром.
The Real Person!
The Real Person!
Clearnet & Onion Bazaar Drugs Marketplace: A New Darknet Platform with Dual Access Bazaar Drugs Marketplace is a new darknet marketplace rapidly gaining popularity among users interested in purchasing pharmaceuticals. Trading is conducted via the Tor Network, ensuring a high level of privacy and data protection. However, what sets this platform apart is its dual access: it is available both through an onion domain and a standard clearnet website, making it more convenient and visible compared to competitors. The marketplace offers a wide range of pharmaceuticals, including amphetamines, ketamine, cannabis, as well as prescription drugs such as alprazolam and diazepam. This variety appeals to both beginners and experienced buyers. All transactions on the platform are carried out using cryptocurrency payments, ensuring anonymity and security. In summary, Bazaar represents a modern darknet marketplace that combines convenience, a broad product selection, and a high level of privacy, making it a notable player in the darknet economy.
The Real Person!
The Real Person!
домашний интернет тарифы
domashij-internet-spb006.ru
интернет домашний санкт-петербург
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-minsk003.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя краснодар
vivod-iz-zapoya-krasnodar005.ru
вывод из запоя круглосуточно краснодар
The Real Person!
The Real Person!
военная служба по контракту Военнослужащие-контрактники: пример для подражания и гордость нации
The Real Person!
The Real Person!
Служба по контракту Служба по контракту: Командный дух и взаимовыручка – основа успешной службы, где каждый готов прийти на помощь товарищу.
Выбор застройщика https://spartak-realty.ru важный шаг при покупке квартиры. Расскажем, как проверить репутацию, сроки сдачи, проектную документацию и избежать проблем с новостройкой.
Смотреть фильмы kinobadi.mom и сериалы бесплатно, самый большой выбор фильмов и сериалов , многофункциональное сортировка, также у нас есть скачивание в mp4 формате
Недвижимость в Балашихе https://balashihabest.ru комфорт рядом с Москвой. Современные жилые комплексы, школы, парки, транспорт. Объекты в наличии, консультации, юридическое сопровождение сделки.
Поставка нерудных материалов https://sr-sb.ru песок, щебень, гравий, отсев. Прямые поставки на стройплощадки, карьерный материал, доставка самосвалами.
Лайфхаки для ремонта https://stroibud.ru квартиры и дома: нестандартные решения, экономия бюджета, удобные инструменты.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk001.ru
вывод из запоя
The Real Person!
The Real Person!
провайдеры по адресу
domashij-internet-ufa004.ru
провайдер по адресу уфа
The Real Person!
The Real Person!
Анонимный вызов нарколога в Красноярске — это необходимый шаг на пути к освобождению от зависимости. Ресурс vivod-iz-zapoya-krasnoyarsk001.ru предлагает наркологическую помощь, включая консультацию нарколога и анонимное лечение; Организация обеспечивает всестороннюю поддержку зависимым и их близким, предоставляя квалифицированную медицинскую помощь в Красноярске. Лечение зависимости требует профессионального подхода. Услуги анонимного нарколога помогут вам обрести необходимую психологическую поддержку и реабилитацию наркоманов. Кризисная служба готова принять ваш звонок на горячую линию помощи наркологов, обеспечивая срочную и качественную помощь. Профилактика зависимостей также важна. Не упустите шанс позвонить за помощью и начать новую жизнь. Помните, что лечение алкоголизма и наркомании возможно, главное — сделать первый шаг и обратится за поддержкой.
Женский журнал https://e-times.com.ua о красоте, моде, отношениях, здоровье и саморазвитии. Советы, тренды, рецепты, вдохновение на каждый день. Будь в курсе самого интересного!
Туристический портал https://atrium.if.ua всё для путешественников: путеводители, маршруты, советы, отели, билеты и отзывы. Откройте для себя новые направления с полезной информацией и лайфхаками.
Женский онлайн-журнал https://socvirus.com.ua мода, макияж, карьера, семья, тренды. Полезные статьи, интервью, обзоры и вдохновляющий контент для настоящих женщин.
Портал про ремонт https://prezent-house.com.ua полезные советы, инструкции, дизайн-идеи и лайфхаки. От черновой отделки до декора. Всё о ремонте квартир, домов и офисов — просто, понятно и по делу.
The Real Person!
The Real Person!
Jogue na plataforma nova pagando fortune tiger e lucre agora!
Всё о ремонте https://sevgr.org.ua на одном портале: полезные статьи, видеоуроки, проекты, ошибки и решения. Интерьерные идеи, советы мастеров, выбор стройматериалов.
The Real Person!
The Real Person!
Ao fazer o download do Google Play Games, você concorda com os Termos de Serviço do Google e do Google Play e reconhece que suas informações vão ser tratadas de acordo com a Política de Privacidade do Google. Ao fazer o download do Google Play Games, você concorda com os Termos de Serviço do Google e do Google Play e reconhece que suas informações vão ser tratadas de acordo com a Política de Privacidade do Google. Confira nosso artigo da Central de Ajuda para saber como instalar e configurar o Google Play Games Beta no PC. Ao fazer o download do Google Play Games, você concorda com os Termos de Serviço do Google e do Google Play e reconhece que suas informações vão ser tratadas de acordo com a Política de Privacidade do Google. O Google Play Games é um aplicativo local do Windows e não ocupa espaço adicional em outros dispositivos, incluindo smartphones.
https://maiitravels.branexitltd.com/plataforma-nova-pagando-aviator-sera-que-e-confiavel-mesmo/
Tiroteio fecha Túnel da Conceição, em Porto Alegre O Spaceman Nielsen é uma ferramenta de gestão de categorias com foco na criação de layouts de prateleiras. Apesar de ser uma solução tradicional, oferece recursos como geração semi-automática de planogramas e sincronização com plantas de lojas. A SeguroBet é uma casa de apostas autorizada a operar no Brasil pelo Ministério da Fazenda, seguindo as normas da nova Lei n° 14.790 2023. Entretanto, não conseguimos aproveitar a funcionalidade. Ao colocar um jogo no modo miniatura, não foi possível manipular o software, apenas alterar o tamanho e posição do slot na tela. O sucesso do Spaceman está na sua simplicidade combinada com a emoção constante. Diferente de jogos complexos com muitas regras, aqui você só precisa de um clique para participar. Além disso, a interação em tempo real e a possibilidade de ganhos altos atraem tanto novatos quanto veteranos dos cassinos online. No Brasil, onde os jogos de azar online estão em alta, o Spaceman se destaca como uma opção divertida e lucrativa.
The Real Person!
The Real Person!
вывод из запоя омск
vivod-iz-zapoya-omsk002.ru
экстренный вывод из запоя
Бюро дизайна https://sinega.com.ua интерьеров: функциональность, стиль и комфорт в каждой детали. Предлагаем современные решения, индивидуальный подход и поддержку на всех этапах проекта.
Портал про ремонт https://techproduct.com.ua для тех, кто строит, переделывает и обустраивает. Рекомендации, калькуляторы, фото до и после, инструкции по всем этапам ремонта.
The Real Person!
The Real Person!
проверить интернет по адресу
domashij-internet-ufa005.ru
провайдер по адресу уфа
Портал о строительстве https://bms-soft.com.ua от фундамента до кровли. Технологии, лайфхаки, выбор инструментов и материалов. Честные обзоры, проекты, сметы, помощь в выборе подрядчиков.
Всё о строительстве https://kinoranok.org.ua на одном портале: строительные технологии, интерьер, отделка, ландшафт. Советы экспертов, фото до и после, инструкции и реальные кейсы.
Ремонт и строительство https://mtbo.org.ua всё в одном месте. Сайт с советами, схемами, расчетами, обзорами и фотоидееями. Дом, дача, квартира — строй легко, качественно и с умом.
The Real Person!
The Real Person!
Заказ нарколога на дому – это оптимальное решение для людей, которые нуждаются в профессиональной помощи в борьбе с зависимостями. Сайт vivod-iz-zapoya-krasnoyarsk002.ru предлагает услуги профессиональных специалистов, которые готовы оказать медицинскую и эмоциональную поддержку. Нарколог на дом проведет диагностику зависимостей, гарантирует анонимное лечение и назначит медикаментозную терапию. Консультация нарколога может предполагать психотерапию в процессе лечения зависимости, что способствует более быстрому восстановлению после курса лечения. Также необходима поддержка для близких, чтобы оказать помощь им справиться с возникшими трудностями. Реабилитация на дому позволяет комфортно пройти лечение алкоголизма и других зависимостей, не покидая привычной обстановки. Квалифицированная помощь ждет каждого, кто готов сделать шаг к новой жизни без зависимости.
Сайт о ремонте https://sota-servis.com.ua и строительстве: от черновых работ до декора. Технологии, материалы, пошаговые инструкции и проекты.
Онлайн-журнал https://elektrod.com.ua о строительстве: технологии, законодательство, цены, инструменты, идеи. Для строителей, архитекторов, дизайнеров и владельцев недвижимости.
Полезный сайт https://quickstudio.com.ua о ремонте и строительстве: пошаговые гиды, проекты домов, выбор материалов, расчёты и лайфхаки. Для начинающих и профессионалов.
Журнал о строительстве https://tfsm.com.ua свежие новости отрасли, обзоры технологий, советы мастеров, тренды в архитектуре и дизайне.
Женский сайт https://7krasotok.com о моде, красоте, здоровье, отношениях и саморазвитии. Полезные советы, тренды, рецепты, лайфхаки и вдохновение для современных женщин.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk003.ru
вывод из запоя цена
The Real Person!
The Real Person!
Teen Patti Gold is a popular Android game app to play the classic version of Indian poker. This application offers a refreshing take on the traditional card game, and lets you play with real players on the internet around the world. Moreover, the game is available in multiple Indian languages, including Hindi, Marathi, Gujarati, etc. See more versions… Disclaimer: Android is a trademark of Google Inc. We ONLY share free apps, we NEVER share paid or modified apps. To report copyrighted content, please contact us. Disclaimer: Android is a trademark of Google Inc. We ONLY share free apps, we NEVER share paid or modified apps. To report copyrighted content, please contact us. Teena – Liked and Shared, Real pretty! Download the latest Windows 10 ISO on your comp… There is a newer version of Teen Patti Gold available. Get the latest apk file now:
https://dtsfinance.com.au/2025/07/09/dragon-tiger-by-tadagaming-a-fresh-look-at-the-classic-online-casino-game/
Create Chicken Road login to unlock bonus offers, play for real money and get fair, big wins. In this game it is absolutely real thanks to high RTP and unique game mechanics. A coefficient is indicated on each lane, which will be the multiplier of your bet. Thus, the amount of your profit will be calculated by multiplying the amount of your bet by the coefficient of the lane where the chick stopped. Let’s also hope for Chicken Cross Free Bets and a Chicken Cross Mobile. 4601 Northfield Road, North Randall, OH 44128 Chicken Road is an exciting crash game by InOut, released on 4 April 2024. In this game, players control a chicken that is trying to reach a golden egg. Players can choose from four difficulty levels, each offering unique challenges. The higher the difficulty, the greater the risk, but the winning potential is also higher.
The Real Person!
The Real Person!
проверить интернет по адресу
domashij-internet-ufa006.ru
провайдеры интернета по адресу уфа
Женские новости https://biglib.com.ua каждый день: мода, красота, здоровье, отношения, семья, карьера. Актуальные темы, советы экспертов и вдохновение для современной женщины.
Все главные женские https://pic.lg.ua новости в одном месте! Мировые и российские тренды, стиль жизни, психологические советы, звёзды, рецепты и лайфхаки.
Сайт для женщин https://angela.org.ua любого возраста — статьи о жизни, любви, стиле, здоровье и успехе. Полезно, искренне и с заботой.
Женский онлайн-журнал https://bestwoman.kyiv.ua для тех, кто ценит себя. Мода, уход, питание, мотивация и женская энергия в каждой статье.
Путеводитель по Греции https://cpcfpu.org.ua города, курорты, пляжи, достопримечательности и кухня. Советы туристам, маршруты, лайфхаки и лучшие места для отдыха.
The Real Person!
The Real Person!
Капельница от запоя – это проверенный метод лечения алкогольной зависимости‚ который обеспечивает возможность комфортного и конфиденциального лечения. Нарколог на дом анонимно обеспечит оперативную медицинскую помощь при запое‚ используя метод капельницы для очищения организма. Ключевые достоинства капельницы заключаются в том‚ что она позволяет быстро устранить симптомы запоя‚ восстанавливает баланс жидкости и электролитов и способствует улучшению общего состояния пациента. Это особенно важно в случае серьезных проявлений алкогольной зависимости‚ когда необходима немедленная помощь.Лечение алкоголизма включает разные методы‚ но капельница выделяется своей эффективностью и скоростью. В отличие от таблеток или инъекций‚ капельница гарантирует непрерывное введение медикаментов в организм‚ что способствует более быстрому восстановлению.Поддержка семьи и психотерапия при алкоголизме также играют важную роль в процессе реабилитации. По завершении этапа детоксикации важно продолжать лечение и работать над предотвращением рецидивов‚ что возможно только при комплексном подходе. Таким образом‚ капельница при запое — это ключевой элемент в борьбе с алкоголизмом‚ предоставляя пациентам эффективное и конфиденциальное лечение с быстрым восстановлением.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-orenburg001.ru
лечение запоя
The Real Person!
The Real Person!
подключить интернет
domashij-internet-volgograd004.ru
подключить интернет в квартиру омск
The Real Person!
The Real Person!
экстренный вывод из запоя минск
vivod-iz-zapoya-minsk001.ru
вывод из запоя круглосуточно минск
The Real Person!
The Real Person!
экстренный вывод из запоя оренбург
vivod-iz-zapoya-orenburg002.ru
вывод из запоя круглосуточно оренбург
The Real Person!
The Real Person!
провайдеры интернета омск
domashij-internet-volgograd005.ru
провайдеры интернета омск
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-minsk002.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-orenburg003.ru
вывод из запоя
The Real Person!
The Real Person!
подключить интернет тарифы омск
domashij-internet-volgograd006.ru
интернет провайдер омск
Портал о строительстве https://ateku.org.ua и ремонте: от фундамента до крыши. Пошаговые инструкции, лайфхаки, подбор материалов, идеи для интерьера.
Строительный портал https://avian.org.ua для профессионалов и новичков: проекты домов, выбор материалов, технологии, нормы и инструкции.
Туристический портал https://deluxtour.com.ua всё для путешествий: маршруты, путеводители, советы, бронирование отелей и билетов. Информация о странах, визах, отдыхе и достопримечательностях.
Открой мир https://hotel-atlantika.com.ua с нашим туристическим порталом! Подбор маршрутов, советы по странам, погода, валюта, безопасность, оформление виз.
Ваш онлайн-гид https://inhotel.com.ua в мире путешествий — туристический портал с проверенной информацией. Куда поехать, что посмотреть, где остановиться.
cialis professional – https://ciltadgn.com/# what is the difference between cialis and tadalafil?
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-minsk003.ru
вывод из запоя цена
The Real Person!
The Real Person!
кайт школа хургада Кайтсерфинг и социальные сети: Делитесь своим опытом
overnight cialis delivery – site cialis coupon 2019
The Real Person!
The Real Person!
аренда инструментов Первомайский р-он, с. Cанниково, Лосихин остров, ул. Зеленая, д. 6 – удобное месторасположение и оперативная доставка оборудования на объект.
The Real Person!
The Real Person!
Химчистка мебели ростов Химчистка замшевой мебели в Ростове – профессиональный уход за замшей.
The Real Person!
The Real Person!
интернет провайдер воронеж
domashij-internet-voronezh004.ru
провайдеры интернета воронеж
Строительный сайт https://diasoft.kiev.ua всё о строительстве и ремонте: пошаговые инструкции, выбор материалов, технологии, дизайн и обустройство.
Журнал о строительстве https://kennan.kiev.ua новости отрасли, технологии, советы, идеи и решения для дома, дачи и бизнеса. Фото-проекты, сметы, лайфхаки, рекомендации специалистов.
Сайт о строительстве https://domtut.com.ua и ремонте: практичные советы, инструкции, материалы, идеи для дома и дачи.
На строительном сайте https://eeu-a.kiev.ua вы найдёте всё: от выбора кирпича до дизайна спальни. Актуальная информация, фото-примеры, обзоры инструментов, консультации специалистов.
Строительный журнал https://inter-biz.com.ua актуальные статьи о стройке и ремонте, обзоры материалов и технологий, интервью с экспертами, проекты домов и советы мастеров.
The Real Person!
The Real Person!
кайт хургада Кайтсерфинг и развитие: Планы на будущее
The Real Person!
The Real Person!
приемка квартир от застройщика Аренда строительных инструментов и оборудования за 5 минут и без залога! – быстрый доступ к необходимому инструменту.
The Real Person!
The Real Person!
где продать лекарства с рук в спб Где можно продать лекарственные препараты? Важно помнить, что продажа лекарственных препаратов без лицензии запрещена и может повлечь за собой юридическую ответственность.
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk005.ru
вывод из запоя круглосуточно смоленск
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk001.ru
вывод из запоя круглосуточно омск
Сайт о ремонте https://mia.km.ua и строительстве — полезные советы, инструкции, идеи, выбор материалов, технологии и дизайн интерьеров.
Сайт о ремонте https://rusproekt.org и строительстве: пошаговые инструкции, советы экспертов, обзор инструментов, интерьерные решения.
Всё для ремонта https://zip.org.ua и строительства — в одном месте! Сайт с понятными инструкциями, подборками товаров, лайфхаками и планировками.
Полезный сайт для ремонта https://rvps.kiev.ua и строительства: от черновых работ до отделки и декора. Всё о планировке, инженерных системах, выборе подрядчика и обустройстве жилья.
Автомобильный портал https://just-forum.com всё об авто: новости, тест-драйвы, обзоры, советы по ремонту, покупка и продажа машин, сравнение моделей.
The Real Person!
The Real Person!
подключить интернет в воронеже в квартире
domashij-internet-voronezh005.ru
подключить интернет воронеж
The Real Person!
The Real Person!
кайт египет Дети ветра кайт школа Хургада: Открой для себя мир кайтсерфинга
Современный женский журнал https://superwoman.kyiv.ua стиль, успех, любовь, уют. Новости, идеи, лайфхаки и мотивация для тех, кто ценит себя и своё время.
Онлайн-портал https://spkokna.com.ua для современных родителей: беременность, роды, уход за малышами, школьные вопросы, советы педагогов и врачей.
Онлайн-журнал https://eternaltown.com.ua для женщин: будьте в курсе модных новинок, секретов красоты, рецептов и психологии.
Сайт для женщин https://ww2planes.com.ua идеи для красоты, здоровья, быта и отдыха. Тренды, рецепты, уход за собой, отношения и стиль.
Сайт для женщин https://womanfashion.com.ua которые ценят себя и своё время. Мода, косметика, вдохновение, мотивация, здоровье и гармония.
The Real Person!
The Real Person!
приемка квартир от застройщика Мощные и надежные инструменты для выполнения различных задач.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-smolensk006.ru
экстренный вывод из запоя смоленск
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk002.ru
вывод из запоя
Женский онлайн-журнал https://abuki.info мода, красота, здоровье, психология, отношения и вдохновение. Полезные статьи, советы экспертов и темы, которые волнуют современных женщин.
Современный авто портал https://simpsonsua.com.ua автомобили всех марок, тест-драйвы, лайфхаки, ТО, советы по покупке и продаже. Для тех, кто водит, ремонтирует и просто любит машины.
Актуальные новости https://uapress.kyiv.ua на одном портале: события России и мира, интервью, обзоры, репортажи. Объективно, оперативно, профессионально. Будьте в курсе главного!
Онлайн авто портал https://sedan.kyiv.ua для автолюбителей и профессионалов. Новинки автоиндустрии, цены, характеристики, рейтинги, покупка и продажа автомобилей, автофорум.
Информационный портал https://mediateam.com.ua актуальные новости, аналитика, статьи, интервью и обзоры. Всё самое важное из мира политики, экономики, технологий, культуры и общества.
The Real Person!
The Real Person!
интернет провайдеры воронеж
domashij-internet-voronezh006.ru
подключение интернета воронеж
The Real Person!
The Real Person!
Клиники предлагают восстановление зависимых, которая включает терапию зависимости и консультации психолога. Процедура кодирования также выступает популярной методом, помогающей снизить тягу к алкоголю. Круглосуточные клиники обеспечивают анонимное лечение и помощь специалистов, что позволяет пациентам ощущать комфортно и безопасно. вывод из запоя круглосуточно Социальная адаптация и поддержка после лечения — важные аспекты, способствующие успешной реинтеграции в общество. Лечение алкоголизма в Туле становится доступным благодаря различным программам и методикам, которые помогают справиться с этой серьезной проблемы.
The Real Person!
The Real Person!
приемка домов Первомайский р-он, с. Cанниково, Лосихин остров, ул. Зеленая, д. 6 – удобное расположение и быстрая доставка.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk003.ru
вывод из запоя омск
The Real Person!
The Real Person!
продать лекарства ростов на дону Продам лекарственные препараты в Москве и других городах России: Что нужно знать? Продажа лекарственных препаратов в России регулируется Федеральным законом “Об обращении лекарственных средств”. Несоблюдение требований закона может повлечь за собой административную и уголовную ответственность. Законные способы продажи: Лицензированные аптеки: Только юридические лица и индивидуальные предприниматели, имеющие лицензию на фармацевтическую деятельность, могут продавать лекарственные препараты. Интернет-аптеки: Продажа лекарств через интернет разрешена только аптекам, имеющим лицензию и соблюдающим требования к доставке и хранению. Незаконные способы продажи: Продажа с рук: Продажа лекарственных препаратов с рук, через объявления или социальные сети является незаконной. Перепродажа рецептурных препаратов: Продажа рецептурных препаратов без рецепта запрещена законом. Риски незаконной продажи: Административная и уголовная ответственность: Нарушение правил продажи лекарственных препаратов может повлечь за собой штрафы, конфискацию товара и даже лишение свободы. Нанесение вреда здоровью: Продажа некачественных или поддельных лекарственных препаратов может нанести серьезный вред здоровью покупателей. Куда обращаться с остатками лекарств: Утилизация: Если у вас остались неиспользованные лекарства, их необходимо правильно утилизировать. Обратитесь в аптеку или медицинское учреждение для получения информации о правилах утилизации.
The Real Person!
The Real Person!
подключение интернета челябинск
domashij-internet-chelyabinsk004.ru
подключить интернет тарифы челябинск
The Real Person!
The Real Person!
Прокат инструментов Тепловизионная диагностика – эффективный способ выявить проблемы с теплоизоляцией, найдите слабые места!
The Real Person!
The Real Person!
Если вам или вашим близким требуется помощь при алкоголизме‚ важно знать‚ как обратиться к нарколога на дом в Туле. Вызов врача нарколога на дом позволяет получить экстренную помощь без нужды в посещении клиники. Это особенно актуально при лечении запоя‚ когда человек нуждается в медицинской помощи немедленно. врач нарколог на дом Первым шагом будет находка услуг нарколога. Можно обратиться в специализированные клиники или воспользоваться онлайн-сервисами для вызова нарколога. Консультация нарколога поможет оценить состояние пациента и определить необходимое лечение зависимости.При вызове врача необходимо сообщить о симптомах запоя‚ чтобы нарколог мог подготовиться к оказанию помощи. Лечение алкоголизма на дому может включать детоксикацию‚ медикаментозное лечение и психологическую поддержку. Конфиденциальная помощь нарколога также доступна‚ что делает процесс более простым для пациента.В Туле есть различные адреса наркологических клиник‚ где можно получить дополнительные услуги и реабилитацию от алкоголя. Не забывайте‚ что медицинская помощь на дому может стать началом к восстановлению и улучшению качества жизни. Если вам требуется экстренная помощь нарколога‚ не теряйте время – здоровье важнее всего!}
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-orenburg001.ru
вывод из запоя цена
Новости Украины https://pto-kyiv.com.ua и мира сегодня: ключевые события, мнения экспертов, обзоры, происшествия, экономика, политика.
Современный мужской портал https://kompanion.com.ua полезный контент на каждый день. Новости, обзоры, мужской стиль, здоровье, авто, деньги, отношения и лайфхаки без воды.
Сайт для женщин https://storinka.com.ua всё о моде, красоте, здоровье, психологии, семье и саморазвитии. Полезные советы, вдохновляющие статьи и тренды для гармоничной жизни.
Новостной портал https://thingshistory.com для тех, кто хочет знать больше. Свежие публикации, горячие темы, авторские колонки, рейтинги и хроники. Удобный формат, только факты.
Следите за событиями https://kiev-pravda.kiev.ua дня на новостном портале: лента новостей, обзоры, прогнозы, мнения. Всё, что важно знать сегодня — быстро, чётко, объективно.
The Real Person!
The Real Person!
подключить домашний интернет в челябинске
domashij-internet-chelyabinsk005.ru
домашний интернет тарифы челябинск
The Real Person!
The Real Person!
Лечение алкогольной зависимости с помощью капельниц на дому – это проверенный способ помощи алкогольной зависимости, который предоставляет возможность пройти детоксикацию без нужды госпитализации. Помощь нарколога предлагают инъекции на дому, что позволяет повышению качества жизни и снижению симптомов абстиненции. Внутривенные инъекции содействуют организму быстрее справляться с токсинами, а также способствуют восстановлению здоровья. Поддержка при отказе от алкоголя имеет большое значение на разных стадиях, включая процесс реабилитации. Предотвращение осложнений также играет ключевую роль в процессе восстановления. Лечение на дому может состоять не только из капельниц, но и консультации специалистов, что способствует более качественному выводу из запоя и успешному лечению. Посетив vivod-iz-zapoya-tula006.ru, вы сможете получить профессиональную поддержку и помощь в борьбе с алкоголизмом.
заказать кейтеринг кофе-брейк на мероприятие
спираль мирена купить спираль мирена цена
The Real Person!
The Real Person!
вывод из запоя круглосуточно оренбург
vivod-iz-zapoya-orenburg002.ru
лечение запоя
Video chat with girl – meet, chat, flirt! Private broadcasts, thousands of users online. No limits, free and no registration. Start a dialogue right now.
Эффективная накрутка ПФ https://nakrutka-pf-seo.ru повышение поведенческих метрик, улучшение ранжирования, увеличение органического трафика. Безопасно, анонимно, с гарантией результата.
ремонт замка стиральной машины ремонт стиральных машин hotpoint
ремонт стиральных машин миле ремонт стиральных машин индезит
ремонт бака стиральной машины мили ремонт стиральных машин миле
The Real Person!
The Real Person!
купить винтовые сваи Купить винтовые сваи: надежный фундамент вашего будущего Винтовые сваи – это современное и экономичное решение для строительства фундамента. Они идеально подходят для различных типов грунтов и позволяют возводить здания даже на сложных участках. Забудьте о долгих и трудоемких земляных работах! Винтовые сваи устанавливаются быстро и легко, обеспечивая надежную опору для вашего дома, бани или хозяйственной постройки.
The Real Person!
The Real Person!
интернет домашний челябинск
domashij-internet-chelyabinsk006.ru
провайдеры интернета челябинск
The Real Person!
The Real Person!
накрутка пф яндекс Накрутка: обман или стратегия? Существуют различные способы накрутки ПФ, от использования ботов до привлечения “живых” пользователей для выполнения определенных действий. Однако, Яндекс постоянно совершенствует свои алгоритмы и успешно выявляет накрутку.
The Real Person!
The Real Person!
Химчистка мебели ростов Химчистка стульев в Ростове – возвращение чистоты и привлекательности.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-orenburg003.ru
лечение запоя оренбург
canadian pharmacy tadalafil 20mg – https://strongtadafl.com/ take cialis the correct way
The Real Person!
The Real Person!
служба по контракту оренбург Служба по контракту: борьба с терроризмом: Военнослужащие участвуют в борьбе с терроризмом, защищая мирных граждан от угроз и насилия. Борьба с терроризмом является одной из важнейших задач, стоящих перед армией.
The Real Person!
The Real Person!
купить профнастил с8 Профнастил С8: экономичное и практичное решение для кровли и ограждений Профнастил С8 – это универсальный материал, который сочетает в себе легкость, прочность и доступную цену. Он идеально подходит для кровли, облицовки стен и строительства заборов. Широкий выбор цветов и покрытий позволяет подобрать оптимальный вариант для любого архитектурного стиля.
The Real Person!
The Real Person!
накрутка пф яндекс Накрутка ПФ Яндекс: Кратчайший путь к санкциям или долгосрочная стратегия? Накрутка поведенческих факторов (ПФ) в Яндексе – это скользкая дорожка. С одной стороны, заманчивая возможность быстро взлететь в ТОП. С другой – высокий риск попасть под санкции и потерять все. Стоит ли игра свеч?
The Real Person!
The Real Person!
Капельницы при алкогольной зависимости – это один из наиболее эффективных методов лечения алкогольной зависимости‚ который предоставляет возможность анонимного и комфортного восстановления. Нарколог на дом анонимно обеспечит оперативную медицинскую помощь при запое‚ используя метод капельницы для очищения организма. Преимущества капельницы заключаются в том‚ что она позволяет быстро устранить симптомы запоя‚ восстановить водно-электролитный баланс и способствует улучшению общего состояния пациента. Это критически важно в случае серьезных проявлений алкогольной зависимости‚ когда необходима немедленная помощь.Лечение алкоголизма включает разные методы‚ но капельница выделяется своей эффективностью и скоростью. В сравнении с таблетками и инъекциями‚ капельница обеспечивает непрерывное поступление лекарств в организм‚ что способствует более быстрому восстановлению.Ключевыми аспектами реабилитации являются поддержка близких и психотерапия в процессе реабилитации. По завершении этапа детоксикации важно продолжать лечение и работать над предотвращением рецидивов‚ что возможно только при системном подходе к лечению. Таким образом‚ капельница при запое — это ключевой элемент в борьбе с алкоголизмом‚ предлагая пациентам надежное анонимное лечение и возможность быстрого восстановления.
pictures of cialis – https://strongtadafl.com/# canadian no prescription pharmacy cialis Este enlace se abrirГЎ en una ventana nueva
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk004.ru
вывод из запоя
резка металла лазер лазерная резка металлопроката
типография сайт спб типография санкт петербург
The Real Person!
The Real Person!
Поддержка близких является важной ролью в восстановлении после запоя. Обсудите с ними свои планы и позвольте о помощи. Сообщества поддержки и обращение к специалистов представляют собой хорошим ресурсом. Не забывайте о профилактике срывов: рекомендации по трезвости и самопомощь при алкоголизме помогут избежать соблазна вернуться к прежнему. вывод из запоя
The Real Person!
The Real Person!
купить металлическую черепицу Купить металочерепицу: элегантная кровля на долгие годы Металочерепица – это современный кровельный материал, имитирующий натуральную черепицу. Она обладает высокой прочностью, устойчивостью к атмосферным воздействиям и привлекательным внешним видом. Металочерепица – это инвестиция в долговечность и эстетику вашей кровли.
The Real Person!
The Real Person!
накрутка пф яндекс Накрутка ПФ: игра с огнем Накрутка ПФ – это рискованная игра, которая может привести к серьезным последствиям. Вместо того, чтобы пытаться обмануть поисковую систему, лучше инвестировать в создание качественного продукта, который будет интересен реальным пользователям. Это – самый надежный способ добиться успеха в SEO-продвижении.
The Real Person!
The Real Person!
В Екатеринбурге огромное количество интернет-провайдеров представляют выгодные предложения на домашний интернет. На сайте domashij-internet-ekaterinburg004.ru можно найти актуальные акции на интернет, которые дают возможность существенно сэкономить. Сопоставление тарифов поможет найти самые выгодные пакеты интернет-услуг по приемлемым ценам. Многие провайдеры предоставляют уникальные предложениявключая уступки на тарифы и интернет безлимитный. Подключение интернета стало проще благодаря широкому ассортименту акциям и promociones de internet, дающим возможность получить надежные услуги связи по приемлемой цене.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-smolensk005.ru
лечение запоя смоленск
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar001.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
служба по контракту оренбург Служба по контракту: мифы и реальность. Развеиваем сомнения: Многие люди имеют ошибочное представление о службе по контракту. Развеем некоторые распространенные мифы и расскажем о реальных преимуществах и возможностях, которые она предоставляет.
The Real Person!
The Real Person!
накрутка пф яндекс Анализ и оптимизация: ключ к успеху Регулярно анализируйте ПФ своего сайта и выявляйте слабые места. Улучшайте контент, оптимизируйте страницы и делайте сайт удобным для пользователей. Это – самый надежный путь к успеху в SEO.
The Real Person!
The Real Person!
В столице России доступный интернет пользуется растущим спросом‚ и провайдеры конкурируют‚ предлагая выгодные тарифы на тарифы. На сайте domashij-internet-ekaterinburg005.ru вы найдете актуальные предложения на тарифы домашнего интернета. Специальные акции позволяют сэкономить на ценах интернет услуг‚ предлагая различные скидки. При сравнении тарифов важно учитывать скорость интернета и условия подключения. Многие провайдеры в Екатеринбурге предлагают выгодные интернет-пакеты с акциями и промокодами‚ которые делают акционные предложения еще более привлекательными. Следите за новыми предложениями‚ чтобы выбрать лучший тариф для себя и своей семьи.
типография заказать типография цены
The Real Person!
The Real Person!
вывод из запоя круглосуточно смоленск
vivod-iz-zapoya-smolensk006.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя краснодар
narkolog-krasnodar002.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
лакомства для собак Хрустящие субпродукты для чистки зубов: Здоровая улыбка вашего питомца Хрустящие сушеные субпродукты – это эффективное и безопасное средство для чистки зубов собаки. Они помогают удалить налет и зубной камень, предотвратить развитие заболеваний десен и сохранить здоровье полости рта вашего питомца.
типография напечатать печать спб типография
The Real Person!
The Real Person!
Выбор интернет-провайдера в Екатеринбурге — задача, требующий внимания к деталям. Для начала необходимо узнать, какие интернет провайдеры в Екатеринбурге по адресу доступны свои услуги. Сравнение провайдеров поможет определить наиболее подходящий тариф для дома или рабочих мест. При выборе провайдера обратите внимание на скорость интернета, доступные варианты и надежные провайдеры в вашем районе. Оптоволокно в Екатеринбурге гарантирует высокую скорость, в то время как кабельный интернет может быть более доступным. Беспроводной интернет удобен для мобильных пользователей. Обратите особое внимание на отзывы о провайдерах, чтобы избежать неприятных сюрпризов. Техническая поддержка провайдера также важна: быстрая помощь в случае неполадок — залог комфортному подключению к сети. Не забудьте ознакомиться с услугами связи, которые предлагает выбранный провайдер, включая телефонную связь и другие опции. Оптимальные предложения интернета помогут вам не только сэкономить, но и получить качественное подключение к интернету.
The Real Person!
The Real Person!
Эффективные рецепты против алкогольной зависимости могут стать полезным дополнением к медицинской помощи в Туле. Преодоление алкогольной зависимости требует всеобъемлющего подхода‚ включая очищение организма и психологическую поддержку.Травы от алкоголизма‚ такие как лекарственные растения‚ могут помочь в борьбе с алкогольной зависимостью. Следует иметь в виду о помощи родных и анонимной терапии. Методы борьбы с зависимостью включают программ реабилитации и методы предотвращения запоев. Рекомендации по отказу от спиртного также играют важную роль в реабилитации после алкоголизма. помощь нарколога тула
услуги типографии типография сайт спб
The Real Person!
The Real Person!
лакомства для собак Как правильно выбирать сушеные лакомства: При выборе сушеных лакомств учитывайте возраст, размер и особенности здоровья вашей собаки. Обращайте внимание на состав, срок годности и рекомендации производителя.
лазерная эпиляция рук лазерная эпиляция зоны
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
narkolog-krasnodar004.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
подключить интернет
domashij-internet-kazan004.ru
провайдеры по адресу
отчет по практике в школе https://gotov-otchet.ru
The Real Person!
The Real Person!
لا شك ان العديد من المراهنين قد خسروا أموالهم بسبب التسرع وعدم التريث في تحليل مباريات ومعرفة ما أهميتها للفريق. لذا من الضروري التحلي بالصبر والإلتزام بالتفكير وتحليل المباريات بشكل جيد قبل وضع الرهان. تدرك الشركات الناشئة في مجال تكنولوجيا التأمين أن العاملين لحسابهم الخاص لديهم مخاطر واحتياجات تأمينية فريدة. على سبيل المثال، تقدم شركات مثل Thimble تغطية تأمينية مرنة مدفوعة الأجر للعاملين لحسابهم الخاص في مختلف الصناعات، مثل المصورين ومنسقي الأغاني ومخططي الأحداث. تسمح هذه السياسات للموظفين المستقلين بشراء التأمين لمشاريع أو فترات زمنية محددة، مما يضمن حمايتهم فقط عندما يحتاجون إليها.
https://lotus-et-nuage.com/%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%84%d8%b9%d8%a8%d8%a9-buffalo-king-megaways-%d9%86%d8%b5%d8%a7%d8%a6%d8%ad-%d9%84%d8%aa%d9%82%d9%84%d9%8a%d9%84-%d8%a7%d9%84%d9%85%d8%ae%d8%a7%d8%b7%d8%b1/
يمكنك هنا تنزيل ملف حزمة تطبيق أندرويد “Time killer Thimbles ” الخاصة بجهازStarmobile Up Selfie مجانًا، نسخة ملف حزمة تطبيق أندرويد – 1.0.2 للتحميل على Starmobile Up Selfie اضغط ببساطة على هذا الزر. إنه سهل وآمن. نحن نقدم فقط ملفات حزمة تطبيق أندرويد الأصلية. إذا انتهكت أية مواد موجودة في الموقع حقوقك قم بإبلاغنا من خلال كانديس سوانبول وهي عارضة أزياء جنوب أفريقية ولدت في يوم20 أكتوبر 1988 في قرية موي ريفر في جنوب أفريقيا عملت مع فيكتوريا سيكريت وفي 2012 أصبحت الفائزة العاشرة في إستطلاع رأي في مجلة فوربس.
The Real Person!
The Real Person!
Алкогольный запой — это серьезная проблема, нуждающаяся в срочном лечении. В Туле доступны различные услуги по анонимному выводу из запоя. Медицинские учреждения предлагают помощь при запоях, включая детоксикацию и психотерапию. вывод из запоя тула Если вам или вашим близким нужна помощь, стоит обратиться в центры лечения зависимостей, где работают выездные наркологи. Также стоит рассмотреть возможность консультаций по алкоголизму и участия в группах поддержки для родственников. В Туле есть реабилитационные программы, включая анонимные группы и восстановление после запоя. Поддержка в трудные времена всегда доступна.
готовые рефераты сколько стоит реферат
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar005.ru
вывод из запоя цена
The Real Person!
The Real Person!
интернет по адресу
domashij-internet-kazan005.ru
подключить интернет по адресу
The Real Person!
The Real Person!
В нашем современном мире проблема зависимости высвечивается все ярче. Если кто-то из ваших близких или ваши близкие испытывают трудности с помощью, не стоит стеснятся. Анонимная консультация нарколога в Туле — это важный шаг на пути к лечению зависимости. На сайте site;com вы можете заказать консультацию нарколога, который предоставит медицинскую помощь и обеспечит полную анонимность пациента.Лечение алкоголизма и реабилитация наркоманов необходимы тщательный и профессиональный подход. В центр по лечению зависимостей вам предложат услуги, включая психотерапию и психологическую поддержку. Вызов врача на дом обеспечит комфортные условия для пациента, что крайне важно в трудные времена. Не откладывайте помощь на потом, обратитесь за анонимной помощью уже сегодня!
The Real Person!
The Real Person!
Капельницы от алкоголя в Туле – это важный этап в лечении алкоголизма. Процедуры детоксикации способствуют справиться с симптомами абстиненции и избавиться от запойного состояния. Лечение в специализированных центрах включает не только капельницы‚ но и поддержку психолога‚ что способствует восстановлению после алкоголя. narkolog-tula001.ru Реабилитация начинается с детоксикации‚ после которой инициируются реабилитационные программы. Поддержка родственников также играет значимую роль в предотвращении срывов. Группы поддержки и обращение к специалиста могут помочь в процессе выздоровления. Не забудьте‚ что лечение алкоголизма требует комплексного подхода.
The Real Person!
The Real Person!
домашний интернет в казани
domashij-internet-kazan006.ru
недорогой интернет казань
The Real Person!
The Real Person!
Наркологическая помощь в владимире доступна 24/7. Если вы подвержен зависимостинеобходимо помнитьчто имеется экстренная медицинская помощь. Наркологическая служба предоставляет вызов нарколога на дома также анонимное лечение алкоголизма и наркомании. Консультация врача-нарколога позволит определить подходящую терапию. Реабилитационный центр обеспечивает детоксикацию организма и помощь пациентам на протяжении всего лечения. Свяжитесь с нами для получения более детальной информации на vivod-iz-zapoya-vladimir004.ru.
The Real Person!
The Real Person!
Как провести ремонт и улучшить свое жилье самостоятельно Если ваш дом требует ремонта, услуги по ремонту могут стать настоящим спасением. На сайте narkolog-tula002.ru можно найти различных мастеров, которые помогут вам в решении проблем. Это может быть как мелкий ремонт, так и более серьезные сантехнические работы или электромонтажные услуги. Для тех, кто решит делать все сам, важно знать основные советы по ремонту. Первый шаг — это планирование: определите, какие именно работы нужно выполнить, и составьте детальную смету. Не забывайте о правилах безопасности, особенно если речь идет о электромонтажных работах. Для декорирования вашего жилья воспользуйтесь услугами опытных мастеров, которые занимаются благоустройством. Они помогут преобразить ваше жилье, сделав его более комфортным и уютным. Помните, что качественный ремонт на дому, это залог вашего комфорта!
The Real Person!
The Real Person!
провайдеры по адресу красноярск
domashij-internet-krasnoyarsk004.ru
провайдер интернета по адресу красноярск
где заказать дипломную работу помощь в написании диплома
дипломная работа на заказ цена помощь в написании диплома
The Real Person!
The Real Person!
Круглосуточные услуги нарколога в владимире – это необходимый элемент в борьбе с наркоманией. В специализированных медицинских учреждениях, таких как vivod-iz-zapoya-vladimir005.ru, возможно получить конфиденциальное лечение и консультации у нарколога. Опытные специалисты предлагает detox-программу, чтобы помочь пациентам преодолеть физическую зависимость. Стационарное лечение и реабилитация наркозависимых включает психотерапевтическую помощь и поддержку для семей пациентов. Организация медицинской помощи в владимире направлена на профилактику наркотиков и быструю реакцию в кризисных ситуациях. Наркологические услуги работают 24 часа в сутки, что позволяет незамедлительно реагировать на любые обращения и предоставлять пациентам необходимую помощь.
The Real Person!
The Real Person!
Вызов нарколога в Туле – важный шаг для людей‚ сталкивающихся с проблемами зависимости. На сайте narkolog-tula003.ru вы имеете возможность получить квалифицированной помощью. Наркологическая помощь включает определение зависимости и конфиденциальное лечение. Если у вас есть алкогольная или наркотическая зависимость‚ неотложный вызов врача поможет избежать последствий. Центры реабилитации предоставляют программы восстановления и поддержку семьи‚ что критично для успешного лечения. Консультация нарколога – это начальный этап на пути к выздоровлению. Не откладывайте‚ просите за медицинской помощью уже сегодня!
The Real Person!
The Real Person!
© 2021 İvme Gümrük Müşavirliği LTD. ŞTİ. Bu promosyonlardan yararlanmak, oyunun keyfini çıkarırken aynı zamanda kazanç ihtimalini artırır. Ancak the woman bonusun belirli kurallara ve çevrim şartlarına sahip olduğunu unutmamak önemlidir. Örneğin, trial modunda oyun deneyimi kazanmak ve oyunun özelliklerini öğrenmek, kazanma ihtimalinizi artırabilir. Ayrıca, casino platformlarının sunduğu bonus ve promosyonlardan yararlanmak, oyun süresini uzatarak daha fazla kazanç şansı sunar. Ancak unutulmamalıdır ki, slot oyunlarında şans faktörü en önemli unsurdur. Birçok casinos sitesinde bulabileceğiniz Sugars Rush slot oyunu, çevrimiçi olarak weil oynanabilir. Contáctanos Calle 126 #46-20 | 404 Sugar Rush Multitude Of Demo: Oyna Türkçe Slot Pragmatic Play Content Sugar Rush Sugar Rush 1000 Position Nedir? Sezon Hakkında Bu Oyunda Nasıl Kazanabilirsin? Sugar Run Demo Oyunun Videosu Sugar Rush Oyununun Videosu Slotun Demo Versiyonunun Avantajları» Sugar Hurry S1 Sezonu Internetten Izleyin, Kiralayın Ya Da Satın Alın «Sugars Rush ( Sugar Rush’ta Oyun Mekaniği
https://www.lesaqo.shop/evoplayin-penalti-cekisme-oyunu-turkiyede-online-casino-dunyasinin-yeni-gozdesi/
Playstore’da ücretsiz şeker acele yuvası demosun Parimatch Casino App Ve Apk Sugar Rush – A Quick Adventure Mod APK 3.17 +91 22 6678 2828 Internationa Legalization and Affidavit registry Agency Wild sembolü bir zencefilli kurabiye adam ile temsil edilir ve kazanan kombinasyonlar oluşturmak için scatter hariç diğer tüm sembollerin yerine geçebilir. Scatter sembolü bir şeker kavanozu ile temsil edilir ve makaralara üç veya daha fazlasını yerleştirmek ücretsiz döndürme bonus turunu tetikler. Şeker Rush 3D çarpıcı grafik ve ilgi çekici bir müzik vardır. Diğer şeker otomobil ile etkisini önleme, sol ve sağ cihazı eğerek, arabanızı hareket ettirin. Resmi Site Para Için Oyna Çok Oyunculu X5000 Content Slotun Demonstration Versiyonunun Avantajları Sugar Rush Ücretsiz Döndürme Turu Demo Oyunu…
The Real Person!
The Real Person!
какие провайдеры интернета есть по адресу красноярск
domashij-internet-krasnoyarsk005.ru
интернет провайдеры по адресу
The Real Person!
The Real Person!
Слив платных прогнозов бесплатно Как отличить надежный источник прогнозов от мошенников? Важно тщательно проверять репутацию источника прогнозов, читать отзывы других пользователей и анализировать статистику прошлых прогнозов. Не стоит доверять обещаниям гарантированной прибыли и слишком заманчивым предложениям.
The Real Person!
The Real Person!
Гражданство, паспорт Италии, Евросоюза, ЕС Гражданство, паспорт Ирландии, Евросоюза, ЕС. Ирландское гражданство – это доступ к англоязычной среде, благоприятному бизнес-климату и высокому уровню жизни в стране с богатой историей и культурой.
пригнать новую машину заказать авто из японии владивосток
пригнать авто из японии пригнать новую машину
The Real Person!
The Real Person!
Использование капельницы при запое – это результативным методом медицинской помощи в случае алкогольной зависимости. Вызов нарколога на дом позволяет быстро надежно получить необходимую помощь при запое. Состав раствора капельницы обычно содержит препараты для очищения организма, такие как физраствор, глюкоза, витамины и антиоксиданты. Действие капельницы сосредоточено на восстановлении баланса жидкости и электролитов, облегчении симптомов абстиненции и улучшение общего состояния пациента. Лечение запоя с помощью капельницы обеспечивает быструю помощь в условиях запоя и способствует восстановлению после запоя. Профессиональная помощь на дому избегает потребности в госпитализации, что делает процесс лечения более комфортным. Важно помнить, что реабилитация после запоя может предусматривать и методы психотерапии для борьбы с алкогольной зависимостью.
The Real Person!
The Real Person!
Слив платных прогнозов бесплатно Будьте осторожны и не попадайтесь на уловки мошенников! Помните, что легких денег не бывает. Чтобы успешно делать ставки, необходимо обладать знаниями, опытом и здравым смыслом.
The Real Person!
The Real Person!
Капельницы для выхода из запоя — это одной из популярных медицинских процедур, которые используются для быстрого выхода из запоя и детоксикации организма. В городе владимир существует множество медицинских учреждений, где предлагают услуги по лечению наркомании и алкоголизма, и стоимость капельниц могут варьироваться в зависимости от уровня сервиса и применяемых лекарств. Лечение алкоголизма обычно начинается с экстренной помощи, и капельницы играют ключевую роль в лечении запоя. Они помогают снять симптомы абстиненции, предоставляя необходимую поддержку при алкогольном запое. Стоимость лечения алкоголизма в владимире может колебаться от 3000 до 10000 рублей в зависимости от выборов учреждения и дополнительных услуг.Экстренный вывод из запоя Медицинские учреждения в владимире предоставляют различные пакеты услуг, включая консультации с наркологом и процесс реабилитации. Необходимо выбирать учреждение, которое предоставляет не только капельницу от запоя, но и комплексный подход к борьбе с алкогольной зависимостью.
The Real Person!
The Real Person!
провайдер по адресу
domashij-internet-krasnoyarsk006.ru
провайдеры интернета в красноярске по адресу
пригнать авто из китая в россию пригнать авто из японии
Как зарегистрировать ООО или ИП https://ifns150.ru в Санкт-Петербурге? Какие документы нужны для ликвидации фирмы? Где найти надежное бухгалтерское сопровождение или помощь со вступлением в СРО?
The Real Person!
The Real Person!
Врач-нарколог на дом в Туле предоставляет квалифицированную помощь тем, кто переживает проблемы с наркоманией и алкогольной зависимостью. Лечение зависимости должно учитывать индивидуальные особенности, и вызов нарколога на дом – это практичное решение для людей, нуждающихся в помощи. Центр лечения зависимостей в Туле предлагает лечение без раскрытия личности и поддержку нарколога. Домашняя реабилитация обеспечивает комфортное прохождение психотерапии при зависимости в комфортной обстановке. Выездная медицинская помощь включает в себя как телесное, так и психотерапевтическое сопровождение. Предотвращение зависимости также является ключевым моментом в борьбе с алкоголизмом и наркоманией. Поддержка алкоголиков и их родных – это работа квалифицированных специалистов, которые готовы протянуть руку помощи в трудную минуту. narkolog-tula005.ru
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar001.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
подключить интернет по адресу
domashij-internet-krasnodar004.ru
провайдеры по адресу дома
buy real viagra online – this viagra buy in uk
Фурнитура MACO https://kupit-furnituru-maco.ru для пластиковых окон — австрийское качество, надёжность и долговечность. Петли, замки, микропроветривание, защита от взлома.
The Real Person!
The Real Person!
Капельница для лечения запоя в домашних условиях — является удобный также эффективный метод помощи в случае алкогольного отравления. В отличие от стационарного детоксикации в стационаре, домашняя терапия запоя дает пациенту возможность находиться в комфортной среде, что уменьшает стресс а также способствует быстрому восстановлению.Врач нарколог на дом Тула осуществляет квалифицированные услуги нарколога, выполняя процедуры по избавлению от зависимости и прекращению запойного состояния. Процедуры капельниц помогают ускоренному устранению симптомов запойного состояния, улучшая самочувствие пациента; Также данные процедуры обеспечивают достаточное увлажнение а также восстановление витаминного баланса. Детоксикация на дому подходит для тех, кто не может или не хочет обращаться в стационар. Однако, при наличии серьезных осложнений необходимо более серьезное медицинское вмешательство. Нарколог на дом поможет организовать реабилитацию пациентов, включая психотерапию при зависимости, что значительно повысит шансы на успешное восстановление после запоя.
доставка ролов https://extrasushi.ru
The Real Person!
The Real Person!
какие провайдеры по адресу
domashij-internet-krasnodar005.ru
проверить провайдера по адресу
viagra sale leeds – https://strongvpls.com/# viagra pfizer 50 mg online
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar002.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
Капельница от запоя – это важный метод лечение алкогольной зависимости, который широко применяется в лечебных учреждениях Тулы. Процедура детоксикации способствует оперативному реабилитации организма после продолжительного употребления алкоголя. Время процедуры обычно варьируется от 1 до 3 часов, в зависимости от индивидуальных потребностей и интенсивности запойного состояния. лечение запоя тула Признаки запоя включает в себя интенсивные головные боли, тошноту и рвоту, обильное потоотделение и тревогу и беспокойство. Медицинская помощь в виде капельницы даёт возможность быстро облегчить похмельные симптомы, вводя жизненно важные вещества для восстановления водно-электролитного баланса и выведения токсинов из организма. Стоимость лечения запоя в Туле различается в зависимости от обраной лечебной организации и количества требуемых процедур. Прием у нарколога поможет определить наилучший курс лечения, включая реабилитацию после запоя. Результативность капельницы доказывается множество положительными отзывами пациентов, что делает данный метод популярным способом борьбы с алкоголизмом.
The Real Person!
The Real Person!
In addition, the region is estimated to recover the worldwide economic recession quicker than the developed countries characterized by encouraging demographic circumstances, built up money reserves, the existence of a robust banking system, bigger authorities expenditure and the mounting craving of traders for rising markets. Music and dance has always been a big part of Mira’s life. She spent her early childhood with her family on the East Coast of the USA, where her family was “infected” by the barbershop. Mira danced classical ballet, played the saxophone and sang in the children’s and youth choir before following her mother into the ranks of the “Harmunichs,” a women’s barbershop choir in Munich, Germany, when she was 15. Here she was involved in the music and choreography team before moving to New York in 2018 to become the director of “Sirens of Gotham.” The success of her quartet “Kickstart” gave her access to Heavy Medal in 2014 where, despite long distance (technology makes it possible!), she is still part of the creative team today.
http://snowcrunch.com/videos/12714-mobile-optimierung-bei-big-bass-splash-wo-uberzeugt-die-umsetzung
Boni sind eine Attraktion für Spieler, casino mit paysafecard einzahlen werden Ihre Gewinne verdoppelt. Die unglückliche Realität ist, würden Sie dann anfangen. Big bass splash awinenmultiplikatoren für ihn selbst erscheint es nur als ein einziges Bild, die Live-Dealer-Websites unterscheiden. Wir empfehlen Ihnen, einige der neueren Spiele auszuführen. Dies ist der Grund, wobei diese in einer bestimmten Reihenfolge ausgeteilt werden. online casino slots strategyWo sehe ich noch Vorteile der Spielgeld Version?Dazu gehört unter anderem,liegt am Gameplay.Gehen wir nun direkt auf das Spiel ein.Mai,mit dem der Spielautomat assoziiert werden kann.online casino mit 400 bonus Roulette Zahlen Addiert Each bet can win up to 1 Prize Drop Seit 2020 läuft die geniale Drops & Wins Promotion von Pragmatic Play. Diese Aktion ist bei Spielern in Online Casinos auf der ganzen Welt super beliebt geworden. Kein Wunder – wer liebt nicht die Chance auf tägliche Geldpreise und spannende Turniere?
The Real Person!
The Real Person!
интернет провайдеры по адресу краснодар
domashij-internet-krasnodar006.ru
какие провайдеры на адресе в краснодаре
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
narkolog-krasnodar003.ru
лечение запоя краснодар
The Real Person!
The Real Person!
пансионат для лежачих после инсульта
pansionat-msk001.ru
пансионат для пожилых после инсульта
Найти фирму по ремонту стиральных машин в Чернигове можно тут –
The Real Person!
The Real Person!
В последние годы новостройки столицы набирают популярность, и с ними растет необходимость в высококачественном доступе к интернету. Современные жилые комплексы предоставляют квартиры с подключением к сети, что является важным критерием при выборе провайдера. Скорость интернета в новостройках зависит от инфраструктуры района района. Многие новые здания оборудованы оптоволоконными линиями, что обеспечивает высокоскоростной доступ в интернет. Это позволяет подобрать тарифы на интернет, соответствующие требованиям пользователей, будь то беспроводной интернет или кабельный интернет. При определении интернет-провайдеров нужно учитывать на доступные пакеты. На сайте domashij-internet-msk004.ru доступна информация о лучших вариантах подключения, а также отзывы клиентов. Плюсы подключения к интернету в новых жилых комплексах очевидны и разнообразны: высокая скорость, стабильное соединение и передовые технологии. Выбор провайдера – это ключевой момент для комфорта в новом доме.
The Real Person!
The Real Person!
La versión en blanco de los SKILLER SGH50 ya cuenta con las nuevas almohadillas para los oídos híbridas recubiertas de piel sintética y tela. Mientras que, para los sonidos más habituales, la versión en negro va equipada con almohadillas para los oídos fabricadas únicamente con piel sintética. Junto a la diadema, que se adapta automáticamente a la forma de la cabeza, el fabricante quiere destacar la comodidad gracias al uso de nuevas almohadillas para los oídos híbridas. Claro está, estas almohadillas híbridas también se incluyen con la versión en negro. La versión en blanco de los SKILLER SGH50 ya cuenta con las nuevas almohadillas para los oídos híbridas recubiertas de piel sintética y tela. Mientras que, para los sonidos más habituales, la versión en negro va equipada con almohadillas para los oídos fabricadas únicamente con piel sintética. Junto a la diadema, que se adapta automáticamente a la forma de la cabeza, el fabricante quiere destacar la comodidad gracias al uso de nuevas almohadillas para los oídos híbridas. Claro está, estas almohadillas híbridas también se incluyen con la versión en negro.
https://edufitindia.com/ventajas-del-juego-balloon-de-smartsoft-en-casinos-online-para-jugadores-chilenos/
Kombatientes kameo Crea o únete a un Clan para jugar con amigos y luchar contra otros clanes juntos y obtener más oro o recursos. Las luchas del Castillo semanales en la guerra de Clanes te mantendrán a ti y a tus amigos ocupados. Solo si trabajan juntos podrán ser más fuertes y poderosos. ¡Muchas batallas PVP a gran escala y precios tentadores te están esperando! Politica de privacidad Pizza Tower 9. Rompecabezas de clasificación de agua Colecciona una colección completa de animales: desde gatos y palomas generados por IA hasta cocodrilos pixelados y bailarines digitales. Cada animal tiene animaciones y efectos de bonificación únicos, y completar la lista completa te otorga enormes bonificaciones de temporada. ¡Video bloggers y opiniones de autores! Nos alegra ver tu contenido sobre TOWERLANDS en tu canal. Nosotros ofrecemos ayuda y soporte a los autores creativos.
The Real Person!
The Real Person!
маркетинг Целевые клиенты Определение целевых клиентов – основа успешной маркетинговой стратегии. Сегментируйте аудиторию по демографическим, психографическим и поведенческим характеристикам. Создавайте персонализированные маркетинговые кампании для каждой группы клиентов.
The Real Person!
The Real Person!
вывод из запоя краснодар
narkolog-krasnodar004.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
пансионат для пожилых с инсультом
pansionat-msk002.ru
пансионат после инсульта
The Real Person!
The Real Person!
https://v-tagile.ru/obschestvo-iyul-5/kak-vybrat-idealnyj-buket-dlya-zhenshchiny-ot-povoda-do-yazyka-tsvetov
The Real Person!
The Real Person!
Подключение интернета в Москве: независимое подключение В современном мире интернет стал важной частью жизни‚ поэтому выбор интернет-провайдеров Москвы имеет большое значение. Чтобы подключить интернет на своем жилье‚ нужно учитывать пакеты на интернет и скорость интернета‚ которые предлагают разные компании. Первым шагом является выбор провайдера. Сравните варианты‚ изучите специальные предложения и скидки провайдеров. После этого соберите необходимые документы для подключения‚ такие как удостоверение личности и документ‚ подтверждающий право пользования помещением. domashij-internet-msk005.ru Для независимого подключения интернета вам понадобятся роутеры и усстройства доступа‚ а также технические условия. Убедитесь‚ что у вас есть все нужные приборы и рекомендации. При настройке Wi-Fi важно правильно сконфигурировать оборудование. Не забудьте протестировать скорость интернета с помощью онлайн-теста‚ чтобы быть уверенным в качестве предоставляемого сервиса. Следуя этим шагам‚ вы сможете легко подключить интернет в своем доме.
The Real Person!
The Real Person!
пансионат инсульт реабилитация
pansionat-msk003.ru
частный пансионат для пожилых
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar005.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
криптообменник Купить криптовалюту за наличные в москве Геолокация сейчас важна, как никогда ранее.
The Real Person!
The Real Person!
пансионат для людей с деменцией в туле
pansionat-tula001.ru
частный пансионат для пожилых
The Real Person!
The Real Person!
Специалист по зависимостям Тула: Квалифицированная поддержка при проблемах с зависимостями Если вы или ваши родные столкнулись с психоактивными веществами‚ необходимо обратиться к профессионалу. Нарколог Тула предлагает квалифицированную медицинскую помощь‚ включая оценку состояния зависимого и индивидуальные консультации нарколога. В наркологической клинике доступны многообразные услуги‚ такие как медикаментозное лечение и психотерапия. Работа с зависимостями – это комплексный и многоступенчатый процесс‚ который требует индивидуального подхода. Программа реабилитации включает в себя этапы‚ направленные на исцеление физического и психологического состояния пациента. Реабилитация наркозависимых также включает поддержку семьи‚ что является ключевой ролью в успешном лечении. Конфиденциальная помощь позволяет пациентам ощущать себя защищенными и свободными от предвзятости. Предотвращение зависимостей и помощь при алкоголизме – важные аспекты работы наркологов. Обратитесь на narkolog-tula001.ru для получения подробностей и записи на консультацию. Помните‚ что своевременное обращение за помощью – гарантия успешного исцеления!
The Real Person!
The Real Person!
домашний интернет тарифы
domashij-internet-nizhnij-novgorod004.ru
провайдеры домашнего интернета нижний новгород
The Real Person!
The Real Person!
пансионат для лежачих пожилых
pansionat-tula002.ru
частный дом престарелых
The Real Person!
The Real Person!
Капельница при алкогольной зависимости – это действующий метод‚ который часто применяется в условиях дома. Если вы ищете нарколог на дом в Туле‚ то вызов врача станет отличным решением. Удобство вызова врача дает возможность получить квалифицированную помощь в уютной обстановке‚ что особенно важно для пациентов‚ находящихся в состоянии алкогольной зависимости.Детоксикация с помощью капельницы способствует быстрому восстановлению здоровья и безопасность для пациента. Врач нарколог на дом осуществит лечение запоя‚ соблюдая конфиденциальность и профессиональную поддержку. врач нарколог на дом тула Домашнее лечение создает уютные условия‚ что положительно сказывается на процессе восстановления после зависимости от алкоголя. Срочная помощь при запое может послужить основой для эффективного выздоровления‚ что делает вызов врача на дом особенно актуальным.
The Real Person!
The Real Person!
متاجر يوباى المشهورة خمن أين الكرة. اللعبة مناسبة للأشخاص ذوي الحدس الجيد Minor bug fixes and improvements. Install or update to the newest version to check it out! بهذه الخطوات ستتمكن من الحصول على حساب predictor aviator مجاني وتشغيل برنامج هلى جهاز والبدء في اللعب. متاجر يوباى المشهورة اتبع هذه الخطوات البسيطة لإنشاء حساب في 1XBet Casino للعب لعبة Aviator Scribe والحصول على ايميل وباسورد predictor aviator. Minor bug fixes and improvements. Install or update to the newest version to check it out! Download APK on Android with Free Online APK Downloader – APKPure يقدم كازينو 1xBet مجموعة متنوعة من الألعاب من مزودي برامج ممتازين مثل predictor aviator و CRASH و Scribe و NetEnt و Microgaming. تتوفر المراهنات الرياضية وخيارات الكازينو المباشر وغير ذلك الكثير في 1xBet.
https://myvietmagazine.com/%d9%85%d8%b1%d8%a7%d8%ac%d8%b9%d8%a9-%d9%84%d8%b9%d8%a8%d8%a9-thimbles-%d9%85%d9%86-evoplay-%d9%81%d9%8a-%d9%83%d8%a7%d8%b2%d9%8a%d9%86%d9%88%d9%87%d8%a7%d8%aa-%d8%a7%d9%84%d8%a5%d9%86%d8%aa%d8%b1-5/
نرجع لك فلوسك على نفس وسيلة الدفع خلال 3 أيام عمل. بسرعة وسهولة! نعم، موقع الكرة يُحدد باستخدام خوارزمية RNG (مولّد الأرقام العشوائية) المعتمدة دوليًا، مما يجعل كل جولة مستقلة تمامًا عن التي قبلها. لا توجد أنماط مخفية أو تغييرات قائمة على رهانك السابق، مما يحافظ على مصداقية تجربة رهان الكشتبانات على الإنترنت بنسبة 100%. يُظهر التصميم اهتماماً ملحوظاً بتجربة المستخدم من البداية حتى نهاية كل جولة. لا توجد عناصر عشوائية أو تأثيرات غير ضرورية. Evoplay اختارت الاعتماد على وضوح الفكرة والأسلوب التفاعلي السريع، مما يجعل لعبة thimbles game واحدة من الألعاب المفضلة لمحبي ألعاب الحظ السريعة.
The Real Person!
The Real Person!
интернет провайдеры в нижнем новгороде по адресу дома
domashij-internet-nizhnij-novgorod005.ru
подключить интернет
The Real Person!
The Real Person!
Casino Simulator İndir Ücretsiz Oyun İndir Ve Oyna!” Content En Iyi Terme Conseillé Oyunlarını Ücretsiz Oynayın En Iyi Canlı Casino Oyunları Bonuslar Sizi Bekliyor Eğlenmek Için Oynayabileceğiniz Ücretsiz Çevrimiçi Slot Türleri En Çok Oynanan Oyunlar Oyunları Gösterme Modunda Deneyin Masrafsız Video Slot Machine Makinelerinde Oynayın Ücretsiz Slotlar Empieza Gerçek Paralı Slotlar Myjackpot Apresentando Ücretsiz Online Casino Simulator İndir Ücretsiz Oyun İndir Ve Oyna!” Content En Iyi Terme Conseillé Oyunlarını Ücretsiz Oynayın En Iyi Canlı Casino Oyunları Bonuslar Sizi Bekliyor Eğlenmek Için Oynayabileceğiniz Ücretsiz Çevrimiçi Slot Türleri En Çok Oynanan Oyunlar Oyunları Gösterme Modunda Deneyin Masrafsız Video Slot Machine Makinelerinde Oynayın Ücretsiz Slotlar Empieza Gerçek Paralı Slotlar Myjackpot Apresentando Ücretsiz Online
https://darshanjkrathod.radardoworks.co.in/index.php/2025/07/16/jak-wygladaja-wyplaty-na-konto-bankowe-z-bizzo-casino_1752669511/
W Vulkan Vegas darmowe spiny kasyno są rozdawane w trochę inny sposób. Skorzystaj z naszego linku, zarejestruj się w kasynie, a już będą czekać na Ciebie darmowe spiny bez depozytu. Taki bonus nie zdarza się za często, więc warto z niego skorzystać. Nasza strona jest prawdziwym partnerem kasyna Vulkan Vegas. Za porozumieniem z kasynem dajemy Osoba do kontakuWojciech Wąsiewicztel.: +48 781 020 214 Really love is actually love: an LGBTQ+ rally weep around the globe â from gay romance novels to movies and… Hesab aktivləşdirildikdən sonra istifadəçilər balanslarını depozit əməliyyatı vasitəsilə maliyyələşdirməlidirlər. Son qədəm platformamızın kazino bölməsinə keçidi dövrə edir, burada axtarış funksiyasından istifadə etməklə Aviator slotunu rahat şəkildə yerləşdirmək olar. Başladıqdan sonra istifadəçilər bu həyəcanlandıran aviasiya temalı oyunda real pul mərc etməyə başlaya bilərlər. Biz istifadəçilərə əvəzsiz demo rejimimiz vasitəsilə oyunun mexanikası, xüsusiyyətləri və ümumi təcrübəsi ilə tanış olmaq imkanı veririk.
The Real Person!
The Real Person!
обмен криптовалюты на рубли rexex.io Погрузитесь в экосистему rexex.io – мир, где технологии встречаются с интуицией. Платформа, созданная для тех, кто ценит свое время и стремится к максимальной эффективности. Анализируйте тренды, совершайте выгодные обмены и управляйте своим криптопортфелем с легкостью. Rexex.io – это не просто инструмент, это ваш интеллектуальный крипто-помощник, который всегда на шаг впереди.
The Real Person!
The Real Person!
пансионат для лежачих пожилых
pansionat-tula003.ru
частный дом престарелых
The Real Person!
The Real Person!
Капельница от алкогольной зависимости – проверенное средство для помощи алкогольной зависимости. Она обеспечивает быстрое вливание различных растворов‚ способствуя детоксикации тела и улучшению состояния здоровья. В учреждениях лечения зависимостей‚ таких как narkolog-tula003.ru‚ квалифицированные специалисты предлагают медицинскую помощь‚ включая инъекции от алкоголизма. Терапия включает не только капельницы‚ но и процессы реабилитации‚ психологическую поддержку при алкогольной зависимости‚ что способствует пациентам возвращаться к нормальной жизни.
The Real Person!
The Real Person!
интернет провайдеры в нижнем новгороде по адресу дома
domashij-internet-nizhnij-novgorod006.ru
провайдеры по адресу нижний новгород
The Real Person!
The Real Person!
вывод из запоя
https://vivod-iz-zapoya-chelyabinsk001.ru
вывод из запоя круглосуточно челябинск
Kazand?ran oyun Sweet Bonanza simdi yay?nda
https://santehmen.ru
The Real Person!
The Real Person!
Как лечат алкоголизм в Туле: лучшие подходы Алкоголизм – это огромная проблема‚ необходима комплексная стратегия. В Туле доступны разнообразные услуги наркологии‚ включая опцию вызова нарколога на дом. Это особенно актуально для тех‚ кто стесняется идти в клиники для лечения зависимости. Первым этапом является детоксикация организма‚ которая позволяет очистить организм от алкоголя и токсинов. После этого начинается терапия алкогольной зависимости. Медикаментозное лечение и психотерапия являются частью программ по лечению алкоголизма. Консультация нарколога поможет выбрать наиболее эффективный путь. Реабилитация зависимых предполагает наличие семейной терапиичто помогает близким людям поддерживать зависимого. Профилактика рецидивов является ключевым моментом‚ который включает занятия в группах поддержки и индивидуальные сессии. Анонимное лечение алкоголизма позволяет пациентам чувствовать себя комфортно. Важно помнить‚ что процесс лечения алкогольной зависимости длительный‚ который требует настойчивости и усилий. Вызвать нарколога на дом – это начало новой жизни без алкоголя.
The Real Person!
The Real Person!
стеклянные перегородки Душевые кабины из стекла — это не только практичное, но и стильное решение для ванных комнат. Они легко вписываются в любой интерьер, от классики до хайтека, создавая современный и чистый вид. Дешевая перегородка
The Real Person!
The Real Person!
Dragon money Графика и звуковое сопровождение создают атмосферу средневекового мира, полного приключений и опасностей. Регулярные обновления и новые функции делают игру интересной и захватывающей.
The Real Person!
The Real Person!
Выбор провайдера домашнего интернета в новосибирске — задача‚ требующая внимательного подхода . На сайте domashij-internet-novosibirsk004.ru можно найти полезные рекомендации . Прежде всего‚ важно учитывать скорость интернет-соединения, которая зависит от выбранного тарифного плана и способа подключения. Провайдеры новосибирска предлагают множество решений: от оптоволоконного интернета до беспроводных технологий. Качество связи также играет важную роль . Читайте отзывы пользователей ‚ чтобы узнать о стабильности работы и наличии технической поддержки . Рассмотрите стоимость подключения и условия контракта , так как они могут различаться. Многим пользователям интересны дополнительные услуги , такие как IP-телефония или телевидение‚ которые могут быть доступны в рамках тарифа. Также не забудьте уточнить‚ какое оборудование необходимо для подключения к интернету. В итоге, собрав все необходимые данные‚ вы сможете сделать обоснованный выбор и наслаждаться стабильным и быстрым интернетом в вашем жилье.
Jogo do Tigrinho com bonus exclusivos para o publico brasileiro
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-chelyabinsk002.ru
вывод из запоя
The Real Person!
The Real Person!
https://remdostavka.ru
The Real Person!
The Real Person!
раздвижные стеклянные перегородки Офисные перегородки из стекла играют важную роль в повышении продуктивности сотрудников. Они позволяют создать комфортные рабочие места, сохраняя при этом возможность общения между сотрудниками. Прозрачные перегородки помогают лучше организовать пространство, разделяя зоны для работы и отдыха. Душевые кабины
Актуальные тренды сегодня источник: фото, видео и медиа. Всё о том, что популярно сегодня — в России и в мире. Мода, визуальные стили, digital-направления и соцсети. Следите за трендами и оставайтесь в курсе главных новинок каждого дня.
Good blog you have here.. It’s obdurate to espy elevated calibre article like yours these days. I really respect individuals like you! Take vigilance!! https://buyfastonl.com/amoxicillin.html
The Real Person!
The Real Person!
Запой – это опасное состояние‚ требующее неотложного вмешательства и поддержки. Народные средства часто воспринимаются как действующие методы лечения‚ но вокруг них существует множество мифов. Например‚ многие считают‚ что травы от алкоголизма способны мгновенно устранить симптомы запоя. Однако реальность таковы‚ что народные рецепты могут лишь помочь в восстановлении после запоя‚ но не вытеснить профессиональную реабилитацию от алкоголя. Существует несколько признанных методов‚ среди которых очищение организма и лечение алкоголизма. Психология зависимости играет ключевую роль в процессе восстановления. Важно понимать‚ что экстренный вывод из запоя требует системного подхода‚ включающего медицинскую помощь и поддержку близких. Не стоит ограничиваться народные средства — это может привести к ухудшению состояния.
The Real Person!
The Real Person!
В столице России огромное количество интернет-провайдеров предлагают выгодные пакеты услуг, включающие услуги интернета и телевидения. Вы можете посетить сайт domashij-internet-novosibirsk005.ru, где вы найдете обзор тарифов на интернет и цифровое телевидение. Высокоскоростной интернет и IPTV в новосибирске становятся стандартом, что позволяет пользователям иметь мультиэкранный доступ к контенту. Большинство провайдеров предлагают акции и скидки, а также пакеты для всей семьи, что обеспечивает оформление подключения к интернету и телевидению более доступным. Услуги связи для корпоративных клиентов также охватывают пакетные решения, позволяя оптимизировать расходы. Выберите лучшее для себя!
Нужен буст в игре? буст dune awakening легендарная броня, костюмы, скины и уникальные предметы. Всё для выживания на Арракисе!
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-chelyabinsk003.ru
вывод из запоя
The Real Person!
The Real Person!
https://uksputnik.ru
Good blog you be undergoing here.. It’s hard to find strong calibre writing like yours these days. I honestly recognize individuals like you! Withstand guardianship!! https://buyfastonl.com/
The Real Person!
The Real Person!
Поддержка близких при алкогольной зависимости Зависимость от алкоголя — это не только неприятность зависимогоно и его семьи. В Туле обращение к специалисту может стать первым шагом к исцелению зависимости. Семейная поддержка играет решающую роль в процессе реабилитации. Близкие могут помочь, предоставляя эмоциональную поддержку и создавая атмосферу сочувствия. В сложной ситуации важно обращаться за профессиональной помощью. Консультация специалиста поможет определить правильный путь лечения алкоголизма. Терапия также может стать важным инструментом для восстановления. вызов нарколога тула Поддержка со стороны общества семьи необходима на всех стадиях лечения, от первой встречи с наркологом до последующей реабилитации. Помощь близких может ускорить выздоровления и вернуть зависимого к нормальной жизни.
The Real Person!
The Real Person!
душевая перегородка из стекла Стеклянные ограждения — это идеальный элемент безопасности и стиля для открытых пространств, таких как балконы и лестницы. Они позволяют наслаждаться панорамными видами, в то время как прочные материалы обеспечивают надежность и долговечность. Эстетика стеклянных ограждений гармонично сочетается с другими элементами интерьера, создавая единое целое. Офисные перегородки
The Real Person!
The Real Person!
Выбор интернет-провайдера в новосибирске, задача не из легких. На сайте domashij-internet-novosibirsk006.ru можно найти разнообразные предложения от многих интернет-провайдеров в новосибирске. Важно обратить внимание на тарифы на интернет, скорость интернета и качество связи. Клиенты часто делятся мнениями о домашнем интернете, рассказывая о надежности соединения и качестве обслуживания. Сравнение провайдеров поможет выбрать оптимальный вариант. Многие компании имеют специальные предложения, что делает интернет по разумной цене еще более привлекательным. При выборе интернет-провайдера важно учитывать все услуги интернет-провайдеров и их предложения. Подумайте о том, что вам важнее: скорость интернета или его стоимость. Правильный набор услуг провайдера обеспечит качественное подключение к интернету для вашего дома.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-cherepovec004.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
https://gidro-vanna-dush.ru
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-cherepovec005.ru
экстренный вывод из запоя череповец
Discover rafting https://www.tara-montenegro-rafting.me – the perfect holiday for nature lovers and extreme sports enthusiasts. The UNESCO-listed Tara Canyon will amaze you with its beauty and energy.
The Real Person!
The Real Person!
домашний интернет в омске
domashij-internet-omsk004.ru
лучший интернет провайдер омск
Гидроизоляция зданий https://gidrokva.ru и сооружений любой сложности. Фундаменты, подвалы, крыши, стены, инженерные конструкции.
The Real Person!
The Real Person!
Капельницы при запое, это критически важный шаг в борьбе с алкоголизмом. Обращение к наркологу на дом в Туле дает возможность получить профессиональную медицинскую помощь. Если появляются симптомы запоя, таких как тремор, потоотделение и тревожность, важно незамедлительно получить помощь. Наркологи осуществляют диагностику алкогольной зависимости и разрабатывают план медикаментозной терапии, включая инфузии для детоксикации. вызов нарколога на дом тула Восстановление после запоя включает семейную поддержку, что значительно увеличивает шансы на успешное восстановление. Предотвращение алкогольной зависимости тоже является важным аспектом. Услуги нарколога, включая вызов врача на дом, помогают решить вопросы, касающиеся алкогольной зависимости, и гарантировать безопасную терапию.
The Real Person!
The Real Person!
Прогулка по Москве и не только – Буду выкладывать свою жизнь, где ел и что и вкусно ли это, какие места увидел новые и свои взгляды на мир и хорошую музыку https://t.me/armanmoscow
The Real Person!
The Real Person!
подшипник для конвейера Купить подшипник для электродвигателя – это важное решение, от которого зависит надежность и долговечность электродвигателя. Выбирайте подшипник, соответствующий техническим характеристикам двигателя и условиям эксплуатации.
The Real Person!
The Real Person!
https://geolexpert.ru
The Real Person!
The Real Person!
лечение запоя череповец
vivod-iz-zapoya-cherepovec006.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
провайдеры омск
domashij-internet-omsk005.ru
домашний интернет тарифы
If you’re looking for the true Grandpashabet experience, this is the verified Instagram profile
The Real Person!
The Real Person!
пансионат для пожилых в москве
pansionat-msk001.ru
пансионат с медицинским уходом
The Real Person!
The Real Person!
Подшипники оптом от производителей Купить подшипники оптом – выгодное решение для предприятий с большим парком техники. Оптовые закупки позволяют существенно сэкономить и обеспечить наличие необходимых подшипников на складе, избегая простоев в производстве.
The Real Person!
The Real Person!
голодание на воде В процессе голодания необходимо пить достаточное количество воды, чтобы избежать обезвоживания.
The Real Person!
The Real Person!
подшипники для пищевой промышленности Цилиндрический подшипник Цилиндрический подшипник — это тип подшипника качения, в котором тела качения имеют форму цилиндров. Он обладает высокой грузоподъемностью и применяется в различных отраслях промышленности, где требуется высокая точность и надежность.
This is the amicable of content I get high on reading. click
Заказать дипломную работу https://diplomikon.ru/ недорого и без стресса. Выполняем работы по ГОСТ, учитываем методички и рекомендации преподавателя.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-irkutsk001.ru
вывод из запоя
… אני לא יכולה … ויקה שם … היא בשבילך.… – ואני רוצה אותך … או שלא אהבת איך אני רוקד? הדבר המוזר ביותר הוא שכפי שאני מעריך את המצב הזה בין באטי לאשתי, אני עצמי מתיישב לבגידה. לא הייתי sneak a peek at this site
The Real Person!
The Real Person!
https://sibgazpribor.ru
The Real Person!
The Real Person!
пансионат для престарелых людей
pansionat-msk002.ru
пансионат для престарелых
I am in point of fact happy to glitter at this blog posts which consists of tons of of use facts, thanks representing providing such data. prednisolona 5 mg para que sirve
The Real Person!
The Real Person!
лечение запоя иркутск
vivod-iz-zapoya-irkutsk002.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
каталог подшипников по размерам Подшипник для конвейера – это важный элемент, обеспечивающий плавную и бесперебойную работу конвейерной системы. Он должен выдерживать высокие нагрузки, вибрации и воздействие абразивных материалов.
The Real Person!
The Real Person!
пансионат для лежачих после инсульта
pansionat-msk003.ru
пансионат после инсульта
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-irkutsk003.ru
вывод из запоя
The Real Person!
The Real Person!
https://altaywater.ru
The Real Person!
The Real Person!
голодание на воде Физиологические аспекты голодания: Что происходит с телом без пищи
The Real Person!
The Real Person!
пансионат для престарелых
pansionat-tula001.ru
дом престарелых в туле
The Real Person!
The Real Person!
Подшипники изготовитель Купить промышленный подшипник Промышленные подшипники – это сердце многих машин и механизмов. Выбор правильного подшипника, соответствующего специфическим требованиям оборудования и условиям эксплуатации, имеет первостепенное значение. Приобретение промышленного подшипника – ответственный шаг, требующий внимательного анализа технических характеристик и надежности поставщика.
The Real Person!
The Real Person!
экстренный вывод из запоя калуга
vivod-iz-zapoya-kaluga004.ru
экстренный вывод из запоя калуга
The Real Person!
The Real Person!
https://personaldliadoma.ru
The Real Person!
The Real Person!
поставщики подшипников в россии Подшипники для сельхозтехники Мы предлагаем широкий выбор подшипников, разработанных специально для сельскохозяйственной техники, обеспечивающих надежность и долговечность в сложных условиях.
Арендуя катер в Адлере вы получаете уникальную возможность насладиться красотой Черного моря: снять яхту в Адлере
The Real Person!
The Real Person!
пансионат для реабилитации после инсульта
pansionat-tula002.ru
пансионат для реабилитации после инсульта
The Real Person!
The Real Person!
вывод из запоя круглосуточно калуга
vivod-iz-zapoya-kaluga005.ru
вывод из запоя
שלו הגיע למקומות הארוגניים ביותר, ובינתיים אצבעותיו משכו את הדגדגן, מה שמספק הנאה נוספת. אממ, המועדון היא השתקפותי במראה. עיניים נוצצות באקסטזה וחיוך רחב. מסקרה דולפת וזרע על השפה. “כן,” נערות מארחות
The Real Person!
The Real Person!
валютные платежи за границу Оплата автомобиля в Китай – это процесс перевода денежных средств китайскому поставщику за автомобиль, приобретенный у него. Он требует соблюдения валютного законодательства и использования надежных систем международных платежей.
The Real Person!
The Real Person!
Ремонт Сочи Подрядчик по ремонту в Сочи – это ваш надежный партнер в реализации любых строительных и ремонтных проектов. Выбирайте профессионалов с опытом и репутацией.
На арендованной яхте в Адлере можно устроить праздник, фотосессию, деловую встречу или просто уединиться с природой в кругу близких https://yachtkater.ru/
The Real Person!
The Real Person!
https://ukhoroshevka.ru
The Real Person!
The Real Person!
дом престарелых в туле
pansionat-tula003.ru
пансионат для лежачих после инсульта
The Real Person!
The Real Person!
платежи за границу Агент по международным платежам – это компания или физическое лицо, которое оказывает услуги по осуществлению международных платежей.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-kaluga006.ru
экстренный вывод из запоя калуга
The Real Person!
The Real Person!
https://uspenka-dom.ru
The Real Person!
The Real Person!
Solpot Casino prioritizes responsible, secure play Discover premium casino gaming now at solpot.xl-gamers.com https://solpot.xl-gamers.com Solpot Casino hosts daily promotions and tournaments: Enjoy new ways to win every day with Solpot Casino’s constant stream of promotions and tournaments!
The Real Person!
The Real Person!
https://mastera-vodonagrevateley-spb.ru
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar001.ru
вывод из запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно челябинск
https://vivod-iz-zapoya-chelyabinsk001.ru
лечение запоя
The Real Person!
The Real Person!
платежный агент по приему платежей Оплата счета за границу – это процесс перевода денежных средств иностранному поставщику на основании выставленного им счета на оплату. Он требует соблюдения валютного законодательства и использования надежных систем международных платежей.
Заказать диплом https://diplomikon.ru быстро, надёжно, с гарантией! Напишем работу с нуля по вашим требованиям. Уникальность от 80%, оформление по ГОСТу.
Отчёты по практике https://gotov-otchet.ru на заказ и в готовом виде. Производственная, преддипломная, учебная.
Оформим реферат https://ref-na-zakaz.ru за 1 день! Напишем с нуля по вашим требованиям. Уникальность, грамотность, точное соответствие методичке.
Диплом под ключ https://diplomnazakaz-online.ru от выбора темы до презентации. Профессиональные авторы, оформление по ГОСТ, высокая уникальность.
The Real Person!
The Real Person!
Шариковый подшипник Подшипники для металлургии Подшипники, используемые в металлургии, должны выдерживать высокие температуры и нагрузки.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar002.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-chelyabinsk002.ru
лечение запоя
The Real Person!
The Real Person!
банк платежи в китай Международные платежи SWIFT – это переводы денежных средств, осуществляемые с использованием системы SWIFT, которая является самой распространенной системой международных платежей.
Заказать диплом https://diplomikon.ru быстро, надёжно, с гарантией! Напишем работу с нуля по вашим требованиям. Уникальность от 80%, оформление по ГОСТу.
Оформим реферат https://ref-na-zakaz.ru за 1 день! Напишем с нуля по вашим требованиям. Уникальность, грамотность, точное соответствие методичке.
Отчёты по практике https://gotov-otchet.ru на заказ и в готовом виде. Производственная, преддипломная, учебная.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar003.ru
лечение запоя
Диплом под ключ https://diplomnazakaz-online.ru от выбора темы до презентации. Профессиональные авторы, оформление по ГОСТ, высокая уникальность.
The Real Person!
The Real Person!
вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk003.ru
вывод из запоя цена
The Real Person!
The Real Person!
https://remmosstroy.ru
The Real Person!
The Real Person!
Solpot Casino pushes the limits of online entertainment Discover premium casino gaming now at solpot.xl-gamers.com https://solpot.xl-gamers.com Get instant payouts at Solpot Casino: Win and receive your winnings instantly! We offer fast and reliable payouts using various payment methods, ensuring you can access your funds quickly and conveniently.
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
https://dacha-ctroi.ru
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-cherepovec004.ru
вывод из запоя череповец
The Real Person!
The Real Person!
https://domsibiri.ru
The Real Person!
The Real Person!
https://dom-servis2.ru
Лучшие и актуальные промокоды на бесплатные ставки в слотах в популярных букмекерских конторах. Бонусы за регистрацию, фрибеты, удвоение депозита. Обновления каждый день.
Купить квартиру или дом недвижимость в Черногории Подберём квартиру, дом или виллу по вашему бюджету. Юридическая проверка, консультации, оформление ВНЖ.
русское порно большие порно массаж
Ваш безопасный портал bitcoin7.ru в мир криптовалют! Последние новости о криптовалютах Bitcoin, Ethereum, USDT, Ton, Solana. Актуальные курсы крипты и важные статьи о криптовалютах. Начните зарабатывать на цифровых активах вместе с нами
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar005.ru
лечение запоя
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-cherepovec005.ru
вывод из запоя круглосуточно череповец
The Real Person!
The Real Person!
https://xplus-master.ru
Продвижение сайтов https://team-black-top.ru под ключ: SEO-оптимизация, технический аудит, внутренняя и внешняя раскрутка. Повышаем видимость и продажи.
esim uk buy esim for switzerland
Всё о металлообработке https://j-metall.ru и металлах: технологии резки, сварки, литья, фрезеровки. Свойства металлов, советы для производства и хобби.
The Real Person!
The Real Person!
Капельница от запоя – данный метод является популярным методом лечения алкоголизма, который внедряется в медицинских учреждениях в Красноярске для очистки организма. Круглосуточное лечение дает возможность эффективно снять симптомы запоя. Тем не менее необходимо учитывать о возможных побочных эффектах, включающих аллергические реакции и нарушение водно-солевого баланса. вывод из запоя круглосуточно Красноярск
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-cherepovec006.ru
вывод из запоя череповец
The Real Person!
The Real Person!
домашний интернет
domashij-internet-perm004.ru
интернет провайдер пермь
This is the gentle of criticism I positively appreciate. https://ursxdol.com/furosemide-diuretic/
The Real Person!
The Real Person!
https://domovodov.ru
Greetings! Very useful recommendation within this article! It’s the little changes which liking espy the largest changes. Thanks a a quantity for sharing! https://prohnrg.com/product/rosuvastatin-for-sale/
В НАШЕМ КАНАЛЕ ТЕБЕ ДОСТУПНО:
?? Рейтинг проверенных LIVE-площадок
?? Зеркала с мгновенным доступом
?? Бонусы до 500% + 200 фриспинов
?? Стратегии для Mega Ball и Deal or No Deal
РЕГИСТРИРУЙСЯ И ВЫВОДИ ВЫИГРЫШИ БЕЗ ВЕРИФИКАЦИИ!
https://t.me/s/iGaming_live/2760
The Real Person!
The Real Person!
Капельница при запое – это значимый метод терапии алкоголизма, который широко применяется в лечебных учреждениях Красноярска. Метод детоксикации способствует оперативному восстановлению организма после длительного употребления алкоголя. Время процедуры обычно составляет от 1 до 3 часов, исходя из индивидуальных потребностей и степени запоя. лечение запоя Красноярск Симптомы алкогольного запоя включает в себя сильные головные боли, тошноту и рвоту, потливость и тревожные состояния. Медицинская помощь в виде капельницы позволяет эффективно снять похмелье, вводя жизненно важные вещества для восстановления водно-электролитного баланса и выведения токсинов из организма. Стоимость лечения запоя в Красноярске варьируется в зависимости от обраной лечебной организации и количества требуемых процедур. Прием у нарколога поможет определить наилучший курс лечения, включая восстановление после запоя. Результативность капельницы доказывается множество положительными отзывами пациентов, что делает ее востребованным способом профилактики с алкоголизмом.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-irkutsk001.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
подключить интернет в перми в квартире
domashij-internet-perm005.ru
интернет провайдер пермь
buy esim for thailand https://esim-buy1.com
I am in point of fact delighted to glitter at this blog posts which consists of tons of profitable facts, thanks towards providing such data. https://prohnrg.com/product/rosuvastatin-for-sale/
The Real Person!
The Real Person!
https://clubdom52.ru
The Real Person!
The Real Person!
Online slots in the US have exploded in popularity, but not every game—or casino—is worth your money. Some slots are known for their huge jackpots, others for frequent payouts, and a few have bonus rounds that keep things exciting. With so many choices, figuring out where to play can feel overwhelming. This guide breaks it all down, so you can focus on playing and—hopefully—winning. Play free Poker with friends and with big! Use Gems to get Good Luck Charms! Unsurprisingly, the Buffalo is the highest paying symbol in the game. According to the paytable, the highest payout you can get when playing Buffalo is 300 coins, a payout you’ll get when you hit five Buffalo symbols. This applies both during the base game and the free spins feature. If you’re really lucky, it’s even possible to hit five buffalos and one or more sunset symbols during free spins, which will each multiply your payout.
https://greenhillsaustralia.com/2025/07/15/how-to-access-sweet-bonanza-free-from-the-uk/
Mission Uncrossable has already caught the attention of a wide range of gamblers. Streams and videos of the game gather millions of views on Youtube, TikTok and other social platforms. Roobet’s product has a huge potential and may well surpass the best representatives of the crash-slots genre. The game hints at the degree of risk visually. By switching modes, you can notice how the number of cars on the road and their speed changes. This helps players navigate which level of risk to choose in Mission Uncrossable Roobet. Mission Uncrossable offers a demo mode that allows players to explore the game freely without placing any bets. This mode preserves the game’s random nature, providing a realistic experience of the gameplay. It’s an excellent opportunity to practice your strategies and familiarize yourself with the game mechanics.
The Real Person!
The Real Person!
Скорая наркологическая помощь — это существенная часть в профилактике зависимостей. На сайте vivod-iz-zapoya-krasnoyarsk003.ru вы можете получить сведения о круглосуточной поддержке, которая предлагает необходимую поддержку. Наркологическая служба предоставляет медицинскую помощь при злоупотреблении наркотиками и алкоголем. Специалисты проводят консультации нарколога, а также предлагают анонимное лечение. В рамках ситуационной помощи возможны программы реабилитации и стационарное лечение. Психологическая поддержка играет ключевую роль в восстановлении после зависимости. Не откладывайте обращение за помощью, помогите себе и своим близким!
The Real Person!
The Real Person!
https://cleaninghouse24.ru
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk002.ru
лечение запоя иркутск
The Real Person!
The Real Person!
домашний интернет в перми
domashij-internet-perm006.ru
подключить интернет пермь
обмен NFT через Web3 кошелек
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-minsk001.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
buy deep groove ball bearings ball bearing: A fundamental component in countless mechanical systems, the ball bearing minimizes friction and enables smooth, efficient rotation. Comprising inner and outer rings, precision balls, and a retainer, ball bearings are engineered for optimal load distribution and durability. Discover the versatility and reliability of ball bearings in various applications, from automotive to aerospace.
The Real Person!
The Real Person!
вывод из запоя круглосуточно иркутск
vivod-iz-zapoya-irkutsk003.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
провайдеры ростов
domashij-internet-rostov004.ru
подключить домашний интернет в ростове
The Real Person!
The Real Person!
https://birvodokanal.ru
The Real Person!
The Real Person!
Продвижение карточек товаров на Wildberries Реклама на wildbеrriеs: Эффективная реклама привлекает новых клиентов и увеличивает узнаваемость бренда. Настройте рекламные кампании, выберите релевантные ключевые слова и отслеживайте результаты.
The Real Person!
The Real Person!
buy deep groove ball bearings pillow block: A fundamental component in mechanical systems, the pillow block provides essential support for rotating shafts, ensuring smooth and stable operation. Our selection encompasses a variety of housing materials and bearing types to cater to diverse application needs. Emphasize proper lubrication and regular inspection for sustained performance and longevity, maximizing the return on your investment.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-minsk002.ru
вывод из запоя цена
The Real Person!
The Real Person!
домашний интернет ростов
domashij-internet-rostov005.ru
недорогой интернет ростов
The Real Person!
The Real Person!
лечение запоя калуга
vivod-iz-zapoya-kaluga004.ru
вывод из запоя цена
The Real Person!
The Real Person!
камень подходит свойства Украшения с доставкой Украшения с доставкой – это удобная услуга, которая позволяет получить выбранное украшение прямо у себя дома. Это особенно удобно для тех, кто живет в отдаленных районах или не имеет времени на посещение магазинов.
тик ток мод последняя версия Скачать Тик Ток Мод Поиск и скачивание Тик Ток мода требуют осторожности. Важно загружать файлы только из проверенных источников, чтобы избежать установки вредоносного программного обеспечения. Перед установкой рекомендуется ознакомиться с отзывами пользователей и убедиться в безопасности файла.
The Real Person!
The Real Person!
bearing manufacturer in Latvia bearing manufacturer in Latvia: Discover a trusted bearing manufacturer in Latvia, offering high-quality products and customized solutions for a wide range of industries. Benefit from local expertise, competitive pricing, and responsive customer service. Support local manufacturing and ensure reliable bearing performance in your applications.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-minsk003.ru
вывод из запоя
Offers fast finality — asset transfers typically complete in 3–10 minutes.
The Real Person!
The Real Person!
https://uraldo.ru
cheating porn buy mephedrone lsd
Нужен дом? https://stroitelstvo-domov-kazan1.ru — от проекта до отделки. Каркасные, кирпичные, брусовые, из газобетона. Гарантия качества, соблюдение сроков, индивидуальный подход.
The Real Person!
The Real Person!
подключить домашний интернет ростов
domashij-internet-rostov006.ru
домашний интернет
Интересная статья: Быстрые блинчики на скорую руку: рецепт нежного теста за 5 минут
Fully decentralized with audited smart contracts and validator network.
Читать полностью: Новый Renault Koleos: Обзор и характеристики кроссовера для опытных водителей
Stake once, earn forever — only on marinade.finance Stake smarter on Solana — use marinade fi via marinade.frnance.com
The Real Person!
The Real Person!
вывод из запоя калуга
vivod-iz-zapoya-kaluga005.ru
вывод из запоя цена
Clean UI that’s intuitive for both newcomers and advanced DeFi users.
The Real Person!
The Real Person!
лечение запоя омск
vivod-iz-zapoya-omsk001.ru
экстренный вывод из запоя омск
Нужен дом? https://stroitelstvo-domov-kazan1.ru — от проекта до отделки. Каркасные, кирпичные, брусовые, из газобетона. Гарантия качества, соблюдение сроков, индивидуальный подход.
The Real Person!
The Real Person!
провайдеры интернета по адресу
domashij-internet-samara004.ru
узнать интернет по адресу
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-kaluga006.ru
экстренный вывод из запоя калуга
The Real Person!
The Real Person!
buy ball bearings ball bearings for sale: Navigate the intricacies of motion with our premium selection of ball bearings. Whether you seek to optimize performance in a high-speed spindle or require robust support for heavy industrial machinery, our catalog presents a spectrum of solutions. From miniature precision bearings to heavy-duty industrial models, discover the ideal match for your specific application needs and operational parameters. Consult our seasoned experts for tailored recommendations and ensure unparalleled performance.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk002.ru
вывод из запоя круглосуточно омск
The Real Person!
The Real Person!
https://ukvd43.ru
The Real Person!
The Real Person!
обучение wildberries Реклама велдбериз: приведите новых клиентов в свой магазин.
Transparent fee structure and gas‑optimized for cost efficiency.
The Real Person!
The Real Person!
какие провайдеры по адресу
domashij-internet-samara005.ru
интернет провайдеры по адресу
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar001.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
изготовление стендов для выставки Изготовление выставочных стендов Москва: Широкий спектр услуг и высокое качество Рынок изготовления выставочных стендов в Москве предлагает широкий спектр услуг, от разработки дизайн-проекта до монтажа и демонтажа готовой конструкции. Важно выбрать компанию, которая предлагает высокое качество работ и индивидуальный подход к каждому клиенту.
The Real Person!
The Real Person!
работа для девушек спб Работа для девушек без опыта Работа для девушек без опыта – это первый шаг к построению карьеры. Не стоит бояться начинать с позиций, не требующих большого опыта. Главное – быть готовым к обучению и проявлять инициативу. Важно помнить, что каждый профессионал когда-то начинал с нуля.
The Real Person!
The Real Person!
работа для девушек без опыта Работа в досуге для девушек: творчество и общение Работа в досуге для девушек – это отличная возможность совместить приятное с полезным. Это может быть работа аниматором, организатором мероприятий, ведущей или танцовщицей. Важно любить общаться с людьми, создавать атмосферу праздника и проявлять творческий подход.
The Real Person!
The Real Person!
проверить провайдеров по адресу самара
domashij-internet-samara006.ru
интернет провайдеры по адресу
The Real Person!
The Real Person!
выставочные стенды москва Изготовление стендов в Москве: Комплексный подход и индивидуальные решения Изготовление стендов в Москве – это комплексный подход, включающий в себя разработку концепции, дизайн, изготовление конструкций, монтаж и оформление. Важно выбрать компанию, которая предлагает индивидуальные решения, учитывающие все ваши пожелания и требования.
The Real Person!
The Real Person!
AME Capitals Отзывы AME Capitals – это команда экспертов, которые всегда готовы помочь. Рекомендую всем!
The Real Person!
The Real Person!
вывод из запоя краснодар
vivod-iz-zapoya-krasnodar002.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
http://xn--80aafabrjladsicc1amg1o4cf1dg.live/
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-orenburg001.ru
вывод из запоя
Retention barely dipped when we switched vendors to buy real tiktok likes; charts in the piece show why.
Authentic vibe: buy real followers on x; slower drip felt safer.
Before depositing money anywhere, I did some research. I wanted something legal, simple, and with quick payouts. Reddit pointed me to the best gambling site.
The Real Person!
The Real Person!
подключить интернет в квартиру санкт-петербург
domashij-internet-spb004.ru
провайдер интернета по адресу санкт-петербург
The Real Person!
The Real Person!
изготовление стендов в москве Выставочный стенд: Эффективный инструмент для продвижения бизнеса Выставочный стенд – это не просто демонстрационная площадка, а ключевой элемент маркетинговой стратегии компании, участвующей в выставке. Он служит визитной карточкой бренда, привлекает внимание потенциальных клиентов и партнеров, способствует укреплению имиджа и увеличению продаж. Грамотно спроектированный и оформленный стенд способен значительно повысить эффективность участия в выставке и обеспечить долгосрочные бизнес-результаты.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar003.ru
вывод из запоя краснодар
Надёжный заказ авто закажи авто купить. Машины с минимальным пробегом, отличным состоянием и по выгодной цене. Полное сопровождение: от подбора до постановки на учёт.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-orenburg002.ru
экстренный вывод из запоя оренбург
Полезная статья: Уход за комнатными растениями осенью: главные советы для здоровых цветов
The Real Person!
The Real Person!
стенд для выставки Выставочные стенды Москва: Разнообразие решений для любого бизнеса Выставочные стенды в Москве представлены в широком ассортименте: от стандартных конструкций до эксклюзивных дизайнерских решений. Важно выбрать стенд, который будет соответствовать вашим целям и бюджету.
Интересная новость: Идеальный педикюр дома: избегаем распространенных ошибок
Читать подробнее: Как подготовить автомобиль к зиме: советы эксперта
The Real Person!
The Real Person!
подключение интернета по адресу
domashij-internet-spb005.ru
подключить интернет санкт-петербург
The Real Person!
The Real Person!
интернет провайдеры санкт-петербург по адресу
domashij-internet-spb006.ru
провайдеры по адресу санкт-петербург
wormhole portal bridge
Читать статью: 5 простых шагов для здоровья мозга: советы для женщин после 50
Новое и актуальное: Мыло для лица: почему стоит отказаться от туалетного мыла для красоты кожи
Статьи обо всем: Топ продуктов, ускоряющих старение: советы врача для женщин о здоровье и молодости
Интересные статьи: Фразы, меняющие жизнь: психология счастья и позитива для женщин
The Real Person!
The Real Person!
интернет по адресу уфа
domashij-internet-ufa004.ru
провайдеры в уфе по адресу проверить
The Real Person!
The Real Person!
Трековые натяжные потолки Парящий натяжной потолок монтаж – требует профессиональных навыков и опыта для обеспечения правильной установки и надежной работы подсветки.
Həftəlik cashback proqramı olan pincoazerbaijan.com saytı mənim seçimimdir – itkilərin bir hissəsi geri qaytarılır.
The Real Person!
The Real Person!
провайдер интернета по адресу уфа
domashij-internet-ufa005.ru
подключение интернета по адресу
Читать полностью: Вкусные салаты с тунцом: простые рецепты для праздничного стола и не только
микрозаймы онлайн взять займы
The Real Person!
The Real Person!
AME Capitals Отзывы AME Capitals – это лучшая инвестиционная платформа, которую я когда-либо использовал.
онлайн займы получить микрозайм
взять деньги онлайн займ получить
The Real Person!
The Real Person!
https://177m.ru
I am in point of fact enchant‚e ‘ to glitter at this blog posts which consists of tons of useful facts, thanks for providing such data. pharmacie en ligne fiable cialis professional
Решили заказать авто из японии с аукциона под ключ: подбор на аукционах, проверка, выкуп, доставка, растаможка и постановка на учёт. Честные отчёты, выгодные цены, быстрая логистика.
Автомобили на заказ заказать авто под заказ. Работаем с крупнейшими аукционами: выбираем, проверяем, покупаем, доставляем. Машины с пробегом и без, отличное состояние, прозрачная история.
Надёжный заказ авто заказать авто доставку с аукционов: качественные автомобили, проверенные продавцы, полная сопровождение сделки. Подбор, доставка, оформление — всё под ключ. Экономия до 30% по сравнению с покупкой в РФ.
Хочешь авто заказать авто из китая? Мы поможем! Покупка на аукционе, проверка, выкуп, доставка, растаможка и ПТС — всё включено. Прямой импорт без наценок.
The Real Person!
The Real Person!
провайдеры по адресу уфа
domashij-internet-ufa006.ru
провайдер по адресу уфа
With thanks. Loads of erudition! https://aranitidine.com/fr/levitra_francaise/
The Real Person!
The Real Person!
подключить интернет в волгограде в квартире
domashij-internet-volgograd004.ru
интернет провайдер омск
The Real Person!
The Real Person!
авто из китая Седаны из Китая: комфорт и элегантность по доступной цене. Китайские седаны предлагают просторный салон, современное оснащение и стильный дизайн, конкурируя с европейскими и японскими брендами.
The Real Person!
The Real Person!
https://frasesmotivacional.com/
defillama swap
manta network
manta airdrop
The Real Person!
The Real Person!
http://xn--80aafabrjladsicc1amg1o4cf1dg.press/
defillama
The Real Person!
The Real Person!
домашний интернет тарифы
domashij-internet-volgograd005.ru
подключить интернет в квартиру омск
defillama tvl
The Real Person!
The Real Person!
подключить домашний интернет омск
domashij-internet-volgograd006.ru
подключить интернет в волгограде в квартире
The Real Person!
The Real Person!
авто из китая Запчасти для китайских авто: где купить и сколько стоит? Рассказываем о том, где купить оригинальные и неоригинальные запчасти для китайских автомобилей и сколько они стоят.
The Real Person!
The Real Person!
домашний интернет
domashij-internet-voronezh004.ru
провайдеры воронеж
Нужна душевая кабина? магазины душевых кабин в минске: компактные и просторные модели, стеклянные и пластиковые, с глубоким поддоном и без. Установка под ключ, гарантия, помощь в подборе. Современный дизайн и доступные цены!
The Real Person!
The Real Person!
офисная мебель купить в москве Офисная мебель купить в Москве – задача, требующая взвешенного подхода. Столица предлагает широкий выбор, от экономичных решений для стартапов до элитной мебели для крупных корпораций. Важно учитывать не только цену, но и функциональность, эргономику и соответствие общему стилю офиса. Грамотно подобранная мебель способствует повышению продуктивности и формирует положительный имидж компании.
The Real Person!
The Real Person!
подключить интернет
domashij-internet-voronezh005.ru
провайдеры домашнего интернета воронеж
Нужна душевая кабина? душевая кабина купить недорого: компактные и просторные модели, стеклянные и пластиковые, с глубоким поддоном и без. Установка под ключ, гарантия, помощь в подборе. Современный дизайн и доступные цены!
The Real Person!
The Real Person!
подключить интернет в воронеже в квартире
domashij-internet-voronezh006.ru
подключить домашний интернет в воронеже
defillama yield farming
defillama analytics
defillama dashboard
Base Bridge
The Real Person!
The Real Person!
http://xn--80aafabrjladsicc1amg1o4cf1dg.world/
The Real Person!
The Real Person!
стол для руководителя “Центр принятия решений”: стол для руководителя – символ власти, комфорта и эффективности, выбор материалов, размера и конфигурации, эргономика и интеграция технологий.
The Real Person!
The Real Person!
создание сайтов для бизнеса Сайт под ключ Сайт под ключ: готовое решение для вашего бизнеса Сайт под ключ – это комплексная услуга, включающая в себя все этапы создания сайта, от анализа требований до запуска и поддержки. Заказывая сайт под ключ, вы получаете готовое решение, которое не требует дополнительных усилий и затрат. Это удобно и выгодно, особенно для тех, кто не имеет опыта в веб-разработке.
The Real Person!
The Real Person!
доставка сборного груза из Китая в Россию Автодоставка из Китая – это удобный способ транспортировки грузов, особенно для больших объемов. Этот метод позволяет доставить товары непосредственно к двери получателя, минуя необходимость перегрузки на другие виды транспорта. Важным аспектом является выбор надежной логистической компании, которая сможет обеспечить безопасность и сохранность груза во время перевозки. Автодоставка экономит время и ресурсы.
Продвижение сайта https://team-black-top.ru в ТОП Яндекса и Google. Комплексное SEO, аудит, оптимизация, контент, внешние ссылки. Рост трафика и продаж уже через 2–3 месяца.
Комедия детства один дома 1 — легендарная комедия для всей семьи. Без ограничений, в отличном качестве, на любом устройстве. Погрузитесь в атмосферу праздника вместе с Кевином!
The Real Person!
The Real Person!
Купить сапёрный противоосколочный костюм с доставкой по России (ПВЗ в ЛНР и ДНР) Саперный костюм: купить с доставкой в ЛНР и ДНР. Гарантированная защита при разминировании.
Нужна душевая кабина? душевая кабина лучшие цены, надёжные бренды, стильные решения для любой ванной. Доставка по городу, монтаж, гарантия. Каталог от эконом до премиум — найдите идеальную модель для вашего дома.
The Real Person!
The Real Person!
Купить сапёрный противоосколочный костюм с доставкой по России (ПВЗ в ЛНР и ДНР) Противоосколочные модульные одеяла для защиты от дронов (из СВМПЭ) с доставкой по России (ПВЗ в ЛНР и ДНР) Защитите свое оборудование и персонал от осколков дронов с помощью противоосколочных модульных одеял из СВМПЭ. Легкие, прочные и надежные, эти одеяла обеспечивают эффективную защиту в различных условиях. Доставка осуществляется по всей России, включая пункты выдачи в ЛНР и ДНР. Обеспечьте безопасность с нашими передовыми решениями.
Нужна душевая кабина? душевая кабина цена лучшие цены, надёжные бренды, стильные решения для любой ванной. Доставка по городу, монтаж, гарантия. Каталог от эконом до премиум — найдите идеальную модель для вашего дома.
https://focusbiathlon.com/ – biathlon news, schedule biathlon world cup and standings
оформить займ мфо взять займ
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Архитектурно-строительное бюро https://arhitektura-peterburg.ru
The Real Person!
The Real Person!
Химчистка мебели ростов
The Real Person!
The Real Person!
Стеклянные перегородки Стеклянные перегородки с триплексом: повышенная безопасность и устойчивость к ударам. Технологии изготовления, применение, монтаж.
The Real Person!
The Real Person!
тепловизоры 2024 тактическая экипировка и снаряжение купить Купить тактическую экипировку и снаряжение можно как в интернет-магазинах, так и в обычных магазинах.
The Real Person!
The Real Person!
можно ли тепловизором тепловизионный прицел 35 Тепловизионный прицел с объективом 35 мм обеспечивает оптимальное соотношение угла обзора и дальности обнаружения.
The Real Person!
The Real Person!
https://servicestat.ru/ Servicestat.ru — это удобный каталог-рейтинг сервисных центров по ремонту электроники. На сайте собраны контакты (адреса, телефоны), отзывы клиентов, акции и скидки, а также оценки качества услуг. Пользователи могут быстро найти проверенные мастерские в своем городе, сравнить рейтинги и выбрать лучший вариант. Полезен для тех, кто хочет отдать технику в надежные руки.
? Поиск сервисов по местоположению и брендам
? Реальные отзывы и оценки клиентов
? Акции, скидки и спецпредложения
? Удобный фильтр для сравнения услуг
Идеальный помощник в поиске надежного ремонта!
The Real Person!
The Real Person!
Pharmaceuticals Bazaar Drugs Marketplace: A New Darknet Platform with Dual Access Bazaar Drugs Marketplace is a new darknet marketplace rapidly gaining popularity among users interested in purchasing pharmaceuticals. Trading is conducted via the Tor Network, ensuring a high level of privacy and data protection. However, what sets this platform apart is its dual access: it is available both through an onion domain and a standard clearnet website, making it more convenient and visible compared to competitors. The marketplace offers a wide range of pharmaceuticals, including amphetamines, ketamine, cannabis, as well as prescription drugs such as alprazolam and diazepam. This variety appeals to both beginners and experienced buyers. All transactions on the platform are carried out using cryptocurrency payments, ensuring anonymity and security. In summary, Bazaar represents a modern darknet marketplace that combines convenience, a broad product selection, and a high level of privacy, making it a notable player in the darknet economy.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Bk8
Bk8
Togelup
The Real Person!
The Real Person!
Развлекитесь на полную катушку, выбрав горящий тур с анимацией. Горящие туры по акции: специальные предложения. Горящие туры по акции – это специальные предложения от туроператоров. Вы можете найти туры по очень выгодным ценам. Горящие туры спецпредложения: уникальные возможности. Горящие туры спецпредложения – это уникальные возможности для отдыха. Вы можете найти туры, которые не предлагаются в обычное время. Горящие туры на несколько дней: короткий отпуск. Горящие туры на несколько дней – это отличная возможность для короткого отпуска. Вы можете выбрать тур на выходные или на несколько дней и отдохнуть от работы. Горящие туры на неделю: полноценный отпуск. Горящие туры на неделю – это отличная возможность для полноценного отпуска. Вы можете выбрать тур на неделю и отдохнуть от работы. Горящие туры на выходные: отдых без отрыва от работы. Горящие туры на выходные – это отличная возможность отдохнуть без отрыва от работы. Вы можете выбрать тур на выходные и восстановить силы перед новой рабочей неделей.
Курс по плазмолифтингу prp терапия в гинекологии обучение в гинекологии: PRP-терапия, протоколы, показания и техника введения. Обучение для гинекологов с выдачей сертификата. Эффективный метод в эстетической и восстановительной медицине.
Курс по плазмотерапии обучение плазмотерапии онлайн с выдачей сертификата. Освойте PRP-методику: показания, противопоказания, протоколы, работа с оборудованием. Обучение для медработников с практикой и официальными документами.
saif zone customs emirates post saif zone post office
Togelup
Интересная статья: Простой способ вернуть блеск вашему автомобилю: советы автовладельцам
Интересная статья: Выращиваем салат дома круглый год: простые советы и лайфхаки
Читать статью: Эффективное похудение: топ-7 доступных продуктов для стройной фигуры
The Real Person!
The Real Person!
кайт Кайтсёрфинг зимой: экстремальные приключения на снегу. Почувствуйте адреналин зимнего кайтинга.
The Real Person!
The Real Person!
кайт лагерь “Ремонт”: Ремонт кайта своими руками
Читать в подробностях: Горячие ванны при поликистозе: польза и риски для здоровья
Интересная новость: Вкусные и полезные супы-пюре: рецепты для здоровья и фигуры
Читать новость: 3 дня полного отдыха: когда забыть о домашних делах
The Real Person!
The Real Person!
кайт лагерь Безопасность в кайтсёрфинге: Прежде всего. Соблюдайте правила, используйте защитное снаряжение и не рискуйте без необходимости.
The Real Person!
The Real Person!
кайтсёрфинг Кайтсерфинг в Доминикане: Кабарете – волны и ветер
Новое на сайте: Симптомы раздраженного кишечника: когда стоит обратиться к врачу?
Editorial take: engagement holds up best when teams buy real twitter followers and avoid sudden surges.
Новое на сайте: Яблочный пирог: рецепт с большим количеством яблок и тонким тестом
The Real Person!
The Real Person!
кайтинг Кайтсёрфинг – это комьюнити, где люди разных возрастов и профессий объединены общей страстью к приключениям и адреналину. Найдите своих единомышленников, путешествуйте вместе и делитесь опытом.
Aw8
The Real Person!
The Real Person!
кайтсёрфинг Погода для кайтсёрфинга: Ветер – ваш друг. Идеальный ветер для кайтинга – ровный и умеренный.
Anyswap
AI platform http://bullbittrade.com for passive crypto trading. Robots trade 24/7, you earn. Without deep knowledge, without constant control. High speed, security and automatic strategy.
Innovative AI platform https://lumiabitai.com/ for crypto trading — passive income without stress. Machine learning algorithms analyze the market and manage transactions. Simple registration, clear interface, stable profit.
Frax Swap
українські фільми 2025 українські фільми онлайн HD
фантастичні фільми 2025 нові фільми 2025 в Україні
фільми 2025 безкоштовно безкоштовне кіно Full HD
Mantle Bridge
The Real Person!
The Real Person!
кайтсёрфинг Страховка в кайтсёрфинге: будьте готовы к неожиданностям. Защитите себя от финансовых потерь.
The Real Person!
The Real Person!
кайтинг Кайтсёрфинг – это динамично развивающийся спорт, постоянно появляются новые трюки, оборудование и стили. Будьте в курсе последних тенденций, посещайте соревнования и общайтесь с другими кайтсёрферами.
Bk8
The Real Person!
The Real Person!
кайтсёрфинг Кайтсёрфинг соревнования: проверьте свои силы. Участвуйте в соревнованиях и почувствуйте дух соперничества.
The Real Person!
The Real Person!
кайт “Неопрен – наше все”: Гидрокостюм: как не замерзнуть и чувствовать себя комфортно в любой воде
The Real Person!
The Real Person!
кайтинг Покупка кайта: новые и б/у варианты
The Real Person!
The Real Person!
кайт Кайтсёрфинг комьюнити: друзья по интересам. Вступите в сообщество кайтсёрферов и обменивайтесь опытом.
matcha swap
Bos88
Bandartogel77
comics 3d online HD comics reader free
read shonen manga free online manga site best
манхва главная манхва с переводом
frax swap yield farming
The Real Person!
The Real Person!
кайт школа “Маврикий – мечта”: Ле Морн: кайтсерфинг на Маврикии
The Real Person!
The Real Person!
Сегодня безопасность персональных данных и конфиденциальность в интернете приобретают необходимыми. Если вы хотите прокапаться анонимно‚ важно использовать анонимные услуги‚ такие как VPN и прокси-сервисы‚ которые помогут скрыть личность и скрыть ваш IP-адрес. Данные инструменты предоставляют шифрование соединения‚ что укрепляет безопасность в интернете и защиту ваших действий. Вы также можете обходить блокировки и доступать к сайтам без идентификации. Защита данных становится критически важным элементом незаметного интернет-серфинга‚ обеспечивая вашу безопасность. vivod-iz-zapoya-krasnoyarsk004.ru
The Real Person!
The Real Person!
Методы детоксикации в себя как медикаментытак и методы, которые можно применять на дому. Важно помнить о поддержке семьи и друзей, которая играет ключевую роль в восстановлении после запоя. Кодирование от алкоголизма может помочь предотвратить рецидивы. vivod-iz-zapoya-krasnoyarsk004.ru Программа трезвости и профилактика рецидивов обеспечат стабильный переход к здоровому образу жизни. Лечение запоя и реабилитация – это комплексный процесс, который требует времени и усилий.
The Real Person!
The Real Person!
кайтинг Кайтсёрфинг – это способ укрепить здоровье, улучшить физическую форму и получить заряд положительных эмоций. Это спорт, который делает вас сильнее и счастливее.
Hometogel
The Real Person!
The Real Person!
кайт Обучение кайтсёрфингу
DefiLlama yield farming guide
The Real Person!
The Real Person!
кайт школа Обучение кайтсёрфингу: от первых шагов до виртуозных трюков. Поэтапное обучение, начиная с основ управления кайтом на берегу и заканчивая сложными прыжками и вращениями в воде.
The Real Person!
The Real Person!
Запойное состояние — это явление‚ характеризующееся продолжительным пьянством‚ способное вызвать опасные для здоровья последствия. Признаки запоя представляют собой острую потребность в спиртном‚ недостаток самоконтроля над объемом алкоголя и физические симптомы‚ такие как тремор и обильное потоотделение. Состояние требует медицинской помощи при запое‚ где нарколог на дом предоставит необходимую помощь. Результаты запойного поведения могут быть самыми разными: от физического истощения до психических расстройств. Зависимость от алкоголя часто сопровождается симптомами абстиненции‚ вызывающим дискомфортные ощущения при остановке употребления. В таких случаях очистка организма с использованием капельниц может значительно улучшить состояние пациента. Терапия запоя предполагает не только лекарственное лечение‚ но и психологическую поддержку со стороны родных‚ что играет важную роль в восстановлении после запоя. Исследования в области психологии зависимости указывают на то‚ что избегать запойного состояния можно‚ используя разнообразные подходы‚ включая профессиональную помощь и изменение образа жизни.
The Real Person!
The Real Person!
кайт лагерь Виды кайтов: баллонные, парафойлы, дельтавидные – сравнение и выбор для стиля
The Real Person!
The Real Person!
Капельница от запоя: экстренная помощь при алкогольном отравлении Капельница содержит жизненно важные вещества для нормализации водно-электролитного состояния и укрепления здоровья больного. Комплексное лечение зависимости включает в себя не только детоксикацию, но и долгосрочную терапию алкоголизма. Необходимо учитывать процесс восстановления после запоя, который может занять определённый период и потребовать усилий со стороны пациента. вывод из запоя круглосуточно Экстренная помощь включает в себя диагностику состояния и индивидуальный подход к каждому пациенту. При выявлении симптомов алкогольного отравления необходимо срочно обратиться за медицинской помощью. Не забывайте, что здоровье является наивысшей ценностью, и профессиональная помощь в наркологической клинике поможет восстановить контроль над своей жизнью.
The Real Person!
The Real Person!
обучение кайтсёрфингу Ремонт кайта: профессиональный ремонт и DIY
The Real Person!
The Real Person!
Эффективная капельница от алкогольной зависимости – это поистине эффективный метод помощи алкогольной зависимости, который можно использовать в городе Красноярск с помощью врачей-наркологов, работающих на выезде. Процедура включает введение определённых растворов, которые помогают организму скорее справится с эффектами употребления алкоголя. При обращении за помощью в случае запойного состояния, вы получите не только капельницу от алкоголизма, также консультацию нарколога. врач нарколог на дом Красноярск Снятие запоя обеспечивает быстрое улучшение состояния пациента, а безопасность лечения капельницы гарантируется опытными специалистами. Помощь нарколога включает детокс-программу, направленную на восстановление организма. Реабилитация зависимых – это важный момент после терапии, который способствует предотвратить возврат к употреблению. В Красноярске предоставляются услуги врача нарколога на дом, что позволяет получить терапию в комфортной обстановке. Не откладывайте, обратитесь за срочной помощью нарколога уже сегодня!
mantcha swap transaction fees
смотреть фильм полностью топ фильмы онлайн
Публичная дипломатия России https://softpowercourses.ru концепции, стратегии, механизмы влияния. От культурных центров до цифровых платформ — как формируется образ страны за рубежом.
Институт государственной службы https://igs118.ru обучение для тех, кто хочет управлять, реформировать, развивать. Подготовка кадров для госуправления, муниципалитетов, законодательных и исполнительных органов.
The Real Person!
The Real Person!
Прокапывание от алкоголизма в городе Красноярске – это важный шаг в борьбе с алкоголизма. Методы детоксикации помогают справиться с симптомами абстиненции и освободиться от пьянства. Медицинская помощь в наркологических клиниках включает не только капельницы‚ но и поддержку психолога‚ что помогает восстановлению после употребления спиртного. vivod-iz-zapoya-krasnoyarsk006.ru Реабилитация начинается с очистки организма‚ после которой инициируются реабилитационные программы. Роль родственников также играет ключевую роль в предотвращении срывов. Группы поддержки и обращение к специалиста вполне способны оказать помощь в процессе реабилитации. Не забудьте‚ что борьба с алкоголизмом требует комплексного подхода.
The Real Person!
The Real Person!
вывод из запоя смоленск
vivod-iz-zapoya-smolensk007.ru
вывод из запоя круглосуточно
Школа бизнеса EMBA https://emba-school.ru программа для руководителей и собственников. Стратегическое мышление, международные практики, управленческие навыки.
Опытный репетитор https://english-coach.ru для школьников 1–11 классов. Подтянем знания, разберёмся в трудных темах, подготовим к экзаменам. Занятия онлайн и офлайн.
The Real Person!
The Real Person!
Dapatkan akses kepada kandungan sebenar hanya di kumpulan mega888
Проходите аттестацию https://prom-bez-ept.ru по промышленной безопасности через ЕПТ — быстро, удобно и официально. Подготовка, регистрация, тестирование и сопровождение.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk008.ru
экстренный вывод из запоя смоленск
Sadece pusulabet’in resmi grubunda guvenilir bilgiye ulas
«Дела семейные» https://academyds.ru онлайн-академия для родителей, супругов и всех, кто хочет разобраться в семейных вопросах. Психология, право, коммуникации, конфликты, воспитание — просто о важном для жизни.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-smolensk007.ru
вывод из запоя круглосуточно смоленск
Трэвел-журналистика https://presskurs.ru как превращать путешествия в публикации. Работа с редакциями, создание медийного портфолио, написание текстов, интервью, фото- и видеоматериалы.
The Real Person!
The Real Person!
https://dtf.ru/id2915936/3931207-promt-dlya-sozdaniya-patcha-iz-kartinki-dlya-odezhdy
jonitogel
Свежие скидки https://1001kupon.ru выгодные акции и рабочие промокоды — всё для того, чтобы тратить меньше. Экономьте на онлайн-покупках с проверенными кодами.
«Академия учителя» https://edu-academiauh.ru онлайн-портал для педагогов всех уровней. Методические разработки, сценарии уроков, цифровые ресурсы и курсы. Поддержка в обучении, аттестации и ежедневной работе в школе.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-smolensk009.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk008.ru
вывод из запоя смоленск
Оригинальный потолок натяжной потолок со световыми линиями со световыми линиями под заказ. Разработка дизайна, установка профиля, выбор цветовой температуры. Идеально для квартир, офисов, студий. Стильно, практично и с гарантией.
?аза? тіліндегі ?ндер Сборник хитов казахских песен ж?рекке жа?ын ?уендер мен ?серлі м?тіндер. ?лтты? музыка мен ?азіргі заман?ы хиттер. Онлайн ты?дау ж?не ж?ктеу м?мкіндігі бар ы??айлы жина?.
Sbototo
The Real Person!
The Real Person!
Лазерная резка Лазерная резка
The Real Person!
The Real Person!
Kat?l, sorular sor ve pusulabet ile ilgili her seyi ilk ogrenen sen ol
?аза? тіліндегі ?ндер Казахские хиты ж?рекке жа?ын ?уендер мен ?серлі м?тіндер. ?лтты? музыка мен ?азіргі заман?ы хиттер. Онлайн ты?дау ж?не ж?ктеу м?мкіндігі бар ы??айлы жина?.
The Real Person!
The Real Person!
варфейс купить оружие Я в восторге от 26 ранга! Очень рекомендую всем!
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-smolensk009.ru
лечение запоя
The Real Person!
The Real Person!
шлюхи донецк Донецк, город с трагической историей, где, несмотря на сложности, жизнь продолжается. И на этом фоне, к сожалению, процветает и теневой рынок интимных услуг. “Проститутки Донецк” – это не просто поисковый запрос, это отражение суровой реальности. За каждой девушкой, предлагающей себя, стоит своя личная история, часто связанная с экономическими трудностями и отсутствием перспектив.
Chia s?, th?o lu?n va tuong tac cung nhom 188bet th?t s?
Th?o lu?n va chia s? cung c?ng d?ng 789bet uy tin
Truy c?p nhom xac minh c?a hi88 d? khong b? l? tin quan tr?ng
Готовый комплект Инсталляция с унитазом в комплекте цена инсталляция и унитаз — идеальное решение для современных интерьеров. Быстрый монтаж, скрытая система слива, простота в уходе и экономия места. Подходит для любого санузла.
Shoestring hack—if ads feel steep, a drip?safe move to buy twitter views cheap can validate hooks quicker.
Hay la m?t ph?n c?a c?ng d?ng chinh th?ng shbet ngay hom nay
The Real Person!
The Real Person!
https://billi-walker.jp
Truy c?p nhanh cac thong bao chinh th?c t? f8bet qua nhom xac minh
D?ng b? l? nh?ng c?p nh?t quan tr?ng t? nhom xac minh new88
The Real Person!
The Real Person!
https://lyncconf.com
The Real Person!
The Real Person!
https://www.saffireblue.ca
Truy c?p nhom 33win d? theo doi cac bai vi?t va thong bao dang tin c?y
Kepritogel
G?p g? cac thanh vien th?c s? trong c?ng d?ng Facebook bk8
The Real Person!
The Real Person!
https://acragencia.es/blog/kak_pravilyno_stavity_celi.html
T?t c? n?i dung chinh th?ng d?u co trong nhom Facebook kubet
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar006.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
лечение запоя
narkolog-krasnodar006.ru
вывод из запоя
The Real Person!
The Real Person!
подключить домашний интернет челябинск
chelyabinsk-domashnij-internet004.ru
домашний интернет подключить челябинск
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
narkolog-krasnodar007.ru
лечение запоя
The Real Person!
The Real Person!
провайдеры домашнего интернета челябинск
chelyabinsk-domashnij-internet005.ru
провайдеры челябинск
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar007.ru
экстренный вывод из запоя краснодар
The original and official plinko website – accept no imitations
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
narkolog-krasnodar008.ru
лечение запоя
Conecte-se com a comunidade oficial do tigrinho e evite sites falsos
The Real Person!
The Real Person!
тарифы интернет и телевидение челябинск
chelyabinsk-domashnij-internet006.ru
домашний интернет тарифы челябинск
The Real Person!
The Real Person!
https://www.bangladeshyp.com
Descubra tudo sobre o Jogo do Tigrinho direto da fonte confiavel
The Real Person!
The Real Person!
лечение запоя
narkolog-krasnodar008.ru
вывод из запоя цена
The Real Person!
The Real Person!
O verdadeiro tigrinho esta aqui – confira o conteudo atualizado e confiavel
The Real Person!
The Real Person!
вывод из запоя цена
narkolog-krasnodar009.ru
лечение запоя
The Real Person!
The Real Person!
Nao caia em imitacoes – o site oficial do Fortune tiger e este
The Real Person!
The Real Person!
В современном мире стабильный интернет в Екатеринбурге — это фундамент для успешного ведения бизнеса. Подключить домашний интернет или интернет для бизнеса требует тщательном подходе. Для офисов необходимы услуги связи‚ которые обеспечивают надежность и максимальную скорость интернета для бизнеса. При подключении интернета в офисе стоит рассмотреть возможности корпоративного интернета с прямым подключением. Это обеспечивает стабильную связь и умопомрачительную скорость‚ что особенно важно для работы с облачными технологиями и VPN для бизнеса.Интернет-провайдеры Екатеринбурга представляют различные тарифы на интернет для компаний‚ включая бизнес-решения для связи‚ которые содействуют улучшить рабочий процесс. Wi-Fi для офиса обязан оставаться надежным и безопасным‚ что обеспечивает онлайн-безопасность для компаний. подключить домашний интернет Екатеринбург Итак‚ выбирая интернет для бизнеса‚ обращайте внимание на надежные интернет-решения‚ которые соответствуют вашим требованиям.
The Real Person!
The Real Person!
Сельхоз доска Размещение бесплатных агро объявлений: секреты привлечения внимания, оптимизация текста и выбор эффективных площадок.
The Real Person!
The Real Person!
Acompanhe as novidades do Jogo do Tigrinho com atualizacoes autenticas
The Real Person!
The Real Person!
https://l-w.jp/art/code_promo_de_bienvenue_1xbet____entrez.html 1xBet bonus promo code 2025: GO333 for VIP bonuses – 300% up to €130 sportsbook offer and up to €1,950 + 150 free spins on casino. 1XBET unveils an exclusive bonus for first-time players in the new year. This promotion is only for new customers aged 18+. Sports offers demand 5x-10x wagering on parlays with 3+ selections at 1.40 odds or above in 30 days.
Use 1xBet welcome bonus code 2025: GO333. Activate this code while registering on the official site, you’ll get a generous 120% bonus up to $200. This offer applies to sports betting. To secure this bonus, register on 1xBet and input it when prompted for a code. Visit 1xBet for rules for bonus conditions.
The Real Person!
The Real Person!
Entre para a comunidade verdadeira do tigrinho atraves da plataforma oficial
The Real Person!
The Real Person!
проститутки индивидуалки донецк Шлюхи Калининский район: Заказать шлюху в Калининском районе Донецка.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar009.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
лечение запоя
narkolog-krasnodar010.ru
вывод из запоя цена
The Real Person!
The Real Person!
Conecte-se ao conteudo oficial do Jogo do Tigrinho em uma pagina verificada
The Real Person!
The Real Person!
В Екатеринбурге интернет-соединение осуществляется через различных провайдеров‚ предлагающих разнообразные тарифы на интернет. Чтобы подключить домашний интернет в Екатеринбурге‚ важно обратить внимание на скорость интернета и качество связи. Беспроводная сеть‚ основанная на точках доступа Wi-Fi‚ позволяет использовать интернет без проводов‚ что удобно для мобильного доступа к сети. При выборе интернет-провайдера стоит сравнить предложения провайдеров‚ чтобы узнать об их услугах связи в Екатеринбурге. Роутеры для Wi-Fi обеспечивают надежное подключение в квартире или доме. Беспроводные технологии набирают популярность благодаря своей удобству и мобильности. Подключение интернета — это важный шаг для обеспечения доступа к информации и развлечениям.
The Real Person!
The Real Person!
Descubra as verdadeiras vantagens do Jogo do Tigrinho no site oficial
The Real Person!
The Real Person!
Visite o tigrinho e conecte-se com a comunidade oficial e ativa
The Real Person!
The Real Person!
Нарколог на дому: профессиональная помощь при зависимости Проблема наркотической зависимости требует внимательного и профессионального подхода. Первый шаг к выздоровлению – это консультация с наркологом. На сайте narkolog-tula008.ru можно получить полную информацию о вызове врача нарколога на дом. Лечение подразумевает проведение детоксикации организма и предоставление психологической помощи. В некоторых случаях необходима реабилитация зависимых, которая включает специализированные программы. Анонимное лечение обеспечивает комфорт и безопасность для пациента. Терапия на дому позволяет обеспечить необходимую помощь на дому, что особенно важно для тех, кто стесняется обращаться в клиники. Алкогольная зависимость может быть успешно лечена на дому, что ускоряет процесс выздоровления. Обращение к квалифицированному специалисту – это залог успешной борьбы с зависимостями.
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar010.ru
вывод из запоя
The Real Person!
The Real Person!
Нарколог на выезде — это вариант для людей, нуждающихся в поддержке, но не может или не хочет посещать клинику. Услуги московского нарколога включают консультацию нарколога на дому, что обеспечивает анонимное лечение и удобные условия для пациента. Процесс лечения зависимостей начинается с диагностики и облегчения симптомов абстиненции. narkolog-tula009.ru Квалифицированные специалисты предлагают помощь нарколога, которая включает медицинскую помощь при алкоголизме и психотерапевтические сеансы при зависимостях. Реабилитация наркозависимых также возможна на выезде, что позволяет вовлечь близких в процесс поддержки. Предотвращение рецидивов и постоянная поддержка имеют решающее значение для успешного выздоровления. Посещение врача-нарколога на дому может стать первым шагом к здоровой жизни.
Сколько бы ни было казино в интернете, редко где встретишь такое сочетание удобства, честности и азарта. Когда решил Vodka Casino скачать, не ожидал, что это будет полноценная замена всему, что пробовал раньше. Тут ты не просто гость, а полноценный участник. Промо-акции, бонусы, турниры — всё в одном месте. И главное — доступно. Даже если блокируют основной домен, зеркало срабатывает автоматически. Не нужно ничего искать. Просто заходишь и играешь. А мобильная версия — отдельная песня. Гладкая, понятная, отзывчивая. Радует, что здесь можно не только поиграть, но и заработать. Без подвоха.
The Real Person!
The Real Person!
Descubra as verdadeiras informacoes sobre o Fortune Tiger com total confianca
The Real Person!
The Real Person!
интернет провайдеры в казани по адресу дома
kazan-domashnij-internet004.ru
подключить домашний интернет казань
The Real Person!
The Real Person!
Алкоголизм — это серьезная проблема, которая касается не только зависимого, но и его близких. Обращение к наркологу на дом в Туле может стать первым шагом к борьбе с алкоголизмом. Обсуждение с наркологом поможет определить уровень зависимости и предложить лечение алкоголизма, включая психотерапию при алкоголизме.Семейные проблемы из-за алкоголя часто приводят к кризису в семье. Помощь со стороны семьи играет ключевую роль в возстановлении здоровья. Семье необходимо знать, как поддержать, и какие советы по борьбе с зависимостью могут быть полезны. Лечение алкоголиков включает в себя не только медицинское вмешательство, но и поддержку социума. Предотвращение алкоголизма начинается с осознания проблемы и желания изменить ситуацию. Возобновление нормальных отношений — это длительный путь, который требует усилий всей семьи. вызов нарколога на дом тула Вызов врача на дом дает возможность воспользоваться помощью в привычной обстановке, что помогает лучше понять информацию и уменьшает стресс. Помощь семье и согласие на лечение — это основные этапы на дороге к восстановлению.
The Real Person!
The Real Person!
провайдеры интернета по адресу
kazan-domashnij-internet005.ru
подключение интернета по адресу
The Real Person!
The Real Person!
No site oficial do Jogo do Tigrinho voce encontra tudo sobre o game
The Real Person!
The Real Person!
Эффективная капельница от алкогольной зависимости – это поистине результативный метод лечения зависимости от алкоголя, что можно использовать в Туле с помощью врачей-наркологов, работающих на выезде. Процедура включает инъекции специальных растворов, которые помогают организму быстрее справится с последствиями алкогольного опьянения. При обращении за помощью в случае запойного состояния, вы получите не только капельницу от алкоголизма, также советы нарколога. врач нарколог на дом тула Снятие запоя обеспечивает оперативное улучшение состояния пациента, а безопасность процедуры капельницы гарантируется опытными специалистами. Помощь нарколога включает детокс-программу, направленную на очищение организма. Реабилитация зависимых – это ключевой момент после лечения, который поможет избежать рецидивов. В Туле доступны услуги врача нарколога на дом, что позволяет пройти лечение в комфортной обстановке. Не медлите, позвоните за экстренной помощью врача-нарколога уже сегодня!
The Real Person!
The Real Person!
Confie no site verdadeiro do tigrinho para novidades e seguranca
The Real Person!
The Real Person!
https://gloexpresslog.com/and-the-day-came-when-the-risk-to-remain-tight-in-a-bud-was-more-painful-than-the-risk-it-took-to-blossom/#comment-1978
The Real Person!
The Real Person!
Ganhe confianca acessando apenas o site verdadeiro do tigrinho
The Real Person!
The Real Person!
Служба наркологии — это необходимая часть системы здравоохраненияобеспечивающая помощь в лечении зависимостей и восстановление. На сайте narkolog-tula012.ru можно найти сведения о наркологических клиниках, где предоставляется помощь при алкогольной зависимости и наркомании. Психотерапевтические методики и программы реабилитации помогают пациентам восстановиться, а поддержка близких играет ключевую роль в этом процессе. Также доступны анонимные консультации и службы экстренной помощи для неотложной помощи. Профилактика наркомании и адаптация в обществе — важные аспекты работы службы наркологии.
The Real Person!
The Real Person!
интернет провайдеры по адресу казань
kazan-domashnij-internet006.ru
провайдер по адресу
The Real Person!
The Real Person!
Participe do Jogo do Tigrinho e aproveite bonus exclusivos
Bosstoto
The Real Person!
The Real Person!
Экстренный вывод из запоя в Туле: как оперативно возвратиться к обычной жизни Когда алкогольная интоксикация становится серьезной проблемой, необходимо знать, что есть шанс получить срочную помощь. Нарколог на дом срочно — это вариант для тех, кто нуждается в неотложной поддержке. Лечение алкоголизма требует квалифицированного подхода, и наркологическая клиника готова предоставить необходимую профессиональную помощь на дому. Поддержка семьи является важной составляющей процесса восстановления. Психотерапия при зависимости от алкоголя поможет справиться с эмоциональными проблемами и предотвратить возврат к прежним привычкам. Обратившись к наркологу на дом, вы делаете первый шаг к избавлению от зависимости и ретурну к нормальной жизни.
The Real Person!
The Real Person!
какие провайдеры на адресе в красноярске
krasnoyarsk-domashnij-internet004.ru
провайдер по адресу
The Real Person!
The Real Person!
Капельное лечение запоя – данный метод является распространенным методом лечения алкоголизма, который применяется в клиниках в Туле для очистки организма. Круглосуточное лечение дает возможность быстро избавиться симптомы запоя. Тем не менее важно помнить о возможных побочных эффектах, включающих аллергические реакции и нарушение водно-солевого баланса. вывод из запоя круглосуточно тула
The Real Person!
The Real Person!
Капельница при алкогольной зависимости — это необходимая первая помощь в случае запоя. При наблюдении симптомов запоя, таких как обнаружите симптомы, такие как интенсивная жажда, боли в голове, тошнота и усталость, обратиться к наркологу на дому в Туле может стать хорошим выходом. Специалист проведет медицинскую помощь и назначит капельницу, которая способствует восстановлению организма. вызвать нарколога на дом тула Преодоление алкогольной зависимости включает вывод из запоя, что способствует значительному улучшению здоровья после запоя. Капельница содействует устранить обезвоживание, восполнить дефицит минералов и улучшить общее состояние пациента. Если требуется можно организовать лечение в домашних условиях, что гарантирует комфорт и безопасность.Наркология в Туле предлагает качественные услуги по реабилитации от алкоголя, что обеспечивает справиться с зависимостью эффективно. Эффекты капельницы заметны быстро: пациенты отмечают улучшение самочувствия и возвращение энергии. Не откладывайте откладывать обращение за помощью, бережное отношение к здоровью — залог вашей жизни!
The Real Person!
The Real Person!
провайдер интернета по адресу красноярск
krasnoyarsk-domashnij-internet005.ru
провайдеры интернета по адресу красноярск
The Real Person!
The Real Person!
Тульская наркологическая служба предлагает разнообразные услуги для помощи людям с зависимостями. В этом городе работают специализированные реабилитационные центры‚ где можно получить анонимное лечение наркозависимости и алкоголизма. Программа лечения алкоголизма включает в себя как медицинские процедуры‚ так и психотерапию. narkolog-tula009.ru Психологическая помощь Тула также играет важную роль в процессе выздоровления. Обратитесь к наркологу позволяет определить уникальные особенности пациента и составить индивидуальный план терапии. Профилактика зависимостей и поддержка родственников зависимых – неотъемлемая часть работы наркологической клиники. Реабилитация зависимых включает как терапевтические меры‚ так и социальное reintegration. Услуги наркологической службы направлены на восстановление здоровья и успешное возвращение к нормальной жизни.
The Real Person!
The Real Person!
Лечение алкогольной интоксикации в Туле: услуги нарколога Алкогольная интоксикация — серьезное состояние, требующее немедленной медицинской помощи. В медицинском центре Тула доступны услуги по лечению алкоголизма, включая детоксикацию и реабилитацию после запоя. Процедуры капельниц помогают очищать организм от токсинов, облегчая симптомы алкогольной интоксикации. Консультация нарколога необходима для индивидуального подхода. Симптомы алкогольной интоксикации включают головную боль, тошноту и слабость. Профессиональная помощь нарколога охватывает как детоксикацию, так и психотерапевтические методы лечения алкоголизма. Комплексный подход к реабилитации алкоголиков и профилактика запоев помогают предотвратить рецидивы. В Туле можно получить качественную медицинскую помощь, что позволяет вернуть здоровье и нормальную жизнь.
The Real Person!
The Real Person!
пансионат для лежачих больных
pansionat-msk004.ru
пансионат с медицинским уходом
Experimente o verdadeiro Fortune Tiger com ganhos instantaneos
Participe do Fortune Tiger com pagamentos rapidos e garantidos
The Real Person!
The Real Person!
https://bs2-bs2site.at
The Real Person!
The Real Person!
пансионат с деменцией для пожилых в москве
pansionat-msk005.ru
частные пансионаты для пожилых в москве
Comece sua sorte no tigrinho com seguranca e bonus reais
The Real Person!
The Real Person!
В современном мире тематика зависимостей становится все более актуальной. Если вы ищете нарколога на дом в Туле, сайт narkolog-tula011.ru предоставляет разнообразные наркологических услуг. Коррекция зависимостей может быть непростым процессом, однако домашний нарколог обеспечивает помощь при алкоголизме и другим зависимостям непосредственно на дому. Первичная консультация – это первый шаг к излечению. Специалисты проведут диагностику зависимости, помогут составить программу реабилитации и предложат методы психотерапии для лечения зависимостей. Анонимное лечение гарантирует полную секретность, что особенно важно для многих пациентов. Медицинская помощь на дому позволяет не только комфортно проходить лечение, но и получать поддержку близких, что играет ключевую роль в процессе восстановления. Профилактика зависимостей и восстановление после наркотиков также входят в комплекс услуг, предлагаемых специалистами. Обращайтесь за помощью на narkolog-tula011.ru, чтобы получить нужную помощь.
The Real Person!
The Real Person!
https://bs2bsme.at
Ganhe premios reais no verdadeiro tigrinho online
The Real Person!
The Real Person!
пансионат инсульт реабилитация
pansionat-msk006.ru
пансионат для престарелых людей
Explore o mundo do tigrinho com total confianca
The Real Person!
The Real Person!
https://bs-bsme.at
Ganhe recompensas no Fortune Tiger confiavel e testado
The Real Person!
The Real Person!
какие провайдеры на адресе в красноярске
krasnoyarsk-domashnij-internet006.ru
подключение интернета по адресу
The Real Person!
The Real Person!
Обращение к наркологам в Туле — необходимая мера, помогающая людям, страдающим от зависимостей. Клиника наркологии в Туле предлагает широкий спектр услуг, включая лечение зависимостей и медицинскую помощь при алкоголизме. Если вы или ваши близкие нуждаетесь в поддержке, обязательно рассмотрите вариант вызова нарколога.Консультация нарколога может стать первым шагом к выздоровлению. Специалисты предоставляют анонимное лечение, чтобы обеспечить комфорт и конфиденциальность пациента. Круглосуточная служба поддержки всегда готова помочь и ответить на ваши вопросы, особенно в экстренных ситуациях. narkolog-tula012.ru Процесс реабилитации для зависимых включает в себя программы лечения зависимостей и психотерапию при зависимости. Услуги по вызову врача-нарколога доступны в любое время, что делает процесс лечения максимально удобным. Не стесняйтесь и не откладывайте помощь, поскольку здоровье — это самое ценное.
The Real Person!
The Real Person!
https://bs2-bs2site.at
The Real Person!
The Real Person!
пансионат для пожилых в туле
pansionat-tula004.ru
пансионаты для инвалидов в туле
Thanks on sharing. It’s outstrip quality. https://ondactone.com/product/domperidone/
The Real Person!
The Real Person!
какие провайдеры на адресе в краснодаре
krasnodar-domashnij-internet004.ru
провайдер интернета по адресу краснодар
The Real Person!
The Real Person!
https://b2best.at
O Jogo do Tigrinho original esta a um clique de distancia
The Real Person!
The Real Person!
Поддержка близких при алкогольной зависимости Алкоголизм — это не только проблема зависимогоно и его близких. В Туле обращение к специалисту может стать первым этапом к лечению зависимости. Семейная поддержка играет ключевую роль в процессе реабилитации. Близкие могут помочь, предоставляя эмоциональную поддержку и создавая атмосферу сочувствия. В кризисной ситуации важно искать за профессиональной помощью. Консультация нарколога поможет найти правильный путь к терапии алкоголизма. Психотерапия также может стать важным инструментом для восстановления. вызов нарколога тула Поддержка со стороны общества семьи необходима на всех этапах лечения, от первого обращения с наркологом до последующей реабилитации. Помощь близких может ускорить процесс выздоровления и вернуть зависимого к нормальной жизни.
The Real Person!
The Real Person!
частный пансионат для пожилых людей
pansionat-tula005.ru
пансионат с деменцией для пожилых в туле
Jogue tigrinho agora e descubra premios reais e diversao garantida
The Real Person!
The Real Person!
провайдеры по адресу краснодар
krasnodar-domashnij-internet005.ru
интернет по адресу
The Real Person!
The Real Person!
https://bs2besd.cc
The Real Person!
The Real Person!
Проблема алкогольной зависимости: важность внимательного подхода. Многие ищут помощь анонимно, чтобы избежать осуждения. Нарколог на дом предлагает услуги по лечению запоя, обеспечивая комфорт и приватность. Обращение к наркологу позволяет получить необходимую медицинскую помощь при запое без ненужных вопросов. Анонимное лечение подразумевает detox-программы, которые включают в себя психологическую поддержку и медикаментозное сопровождение. Это позволяет избежать рецидива алкоголизма и восстановиться после запоя. Кризисная помощь помогает преодолеть острые состояния, предоставляя необходимые ресурсы. Таким образом, анонимный вывод из запоя – это вполне реальная возможность для тех, кто ищет помощь.
The Real Person!
The Real Person!
узнать провайдера по адресу краснодар
krasnodar-domashnij-internet005.ru
какие провайдеры на адресе в краснодаре
A sorte esta com voce no Fortune tiger legitimo
A sorte esta ao seu lado no Fortune Tiger – venha girar agora
Tudo sobre o tigrinho esta aqui – acesse agora e explore
The Real Person!
The Real Person!
пансионат для лежачих тула
pansionat-tula006.ru
пансионат для лежачих пожилых
The Real Person!
The Real Person!
провайдер интернета по адресу краснодар
krasnodar-domashnij-internet006.ru
подключение интернета по адресу
The Real Person!
The Real Person!
Врач-нарколог на дом в Туле предоставляет квалифицированную помощь тем, кто борется с наркоманией и алкоголизмом. Лечение зависимости должно учитывать индивидуальные особенности, и вызов нарколога на дом – это практичное решение для людей, нуждающихся в помощи. Центр лечения зависимостей в Туле предлагает анонимное лечение и профессиональные консультации. Реабилитация на дому даёт возможность пройти психотерапию при зависимости в домашней обстановке. Медицинская помощь на дому включает в себя как телесное, так и психологическое сопровождение. Предотвращение зависимости также играет важную роль в противостоянии алкоголизму и наркомании. Поддержка алкоголиков и их родных – это задача профессионалов, которые готовы протянуть руку помощи в трудную минуту. narkolog-tula014.ru
The Real Person!
The Real Person!
https://abs2best.at
goltogel
Tudo sobre tigrinho voce encontra no site original
The Real Person!
The Real Person!
лечение запоя тула
tula-narkolog001.ru
вывод из запоя круглосуточно тула
The Real Person!
The Real Person!
https://bsmc.at
O unico Jogo do Tigrinho confiavel esta aqui – aproveite
The Real Person!
The Real Person!
В Москве количество интернет-провайдеров огромен, и множество компаний представляют пакеты услуг интернета и телевидения. Связь в столице предлагают как высокоскоростной интернет, так и цифровое телевидение. При выборе стоит обратить внимание на тарифы на интернет и телевидение, чтобы подобрать выгодные варианты.Анализ цен позволит найти выгодные предложения. Некоторые компании предлагают специальные предложения, что позволяет значительно сэкономить. Спутниковое телевидение и IPTV также рассматриваются как доступными опциями для жителей Москвы.На сайте msk-domashnij-internet004.ru представлены актуальными предложениями по интернету и телевидению, доступными в столице. Найдите оптимальный вариант и наслаждайтесь высоким качеством контента!
The Real Person!
The Real Person!
пансионат для лежачих москва
pansionat-msk004.ru
пансионат для престарелых людей
totojitu
The Real Person!
The Real Person!
лечение запоя тула
tula-narkolog002.ru
вывод из запоя цена
The Real Person!
The Real Person!
https://bs2bet.at
The Real Person!
The Real Person!
Выбор домашнего интернета в Москве – вопрос не из легких. С множество интернет-провайдеров‚ которые предлагают различные тарифы на интернет‚ важно ориентироваться на мнения клиентов. Они помогают в оценке качества связи и скорости интернета‚ что критично для удобного серфинга. msk-domashnij-internet005.ru При выборе провайдеров обратите внимание на качество соединения и доступный интернет. Ознакомьтесь с акционные предложения и скидки‚ чтобы выбрать лучшее решение по цене и качеству. Не забудьте учесть качество техподдержки‚ ведь она необходима для оперативного устранения неполадок с подключением интернета.
The Real Person!
The Real Person!
дом престарелых
pansionat-msk005.ru
частный пансионат для пожилых
The Real Person!
The Real Person!
https://bsme-at.at
The Real Person!
The Real Person!
вывод из запоя тула
tula-narkolog003.ru
экстренный вывод из запоя тула
Ganhe de verdade no site original do tigrinho
The Real Person!
The Real Person!
В современном бизнесе надежное интернет-соединение является. Провайдеры для бизнеса предоставляют широкий выбор тарифов на интернет‚ в т.ч. бизнес-пакеты и корпоративные тарифы. Качество интернет-соединения играют ключевую роль в эффективности работы. На сайте msk-domashnij-internet006.ru можно найти выгодные предложения от ведущих провайдеров‚ включая хостинг и беспроводной интернет. Выбирая провайдера‚ принимайте во внимание свои нужды и возможности подключения к интернету‚ чтобы обеспечить стабильные услуги связи для вашей организации.
O Jogo do Tigrinho verdadeiro esta disponivel neste site
The Real Person!
The Real Person!
пансионат для пожилых с деменцией
pansionat-msk006.ru
пансионат для пожилых с инсультом
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-chelyabinsk004.ru
лечение запоя
Descubra como jogar tigrinho de forma confiavel e facil
The Real Person!
The Real Person!
https://abs2best.at
The Real Person!
The Real Person!
провайдер по адресу
nizhnij-novgorod-domashnij-internet004.ru
подключение интернета нижний новгород
The Real Person!
The Real Person!
When selecting Christmas Big Bass Bonanza, you’ll have wagering options that alternate between $0.10 to $250.00 per spin. The amount wagered won’t affect the player’s capability at triggering bonus features & winning payout combinations. However, wagering values affect the payouts valuation. For instance, $50,000 can exclusively be awarded when punters have staked the maximum bet. Just remember that Wilds, Free Spins, and Multipliers are available to gamblers regardless of their wager. Gamblers that interact with Christmas Big Bass Bonanza will maintain enjoyable gameplay constructed through the Pragmatic Play Software & Reel Kingdom Development House. The experiences these manufacturers have built exceed expectations, as 3D Animations are matched alongside the gameplay mechanics. That’ll mean payout symbols will double in their size & glisten with shining lights as winning combinations are awarded onto players.
https://www.system-print.de/no-time-to-waste-jump-into-the-action-instantly-with-aviator-by-spribe/
Max win potential ranges from 2,100 x bet at the bottom end with the debut Big Bass Bonanza, Christmas Big Bass Bonanza and most recently Big Bass Halloween right up the largest Big Bass max win of 20,000 x bet available on Big Bass Bonanza 1000 and Big Bass Hold and Spinner Megaways. Big Time Gaming are famous for their Megaways casino series. Play Bonanza, Chocolates, and Pop for real money bets at MrQ online casino. The big real money prizes on tap make jackpot slots almost appealing…at least on the surface. But on average, progressive slots feature the lowest RTPs in gambling, so the odds of winning aren’t so great. On top of that, you always need to bet the maximum to access the top jackpot prizes – meaning you might end up spending more money than you intend to. There is no shortage of bonuses, either. There are free spins and multipliers as well as free games within the game. If you like big scores, you’re in luck because the maximum jackpot is 50,000 times your wager.
The Real Person!
The Real Person!
пансионат с медицинским уходом
pansionat-tula004.ru
пансионат для лежачих после инсульта
The Real Person!
The Real Person!
вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk005.ru
вывод из запоя круглосуточно челябинск
The Real Person!
The Real Person!
https://bs2vveb.at
The Real Person!
The Real Person!
подключить интернет в нижнем новгороде в квартире
nizhnij-novgorod-domashnij-internet005.ru
подключить интернет в квартиру нижний новгород
The Real Person!
The Real Person!
частный пансионат для пожилых
pansionat-tula005.ru
пансионат для престарелых
tron bridge
The Real Person!
The Real Person!
https://bsme-at.at
The Real Person!
The Real Person!
провайдер по адресу нижний новгород
nizhnij-novgorod-domashnij-internet006.ru
какие провайдеры на адресе в нижнем новгороде
The Real Person!
The Real Person!
лечение запоя череповец
vivod-iz-zapoya-cherepovec007.ru
лечение запоя
The Real Person!
The Real Person!
пансионат для пожилых после инсульта
pansionat-tula006.ru
пансионат для пожилых с инсультом
The Real Person!
The Real Person!
https://bs2-bs2site.at
ton bridge
The Real Person!
The Real Person!
Ищете доступный интернет в новосибирске следует обратить внимание на несколько моментов. Для выбора интернет-провайдераначните с рассмотрения мнений пользователей о провайдерах на сайте novosibirsk-domashnij-internet004.ru. Сравнив тарифы, вы сможете найти оптимальные предложения на интернетвключая доступные тарифы и безлимитные опции в новосибирске. Обратите внимание на скорость интернета в новосибирске. Комфортное пользование требует наличия высокоскоростного интернета. Изучите акции и скидкикоторые предлагают разные провайдеры. Онлайн-подключение интернета сделает процесс гораздо проще.
The Real Person!
The Real Person!
экстренный вывод из запоя череповец
vivod-iz-zapoya-cherepovec008.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя круглосуточно тула
tula-narkolog001.ru
лечение запоя
The Real Person!
The Real Person!
Los reembolsos están disponibles una vez por semana y caducan después de siete días, ya que requiere que el servicio de atención al cliente de Skrill elimine un correo electrónico. Cada día obtienes 10 giros gratis, pero siempre quisiste tener una experiencia de juego de cricket en tu propia vida. ¿Cuáles Son Los Mejores Bonos De Narcos Mexico Para Nuevos Clientes? Ganar en este juego base no es tan emocionante como los extras, recibí la información de que el retiro solo puede realizarse si hago un depósito con mi nueva billetera electrónica. Ganar un increíble premio en efectivo se paga a todos los jugadores que se conectan con éxito con los fabulosos juegos de casino con depósitos lo más grandes posible no son nuevos en este casino, monedas en big bass bonanza más conocidas como máquinas de póquer. Hold and Win y muchas otras tragamonedas, tras nuestra revisión.
https://noperti.com/analisis-de-la-popularidad-de-sugar-rush-de-pragmatic-play-en-espana/
La esencia de privacidad de ‘spin’. Echa un jugador; eres solo un vistazo a nuestras slots, una de las ganancias. Sumérgete en nuestro lanzamiento más dulce del azúcar personalizados, una fantástica. Sumérgete en sugar rush pragmatic play. Echa un emocionante viaje de tiradas gratuitas durante el creador y tema sugar rush tragamonedas de jugadores que se convierte en modo demopor pragmatic play. Tienes alguna sugerencia o más populares de cartas, tu aventura de internet. Para obtener ganancias. De sugar rush 1000 gratis en nuestra guía sobre las preferidas. El Maxi Sugar Rush de Scheepjes es un hilo de peso de encaje, de alta torsión que cuenta con un lujoso brillo. La Sugar Rush 1000 slot se ha convertido en una de las grandes novedades del catálogo de YoCasino y podemos acceder a este título con la única condición de registrarnos previamente en la plataforma. Esto nos da acceso a una tragaperras vanguardista, que aprovecha el nuevo formato para pulir detalles imprescindibles.
The Real Person!
The Real Person!
В новосибирске разнообразие поставщиков интернета достаточно обширно, но для геймеров критически важно выбрать провайдера с низким пингом и стабильным соединением. Высокоскоростной интернет обязателен для игр в сети, чтобы избежать лагов. Цены на интернет-услуги разнятся, поэтому стоит тщательно рассмотреть предложения. Оптоволоконный интернет гарантирует большую скорость соединения и минимальную задержку. Кабельные технологии может быть хорошим вариантом, но важно ознакомится с отзывами о провайдерах на сайте novosibirsk-domashnij-internet005.ru. Советуем сделать тест скорости интернета перед подключением. Выбирайте провайдера, который гарантирует стабильное соединение для комфортной игры!
The Real Person!
The Real Person!
вывод из запоя череповец
vivod-iz-zapoya-cherepovec009.ru
лечение запоя череповец
The Real Person!
The Real Person!
https://bs-bsme.at
The Real Person!
The Real Person!
вывод из запоя круглосуточно тула
tula-narkolog002.ru
вывод из запоя цена
The Real Person!
The Real Person!
Столица России стала центром медиа-влияния, где ТВ и интернет служат мощными инструментами пропаганды. Средства массовой информации формируют восприятие людей, контролируя данные. На novosibirsk-domashnij-internet006.ru можно наблюдать, как рекламные кампании и содержимое манипулируют сознанием аудитории. Социальные сети добавляют дополнительный слой влияния, позволяя делиться новости мгновенно. Политтехнологии используют эти инструменты для контроля восприятием. Цензура в средствах массовой информации создает барьеры, ограничивая свободу слова. Связь с обществом через коммуникацию становится ключевой для определения мнений. В конечном итоге, влияние медиа на общество в столице невозможно переоценить.
The Real Person!
The Real Person!
лечение запоя иркутск
vivod-iz-zapoya-irkutsk004.ru
вывод из запоя иркутск
The Real Person!
The Real Person!
https://bsmc.at
Hand Sanitisers
The Real Person!
The Real Person!
вывод из запоя круглосуточно
tula-narkolog003.ru
вывод из запоя цена
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-irkutsk005.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
провайдеры интернета в омске
omsk-domashnij-internet004.ru
подключить домашний интернет в омске
The Real Person!
The Real Person!
https://abs2best.at
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-chelyabinsk004.ru
лечение запоя
Greetings! Extremely productive advice within this article! It’s the scarcely changes which liking obtain the largest changes. Thanks a lot in the direction of sharing! where to get cheap motilium online
The Real Person!
The Real Person!
экстренный вывод из запоя иркутск
vivod-iz-zapoya-irkutsk006.ru
вывод из запоя
The Real Person!
The Real Person!
домашний интернет омск
omsk-domashnij-internet005.ru
подключить интернет в квартиру омск
The Real Person!
The Real Person!
https://bs2bet.at
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-chelyabinsk005.ru
лечение запоя челябинск
The Real Person!
The Real Person!
лечение запоя калуга
vivod-iz-zapoya-kaluga007.ru
вывод из запоя круглосуточно калуга
The Real Person!
The Real Person!
https://bs2-bs2site.at
The Real Person!
The Real Person!
подключить интернет тарифы омск
omsk-domashnij-internet006.ru
домашний интернет омск
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-kaluga008.ru
вывод из запоя круглосуточно калуга
The Real Person!
The Real Person!
вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk006.ru
вывод из запоя
Мы предлагаем уборка офиса в Москве и области, обеспечивая высокое качество, внимание к деталям и индивидуальный подход. Современные технологии, опытная команда и прозрачные цены делают уборку быстрой, удобной и без лишних хлопот.
методы лечения зависимости лечение лудомании
Мы предлагаем поиск протечки в СПб и области с гарантией качества и соблюдением всех норм. Опытные мастера, современное оборудование и быстрый выезд. Честные цены, удобное время, аккуратная работа.
The Real Person!
The Real Person!
интернет провайдеры пермь
perm-domashnij-internet004.ru
провайдеры домашнего интернета пермь
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-kaluga009.ru
лечение запоя калуга
клиника лечения зависимостей лечение игровой зависимости
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-cherepovec007.ru
лечение запоя
The Real Person!
The Real Person!
домашний интернет
perm-domashnij-internet005.ru
домашний интернет
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-krasnodar006.ru
вывод из запоя цена
The Real Person!
The Real Person!
интернет тарифы пермь
perm-domashnij-internet006.ru
провайдеры интернета пермь
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar008.ru
вывод из запоя цена
The Real Person!
The Real Person!
домашний интернет подключить ростов
rostov-domashnij-internet004.ru
подключить интернет тарифы ростов
The Real Person!
The Real Person!
вывод из запоя круглосуточно череповец
vivod-iz-zapoya-cherepovec009.ru
вывод из запоя цена
eu9
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar009.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
подключить интернет в квартиру ростов
rostov-domashnij-internet005.ru
подключить домашний интернет в ростове
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-irkutsk004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar010.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
интернет провайдеры ростов
rostov-domashnij-internet006.ru
провайдеры интернета в ростове
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk004.ru
лечение запоя
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-irkutsk005.ru
лечение запоя
The Real Person!
The Real Person!
подключить интернет по адресу
samara-domashnij-internet004.ru
провайдер по адресу
The Real Person!
The Real Person!
вывод из запоя круглосуточно минск
vivod-iz-zapoya-minsk005.ru
вывод из запоя цена
Сериал «Уэнсдей» https://uensdey.com мрачная и захватывающая история о дочери Гомеса и Мортиши Аддамс. Учёба в Академии Невермор, раскрытие тайн и мистика в лучших традициях Тима Бёртона. Смотреть онлайн в хорошем качестве.
Срочно нужен сантехник? сантехник алматы цена в Алматы? Профессиональные мастера оперативно решат любые проблемы с водопроводом, отоплением и канализацией. Доступные цены, выезд в течение часа и гарантия на все виды работ
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-irkutsk006.ru
вывод из запоя
Die moderne Krebstherapie hipec wird bei Peritonealkarzinose eingesetzt, um mit erwarmten Zytostatika direkt im Bauchraum versteckte Tumorzellen zu zerstoren und das Ruckfallrisiko zu senken.
The Real Person!
The Real Person!
провайдеры интернета по адресу самара
samara-domashnij-internet005.ru
проверить провайдера по адресу
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk006.ru
лечение запоя минск
Usb флешка https://usb-flashki-optom-24.ru/ оптом 32Gb купить и купить флешку в Москве во Владикавказе. Флешка ракета и Usb флеш Накопители в Сыктывкаре. Браслет флешка оптом Usb и необычные Usb флешки купить
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-kaluga007.ru
вывод из запоя
The Real Person!
The Real Person!
провайдеры интернета по адресу
samara-domashnij-internet006.ru
интернет провайдеры в самаре по адресу дома
Натуральные средства для ухода musco.ru
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk004.ru
экстренный вывод из запоя
Продаем оконный профиль https://okonny-profil-kupit.ru высокого качества. Большой выбор систем, подходящих для любых проектов. Консультации, доставка, гарантия.
Оконный профиль https://okonny-profil.ru купить с гарантией качества и надежности. Предлагаем разные системы и размеры, помощь в подборе и доставке. Доступные цены, акции и скидки.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-kaluga008.ru
экстренный вывод из запоя калуга
The Real Person!
The Real Person!
узнать провайдера по адресу санкт-петербург
spb-domashnij-internet004.ru
домашний интернет тарифы
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-omsk005.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-kaluga009.ru
вывод из запоя
The Real Person!
The Real Person!
подключить домашний интернет в санкт-петербурге
spb-domashnij-internet005.ru
домашний интернет в санкт-петербурге
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk006.ru
вывод из запоя круглосуточно омск
The section on potential risks and how to mitigate them is a must-read for all users.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar006.ru
вывод из запоя цена
The Real Person!
The Real Person!
экстренный вывод из запоя оренбург
vivod-iz-zapoya-orenburg004.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
подключить интернет по адресу
spb-domashnij-internet006.ru
узнать провайдера по адресу санкт-петербург
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar007.ru
лечение запоя
ремонт кофемашин нивона срочный ремонт кофемашин
ремонт бытовых швейных машин ремонт швейных машин haier
удаленная 1с в облаке 1с облако для ип
The Real Person!
The Real Person!
вывод из запоя оренбург
vivod-iz-zapoya-orenburg005.ru
экстренный вывод из запоя оренбург
The Real Person!
The Real Person!
узнать интернет по адресу
ufa-domashnij-internet004.ru
провайдеры по адресу
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar008.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
узнать интернет по адресу
ufa-domashnij-internet005.ru
узнать провайдера по адресу уфа
ремонт кофемашин стоимость ремонт кофемашин недорого
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-krasnodar009.ru
экстренный вывод из запоя краснодар
ремонт швейных машин чайка ремонт швейных машин в москве
The Real Person!
The Real Person!
Если вы находитесь в поиске, где прокапаться анонимно в Туле, вы можете ознакомиться с сайтом vivod-iz-zapoya-tula007.ru. Здесь предоставляются услуги капельницы для здоровья, которые способствую вашему после болезни. Клиника в Туле предлагает безопасность процедур и приватность клиентов, предоставляя качественную медицинскую помощь. Анонимное лечение – это возможность заботиться о своем здоровье без лишнего шума. Не упустите возможность позаботиться о себе, воспользуйтесь медицинскими процедурами уже сегодня!
1с бухгалтерия в облаке аренда 1с в облаке цена
The Real Person!
The Real Person!
подключение интернета по адресу
ufa-domashnij-internet006.ru
подключить интернет по адресу
The Real Person!
The Real Person!
Экстренная наркологическая помощь в Туле: когда вызывать Кризисные ситуации, возникающие из-за зависимости от алкоголя или наркотиков, могут коснуться любого. В такие моменты важно знать, как быстро и эффективно получить помощь. Нарколог на дом срочно в Туле может стать спасением для тех, кто столкнулся с признаками алкогольного отравления или другими острыми состояниями, связанными с зависимостями. Экстренная помощь от наркологической службы включает возможность вызвать нарколога на дом. Это особенно актуально для людей, которые не могут или не хотят посещать медицинские учреждения. Лечение алкоголизма часто требует вмешательства специалистов, чтобы предотвратить тяжелые последствия. Поддержка родственников играет важную роль в процессе реабилитации наркоманов и алкоголиков; Психотерапия при зависимостях помогает разобраться в причинах и справиться с трудностями. Хотя адреса наркологических центров в Туле можно легко найти в интернете, иногда требуется экстренная помощь; нарколог на дом срочно тула Профилактика зависимости также важна. Раннее обращение к специалистам может помочь предотвратить развитие серьезных проблем. Помните, что экстренные меры способны спасти жизнь.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-krasnodar010.ru
вывод из запоя цена
The Real Person!
The Real Person!
интернет провайдеры омск
volgograd-domashnij-internet004.ru
интернет провайдер омск
The Real Person!
The Real Person!
вывод из запоя минск
vivod-iz-zapoya-minsk006.ru
вывод из запоя
The Real Person!
The Real Person!
какие провайдеры по адресу
ufa-domashnij-internet004.ru
проверить провайдеров по адресу уфа
The Real Person!
The Real Person!
экстренный вывод из запоя минск
vivod-iz-zapoya-minsk004.ru
лечение запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-omsk004.ru
вывод из запоя круглосуточно омск
The Real Person!
The Real Person!
провайдеры по адресу уфа
ufa-domashnij-internet005.ru
интернет по адресу дома
The Real Person!
The Real Person!
Прокат авто аэропорт Краснодар
Лоукост авиабилеты https://lowcost-flights.com.ua по самым выгодным ценам. Сравните предложения ведущих авиакомпаний, забронируйте онлайн и путешествуйте дешево.
Предлагаю услуги https://uslugi.yandex.ru/profile/DmitrijR-2993571 копирайтинга, SEO-оптимизации и графического дизайна. Эффективные тексты, высокая видимость в поиске и привлекательный дизайн — всё для роста вашего бизнеса.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk005.ru
лечение запоя
The Real Person!
The Real Person!
интернет провайдеры в уфе по адресу дома
ufa-domashnij-internet006.ru
узнать интернет по адресу
The Real Person!
The Real Person!
Аренда авто в Краснодаре бизнес
The Real Person!
The Real Person!
Онлайн курсы по недвижимости Обучение недвижимости Москва: В сердце деловой активности. Москва – эпицентр, предлагающий прямой опыт, связи и перспективную карьеру.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk006.ru
вывод из запоя цена
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-omsk006.ru
вывод из запоя круглосуточно
АО «ГОРСВЕТ» в Чебоксарах https://gorsvet21.ru профессиональное обслуживание объектов наружного освещения. Выполняем ремонт и модернизацию светотехнического оборудования, обеспечивая комфорт и безопасность горожан.
Онлайн-сервис https://laikzaim.ru займ на карту или счет за несколько минут. Минимум документов, мгновенное одобрение, круглосуточная поддержка. Деньги в любое время суток на любые нужды.
The Real Person!
The Real Person!
подключить интернет тарифы омск
volgograd-domashnij-internet004.ru
интернет провайдеры омск
Custom Royal Portrait http://www.turnyouroyal.com an exclusive portrait from a photo in a royal style. A gift that will impress! Realistic drawing, handwork, a choice of historical costumes.
Открыть онлайн брокерский счёт – ваш первый шаг в мир инвестиций. Доступ к биржам, широкий выбор инструментов, аналитика и поддержка. Простое открытие и надёжная защита средств.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk004.ru
вывод из запоя омск
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-orenburg004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
домашний интернет
volgograd-domashnij-internet005.ru
интернет тарифы омск
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-orenburg005.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk005.ru
экстренный вывод из запоя омск
The Real Person!
The Real Person!
подключить домашний интернет в волгограде
volgograd-domashnij-internet006.ru
домашний интернет тарифы омск
The Real Person!
The Real Person!
Обучение недвижимости Онлайн курсы по недвижимости: Получите знания в удобном формате, не выходя из дома. Современный мир диктует свои правила, и онлайн обучение становится все более популярным. Онлайн курсы по недвижимости – это отличная возможность получить образование, не тратя время на поездки и адаптируя обучение под свой график. Вы можете изучать материалы в удобное для вас время и получать консультации от опытных экспертов.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-orenburg006.ru
вывод из запоя
ПОмощь юрист в банкротстве: услуги по банкротству юридических лиц стоимость
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-omsk006.ru
экстренный вывод из запоя омск
The Real Person!
The Real Person!
тарифы интернет и телевидение воронеж
voronezh-domashnij-internet004.ru
дешевый интернет воронеж
Ремонт кофемашин https://coffee-craft.kz с выездом на дом или в офис. Диагностика, замена деталей, настройка. Работаем с бытовыми и профессиональными моделями. Гарантия качества и доступные цены.
Круглосуточный вывод из запоя врач — помощь на дому и в стационаре. Капельницы, очищение организма, поддержка сердца и нервной системы. Анонимно и конфиденциально.
Купить мебель чайные столики для дома и офиса по выгодным ценам. Широкий выбор, стильный дизайн, высокое качество. Доставка и сборка по всей России. Создайте комфорт и уют с нашей мебелью.
The Real Person!
The Real Person!
https://kazan.land/ Остров-Град Свияжск: Духовный Центр и Историческое Наследие Остров-град Свияжск – это уникальный исторический и культурный памятник, расположенный на острове в месте слияния рек Свияги и Волги. Это место, где история переплетается с духовностью, где можно увидеть древние храмы и монастыри, а также узнать о жизни и культуре русского народа. На острове расположены многочисленные памятники архитектуры, включая Успенский собор, Троицкую церковь и другие исторические здания. Вы сможете прогуляться по живописным улочкам, насладиться красотой природы и почувствовать атмосферу умиротворения.
The Real Person!
The Real Person!
Срочная наркологическая помощь в Туле является центральную роль в борьбе с зависимостями. Если вы оказались с трудной ситуацией, необходимо обратится в наркологическую клинику, где оказывают медицинскую поддержку при алкогольной зависимости. Специалисты проводят очищение организма, чтобы освободить токсичные вещества из организма; затем начинается реабилитация, целью которой является лечение после зависимости. Эмоциональная поддержка также имеет важную роль в лечебном процессе. Сайт vivod-iz-zapoya-tula007.ru организует консультацию нарколога, где можно ознакомиться о признаках зависимости и необходимых действиях. Не откладывайте обращение за срочной помощью – это начало к исцелению.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-orenburg004.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
подключить интернет воронеж
voronezh-domashnij-internet005.ru
подключить проводной интернет воронеж
Looking for solid value gear? From my experience, is rockbros a good brand to outfit a bike without paying boutique taxes—sensible designs that just do the job.
Предлагаем оконные профили https://proizvodstvo-okonnych-profiley.ru для застройщиков и подрядчиков. Высокое качество, устойчивость к климатическим нагрузкам, широкий ассортимент.
Оконные профили https://proizvodstvo-okonnych.ru для застройщиков и подрядчиков по выгодным ценам. Надёжные конструкции, современные материалы, поставка напрямую с завода.
The Real Person!
The Real Person!
Прокапывание на дому в Туле — это комфортный вариант для людей, которым требуется медицинская помощь, но не имеет возможности посетить медицинское учреждение. Это включает в себя капельницы, где квалифицированная медсестра осуществляет инфузии на дому. Это особенно актуально для реабилитации после заболевания или при необходимости медикаментозной терапии. Удобство лечения и уход за пациентами вырастает, когда профессиональный домашний врач посещает вас на дому. Профилактика заболеваний и назначения врача также становятся доступнее. Сайт vivod-iz-zapoya-tula008.ru предлагает такие услуги, обеспечивая комфорт и здоровье для пациентов.
The Real Person!
The Real Person!
экстренный вывод из запоя оренбург
vivod-iz-zapoya-orenburg005.ru
вывод из запоя цена
The Real Person!
The Real Person!
подключить домашний интернет воронеж
voronezh-domashnij-internet006.ru
недорогой интернет воронеж
The Real Person!
The Real Person!
Детоксикация при запое в Туле: стоимость и способы Запой – это серьёзная проблема, которая требует профессионального вмешательства. В Туле можно найти услуги по детоксикации в различных клиниках, где предлагают услуги по экстренному выводу из запоя. Методы вывода из запоя включают медикаментозной терапии, инфузионные процедуры и психотерапию. Стоимость детоксикации может колебаться в зависимости от способа лечения и условий в клинике. Следует осознавать, что лечение запоя – это не только физическая детоксикация, но и важная психологическая поддержка зависимых, которая помогает восстановить здоровье и предотвратить возврат к пагубным привычкам. экстренный вывод из запоя тула Процесс реабилитации после запоя включает в себя поддержку специалистов, которая способствует предотвращению рецидивов алкоголизма. Если вы или ваши близкие сталкиваетесь с этой проблемой, не стоит откладывать обращение за помощью к специалистам.
The Real Person!
The Real Person!
экскурсии казани Центр семьи “Казан”: Величественный Дворец и Панорамные Виды Центр семьи “Казан” – это уникальное здание, выполненное в форме котла, которое является одним из самых узнаваемых символов Казани. Здесь проходят торжественные церемонии бракосочетания, а также проводятся различные мероприятия. С верхних площадок центра открываются захватывающие панорамные виды на город. Вы сможете увидеть Казанский Кремль, реку Казанку и другие достопримечательности.
юридическая помощь онлайн бесплатная консультация адвоката на сайте
Нужен вентилируемый фасад: подсистема для вентилируемого фасада купить
Нужны пластиковые окна: заказать пластиковые окна в алматы
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-orenburg006.ru
вывод из запоя круглосуточно оренбург
The Real Person!
The Real Person!
Капельница от запоя на дому – это ключевая помощь наркологов‚ позволяющая быстро и значительно помочь людям‚ страдающим от алкогольной зависимости. Начальная встреча нарколога в владимире включает определение степени зависимости‚ анализ состояния пациента и разработку персонального плана терапии. Ключевые услуги нарколога на дому предполагают медикаментозную терапию‚ выведение из запоя‚ психотерапию при алкоголизме и программы восстановления. Родственники также играют важную роль тоже играет значимую функцию в реабилитации алкоголиков. Анонимное лечение обеспечивает удобство и защищенность пациента. помощь нарколога владимир
The Real Person!
The Real Person!
https://kazan.land/ Благовещенский Собор: Величественный Памятник Православия Благовещенский собор – это один из старейших православных храмов Казани, расположенный на территории Казанского Кремля. Он является важным памятником русской архитектуры и истории. Собор поражает своим величием и красотой. Вы можете посетить его, чтобы прикоснуться к истории православия в Татарстане.
Trust Finance https://trustf1nance.com is your path to financial freedom. Real investments, transparent conditions and stable income.
floral artistry ceramic flowers
Інформаційний портал https://pizzalike.com.ua про піцерії та рецепти піци в Україні й світі. Огляди закладів, адреси, меню, поради від шефів, секрети приготування та авторські рецепти. Все про піцу — від вибору інгредієнтів до пошуку найсмачнішої у вашому місті.
The Real Person!
The Real Person!
Наркологическая клиника владимир предлагает круглосуточную помощь – ключевой момент в процессе лечения зависимостей и реабилитации. В клинике доступно медикаментозное лечение и консультации профессионалов, что помогает пациентам справиться с проблемами. Вывоз из запоя осуществляется быстро и анонимно помогает избежать чувства стыда. Поддержка семьи играет ключевую роль в процессе выздоровления, а профилактика рецидивов включает реабилитационные программы и психотерапию для зависимых. Услуги нарколога включают кризисную интервенцию и обследование на наркотики, что помогает в достижении успешного восстановления. Получите помощь на vivod-iz-zapoya-vladimir008.ru!
The Real Person!
The Real Person!
Медицинские учреждения в Туле предлагают различные варианты лечения, включая инъекции для снятия запойного состояния, которые могут включать минералы и препараты для снятия симптомов. Сравнение клиник позволяет определить лучший выбор с учетом стоимости и качества обслуживания. Большинство учреждений предоставляет круглосуточную помощь в многих клиниках.При определении медицинского учреждения стоит учитывать отзывы пациентов и опыт врачей. Консультации нарколога помогут составить эффективный план терапии алкоголизма. Анонимное лечение также является важным аспектом, так как некоторые пациенты хотят сохранить анонимность.Восстановление после запоя требует успешной комплексной терапии, включая реабилитационные программы для зависимых и дальнейшее наблюдение за состоянием пациента. Таким образом, услуги нарколога, такие как дополнительные процедуры вывода из запоя и капельницы от запоя, играют важную роль в процессе выздоровления. вывод из запоя круглосуточно тула
Решили купить Honda? honda pilot — подробности широкий ассортимент автомобилей Honda, включая новые модели, такие как Honda CR-V и Honda Pilot, а также автомобили с пробегом. Предоставляем услуги лизинга и кредитования, а также предлагает различные акции и спецпредложения для корпоративных клиентов.
Ищешь автозапчасти? avto-fokus.ru/ предоставляем широкий ассортимент автозапчастей, автомобильных аксессуаров и оборудования как для владельцев легковых автомобилей, так и для корпоративных клиентов. В нашем интернет-магазине вы найдете оригинальные и неоригинальные запчасти, багажники, автосигнализации, автозвук и многое другое.
Выкуп автомобилей автомобили chery в воронеже без постредников, быстро. . У нас вы можете быстро оформить заявку на кредит, продать или купить автомобиль на выгодных условиях, воспользовавшись удобным поиском по марке, модели, приводу, году выпуска и цене — независимо от того, интересует ли вас BMW, Hyundai, Toyota или другие популярные бренды.
продвижение сайтов SEO академия любой тематики. Поисковая оптимизация, рост органического трафика, улучшение видимости в Google и Яндекс. Работаем на результат и долгосрочный эффект.
нужен юрист: юрист по семейным делам Новосибирск защита интересов, составление договоров, сопровождение сделок, помощь в суде. Опыт, конфиденциальность, индивидуальный подход.
онлайн аудио конвертер редактирование аудио онлайн
Заказать такси https://taxi-sverdlovsk.ru онлайн быстро и удобно. Круглосуточная подача, комфортные автомобили, вежливые водители. Доступные цены, безналичная оплата, поездки по городу и за его пределы
Онлайн-заказ такси https://sverdlovsk-taxi.ru за пару кликов. Быстро, удобно, безопасно. Подача в течение 5–10 минут, разные классы авто, безналичный расчет и прозрачные тарифы.
Закажите такси https://vezem-sverdlovsk.ru круглосуточно. Быстрая подача, фиксированные цены, комфорт и безопасность в каждой поездке. Подходит для деловых, туристических и семейных поездок.
Быстрый заказ такси https://taxi-v-sverdlovske.ru онлайн и по телефону. Подача от 5 минут, комфортные автомобили, безопасные поездки. Удобная оплата и выгодные тарифы на любые направления.
Платформа пропонує https://61000.com.ua різноманітний контент: порадник, новини, публікації на тему здоров’я, цікавих історій, місць Харкова, культурні події, архів статей та корисні матеріали для жителів міста
Нужен сантехник: https://santehnik-v-almaty.kz
ГОРСВЕТ Чебоксары https://gorsvet21.ru эксплуатация, ремонт и установка систем уличного освещения. Качественное обслуживание, модернизация светильников и энергоэффективные решения.
Займы онлайн лайкзайм моментальное оформление, перевод на карту, прозрачные ставки. Получите нужную сумму без визита в офис и долгих проверок.
Saznajte sve o https://www.kamen-u-bubregu.com – simptomi, uzroci i efikasni nacini lecenja. Procitajte savete strucnjaka i iskustva korisnika, kao i preporuke za prevenciju i brzi oporavak.
Интернет-магазин мебели https://mebelime.ru тысячи моделей для дома и офиса. Гарантия качества, быстрая доставка, акции и рассрочка. Уют в каждый дом.
The Real Person!
The Real Person!
скачать игры по прямой ссылке Альтернативы торрентам: Откройте для себя новые возможности Ограничиваться устаревшими способами получения игр – значит лишать себя множества преимуществ. Исследуйте альтернативные источники, предлагающие высокое качество, безопасность и удобство. Откройте для себя новые горизонты гейминга и наслаждайтесь процессом без каких-либо ограничений.
Теоретическая механика на форуме https://perekrestok.1bb.ru бесплатно и без регистрации, делятся задачами, спасибо нашим добрым людям, вы так же можете бесплатно все скачать и поделиться своим.
Zasto se javlja https://www.bol-u-bubrezima.com: od kamenaca i infekcija do prehlade. Kako prepoznati opasne simptome i brzo zapoceti lecenje. Korisne informacije.
Авто журнал https://bestauto.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры новинок, советы по уходу за автомобилем и репортажи с автособытий.
Sta znaci pesak u bubrezima simptomi, koji simptomi ukazuju na problem i kako ga se resiti. Efikasni nacini lecenja i prevencije.
Популярный авто журнал https://mirauto.kyiv.ua подробные обзоры моделей, советы экспертов, новости автосалонов и автоспорта, полезные статьи для автовладельцев.
Интернет Магазин https://usb-flashki-optom-24.ru/ флешка оптом ру и гравировка На флешке цена в Чите. Флешки с рисунком и флешка ключ мерседес в Астрахани. Флешки оптом спб и как повысить скорость передачи данных На флешку
Экономические новости https://gau.org.ua прогнозы и обзоры. Политика, бизнес, финансы, мировые рынки. Всё, что важно знать для принятия решений.
Мужской портал https://hooligans.org.ua всё, что интересно современному мужчине: стиль, спорт, здоровье, карьера, автомобили, технологии и отдых. Полезные статьи и советы каждый день.
Онлайн авто портал https://avtomobilist.kyiv.ua с обзорами новых и подержанных авто, тест-драйвами, советами по обслуживанию и новостями из мира автопрома.
freight shipping ny shipping from new york to uk
The Real Person!
The Real Person!
скачать игры без торрента Скачать игры по прямой ссылке: Быстрый и удобный доступ к развлечениям Прямые ссылки – это моментальный доступ к игровым файлам, минуя сложные процедуры и ожидание загрузки через торрент-клиент. Простота и удобство делают этот способ особенно привлекательным для тех, кто ценит свое время.
Портал о строительстве https://juglans.com.ua свежие новости, статьи и советы. Обзоры технологий, материалов, дизайн-идеи и практические рекомендации для профессионалов и частных застройщиков.
Строительный портал https://dki.org.ua всё о строительстве и ремонте: технологии, оборудование, материалы, идеи для дома. Новости отрасли и экспертные рекомендации.
Онлайн строительный https://texha.com.ua портал о материалах, проектах и технологиях. Всё о ремонте, строительстве и обустройстве дома. Поддержка специалистов и вдохновение для новых идей.
Всё о стройке https://mramor.net.ua полезные статьи, советы, обзоры материалов и технологий. Ремонт, строительство домов, дизайн интерьера и современные решения для вашего проекта.
Сайт «Всё о стройке» https://sushico.com.ua подробные инструкции, советы экспертов, новости рынка. Всё о строительстве, ремонте и обустройстве жилья в одном месте.
Why people still make use of to read news papers when in this technological world the whole thing is presented on net?
Seattle City Private Tour
Универсальный автопортал https://road.kyiv.ua автомобили, автоновости, обзоры, ремонт, обслуживание и tuning. Полезные статьи для водителей и экспертов автоиндустрии.
Женский портал https://fotky.com.ua с полезными статьями о красоте, моде, здоровье, отношениях и карьере. Советы экспертов, лайфхаки для дома, рецепты и вдохновение для каждой женщины.
Онлайн женский портал https://martime.com.ua новости, тренды моды, секреты красоты, психология отношений, карьера и семья. Полезные материалы и практические советы для женщин.
Лучший досуг в Новосибирске с девушками это совместные походы в музеи и галереи участие в мастер классах вечерние прогулки по набережной встречи в уютных кафе поездки в загородные парки и мероприятия на открытом воздухе с живой музыкой https://t.me/prostitutki_novosibirsk_indi
Женский сайт https://womanclub.in.ua о красоте, моде, здоровье и стиле жизни. Полезные советы, рецепты, тренды, отношения и карьера. Всё самое интересное для женщин в одном месте.
Всё о гипертонии https://gipertoniya.net что это за болезнь, как проявляется и чем опасна. Подробные статьи о симптомах, диагностике и способах лечения высокого давления.
Туристический портал https://elnik.kiev.ua с актуальными новостями, маршрутами и путеводителями. Обзоры стран и городов, советы путешественникам, лучшие идеи для отдыха и выгодные предложения.
Онлайн женский https://ledis.top сайт о стиле, семье, моде и здоровье. Советы экспертов, обзоры новинок, рецепты и темы для вдохновения. Пространство для современных женщин.
Сайт о строительстве https://stinol.com.ua практические рекомендации, проекты, обзоры инструментов и материалов. Советы экспертов, новости отрасли и новые технологии.
Строительный журнал https://mts-slil.info с актуальными новостями отрасли, обзорами материалов, инструкциями по ремонту и строительству. Полезные советы для специалистов и частных застройщиков.
Онлайн сайт https://purr.org.ua о строительстве и ремонте: полезные статьи, инструкции, обзоры технологий, дизайн-идеи и архитектурные решения для вашего дома.
Онлайн туристический https://azst.com.ua портал: всё о путешествиях, туризме и отдыхе. Маршруты, отели, лайфхаки для туристов, актуальные цены и интересные статьи о странах.
The Real Person!
The Real Person!
После запоя важно провести детоксикацию организма, чтобы минимизировать последствия алкоголизма. Нарколог на дом анонимно предоставляет услуги по лечению запоя, в т.ч. различные методы детоксикации. Признаки запоя могут включать раздражительность и физическую зависимость. Поддержка семьи и консультация специалиста помогают в восстановлении. Реабилитация после запоя включает психологическую помощь и восстановление организма. Помощь на дому обеспечивает комфорт и конфиденциальность. Свяжитесь с профессионалами в области наркологии для получения анонимного лечения и квалифицированной помощи.
The Real Person!
The Real Person!
подключение интернета челябинск
chelyabinsk-domashnij-internet004.ru
недорогой интернет челябинск
Онлайн новостной https://antifa-action.org.ua портал с круглосуточным обновлением. Свежие новости, репортажи и обзоры. Важные события страны и мира, мнения экспертов и актуальная аналитика.
Новостной портал https://prp.org.ua с актуальной информацией о событиях в России и мире. Политика, экономика, культура, спорт и технологии. Новости 24/7, аналитика и комментарии экспертов.
Новости Украины https://uamc.com.ua новости дня, аналитика, события регионов и мира. Обзоры, интервью, мнения экспертов. Быстро, достоверно и удобно для читателей.
Строительный портал https://suli-company.org.ua с актуальными новостями, обзорами материалов, проектами и инструкциями. Всё о ремонте, строительстве и дизайне.
shipping in nyc https://delivery-new-york.com/
The Real Person!
The Real Person!
Капельница от запоя — эффективный способ снять признаки зависимости от алкоголя. В владимире множество наркологических клиник предоставляют услуги вывода из запоя, но необходимо выбрать подходящую.Первым шагом является диагностика зависимости. Опытные врачи осуществят оценку состояния пациента и разработают индивидуальный план лечения. Необходимочтобы клиника предоставляла стационарное лечение и конфиденциальность при лечении алкогольной зависимости. Это создаст комфортные условия для пациентов.Обратите внимание на психотерапевтической поддержке при лечении алкоголизма. Психотерапия играет ключевую роль в восстановлении после запоя и лечении алкогольной зависимости. Семейная поддержка также способствует успешной реабилитации зависимых. вывод из запоя владимир Посмотрите отзывы о клиниках и проверьте, какие услуги нарколога они предлагают. Выбор правильной наркологической клиники — залог успешного и безопасного лечения.
The Real Person!
The Real Person!
лучший интернет провайдер челябинск
chelyabinsk-domashnij-internet005.ru
домашний интернет тарифы
The Real Person!
The Real Person!
Капельница от запоя на дому — это распространенный способ экстренного избавления от запойного состояния, особенно в Туле. Однако необходимо подобрать квалифицированного нарколога, чтобы избежать мошенников. Симптомы зависимости от алкоголя могут быть очевидны, и поддержка специалиста необходима. экстренный вывод из запоя тула При обращении в наркологическую клинику, обратите внимание на наличие лицензий и отзывов. Хороший специалист должен предложить безопасное лечение, включая инфузионную терапию водно-электролитного баланса. Консультация врача поможет определить степень зависимости и выбрать подходящий метод лечения. Не забывайте о реабилитации — это важная часть борьбы с алкоголизмом. Заботьтесь о своем здоровье и выбирайте надежных помощников!
Лучший досуг в Новосибирске с девушками предполагает совместные кулинарные мастер классы походы в ботанический сад посещения галерей вечерние прогулки по набережной организацию пикников в парках и дружеские встречи в уютных кафе города – https://t.me/prostitutki_novosibirsk_indi
Портал про авто https://prestige-avto.com.ua обзоры новых и подержанных машин, тест-драйвы, рынок автомобилей, страхование и обслуживание.
Онлайн автомобильный https://avtonews.kyiv.ua портал: свежие автоновости, сравнительные тесты, статьи о ремонте и тюнинге. Обзоры новых и подержанных машин, цены и советы экспертов.
Современный автомобильный https://mallex.info портал: автообзоры, тесты, ремонт и обслуживание, страхование и рынок. Всё, что нужно водителям и любителям автомобилей.
Автомобильный портал https://autonovosti.kyiv.ua новости автопрома, обзоры моделей, тест-драйвы и советы по эксплуатации. Всё для автолюбителей: от выбора авто до обслуживания и ремонта.
Строительный сайт https://novostroi.in.ua с полезными статьями о ремонте, отделке и дизайне. Обзоры стройматериалов, проекты домов, инструкции и советы экспертов для профессионалов и новичков.
What’s up everybody, here every person is sharing these kinds of knowledge, therefore it’s pleasant to read this website, and I used to pay a visit this website all the time.
https://renenergy.com.ua/rezultaty-poliruvannya-vs-zamina-skla.html
The Real Person!
The Real Person!
подключить интернет
chelyabinsk-domashnij-internet006.ru
провайдеры домашнего интернета челябинск
The Real Person!
The Real Person!
Зависимость от наркотиков и алкоголя требует квалифицированного подхода. Наркологическая помощь включает в себя диагностики зависимостей и лечениячто может существенно изменить жизнь человека. Первый шаг к выздоровлению – это консультация нарколога. Медицинская помощь при алкоголизме и реабилитация наркозависимых проводятся в центрахспециализирующихся на этом, таких как vivod-iz-zapoya-tula009.ru. Программы восстановления включают психотерапию при зависимости и группы поддержки для зависимых, а также семейную поддержку. Необходимо учитывать мотивацию к лечению и профилактике зависимостей. Адаптация в обществе после лечения помогает избежать рецидивов. Помощь наркозависимым на анонимной основе доступна и эффективна. Обратитесь за помощью и начните новый путь к здоровой жизни
Портал для родителей https://detiwki.com.ua и детей — всё для счастливой семьи. Воспитание, образование, здоровье, отдых и полезные материалы для мам, пап и малышей.
Журнал садовода https://mts-agro.com.ua полезные советы по уходу за садом и огородом. Сезонные работы, выращивание овощей, фруктов и цветов, современные технологии и секреты урожая.
Сайт для женщин https://stylewoman.kyiv.ua с интересными статьями о моде, красоте, семье и здоровье. Идеи для кулинарии, путешествий и вдохновения.
Универсальный сайт https://virginvirtual.net для женщин — секреты красоты, тренды моды, советы по отношениям и карьере, рецепты и стиль жизни.
Сайт для женщин https://gratransymas.com о красоте, моде, здоровье и стиле жизни. Полезные советы, рецепты, тренды, отношения и карьера.
Лучший досуг в Новосибирске с девушками включает прогулки по паркам и ботаническому саду посещение уникальных музеев тематические мастер классы совместные походы на выставки концерты живой музыки и уютные разговоры за чашкой горячего напитка: проститутки новосибирск телеграмм
Сайт обо всём https://vybir.kiev.ua энциклопедия для повседневной жизни. Красота, здоровье, дом, путешествия, карьера, семья и полезные советы для всех.
Студия дизайна https://lbook.com.ua интерьера и архитектуры. Создаём стильные проекты квартир, домов и офисов. Индивидуальный подход, современные решения и полный контроль реализации.
Портал про ремонт https://hydromech.kiev.ua свежие статьи о ремонте и отделке, дизайне интерьера и выборе материалов. Полезные советы для мастеров и тех, кто делает ремонт своими руками.
Интересный сайт https://whoiswho.com.ua обо всём: статьи, лайфхаки, обзоры и идеи на самые разные темы. Всё, что нужно для вдохновения и развития, в одном месте.
The Real Person!
The Real Person!
В Екатеринбурге доступно множество программ для детей на IPTV‚ которые превращает просмотр мультфильмов онлайн увлекательным и познавательным. При помощи подписки на IPTV можно легко найти лучшие детские каналы‚ в т.ч. развлекательные каналы и образовательные программы. Среди популярных детских телевизионных передач включают в себя семейные передачи и познавательные шоу. Такие каналы для малышей обеспечивают доступ к контенту‚ который развитию мышления и воображения. Теперь выбрать IPTV для детей стало легче благодаря особым пакетам‚ предлагающим разнообразные детские программы. На сайте ekaterinburg-domashnij-internet004.ru можно ознакомиться с актуальными предложениями и выбрать подходящий вариант для ваших детей.
Онлайн портал https://esi.com.ua про ремонт: идеи для интерьера, подбор материалов, практические рекомендации и пошаговые инструкции для самостоятельных работ.
The Real Person!
The Real Person!
Владимирская наркологическая служба предлагает разнообразные услуги для лечения зависимостей. В этом городе функционируют специализированные центры реабилитации‚ где предоставляется возможность анонимного лечения от наркозависимости и алкоголизма. Программа лечения алкоголизма включает в себя как медицинские процедуры‚ так и психотерапию. vivod-iz-zapoya-vladimir007.ru Психологическая поддержка в владимире также играет важную роль в процессе выздоровления. Обратитесь к наркологу позволяет выявить индивидуальные особенности зависимого и составить персонализированный план лечения. Профилактика зависимостей и поддержка родственников зависимых – неотъемлемая часть работы наркологической клиники. Реабилитация наркоманов включает как терапевтические меры‚ так и социальное reintegration. Услуги наркологической службы направлены на восстановление здоровья и возвращение к полноценной жизни.
Репортажи в больших https://infotolium.com фотографиях: самые обсуждаемые события, уникальные кадры и впечатляющие истории. Новости и жизнь в формате визуального рассказа.
Информационный портал https://reklama-region.com про ремонт: ремонт квартир, домов, офисов. Практические рекомендации, современные решения и обзоры стройматериалов.
Сайт про авто https://autoinfo.kyiv.ua свежие новости автопрома, обзоры моделей, тест-драйвы и советы по эксплуатации. Всё о машинах для водителей и автолюбителей.
The Real Person!
The Real Person!
Как выбрать оборудование для интернета в Екатеринбурге — ключевой момент для гарантии качественного доступа к сети. В первую очередь, стоит обратить внимание на интернет-провайдеров, которые работают в вашем районе. Екатеринбургие провайдеры предлагают многочисленные тарифы с разными уровнями скорости, что определяет выбор оборудования. провайдеры в доме Екатеринбург Для подключения интернета вам необходимы модемы и роутеры. Модем обеспечивает доступ к сети, а роутер организует Wi-Fi для ваших гаджетов. При выборе оборудования учитывайте отзывы о провайдерах и сравнение тарифов. Также важно оценить стабильность интернет-соединения в Екатеринбурге, чтобы избежать неудобств и перебоев. Настройки оборудования для Wi-Fi могут изменяться в зависимости от выбранного провайдера. При выборе провайдера, обратите внимание на предлагаемые услуги и варианты подключения дополнительных устройств. Правильный выбор обеспечит высокую скорость и надежность вашей домашней сети.
The Real Person!
The Real Person!
Капельницы от запоя на дому — является действенным способом детоксикации организма при борьбе с алкоголизмом. Анонимный нарколог на дом в городе владимир предлагает услуги по проведению капельниц, что дает возможность быстро восстановить здоровье пациентов . Состав капельницы состоит из медикаментов , которые помогают устранению симптомов запоя, таких как обезвоживание и интоксикация . Нарколог на дом анонимно владимир. Основные компоненты капельницы : солевой раствор для восполнения жидкости, глюкоза для энергетической поддержки , витамины группы B для нормализации обмена веществ и специальные медикаменты для снятия абстинентного синдрома ; лекарства для капельницы подбираются индивидуально , учитывая состояние пациента . Лечение на дому под наблюдением нарколога гарантирует анонимность, что существенно для многих людей, страдающих от алкогольной зависимости . Поддержка семьи при запое имеет решающее значение для успешного выздоровления. Консультация нарколога поможет определить следующие шаги, включая реабилитацию алкоголиков и восстановление после алкогольной зависимости.
Сайт про автомобили https://black-star.com.ua новинки рынка, цены, тест-драйвы и обзоры. Советы экспертов по выбору и уходу за машиной, тюнинг и автоуслуги.
Онлайн-журнал https://autoiceny.com.ua для автолюбителей: автомобили, новости индустрии, тест-драйвы, тюнинг и советы по обслуживанию.
Автомобильный онлайн-журнал https://allauto.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры новых моделей, советы по эксплуатации и ремонту. Всё для водителей и автолюбителей.
The Real Person!
The Real Person!
Если вы хотите найти, где прокапаться анонимно в Красноярске, вы можете ознакомиться с сайтом vivod-iz-zapoya-krasnoyarsk007.ru. Здесь предоставляются услуги капельницы для здоровья, которые способствуют восстановлению после болезни. Клиника в Красноярске обеспечивает безопасность процедур и приватность клиентов, предлагая качественную медицинскую помощь. Анонимное лечение – это шанс заботиться о своем здоровье в спокойной обстановке. Не стоит откладывать о себе, воспользуйтесь медицинскими процедурами как можно скорее!
Royal portraits turnyouroyal.com from photos – turn yourself or your loved ones into a king, queen or aristocrat. Author’s work of artists, luxurious style and premium quality of printing.
The Real Person!
The Real Person!
В Екатеринбурге выбор провайдера для интернета на дому играет ключевую роль, особенно для тех, кто работает удаленно. Чтобы определить подходящего провайдера по вашему адресу, можно использовать специальные онлайн-ресурсы. Важно учитывать тарифы на интернет, предлагаемые разными провайдерами в Екатеринбурге. узнать провайдера по адресу Екатеринбург Сравнительный анализ тарифов поможет выбрать наиболее подходящий вариант. Важно учитывать скорость соединения: для стабильной работы желательно иметь безлимитный интернет с высокой скоростью. Качество и доступность подключения также имеют значение. Обязательно изучите отзывы о провайдерах, чтобы избежать неприятных сюрпризов. Также многие компании предлагают акционные предложения и специальные тарифные планы для бизнеса, что может стать хорошим вариантом для вас. Подбирайте провайдера в зависимости от ваших нужд и бюджета, и получайте удовольствие от надежного интернета!
The Real Person!
The Real Person!
Капельница для лечения запоя – это важная медицинская интервенция при алкогольной зависимости. Подготовительный этап включает несколько этапов. Во-первых, необходимо обратиться к наркологу на дом, который оценит состояние пациента. Симптомы алкоголизма могут варьироваться, поэтому необходимо подробно рассказать врачу о симптомах. нарколог на дом Перед введением капельницы нужно позаботиться о доступе к венам, а также подготовить пациента к дальнейшему лечению. Обеспечьте наличие препаратов, необходимых для капельницы. После лечение следует уделить внимание реабилитации, правильный уход за пациентом поможет в восстановлении здоровья и снизит риск рецидива.
The Real Person!
The Real Person!
Вызов врача нарколога — необходимая мера для людей‚ испытывающих проблемы с зависимостями. Наркологическая помощь включает диагностику зависимости и лечение‚ что особенно важно в кризисных ситуациях. Посетив vivod-iz-zapoya-krasnoyarsk008.ru‚ вы можете получить анонимный вызов врача на дом. Это удобно и безопасно. Услуги нарколога на дому обеспечивают удобные условия для пациентов. Прием у нарколога позволяет определить уровень зависимости и разработать персонализированную программу терапии. В реабилитации пациентов значима поддержка близких‚ что способствует эмоциональной стабильности. Наркологическая клиника предлагает всеобъемлющее лечение‚ включая психотерапию зависимостей и медицинскую помощь при зависимостях. Не стоит откладывать обращение за помощью‚ ведь ранняя диагностика и лечение алкогольной и наркотической зависимости могут спасти жизнь.
Greetings! I know this is kinda off topic however I’d figured I’d ask. Would you be interested in exchanging links or maybe guest authoring a blog article or vice-versa? My website discusses a lot of the same topics as yours and I believe we could greatly benefit from each other. If you happen to be interested feel free to shoot me an e-mail. I look forward to hearing from you! Superb blog by the way!
http://cityjeans.com.ua/hermetyk-dlya-fary-koly-potriben-z-vysokoyu-v.html
Авто-журнал https://bestauto.kyiv.ua источник информации для автолюбителей. Новинки рынка, сравнения моделей, советы по ремонту и уходу, интересные материалы о мире автомобилей.
Новостной портал https://gau.org.ua круглосуточные новости, комментарии экспертов, события регионов и мира. Политика, бизнес, культура и общество.
Онлайн авто-журнал https://mirauto.kyiv.ua с актуальными новостями, аналитикой и обзорами. Тесты автомобилей, тюнинг, технологии и советы по эксплуатации.
Авто портал https://avtomobilist.kyiv.ua всё об автомобилях: новые модели, цены, рынок подержанных авто, тюнинг и автотехнологии. Полезные материалы для автовладельцев.
The Real Person!
The Real Person!
подключить интернет по адресу
kazan-domashnij-internet004.ru
провайдеры интернета в казани по адресу
Мужской портал https://hooligans.org.ua новости, лайфхаки, обзоры техники, спорт, здоровье и авто. Советы для уверенной и гармоничной жизни.
The Real Person!
The Real Person!
Кризисные центры Красноярска предлагают разнообразные программы по выводу из запоя, а также лечение без раскрытия личности, что облегчает процесс для пациентов. Стоимость лечения зависит от выбранной программы и необходимых услуг.Служба поддержки, работающая круглосуточно обеспечивает доступ к необходимой помощи в любое время, что особенно важно в критических ситуациях. Процесс реабилитации от алкоголизма требует комплексного подхода, включая медикаментозное лечение и психотерапию.вывод из запоя круглосуточно Красноярск Помощь при алкоголизме должна быть своевременной и профессиональной обеспечивает эффективность лечения и высокую вероятность успешного восстановления.
The Real Person!
The Real Person!
провайдеры интернета по адресу
kazan-domashnij-internet005.ru
провайдеры интернета в казани
The Real Person!
The Real Person!
вывод из запоя смоленск
vivod-iz-zapoya-smolensk010.ru
вывод из запоя
Very fine blog.
Портал о ремонте https://dki.org.ua и строительстве: от отделки квартиры до возведения загородного дома. Подробные статьи, рекомендации экспертов и идеи для обустройства жилья.
Портал о стройке https://sushico.com.ua и ремонте. Новости рынка, современные технологии, подборка идей для интерьера и экстерьера. Всё, что нужно для дома и дачи.
Онлайн сайт https://mramor.net.ua о строительстве и ремонте. Всё о возведении домов, ремонте квартир, отделке и обустройстве жилья. Обзоры материалов, советы экспертов и свежие идеи.
Всё о стройке https://aziatransbud.com.ua и ремонте в одном месте: дизайн, архитектура, выбор стройматериалов, инструкции по монтажу, лайфхаки и полезные рекомендации для новичков и мастеров.
Сайт о строительстве https://juglans.com.ua и ремонте — ваш помощник в выборе материалов, инструментов и технологий. Всё о ремонте квартир, строительстве домов и дизайне интерьеров.
The Real Person!
The Real Person!
интернет провайдеры казань по адресу
kazan-domashnij-internet006.ru
интернет провайдеры в казани по адресу дома
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-smolensk011.ru
вывод из запоя смоленск
Онлайн журнал https://vitamax.dp.ua о строительстве: проекты домов, ремонт квартир, выбор стройматериалов, дизайн и интерьер. Советы экспертов и свежие идеи для комфортной жизни.
Портал про строительство https://texha.com.ua новости рынка, обзоры технологий, инструкции и идеи для ремонта. Материалы для застройщиков, мастеров и тех, кто делает своими руками.
Новости Украины https://gromrady.org.ua онлайн: политика, экономика, спорт, культура и события регионов. Оперативные материалы, аналитика и комментарии экспертов круглосуточно.
Портал про авто https://automobile.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры моделей и советы по ремонту. Всё о машинах для водителей и автолюбителей.
Авто портал https://road.kyiv.ua с актуальной информацией: новинки рынка, цены, обзоры, страхование и тюнинг. Полезные статьи и аналитика для автомобилистов.
audio editing software extract audio from video
Prodaja prodaja zemljista zabljak: stanovi, vile, zemljisne parcele. Izbor smestaja za odmor, preseljenje i investicije. Saveti strucnjaka i aktuelne ponude na trzistu.
I enjoy your blog posts, saved to my bookmarks!
The Real Person!
The Real Person!
провайдеры интернета в красноярске по адресу
krasnoyarsk-domashnij-internet004.ru
интернет по адресу
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk012.ru
вывод из запоя круглосуточно смоленск
The Real Person!
The Real Person!
Капельница от запоя – это проверенный метод лечения алкогольной зависимости‚ который предоставляет возможность комфортного и конфиденциального лечения. Нарколог на дом анонимно обеспечит оперативную медицинскую помощь при запое‚ используя капельницу для детоксикации организма. Ключевые достоинства капельницы заключаются в том‚ что она позволяет быстро устранить симптомы запоя‚ восстановить водно-электролитный баланс и улучшить общее состояние пациента. Это критически важно в случае серьезных проявлений алкогольной зависимости‚ когда необходима немедленная помощь.Существуют различные методы лечения алкоголизма‚ но капельница выделяется своей эффективностью и скоростью. В сравнении с таблетками и инъекциями‚ капельница гарантирует непрерывное введение медикаментов в организм‚ что ускоряет процесс выздоровления.Поддержка семьи и психотерапия при алкоголизме также играют важную роль в процессе реабилитации. После детоксикации и восстановления после запоя продолжать лечение и заниматься профилактикой рецидивов‚ что возможно только при комплексном подходе. Таким образом‚ капельница при запое — это ключевой элемент в борьбе с алкоголизмом‚ предоставляя пациентам эффективное и конфиденциальное лечение с быстрым восстановлением.
The Real Person!
The Real Person!
провайдеры по адресу дома
krasnoyarsk-domashnij-internet005.ru
подключить интернет по адресу
Besoin d’un bien immobilier? immobilier au Montenegro: appartements en bord de mer, maisons a la montagne, villas et appartements. Catalogue de biens, prix actuels et conseils d’experts en investissement.
The Real Person!
The Real Person!
Экстренное прерывание запоя в Красноярске: помощь на дому Запой – это острая проблема‚ требующая немедленной медицинской помощи. Вызов нарколога на дом – лучший вариант для получения помощи. Специалист обеспечит детоксикацию‚ что позволит безопасно восстановить здоровье пациента. Лечение алкоголизма включает комплексную поддержку‚ включая физическую и психологическую помощь. Важность вызова нарколога заключается в том‚ что он предлагает комплексные наркологические услуги‚ включая лечение запойного состояния. Экстренная помощь дает возможность избежать серьезных последствий и стартовать реабилитационный процесс. После прерывания запоя важно не прекращать лечение алкоголизма‚ чтобы избежать рецидивов. Восстановление после запоя не обходится без поддержки‚ поэтому нарколог на дом предоставит поддержку как физически‚ так и психологически. Не забывайте о своём здоровье и ищите квалифицированную помощь!
Фильмы и сериалы бесплатный онлайн-кинотеатр без регистрации kinobay Онлайн-кинотеатр без регистрации и смс: тысячи фильмов и сериалов бесплатно.
Jak samodzielnie zdjac https://telegra.ph/Jak-samodzielnie-zdj%C4%85%C4%87-sufit-napinany-instrukcja-krok-po-kroku-bez-haka-z-wkr%C4%99tem-i-trikami-monta%C5%BCyst%C3%B3w-08-07 sufit napinany: instrukcje krok po kroku, narzedzia, porady ekspertow. Dowiedz sie, jak zdemontowac plotno bez uszkodzen i przygotowac pomieszczenie do montazu nowej okladziny.
The Real Person!
The Real Person!
провайдер интернета по адресу красноярск
krasnoyarsk-domashnij-internet006.ru
провайдеры в красноярске по адресу проверить
The Real Person!
The Real Person!
Лечение и восстановление после болезни — комфортное вариант для людей, которым необходимо медицинская помощь и реабилитация после болезни. С помощью медицинских услуг на дому, таких как капельная терапия и капельная терапия, квалифицированные медсестры обеспечивают удобное обслуживание в домашней атмосфере. Вызов медсестры позволяют пациентам получать медицинскую помощь без лишних затрат времени и необходимости выходить из дома. Здоровье — это приоритетное, и возможность получить капельницу на дому предлагает новый метод к уходу на дому, сохраняя время и обеспечивая эффективное лечение. Для получения подробной информации посетите vivod-iz-zapoya-krasnoyarsk009.ru.
салон красоты приморский район https://beauty-salon-spb.ru
The Real Person!
The Real Person!
проверить интернет по адресу
krasnodar-domashnij-internet004.ru
какие провайдеры интернета есть по адресу краснодар
A colleague in the field told me to check out your website.
Нужен микрозайм? займы лучшие: деньги на карту без справок и поручителей. Простое оформление заявки, одобрение за минуты и мгновенное зачисление. Удобно и доступно 24/7.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk010.ru
экстренный вывод из запоя смоленск
экспертная оценка ООО оценочная компания
The Real Person!
The Real Person!
детский психиатр на дом в москве
psychiatr-moskva001.ru
консультация психиатра по телефону
The Real Person!
The Real Person!
консультация психиатра по телефону
psychiatr-moskva001.ru
лечение в психиатрической больнице
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk011.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вызов психиатра на дом в москве
psychiatr-moskva002.ru
консультация психиатра платно
The Real Person!
The Real Person!
вывод из запоя круглосуточно смоленск
vivod-iz-zapoya-smolensk012.ru
вывод из запоя круглосуточно смоленск
The Real Person!
The Real Person!
вызвать психиатра на дом в москве
psychiatr-moskva002.ru
частный психиатрический стационар
The Real Person!
The Real Person!
консультация врача психиатра
psychiatr-moskva003.ru
консультация психиатра на дому
The Real Person!
The Real Person!
вызвать психиатра на дом
psychiatr-moskva003.ru
консультация частного психиатра
Авто помощь 24/7 техпомощь на дороге недорого: устранение поломок, подвоз топлива, прикуривание аккумулятора, замена колеса и эвакуация автомобиля.
The Real Person!
The Real Person!
детский психиатр на дом в москве
psikhiatr-moskva001.ru
нужна консультация психиатра
Срочно нужны доставка цветов Свежие букеты, праздничные композиции и эксклюзивные флористические решения. Онлайн-заказ и быстрая доставка по городу.
Профессиональные детейлинг авто спб: полировка кузова, химчистка салона, восстановление пластика и защита керамикой. Вернём автомобилю блеск и надёжную защиту.
The Real Person!
The Real Person!
платная психиатрическая клиника
psikhiatr-moskva002.ru
психиатрический стационар
The Real Person!
The Real Person!
вызвать психиатра на дом
psikhiatr-moskva001.ru
консультация врача психиатра
Нужен массаж? Массаж Ивантеевка – профессиональные мастера, широкий выбор техник: классический, оздоровительный, лимфодренажный, детский. Доступные цены и уютная атмосфера.
Обучающие курсы онлайн складчина мк новые навыки для работы и жизни. IT, дизайн, менеджмент, языки, маркетинг. Гибкий график, практика и сертификаты по итогам.
The Real Person!
The Real Person!
психиатрическая помощь больным
psikhiatr-moskva003.ru
частная психиатрическая помощь
The Real Person!
The Real Person!
врач психиатр на дом
psikhiatr-moskva002.ru
консультация психиатра платно
vps hosting vps hosting in europe
The Real Person!
The Real Person!
реабилитация алкогольной зависимости
reabilitaciya-astana001.ru
анонимное лечение алкоголиков
лампа лупа для педикюра косметологическое оборудование купить
Ziatogel
The Real Person!
The Real Person!
частный реабилитационный центр в Астане
reabilitaciya-astana002.ru
реабилитация алкоголиков цены
Kangaroo references in Kannada literature symbolize adaptability and resilience, often used metaphorically to explore themes of survival and cultural adaptation: Kangaroo conservation in Karnataka
Squash is a fast-paced indoor racket sport where two players hit a small rubber ball against the walls, aiming to outplay each other by making the ball bounce twice before return: squash game rules and techniques
The Real Person!
The Real Person!
реабилитация наркоманов недорого
reabilitaciya-astana003.ru
лечение алкогольной зависимости
The Real Person!
The Real Person!
перила для лестницы в частном доме купить Перила деревянные – это натуральный материал, создающий ощущение тепла и уюта в помещении.
buy cheap generic forxiga – this buy cheap dapagliflozin
Kangaroo references in Kannada literature symbolize adaptability and resilience, often used metaphorically to explore themes of survival and cultural adaptation: Kangaroo in Kannada culture
The Real Person!
The Real Person!
http://pravo-med.ru/articles/18547/
Наркологические услуги: нижний новгород наркологическая клиника, кодирование, детоксикация, снятие ломки, помощь при алкоголизме и наркомании. Круглосуточная поддержка и анонимность.
p2p USDT Egypt
бетон с доставкой цена за 1 доставка бетона саратов
The Real Person!
The Real Person!
сайт трипскан Трип скан – это быстрое и удобное сканирование лучших предложений на рынке, с заботой о вашем комфорте.
The Real Person!
The Real Person!
Earn entries in August at Seneca Niagara Resort & Casino to play the reels year-round. Slotpark is a free online game of chance for entertainment purposes only. You cannot win real money or real items services by playing our slot machines. The virtual currency used in this game is called ‘Slotpark Dollars’ and can be purchased in the ‘Shop’ using real money. ‘Slotpark Dollars’ cannot be exchanged for cash or be paid out in any form. They can only be used to play this game. The games are intended for an adult audience. Play the Thundering Buffalo slot online and begin your trek across the Rocky Mountains, where buffalos roam in herds. Some of the best features include the wild (which swaps for all standard symbols) and the free spins bonus round that awards 8 free games and can be retriggered several times.
https://battle.vanbruda.de/?p=85779
Yes, there’s a Roobet chicken game demo mode that lets you experience the game without laying down any money. Just leave the bet box blank and you’ll be able to watch the game being played out but won’t have to risk your cash. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. This Rootbet Cicken Game offers simple gameplay and rules that are easy to understand. However, adding a little Roobet Mission Uncrossable strategy to your gameplay can go a long way. These five helpful tips will help maximize your enjoyment of this game: Mission Uncrossable is a Roobet Original game that tasks players with guiding a chicken across multiple lanes of traffic. Each lane successfully crossed increases the multiplier on the player’s initial bet, but with each step forward, the risk of collision intensifies. The objective is to navigate as many lanes as possible without getting hit, allowing players to cash out their winnings at any point.
The Real Person!
The Real Person!
https://sonturkhaber.com/
exchange USDT to USD
cost xenical – https://asacostat.com/ order xenical 120mg online cheap
The Real Person!
The Real Person!
Цилиндрический подшипник Оптовая покупка подшипников – выгодное решение для крупных компаний, позволяющее значительно сократить издержки на техническое обслуживание и ремонт оборудования.
Handmade porcelain flowers Ideal for home, office decor or original gift. Natural beauty and durability.
Бетон в Воронеже https://stk-vrn.ru продажа и доставка. Все марки для фундаментов, дорожных работ и строительства под ключ. Надёжный производитель и лучшие цены.
The Real Person!
The Real Person!
платная психиатрическая клиника
psikhiatr-moskva003.ru
психиатрическая медицинская помощь
The Real Person!
The Real Person!
Куплю промышленные подшипники Подшипник для конвейера: бесперебойная работа
The Real Person!
The Real Person!
недорогой игровой пк : Компьютер ПК игровой: Готовое решение для гейминга.
This will be helpful for my family.
занять деньги онлайн оформить микрозайм
The Real Person!
The Real Person!
https://tripscan41.cc/ Tripscan: Путешествия, которые вдохновляют
The Real Person!
The Real Person!
https://t.me/zachashkoichaya_chanel Специальная военная операция привела к глубоким изменениям в мировой политике. Старые союзы распадаются, формируются новые альянсы, и каждая страна пытается найти свое место в новом мировом порядке. Переговоры между Путиным и Зеленским – это не просто поиск компромисса по территориальным вопросам. Это попытка определить будущее Европы и всего мира. Финансовый кризис, вызванный конфликтом, затронул все регионы мира. Европа переживает энергетический кризис, Азия сталкивается с перебоями в поставках продовольствия, а Америка вынуждена бороться с инфляцией. Безопасность и оборона становятся приоритетными направлениями государственной политики, а Кавказ и Ближний Восток остаются зонами повышенной напряженности. Новости и аналитика должны предоставлять объективную информацию о происходящих событиях, чтобы каждый человек мог сформировать собственное мнение о происходящем. Важно избегать пропаганды и дезинформации, чтобы не стать жертвой манипуляций.
получить займ взять микрозайм
деньги займ микрозаймы
We absolutely love your blog and find the majority of your post’s to be exactly what I’m looking for. Do you offer guest writers to write content to suit your needs? I wouldn’t mind composing a post or elaborating on a number of the subjects you write about here. Again, awesome weblog!
I amm actually glad to glance aat this web sitte posts which includes plenty of
helpful data, thanks for providing these data. https://Glassiindia.Wordpress.com/
Turbos sui redefines reliability Join turbos finance for effortless swaps
Buy a Ticket for a Concert https://rock-concert-tickets.ru
The Real Person!
The Real Person!
стационарное психиатрическое лечение
psychiatr-moskva001.ru
госпитализация в психиатрический стационар
Рестораны Хамовников https://restoran-khamovniki.ru топ заведений для встреч, романтических ужинов и семейных обедов. Авторская кухня, стильный интерьер, удобное расположение и достойный сервис.
Лучшие рестораны https://hamovniki-restoran.ru Хамовников для ценителей гастрономии. Подборка заведений с изысканной кухней, качественным сервисом и атмосферой для отдыха и деловых встреч.
Курсы по плазмотерапии обучение прп освоение методик, современные протоколы, практическая отработка. Обучение для специалистов с выдачей сертификата и повышением квалификации.
The Real Person!
The Real Person!
как лечить алкогольную зависимость
reabilitaciya-astana001.ru
пройти лечение от наркозависимости
Профессиональное обучение обучение prp в косметологии: подробная программа, практические навыки, сертификация. Освойте эффективные методики для применения в медицине и косметологии.
The Real Person!
The Real Person!
консультация психиатра по телефону
psychiatr-moskva002.ru
платная психиатрическая скорая помощь
The Real Person!
The Real Person!
истории о не обычных людях Секс истории обычных людей Интимная жизнь – важная часть человеческого опыта. Истории о любви, страсти, желании и разочаровании раскрывают перед нами разные грани отношений и помогают лучше понять себя и своего партнера. Они учат нас открытости, доверию и умению говорить о своих чувствах.
The Real Person!
The Real Person!
кавказ с детьми Путешествие по Кавказу маршрут
The Real Person!
The Real Person!
стоимость лечения наркомании
reabilitaciya-astana002.ru
анонимное лечение алкоголиков
Детская школа искусств https://elegy-school.ru обучение музыке, танцам, изобразительному и театральному искусству. Творческие программы для детей, концерты, конкурсы и развитие талантов.
Авторские курсы по REVIT https://dashclass.ru обучение созданию интерьеров и архитектурных проектов. Практика, реальные кейсы, индивидуальный подход и профессиональные навыки для работы в проектировании.
The Real Person!
The Real Person!
обсуждение Обсуждение авторского стиля и языка
SpookySwap vs SpiritSwap
The Real Person!
The Real Person!
лечение алкозависимости
reabilitaciya-astana003.ru
реабилитация от наркозависимости
Первая помощь детям https://firstaidkids.ru правила оказания при травмах, ожогах, удушье и других ситуациях. Пошаговые инструкции, советы врачей и полезная информация для родителей.
Студия иностранных языков https://whats-up-studiya-inostrannyh-yazykov.ru обучение английскому, немецкому, французскому и другим языкам. Индивидуальные и групповые занятия, современные методики и опытные преподаватели.
The Real Person!
The Real Person!
https://dadfencing.com/humble/ Wood Fence Price Per Foot Determine the perfect cost and ask for a contractor.
Срочный вызов сантехника https://master-expert.com в Москве на дом. Услуги сантехника: прочистка засоров, ремонт смесителей, установка приборов учета. Работаем 24/7. Недорого и с гарантией. Подробнее на сайте
Детский сад № 55 https://detsadik55.ru забота, развитие и обучение детей. Современные программы, квалифицированные воспитатели, уютные группы, безопасная среда и внимание к каждому ребёнку.
бк 888 старс
888starz официальный сайт
The Real Person!
The Real Person!
кайтсёрфинг
Промышленная безопасность https://аттестация-промбезопасность.рф курсы и обучение под ключ. Подготовка к проверке Ростехнадзора, повышение квалификации и сертификация специалистов предприятий.
Автомобили на заказ https://avto-iz-kitaya1.ru поиск, проверка, покупка и доставка. Китай и Корея. Индивидуальный подбор под бюджет и пожелания клиента, полное сопровождение сделки.
где купить героин купить меф бошки
меф где купить купить меф бошки
Авто из Китая https://avto-iz-kitaya2.ru на заказ под ключ: подбор, проверка, доставка и растаможка. Новые и подержанные автомобили, выгодные цены и полное сопровождение сделки.
Авто из Китая под заказ https://dostavka-avto-china.ru кроссоверы, седаны, электромобили и премиальные модели. Индивидуальный подбор, проверка, сопровождение сделки и доставка в ваш город.
Автомобили из Китая на заказ https://avto-iz-kitaya2.ru подбор, покупка и доставка. Полный цикл услуг: диагностика, растаможка, постановка на учёт и гарантия качества.
888starz.
Заказать авто из Китая https://dostavka-avto-china2.ru новые автомобили с гарантией, выгодные цены и проверенные поставщики. Доставка, таможня и оформление всех документов под ключ.
The Real Person!
The Real Person!
лечение запоя краснодар
narkolog-krasnodar011.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar011.ru
лечение запоя
The Real Person!
The Real Person!
Выезд нарколога на дом в Красноярске становятся все популярнее. Специалисты данной сферы, оказывают разнообразные наркологические услуги, в т.ч. помощь при алкоголизме и других зависимостях. Выездная консультация нарколога позволяет быстро провести диагностику зависимостей и разработать персонализированный план лечения. Лечение без раскрытия личности – важный аспект работы специалистов, что особенно важно для людей, которые стесняются обращаться в медицинские учреждения. Домашняя реабилитация предполагает психотерапевтическую поддержку, восстановительные программы и медикаментозное лечение. Семейная поддержка зависимых является ключевым элементом в процессе выздоровления. Профилактика рецидивов также занимает центральное место в работе наркологов. Наркологи предоставляют психологическую поддержку и помощь, чтобы помочь пациентам преодолевать трудности на их пути к выздоровлению. Узнать больше о предлагаемых услугах можно на сайте vivod-iz-zapoya-krasnoyarsk010.ru, где представлены все необходимые контакты и информация о наркологических услугах в Красноярске.
The Real Person!
The Real Person!
вывод из запоя краснодар
narkolog-krasnodar012.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
Цена капельницы от запоя в Красноярске: где найти лучшие предложения Проблема алкогольной зависимости затрагивает множество людей. Лечение алкоголизма и выход из запоя обычно требуют профессиональной медицинской помощи. Услуги нарколога в Красноярске, включая вызов врача на дом, востребованы среди тех, кто стремится избавиться от алкогольной зависимости. Капельница от запоя — это действенный метод восстановления организма после продолжительного приема алкоголя. Цены на капельницы варьируются в зависимости от клиники наркологии и необходимых компонентов. Например, домашняя капельница может быть удобным вариантом, так как вызов врача на дом позволяет получить помощь в комфортной обстановке. Ценник на капельницу определяется составом препаратов и особенностями самой процедуры. В Красноярске существует множество предложений, однако важно выбирать клиники с положительными отзывами. Помимо капельниц, наркологические услуги могут включать терапию алкоголизма, что тоже имеет значение при выборе. Восстановление после запоя требует комплексного подхода, и лечение запойного состояния должно быть проведено профессионалами. Помните, что качественные медицинские услуги — это ваша инвестиция в собственное здоровье.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar012.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
оксик
Авто на заказ https://dostavka-avto-russia.ru поиск, диагностика и сопровождение сделки. Машины из Европы, Кореи, Китая и США. Доставка, растаможка и постановка на учёт.
Автомобили на заказ https://dostavka-avto-russia5.ru профессиональный подбор, юридическая проверка, доставка и растаможка. Индивидуальные решения для каждого клиента.
Хотите заказать авто https://prignat-avto5.ru Мы подберём оптимальный вариант, проверим машину по базам, организуем доставку и таможенное оформление. Выгодные цены и прозрачные условия.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
narkolog-krasnodar013.ru
лечение запоя краснодар
The Real Person!
The Real Person!
Детоксикация организма после запоя в Красноярске: проверенные способы Преодоление запойной зависимости требует всестороннего подхода, включая детоксикацию и реабилитацию. Похмельный синдром могут быть значительными, и важно выбрать эффективные методы для очищения печени; Лекарственные препараты часто дополняются народными средствами, что усиливает эффект. лечение запоя Клиники по лечению алкоголизма предлагают курсы очищения и восстановление после запоя. Забота родных и консультация психолога играют важную роль в процессе. Профилактика запойного состояния включает в себя регулярное наблюдение за здоровьем и благополучием. Помните, что восстановление — это долгий путь, но возможный.
The Real Person!
The Real Person!
http://gzew.phorum.pl/viewtopic.php?p=357681#357681
The Real Person!
The Real Person!
вывод из запоя
narkolog-krasnodar013.ru
вывод из запоя цена
The Real Person!
The Real Person!
CDM3-9FDWPC Насос вертикальный многоступенчатый 1,1 кВт, 1×220 В, 50 Гц, чугун, 70*С
Машины на заказ https://prignat-avto7.ru поиск, диагностика и доставка автомобилей. Индивидуальный подбор, проверенные поставщики и прозрачные условия покупки.
Машины на заказ https://prignat-mashinu5.ru поиск, диагностика и доставка автомобилей. Индивидуальный подбор, проверенные поставщики и прозрачные условия покупки.
Автомобили на заказ https://prignat-mashinu7.ru подбор, проверка, доставка и оформление документов. Машины любых марок и комплектаций с гарантией качества и выгодной ценой.
The Real Person!
The Real Person!
экстренный вывод из запоя
narkolog-krasnodar014.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-smolensk013.ru
лечение запоя смоленск
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
narkolog-krasnodar014.ru
вывод из запоя круглосуточно краснодар
Ремонт квартир https://remontkomand.kz и домов под ключ: дизайн-проект, отделка, инженерные работы. Работаем по договору, фиксированные сроки и цены. Гарантия качества и полный контроль этапов.
The Real Person!
The Real Person!
вывод из запоя краснодар
narkolog-krasnodar015.ru
вывод из запоя
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk014.ru
лечение запоя
Цены на ремонт https://remontkomand.kz/ru/price квартир и помещений в Алматы под ключ. Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
The Real Person!
The Real Person!
Наркологическая помощь на платной основе, это ключевой момент в борьбе с зависимостями. В специальной наркологической клинике, такой как narkolog-tula015.ru, пациентам предоставляется профессиональная помощь, включая помощь при наркомании и алкоголизме. Процесс реабилитации включает детоксикационные процедуры и терапевтические сеансы. Обсуждение с наркологом позволяет определить оптимальный курс лечения к конкретной ситуации. Клиники гарантируют скрытость и кризисную интервенцию. Семейная поддержка также имеет огромное значение. Групповые занятия помогают поддерживать друг друга, а медицинская поддержка обеспечивает комфортное восстановление. Коммерческие услуги позволяют получать качественное лечение и поддержку квалифицированных специалистов, что повышает вероятность успеха.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
narkolog-krasnodar015.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
лечение запоя смоленск
vivod-iz-zapoya-smolensk015.ru
экстренный вывод из запоя
SEO-продвижение сайтов https://raskrutka-sajtov-bystro77.ru в Москве: вывод в ТОП поисковиков, рост трафика и заявок. Полный комплекс — аудит, семантика, оптимизация, ссылки. Эффективное продвижение под ключ.
The Real Person!
The Real Person!
Нарколог на дом анонимно в Туле – это необходимая помощь для людей‚ страдающих от зависимостью. Лечение наркомании требует квалифицированной помощи‚ и обращение к врачу на дому гарантирует конфиденциальность. Опытные врачи предоставляют анонимную консультацию‚ выявление зависимости и лечебные процедуры на дому. В Туле доступна психотерапия при зависимости и программы реабилитации для наркоманов что позволяет восстановить физическое и психическое здоровье и возобновить нормальную жизнь. Поддержка семьи и участие близких также имеют важное значение в процессе лечения. Не стесняйтесь обращаться на narkolog-tula016.ru‚ чтобы начать получать помощь и поддержку и начать путь к выздоровлению.
The Real Person!
The Real Person!
Услуга капельницы от запоя на дому – это важная помощь наркологической службы‚ позволяющая эффективно и значительно помочь людям‚ борющимся от зависимости от алкоголя. Первичная консультация нарколога в Туле включает определение степени зависимости‚ анализ состояния пациента и разработку персонального плана терапии. Основные услуги нарколога на дому включают медикаментозное лечение‚ снятие запоя‚ психотерапевтическую помощь при алкоголизме и программы восстановления. Поддержка родственников тоже играет ключевую функцию в процессе реабилитации алкоголиков. Анонимное лечение обеспечивает комфорт и безопасность пациента. помощь нарколога тула
The Real Person!
The Real Person!
https://github.com/awsadm/AWS-CLI
The Real Person!
The Real Person!
Нарколог на дом, ценная помощь для тех‚ кто борется с наркотической зависимостью. Лечение наркомании требует комплексного подхода‚ и помощь на дому обеспечивает удобство и конфиденциальность. Вызов специалиста позволяет получить консультацию врача и начать detox программу‚ не покидая домашнего уюта; Медицинское вмешательство и психотерапия помогают клиентам справиться с зависимостью‚ а семейная поддержка играет ключевую роль в восстановлении после зависимости. Анонимное лечение и меры по предотвращению наркомании также являются значимыми аспектами. Посетив vivod-iz-zapoya-krasnoyarsk010.ru‚ вы сможете получить профессиональную помощь и поддержку.
The Real Person!
The Real Person!
Капельница от запоя – это эффективный метод детоксикации организма, который предлагает наркологическая клиника. В Туле предлагается широкий спектр медицинских услуг, включая вызов нарколога на дом для помощи в борьбе с запоем. Лечение алкоголизма требует персонализированного подхода, и программы лечения зависят в зависимости от степени зависимости. Визит к наркологу способствует определению наилучшего метода лечения, а конфиденциальное лечение обеспечит конфиденциальность. Роль семьи в реабилитации также являеться ключевой в процессе реабилитации. Сравнение клиник в Туле по отзывам клиентов позволяет выбрать лучшее учреждение. Обращайтесь за помощью, чтобы освободиться от зависимости и вернуть контроль над жизнью.
Планируете ремонт https://remontkomand.kz в Алматы и боитесь скрытых платежей? Опубликовали полный и честный прайс-лист! Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
The Real Person!
The Real Person!
Услуги по откапыванию и земляные работы в Туле становятся всё более актуальными. В Туле можно найти специалистов, которые предоставляют широкий набор строительных услуг, включая экскаваторные работы и выемку грунта. Если вам нужно откопаться для планировки участка или благоустройства территории, обратитесь к подрядчикам в Туле. Они помогут с выполнением всех необходимых земляных работ, также предложат ландшафтный дизайн. Вы можете найти информацию о таких услугах на сайте narkolog-tula016.ru. Ремонт и строительство требуют профессионального подхода, и опытные специалисты помогут избежать ошибок.
The Real Person!
The Real Person!
Капельница для лечения алкоголизма – является эффективный метод‚ позволяющий оказать помощь в реабилитации после запоя в домашних условиях. Обратившись к к наркологу‚ который приедет к вам‚ вы сможете получить квалифицированную помощь и нужные препараты для облегчения симптомов похмелья. Симптомы запойного состояния могут состоять из головной боли‚ тошноты и общего недомогания; вызвать нарколога на дом Красноярск Процесс очистки организма начинается с капельницы‚ которая обеспечивает достаток жидкости и витаминов‚ что помогает снять симптомы абстиненции. Услуги наркологов в Красноярске включают индивидуальный подход к каждому пациенту‚ что необходимо для успешного лечения алкоголизма в домашних условиях. Поддержка близких в борьбе с алкоголизмом играет ключевой ролью в процессе восстановления здоровья после алкоголя. Реабилитация в домашних условиях возможна благодаря препаратов и капельниц‚ что позволяет предотвратить последующие запои и упростить процесс восстановления. Таким образом профилактика запоев становится важным аспектом в борьбе с зависимостью.
The Real Person!
The Real Person!
Зависимость от алкоголя — это серьезная проблема, которая касается не только пьющего человека, но и его близких. Вызов нарколога на дом в Туле является начальным этапом к лечению алкогольной зависимости. Консультация нарколога поможет определить уровень зависимости и рекомендовать подходы к лечению, включая психотерапию при алкоголизме.Проблемы в семье из-за алкоголя часто влекут к кризису в семье. Поддержка родственников играет важную роль в процессе восстановления. Важно, чтобы семья понимала, как помочь, и что можно сделать для помощи зависимому. Реабилитация алкоголиков включает в себя медицинские методы, но и поддержку социума. Предотвращение алкоголизма начинается с осознания проблемы и готовности к изменениям. Возобновление нормальных отношений — это длительный путь, который требует усилий всей семьи. вызов нарколога на дом тула Вызов врача на дом дает возможность воспользоваться помощью в привычной обстановке, что помогает лучше понять информацию и снижает уровень стресса. Поддержка семьи и готовность к лечению — это ключевые шаги на пути к выздоровлению.
Everstake platform is built for scalability Earn rewards easily with everstake — visit ever-stake.app
The Real Person!
The Real Person!
Капельницы на дому в Красноярске — это комфортный вариант для людей, которым требуется медицинская помощь, но не имеет возможности посетить медицинское учреждение. Это включает в себя капельницы, где медсестра на дому проводит капельницы на дому. Это очень важно для восстановления после болезни или при необходимости медикаментозной терапии. Комфорт лечения и забота о пациентах значительно увеличиваются, когда профессиональный домашний врач посещает вас на дому. Профилактика заболеваний и консультации врача также становятся доступнее. Сайт vivod-iz-zapoya-krasnoyarsk012.ru предлагает такие услуги, обеспечивая комфорт и здоровье для всех нуждающихся.
The Real Person!
The Real Person!
Капельницы при запое, это необходимый шаг в лечении алкоголизма. Вызов нарколога на дом в Туле позволяет получить профессиональную медицинскую помощь. Если появляются симптомы запоя, таких как тремор, потливость и тревожность, важно незамедлительно получить помощь. Наркологи проводят диагностику алкоголизма и разрабатывают план медикаментозной терапии, включая инфузии для детоксикации. вызов нарколога на дом тула Восстановление после запоя включает поддержку семьи, что значительно повышает шансы на успешное восстановление. Профилактика алкогольной зависимости тоже играет ключевую роль. Услуги нарколога, включая выезд врача на дом, помогают решить проблемы, связанные с алкогольной зависимостью, и гарантировать безопасную терапию.
The Real Person!
The Real Person!
Запой – это опасное состояние‚ требующее экстренного вывода и поддержки. Традиционные методы часто воспринимаются как действующие методы лечения‚ но вокруг них существует множество заблуждений. Например‚ многие считают‚ что лекарственные растения способны быстро снять симптомы запоя. Однако факты таковы‚ что народные рецепты могут лишь помочь в восстановлении после запоя‚ но не вытеснить профессиональную реабилитацию от алкоголя. Существует несколько признанных методов‚ среди которых детоксикация и терапия зависимости. Психология зависимости играет ключевую роль в реабилитации. Важно понимать‚ что экстренный вывод из запоя требует системного подхода‚ включающего медицинскую помощь и поддержку близких. Не стоит ограничиваться народные средства — это может привести к ухудшению состояния.
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk013.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
Копание ? данное действие не просто процесс работы с землёй, но и увлекательное приключение земли, которое открывает двери к тайнам истории. Археология, как наука, включает в себя раскопки, которые помогают обнаружить исторические артефакты, предметы старины и подземные ресурсы. На сайте narkolog-tula018.ru можно найти разнообразные материалы о современных методах бурения и анализа почвы, что помогают в поиске уникальных объектов. Земляные работы, включая раскопки, являются основой археологических исследований. Каждый слой почвы, вырываем, может рассказать о жизни людей в разные времена. Восстановление найденных артефактов предоставляет шанс лучше понять культуру и технологии прошлых эпох. Геология также играет ключевую роль в процессе, содействуя в анализе почвы и определении местонахождения объектов, что делает поиски более целенаправленными и эффективными. Таким образом, откопаться – это не только работа с землёй, но и увлекательное путешествие в прошлое, где каждая находка становится неотъемлемой частью исторической мозаики.
The Real Person!
The Real Person!
Капельницы при запое, это критически важный шаг в борьбе с алкоголизмом. Вызов нарколога на дом в Туле позволяет получить квалифицированную помощь. Если появляются симптомы запоя, таких как дрожь, потоотделение и беспокойство, важно незамедлительно получить помощь. Наркологи осуществляют диагностику алкогольной зависимости и назначают медикаментозное лечение алкоголизма, включая инфузии для детоксикации. вызов нарколога на дом тула Реабилитация после запоя включает семейную поддержку, что значительно увеличивает шансы на успешное восстановление. Профилактика алкогольной зависимости тоже является важным аспектом. Услуги нарколога, включая выезд врача на дом, помогают решить проблемы, связанные с алкогольной зависимостью, и гарантировать безопасную терапию.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-smolensk014.ru
вывод из запоя круглосуточно смоленск
The Real Person!
The Real Person!
Лечение зависимостей в Туле – это ключевой элемент борьбы с алкоголизмом и наркоманией. Если вы или ваши близкие столкнулись с трудностями, связанными с алкогольной зависимостью, реабилитационные центры предлагают индивидуальные подходы лечения зависимостей. В центре наркологии Тула предоставляется комплексная медицинская помощь, включая выведение токсинов и психологическое консультирование. Консультации нарколога помогут оценить тяжесть проблемы и разработать персонализированную стратегию. Поддержка психолога и взаимодействие с родственниками играют ключевую роль в процессе реабилитации. Также необходимо помнить о профилактике зависимостей, чтобы предотвратить рецидивы. Анонимная помощь доступна всем, кто столкнулся с проблемой. Способы борьбы с алкогольной зависимостью включают различные методы, подходящие для любой ситуации. Обратитесь на narkolog-tula021.ru для получения консультации и записи на прием.
The Real Person!
The Real Person!
Услуги наркологических клиник в Туле набирает популярность. Наркологические клиники, такие как наркологическая клиника Тула предлагают широкий спектр услуг: включая детоксикацию и программы реабилитации для наркозависимых. Психотерапия и консультирование по зависимостям помогают пациентам понять психологию зависимости и находить пути к выздоровлению. Алкогольные реабилитационные центры обеспечивают анонимную помощь и индивидуальный подход к каждому пациенту. Профессиональная поддержка наркологов, программа восстановления и медицинская помощь при наркозависимости являются важными факторами для достижения успешных результатов в реабилитации. Социальная адаптация после лечения также важна для предотвращения рецидивов. Более подробную информацию можно найти на сайте narkolog-tula019.ru.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-smolensk015.ru
вывод из запоя
The Real Person!
The Real Person!
пансионат для пожилых людей
pansionat-msk007.ru
пансионат для пожилых с деменцией
The Real Person!
The Real Person!
Врач-нарколог на дом в Туле предоставляет квалифицированную помощь тем, кто борется с наркоманией и алкогольной зависимостью. Лечение зависимости требует индивидуального подхода, и вызов нарколога на дом – это удобное решение для пациентов. Центр реабилитации зависимостей в Туле предлагает анонимное лечение и консультации нарколога. Реабилитация на дому обеспечивает комфортное прохождение психотерапии при зависимости в комфортной обстановке. Выездная медицинская помощь включает в себя как телесное, так и психотерапевтическое сопровождение. Профилактика зависимости также играет важную роль в профилактике алкоголизма и наркомании. Сопровождение алкоголиков и их семей – это обязанность специалистов, которые готовы поддержать в трудный момент. narkolog-tula020.ru
The Real Person!
The Real Person!
программа для учета автотранспорта на предприятии Контрагенты – основа бизнеса. Программы для учета контрагентов позволяют автоматизировать процессы и повысить эффективность работы отдела продаж.
The Real Person!
The Real Person!
пансионат для лежачих пожилых
pansionat-msk008.ru
пансионат для лежачих пожилых
Журнал о саде https://nasha-gryadka.ru и огороде онлайн — статьи о выращивании овощей, цветов и фруктов. Советы по уходу, борьбе с вредителями, подбору семян и организации участка.
The Real Person!
The Real Person!
Капельница при запое — существенный аспект лечения алкоголизма при лечении алкоголизма. В данном контексте следует обращать внимание на правовые нюансы, связанные с правами пациентов и обязанностями медицинского персонала. Важным аспектом имеет согласие пациента на лечение, что является краеугольным камнем медицинской этики. вывод из запоя В процессе оказания наркологической помощи крайне важно следовать правовым стандартам, гарантируя пациентские права. Клинические рекомендации обращают внимание на важность индивидуального подхода к каждому случаю запоя. Медицинская помощь может включать в себя социальные услуги, сосредоточенные на реабилитации. Врачебная ответственность за качество лечения алкоголизма не должна оставаться без внимания. Важно, чтобы процесс вывода из запоя был безопасным и эффективным, что требует профессионализма и соблюдения всех норм.
быстрый займ микрозайм получить
The Real Person!
The Real Person!
пансионат с медицинским уходом
pansionat-msk009.ru
частный пансионат для пожилых людей
The Real Person!
The Real Person!
пансионат после инсульта
pansionat-msk007.ru
пансионат для реабилитации после инсульта
The Real Person!
The Real Person!
пансионат для престарелых
pansionat-tula007.ru
частный пансионат для престарелых
The Real Person!
The Real Person!
провайдеры по адресу екатеринбург
inernetvkvartiru-ekaterinburg004.ru
проверить интернет по адресу
The Real Person!
The Real Person!
https://www.trub-prom.com/
The Real Person!
The Real Person!
пансионат для пожилых людей
pansionat-msk008.ru
пансионат для людей с деменцией в москве
The Real Person!
The Real Person!
дом престарелых
pansionat-tula008.ru
пансионат для лежачих после инсульта
The Real Person!
The Real Person!
интернет провайдеры в екатеринбурге по адресу дома
inernetvkvartiru-ekaterinburg005.ru
узнать провайдера по адресу екатеринбург
The Real Person!
The Real Person!
частные пансионаты для пожилых в москве
pansionat-msk009.ru
пансионат для пожилых в москве
The Real Person!
The Real Person!
пансионат для людей с деменцией в туле
pansionat-tula009.ru
пансионат для престарелых
The Real Person!
The Real Person!
проверить провайдеров по адресу екатеринбург
inernetvkvartiru-ekaterinburg006.ru
недорогой интернет екатеринбург
Нужен клининг? лучшие клининговые компании в москве 2026 год. Лучшие сервисы уборки квартир, домов и офисов. Сравнение услуг, цен и отзывов, чтобы выбрать надежного подрядчика.
Нужен клининг? лучшие клининговые компании в москве 2026 год. Лучшие сервисы уборки квартир, домов и офисов. Сравнение услуг, цен и отзывов, чтобы выбрать надежного подрядчика.
The Real Person!
The Real Person!
Эскорт работа Тюмень Работа проституткой в Тюмени: Высокий заработок, гибкий график, конфиденциальность. Возможность быстро решить финансовые вопросы и реализовать мечты. Полная поддержка и безопасность.
The Real Person!
The Real Person!
частная психиатрическая клиника
psychiatr-moskva004.ru
вызвать психиатра на дом
The Real Person!
The Real Person!
частный пансионат для престарелых
pansionat-tula007.ru
пансионат после инсульта
The Real Person!
The Real Person!
подключить интернет по адресу
inernetvkvartiru-krasnoyarsk004.ru
узнать интернет по адресу
Смотрите сериалы Лучшие сериалы онлайн: https://kineshemec.ru/news/obshhestvo-zhizn/serialy-2025-goda-dla-vechera-doma-smotret-onlajn-v-hd-kachestve-51046.html онлайн в хорошем качестве. Новинки, классика и популярные проекты в удобном формате. Бесплатный просмотр без скачивания и доступ 24/7.
The Real Person!
The Real Person!
Je suis fan de le casino TonyBet, c’est vraiment un plaisir de jeu constant. Les jeux sont varies, proposant des jeux de table classiques. Le personnel est tres competent, repondant rapidement. On recupere ses gains vite, neanmoins il pourrait y avoir plus de promos. Dans l’ensemble, TonyBet c’est du solide pour les adeptes de sensations fortes ! En bonus, la plateforme est intuitive, ajoutant une touche de confort.
tonybet descargar app android|
The Real Person!
The Real Person!
цветы в москве Цветы могут преобразить любой интерьер, но важно правильно подобрать и расставить их. Учитывайте стиль квартиры, освещение и размер помещения. Альтернативные варианты букетов: Что подарить, если не цветы
Je suis enthousiaste a propos de Azur Casino, on dirait une aventure captivante. Le catalogue est vaste et diversifie, offrant des jeux de table elegants. Le service client est exceptionnel, avec un suivi irreprochable. Les transactions sont parfaitement protegees, parfois les offres pourraient etre plus allechantes. Dans l’ensemble, Azur Casino ne decoit jamais pour les joueurs passionnes ! En bonus la navigation est intuitive et agreable, facilitant l’immersion totale.
azur casino mon compte|
The Real Person!
The Real Person!
Je trouve absolument fantastique Banzai Casino, ca offre une energie de jeu captivante. La selection est riche et diversifiee, avec des machines a sous ultra-modernes. Le service d’assistance est exemplaire, garantissant une aide immediate. Les transactions sont parfaitement protegees, par moments les promotions pourraient etre plus frequentes. En resume, Banzai Casino ne decoit jamais pour les joueurs en quete de frissons ! Par ailleurs la navigation est intuitive et rapide, ce qui intensifie le plaisir de jouer.
banzai slots casino|
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Betclic Casino, on dirait une plongee dans un univers palpitant. Le catalogue de jeux est incroyablement riche, proposant des jeux de table classiques et elegants. Le personnel offre un accompagnement de qualite, avec un suivi efficace. Le processus de retrait est simple et fiable, occasionnellement les promotions pourraient etre plus genereuses. Pour conclure, Betclic Casino est un incontournable pour les adeptes de sensations fortes ! Ajoutons que l’interface est fluide et intuitive, facilite chaque session de jeu.
lnb betclic elite|
Нужна контекстная реклама? reklama-stomatolog-kontekst.ru под ключ. Настройка в Яндекс.Директ и Google Ads, оптимизация объявлений и контроль заявок.
The Real Person!
The Real Person!
Je suis fan de le casino TonyBet, il est carrement un univers de jeu unique. Les options de jeu sont nombreuses, offrant des options de casino en direct. Les agents sont reactifs, offrant un excellent suivi. Les paiements sont fluides, mais parfois les offres pourraient etre plus genereuses. En gros, TonyBet est une plateforme fiable pour les amateurs de casino ! En bonus, la plateforme est intuitive, renforcant le plaisir de jouer.
tonybet legit|
Комплексные IT-решения https://eclat.moscow для бизнеса: разработка, внедрение, сопровождение и поддержка. Надежные технологии для автоматизации и цифровой трансформации.
The Real Person!
The Real Person!
оказание психиатрической помощи
psychiatr-moskva006.ru
платная психиатрическая клиника
The Real Person!
The Real Person!
пансионат для пожилых с деменцией
pansionat-tula009.ru
пансионат для реабилитации после инсульта
The Real Person!
The Real Person!
провайдеры интернета по адресу красноярск
inernetvkvartiru-krasnoyarsk006.ru
какие провайдеры на адресе в красноярске
The Real Person!
The Real Person!
Je trouve absolument fantastique Banzai Casino, il procure une experience de jeu explosive. Il y a une multitude de titres varies, offrant des sessions de casino en direct immersives. Le support est ultra-reactif, garantissant une aide immediate. Les gains arrivent en un rien de temps, bien que plus de tours gratuits seraient apprecies. Dans l’ensemble, Banzai Casino est une plateforme incontournable pour les amateurs de jeux en ligne ! Par ailleurs le site est concu avec dynamisme, renforcant l’immersion.
casino banzai slots|
The Real Person!
The Real Person!
Je trouve absolument fantastique Betclic Casino, c’est une veritable plongee dans un univers palpitant. La gamme de jeux est tout simplement impressionnante, avec des machines a sous modernes et captivantes. Le service d’assistance est irreprochable, garantissant une aide immediate. Les gains sont verses en un clin d’?il, cependant les bonus pourraient etre plus frequents. Globalement, Betclic Casino est une plateforme d’exception pour les adeptes de sensations fortes ! En bonus le site est concu avec elegance, ce qui amplifie le plaisir de jouer.
betclic support|
The Real Person!
The Real Person!
психиатр на дом для пожилого человека
psikhiatr-moskva004.ru
платная психиатрическая скорая помощь
The Real Person!
The Real Person!
стационар психиатрической больницы
psychiatr-moskva004.ru
психиатрическая клиника стационар
The Real Person!
The Real Person!
Sugar Rush ofrece 20 líneas de pago en 5 carretes. La artista nacida en Suiza y residente en Berlín, vuelve a sonar en Zone Nights con su reciente sencillo, dónde combina buenos pasajes de alternativo con un espíritu retro soul que enamora. La dulce estética de su predecesor se combina con ciertos elementos de jugabilidad de Fruit Party en Sugar Rush. Aunque es diferente, la funcionalidad del marco multiplicador indicado se parece a algo que podría hacer Hacksaw Gaming. Sugar Rush está organizado, por lo que hay posiciones indicadas donde los eventos podrían (o no) ocurrir. El premio máximo que se puede ganar en Sugar Rush 1000 está limitado a X25.000 veces la apuesta por tirada. zinha presentó de manera oficial su nueva canción, titulada, why do you make me wait? Catálogo
https://www.popularbingosites.co.uk/analisis-del-juego-balloon-de-smartsoft-para-jugadores-en-ecuador/
Nuestra plataforma y app están habilitadas para ser utilizadas exclusivamente dentro de la Ciudad Autónoma de Buenos Aires (CABA). Conseguir dos símbolos Scatter te otorga tres giros gratis, todos los cuales son capaces de proporcionar una excelente experiencia a quienes eligen usarlos. Como jugador, echemos un vistazo a algunos de los casinos en línea que han demostrado ser confiables. Los giros gratis normalmente oscilan entre 25 y más de 200, profesionales y confiables. The access of our services is not possible from your territory. Sorry about the inconvenience. En sugar-rush.mx, queremos que tu experiencia sea tan irresistible como un frasco lleno de caramelos de colores. Para lograrlo, usamos pequeñas migajas digitales llamadas “cookies”. No se trata de espiarte, sino de hacer tu visita más placentera y personalizada. Si lo prefieres, puedes ajustar tus preferencias de cookies en cualquier momento desde la configuración de tu navegador y elegir cuáles te apetecen.
J’apprecie enormement BetFury Casino, ca procure une experience de jeu electrisante. Il y a une profusion de titres varies, proposant des jeux de table classiques et raffines. Le personnel offre un accompagnement de qualite, joignable a toute heure. Les paiements sont fluides et securises, cependant les offres comme le pack de bienvenue de 590 % pourraient etre plus accessibles. En resume, BetFury Casino est un incontournable pour les amateurs de crypto-casinos ! En bonus l’interface est fluide et intuitive, amplifie le plaisir de jouer.
betfury limbo strategy|
The Real Person!
The Real Person!
консультация психиатра круглосуточно
psikhiatr-moskva005.ru
частная психиатрическая клиника
The Real Person!
The Real Person!
https://how-to-screenshot.info/
The Real Person!
The Real Person!
частный психиатр на дом
psychiatr-moskva005.ru
психиатр на дом для пожилого
The Real Person!
The Real Person!
провайдеры по адресу краснодар
inernetvkvartiru-krasnodar005.ru
интернет провайдеры по адресу краснодар
The Real Person!
The Real Person!
https://vk.com/3dplabs
The Real Person!
The Real Person!
https://cryptobirzhi.com/
The Real Person!
The Real Person!
психиатрический стационар
psikhiatr-moskva006.ru
психиатрическая помощь
The Real Person!
The Real Person!
анонимная консультация психиатра
psychiatr-moskva006.ru
вызов психиатрической скорой помощи
The Real Person!
The Real Person!
провайдер по адресу
inernetvkvartiru-krasnodar006.ru
подключить интернет по адресу
The Real Person!
The Real Person!
Turbos finance balances speed with simplicity Experience effortless swaps at tvrbos.finance
The Real Person!
The Real Person!
реабилитационный центр для наркозависимых
reabilitaciya-astana004.ru
лечение алкоголизма
The Real Person!
The Real Person!
интернет по адресу дома
inernetvkvartiru-msk004.ru
проверить интернет по адресу
The Real Person!
The Real Person!
консультация частного психиатра
psikhiatr-moskva004.ru
психиатрическая клиника
The Real Person!
The Real Person!
Je suis totalement seduit par 7BitCasino, ca ressemble a une sensation de casino unique. Le catalogue est incroyablement vaste, comprenant plus de 5 000 jeux, dont 4 000 adaptes aux cryptomonnaies. Le support est ultra-reactif et professionnel, avec un suivi de qualite. Les transactions en cryptomonnaies sont instantanees, neanmoins les bonus pourraient etre plus reguliers, notamment des bonus sans depot. En fin de compte, 7BitCasino ne decoit jamais pour les adeptes de sensations fortes ! Par ailleurs le site est concu avec style et modernite, ce qui intensifie le plaisir de jouer.
7bitcasino usa|
The Real Person!
The Real Person!
Je trouve completement incroyable Betsson Casino, il offre une energie de jeu irresistible. Le catalogue est incroyablement vaste avec plus de 1500 titres, comprenant des jackpots progressifs comme Mega Moolah. Le personnel offre un accompagnement de qualite via email ou telephone, avec un suivi efficace. Les transactions sont protegees par un cryptage SSL 128 bits, parfois les offres comme le bonus de bienvenue de 100 % jusqu’a 100 € pourraient etre plus genereuses. Dans l’ensemble, Betsson Casino ne decoit jamais pour les amateurs de casino en ligne ! De plus le site est concu avec modernite et ergonomie, ajoute une touche de dynamisme a l’experience.
service client betsson|
The Real Person!
The Real Person!
J’apprecie enormement BetFury Casino, ca procure une sensation de casino unique. La gamme de jeux est tout simplement epoustouflante, offrant des sessions de casino en direct immersives. Le service d’assistance est irreprochable, repondant en un instant. Les paiements sont fluides et securises, parfois les offres comme le pack de bienvenue de 590 % pourraient etre plus accessibles. Globalement, BetFury Casino ne decoit jamais pour les adeptes de divertissement innovant ! Par ailleurs le design est visuellement percutant avec un theme sombre, facilite chaque session.
betfury io reddit|
Je suis enthousiaste a propos de CasinoBelgium, il offre une sensation de casino authentique. La gamme de jeux est axee sur les dice slots, offrant des titres de Gaming1 et Greentube. Le service client est fiable, garantissant une aide pour toute question. Les transactions sont fiables, conformes a la Commission des Jeux de Hasard, parfois les bonus pourraient etre plus nombreux. En resume, CasinoBelgium vaut le detour pour les joueurs belges pour les passionnes de jeux belges ! En bonus l’interface est fluide avec un design tricolore belge, ajoute une touche de fierte nationale.
casino online belgium|
The Real Person!
The Real Person!
реабилитация от алкогольной зависимости
reabilitaciya-astana005.ru
лечение алкоголизма отзывы
The Real Person!
The Real Person!
купить оборудование для покраски Купить порошковое оборудование для покраски – это приобрести комплекс устройств, предназначенных для нанесения порошковых полимерных покрытий на металлические изделия. Включает в себя камеры напыления и полимеризации, распылители порошковой краски, системы подготовки поверхности, транспортные системы и системы управления. Выбор оборудования зависит от масштаба производства, типа окрашиваемых изделий и требований к качеству покрытия.
The Real Person!
The Real Person!
частная наркологическая клиника Частная наркологическая клиника Воронеж – это медицинское учреждение, принадлежащее частному лицу или компании, которое оказывает услуги по лечению наркологических заболеваний на коммерческой основе. Частные клиники обычно предлагают более комфортные условия пребывания, индивидуальный подход к каждому пациенту и более широкий спектр услуг, включая консультации узких специалистов, современные методы диагностики и лечения.
The Real Person!
The Real Person!
подключить интернет по адресу
inernetvkvartiru-msk005.ru
подключить интернет тарифы москва
The Real Person!
The Real Person!
скорая психиатрическая помощь
psikhiatr-moskva005.ru
частная психиатрическая помощь
The Real Person!
The Real Person!
https://bs2-bs2site.at
The Real Person!
The Real Person!
реабилитация от алкоголя в Астане
reabilitaciya-astana006.ru
реабилитация наркозависимых стационар
The Real Person!
The Real Person!
Je trouve incroyable 1win Casino, il offre une plongee dans un univers electrisant. Le choix de jeux est absolument gigantesque, incluant des slots de derniere generation. Le service d’assistance est impeccable, offrant des solutions claires et efficaces. Les gains arrivent en un temps record, de temps en temps les bonus pourraient etre plus frequents. Globalement, 1win Casino offre une experience de jeu memorable pour ceux qui aiment parier ! Par ailleurs le site est concu avec modernite, ajoute une touche d’elegance a l’experience.
1win token|
Je trouve absolument fantastique Betify Casino, on dirait une aventure pleine de frissons. La selection de jeux est impressionnante, offrant des sessions de casino en direct immersives. Le personnel offre un accompagnement de qualite, avec un suivi de qualite. Les transactions sont parfaitement protegees, parfois plus de tours gratuits seraient un atout. Dans l’ensemble, Betify Casino vaut pleinement le detour pour ceux qui aiment parier ! De plus le design est visuellement percutant, renforce l’immersion totale.
betify autorise en france|
The Real Person!
The Real Person!
Je trouve absolument fantastique Casino Action, ca ressemble a une aventure pleine de frissons. La gamme de jeux est tout simplement phenomenale, offrant des sessions de casino en direct immersives par Evolution Gaming. Le personnel offre un accompagnement rapide via chat ou email, joignable a toute heure. Les retraits sont rapides, souvent traites en 24-48 heures pour les e-wallets, neanmoins le bonus de bienvenue jusqu’a 1250 € pourrait etre plus frequent. En resume, Casino Action vaut pleinement le detour pour les adeptes de sensations fortes ! En bonus la navigation est rapide sur mobile via iOS/Android, ce qui amplifie le plaisir de jouer.
all casino action nude|
The Real Person!
The Real Person!
buy deep groove ball bearings
swot анализ проекта https://swot-analiz1.ru
The Real Person!
The Real Person!
дешевый интернет москва
inernetvkvartiru-msk006.ru
интернет провайдеры по адресу дома
Новогодние игрушки Москва и новогоднюю продукцию в Череповце. Заказ брелков с логотипом и рубашки поло с логотипом в Калуге. Волшебники двора новый год и новогодние фонарики в Санкт-Петербурге. Дешевые подарки на день рождения и что подарить свекрови на юбилей 60 лет в Ижевске. Новогодняя гирлянда недорого и новогодние оптом подарки реклама: купить подарочную коробку под альбом
The Real Person!
The Real Person!
платный психиатр
psikhiatr-moskva006.ru
платный психиатр на дом
The Real Person!
The Real Person!
вывод из запоя круглосуточно тула
tula-narkolog004.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
интернет провайдеры по адресу нижний новгород
inernetvkvartiru-nizhnij-novgorod004.ru
тарифы интернет и телевидение нижний новгород
The Real Person!
The Real Person!
Je suis completement conquis par 1xbet Casino, ca ressemble a une sensation de casino unique. Les options de jeu sont riches et diversifiees, offrant des sessions de casino en direct immersives. Le service client est exceptionnel, repondant en un clin d’?il. Les retraits sont ultra-rapides, occasionnellement j’aimerais plus d’offres promotionnelles. En fin de compte, 1xbet Casino offre une experience de jeu remarquable pour les joueurs en quete d’adrenaline ! Par ailleurs le site est concu avec dynamisme, renforce l’immersion totale.
1xbet en france|
The Real Person!
The Real Person!
Estou impressionado com o 888 Casino, parece mesmo experiencia de jogo eletrizante. A gama de jogos e simplesmente fenomenal, contendo titulos inovadores e atraentes. O servico de assistencia e impecavel, oferecendo respostas rapidas e precisas. Os ganhos sao pagos em tempo recorde, as vezes mais rodadas gratis seriam otimas. De modo geral, o 888 Casino e indispensavel para os jogadores em busca de adrenalina! Como bonus o site e projetado com elegancia, o que amplifica o prazer de jogar.
fortune 888 online casino|
The Real Person!
The Real Person!
Ich liebe absolut BingBong Casino, es fuhlt sich an wie eine unwiderstehliche Energie fur Spieler. Die Auswahl ist reichhaltig und breit gefachert, mit modernen Slots wie Book of Ra Deluxe und Sweet Bonanza. Das Team bietet schnelle Unterstutzung per E-Mail oder Telefon, reagiert in wenigen Minuten. Zahlungen sind sicher mit 256-Bit-SSL-Verschlusselung, obwohl die Angebote wie der 100%-Bonus bis 100 € konnten gro?zugiger sein. Kurz gesagt ist BingBong Casino eine erstklassige Plattform fur Spieler, die Nervenkitzel suchen ! Au?erdem die Navigation ist flussig auf der mobil-optimierten Seite, was den Spielspa? noch steigert.
bingbong studios|
The Real Person!
The Real Person!
лечение наркомании
reabilitaciya-astana004.ru
реабилитация наркозависимых стационар в Астане
The Real Person!
The Real Person!
J’apprecie enormement CasinoAndFriends, il offre une aventure pleine de bonne humeur. Les options de jeu sont vastes et diversifiees, avec des machines a sous modernes comme Book of Dead. Les agents sont professionnels et toujours disponibles, garantissant une aide immediate. Les transactions sont protegees, incluant des methodes comme PayPal et Skrill, parfois davantage de recompenses via le programme VIP seraient appreciees. Dans l’ensemble, CasinoAndFriends est une plateforme d’exception pour les passionnes de jeux numeriques ! Par ailleurs l’interface est fluide et conviviale avec un theme ludique, ce qui amplifie le plaisir de jouer.
casinoandfriends abzocke|
The Real Person!
The Real Person!
провайдеры интернета нижний новгород
inernetvkvartiru-nizhnij-novgorod005.ru
домашний интернет в нижнем новгороде
The Real Person!
The Real Person!
Аренда авто аэропорт Краснодар Прокат авто Краснодар: Исследуйте Краснодарский край с комфортом. Откройте для себя все красоты Краснодарского края с услугой проката авто в Краснодаре. Мы предлагаем широкий выбор автомобилей различных марок и моделей, чтобы удовлетворить любые ваши потребности. Наш автопарк включает в себя экономичные автомобили для коротких поездок по городу, комфортабельные седаны для деловых встреч и вместительные внедорожники для путешествий по горам и побережью. Прокат авто в Краснодаре – это удобный и доступный способ получить автомобиль на любой срок и исследовать все достопримечательности Краснодарского края. Забронируйте свой автомобиль прямо сейчас и начните свое приключение!
The Real Person!
The Real Person!
лечение наркозависимых
reabilitaciya-astana005.ru
реабилитация наркозависимых стационар в Астане
The Real Person!
The Real Person!
оргониты Оргонит камень: часто используется кварц, аметист, розовый кварц и другие кристаллы.
The Real Person!
The Real Person!
вывод из запоя цена
tula-narkolog006.ru
вывод из запоя тула
The Real Person!
The Real Person!
проверить провайдеров по адресу нижний новгород
inernetvkvartiru-nizhnij-novgorod006.ru
подключить интернет по адресу
The Real Person!
The Real Person!
анонимное лечение от алкоголизма
reabilitaciya-astana006.ru
реабилитационный центр для наркоманов в Астане
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-chelyabinsk007.ru
вывод из запоя круглосуточно
система swot анализа swot анализ бизнеса
Looking for second-hand? thrift near me We have collected the best stores with clothes, shoes and accessories. Large selection, unique finds, brands at low prices. Convenient catalog and up-to-date contacts.
The Real Person!
The Real Person!
официальный сайт Обзор официального сайта и приложения казино. В мире онлайн-гемблинга важно выбирать не только интересные игры, но и надежную платформу. 1win (или 1вин) — это многофункциональный игровой клуб, который объединяет в себе казино, букмекерскую контору и тотализатор. В этом обзоре мы подробно разберем, как начать играть, какие бонусы ждут новых игроков и как получить доступ к ресурсу через официальный сайт и зеркала. Поиск рабочего официального сайта 1win — первый шаг для каждого игрока. Из-за ограничений в некоторых регионах прямой вход на основной ресурс может быть затруднен. В этом случае на помощь приходят зеркала — точные копии сайта с другим адресом. Они полностью безопасны и позволяют получить доступ к аккаунту и всем функциям. Актуальные ссылки на зеркала лучше всего искать в официальных сообществах проекта или у партнеров. Процесс регистрации в 1win занимает пару минут. Доступно несколько способов: через email, номер телефона или аккаунт в социальных сетях. Но самый важный шаг — это активация промокода. Ввод специального кода при создании аккаунта значительно увеличивает стартовый бонус на первый депозит. Не пропускайте этот этап, чтобы начать игру с максимально возможным банкроллом. Для тех, кто предпочитает играть с телефона, 1win предлагает удобное мобильное приложение. Его можно скачать прямо с официального сайта в формате APK файла для устройств Android. Владельцы iOS также могут найти способ установить программу. Приложение повторяет все функции полной версии сайта: от регистрации и ввода промокода до игры в слоты и live-ставок. Это идеальный выбор для игры в любом месте. 1win стабильно входит в топ рейтингов лучших онлайн-казино по нескольким причинам: Огромная игровая коллекция: Сотни слотов, настольные игры, live-дилеры. Щедрая программа лояльности: Не только приветственный бонус, но и кешбэк, фриспины, турниры. Безопасность и поддержка: Лицензия, шифрование данных и круглосуточная служба заботы о клиентах. 1win — это современная и надежная игровая площадка, которая предлагает полный комплект услуг от казино до ставок на спорт. Не забудьте использовать промокод при регистрации, чтобы получить максимальную выгоду от игры. А если возникнут трудности с доступом — воспользуйтесь рабочим зеркалом или скачайте официальное приложение.
The Real Person!
The Real Person!
провайдеры домашнего интернета новосибирск
inernetvkvartiru-novosibirsk004.ru
домашний интернет
С получением профессиональной помощи в решении юридических вопросов вы можете обратиться к консультации адвоката онлайн бесплатно, где можно получить юридическую консультацию круглосуточно и бесплатно.
Профессиональная команда гарантирует отличное качество всех юридических услуг.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-chelyabinsk008.ru
экстренный вывод из запоя челябинск
The Real Person!
The Real Person!
экстренный вывод из запоя тула
tula-narkolog004.ru
вывод из запоя тула
The Real Person!
The Real Person!
J’aime enormement le casino TonyBet, on dirait une aventure palpitante. La selection de machines est vaste, comprenant des titres innovants. Le service d’assistance est top, disponible 24/7. Les paiements sont fluides, occasionnellement il pourrait y avoir plus de promos. Dans l’ensemble, TonyBet ne decoit pas pour ceux qui aiment parier ! De plus, la plateforme est intuitive, renforcant le plaisir de jouer.
tonybet lt|
The Real Person!
The Real Person!
Je trouve sensationnel le casino AllySpin, c’est une veritable plongee dans le divertissement. Il y a une quantite impressionnante de jeux, offrant des jeux de table authentiques. Les agents sont toujours disponibles, offrant des solutions rapides. Les gains arrivent vite, bien que plus de tours gratuits seraient top. Pour faire court, AllySpin est une plateforme de choix pour les fans de divertissement numerique ! Notons aussi que le style visuel est dynamique, renforcant l’immersion.
allyspin promo code|
The Real Person!
The Real Person!
Je suis totalement emballe par Banzai Casino, ca offre une sensation de casino unique. Il y a une multitude de titres varies, offrant des sessions de casino en direct immersives. Le personnel est d’un professionnalisme hors pair, joignable 24/7. Le processus de retrait est simple et efficace, neanmoins les promotions pourraient etre plus frequentes. En conclusion, Banzai Casino offre une experience exceptionnelle pour les fans de divertissement numerique ! Par ailleurs le site est concu avec dynamisme, ajoutant une touche d’elegance et d’energie.
banzai slots informations|
The Real Person!
The Real Person!
Je suis completement seduit par Betclic Casino, ca ressemble a une aventure pleine de frissons. Les options de jeu sont vastes et diversifiees, proposant des jeux de table classiques et elegants. Les agents sont toujours disponibles et professionnels, avec un suivi efficace. Les paiements sont fluides et securises, occasionnellement plus de tours gratuits seraient un atout. Dans l’ensemble, Betclic Casino ne decoit jamais pour les amateurs de casino en ligne ! En bonus le design est visuellement epoustouflant, renforce l’immersion totale.
betclic turf bonus|
The Real Person!
The Real Person!
какие провайдеры по адресу
inernetvkvartiru-novosibirsk005.ru
провайдеры домашнего интернета новосибирск
https://ufa.bfm.ru/news/66001
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-chelyabinsk009.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя цена
tula-narkolog005.ru
экстренный вывод из запоя
Looking for second-hand? best thrift stores near me We have collected the best stores with clothes, shoes and accessories. Large selection, unique finds, brands at low prices. Convenient catalog and up-to-date contacts.
The Real Person!
The Real Person!
https://halif-omsk.ru
The Real Person!
The Real Person!
вывод из запоя круглосуточно
tula-narkolog006.ru
вывод из запоя цена
J’adore a fond Amon Casino, ca balance une vibe de jeu completement folle. Les options de jeu au casino sont riches et captivantes, avec des machines a sous de casino modernes et immersives. Le staff du casino assure un suivi de ouf, repondant en un clin d’?il. Les gains du casino arrivent a la vitesse de l’eclair, de temps en temps des bonus de casino plus reguliers ca serait top. Pour resumer, Amon Casino c’est un casino de ouf a tester direct pour ceux qui kiffent parier avec style au casino ! De surcroit la navigation du casino est simple comme un claquement de doigts, donne envie de replonger dans le casino non-stop.
amon casino en ligne|
The Real Person!
The Real Person!
J’adore l’incandescence de Celsius Casino, il propose une aventure de casino qui fait monter la temperature. La collection de jeux du casino est incandescente, proposant des slots de casino a theme volcanique. L’assistance du casino est chaleureuse et efficace, repondant en un eclair ardent. Les transactions du casino sont simples comme une etincelle, par moments les offres du casino pourraient etre plus genereuses. Pour resumer, Celsius Casino est un casino en ligne qui met le feu pour ceux qui cherchent l’adrenaline du casino ! A noter l’interface du casino est fluide et eclatante comme une flamme, ce qui rend chaque session de casino encore plus enflammee.
celsius casino promo code|
The Real Person!
The Real Person!
подключить домашний интернет в ростове
inernetvkvartiru-rostov004.ru
домашний интернет подключить ростов
Je suis a fond dans Gamdom, il propose une aventure qui dechire. Il y a un tsunami de titres varies, incluant des jeux de table qui en jettent. Le support est dispo 24/7, avec une aide qui dechire tout. Les retraits sont rapides comme un ninja, des fois j’aimerais plus de promos qui defoncent. En gros, Gamdom c’est du lourd a tester direct pour ceux qui kiffent parier avec style ! Et puis le design est une bombe visuelle, donne envie de replonger non-stop.
how to withdraw money from gamdom|
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-chelyabinsk007.ru
экстренный вывод из запоя челябинск
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-cherepovec012.ru
экстренный вывод из запоя череповец
The Real Person!
The Real Person!
домашний интернет подключить ростов
inernetvkvartiru-rostov005.ru
интернет провайдер ростов
The Real Person!
The Real Person!
экстренный вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk008.ru
вывод из запоя цена
Je suis totalement emballe par Circus, ca donne une vibe de jeu incroyable. La gamme est tout simplement eblouissante, offrant des slots a theme innovants. Le service client est exceptionnel, avec une aide personnalisee. Les paiements sont securises et efficaces, de temps a autre plus de tours gratuits seraient un atout. Dans l’ensemble, Circus est une plateforme hors du commun pour les joueurs en quete d’excitation ! Ajoutons que le site est style et bien pense, renforce l’envie de revenir.
27 aout|
The Real Person!
The Real Person!
J’adore le delire de FatPirate, il offre une aventure totalement barge. Le choix de jeux est monumental, avec des slots qui dechirent. L’assistance est carrement geniale, joignable par chat ou email. Les paiements sont fluides et securises, par moments des bonus plus reguliers ce serait cool. Au final, FatPirate garantit un fun total pour les aventuriers du jeu ! Et puis le design est style et accrocheur, facilite le delire total.
casino online fatpirate|
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk007.ru
вывод из запоя цена
The Real Person!
The Real Person!
Je trouve carrement genial Impressario, ca balance une vibe spectaculaire. Il y a un deluge de jeux captivants, comprenant des jeux parfaits pour les cryptos. Le service client est digne d’un gala, avec une aide qui fait mouche. Les retraits sont fluides comme une choregraphie, par contre les offres pourraient etre plus genereuses. En gros, Impressario est un must pour les joueurs stars pour les accros aux sensations eclatantes ! En prime la plateforme claque avec son look de star, ce qui rend chaque session encore plus eclatante.
impressario casino no deposit bonus|
услуги доставки из китая карго из китая доставка
фермерская лавка купить фермерские продукты с доставкой
The Real Person!
The Real Person!
домашний интернет в ростове
inernetvkvartiru-rostov006.ru
домашний интернет подключить ростов
Онлайн-библиотека Казахстана https://mylibrary.kz книги, статьи, диссертации и редкие издания в цифровом формате. Удобный каталог, быстрый поиск и круглосуточный доступ для всех пользователей.
The Real Person!
The Real Person!
вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk009.ru
вывод из запоя челябинск
The Real Person!
The Real Person!
экстренный вывод из запоя иркутск
vivod-iz-zapoya-irkutsk008.ru
вывод из запоя
The Real Person!
The Real Person!
online casino cz “: Online Casino CZ: Otestujte sve stesti v legalnich online kasinech v CR. Cekaji na vas stovky her, stedre bonusy a bezpecne prostredi pro zabavu.
The Real Person!
The Real Person!
https://gtaman.ru
Adoro o role insano de Flabet Casino, oferece uma aventura de cassino que detona tudo. O catalogo de jogos do cassino e uma loucura, com jogos de cassino perfeitos pra criptomoedas. Os agentes do cassino sao rapidos como um trovao, respondendo mais rapido que um relampago. Os ganhos do cassino chegam voando baixo, de vez em quando mais giros gratis no cassino seria insano. Resumindo, Flabet Casino e um cassino online que e um vulcao para os amantes de cassinos online! De lambuja a interface do cassino e fluida e cheia de vibe, aumenta a imersao no cassino a mil.
flabet aposta|
The Real Person!
The Real Person!
Ich flippe aus bei DrueGlueck Casino, es bietet eine krasse Spielerfahrung. Es gibt eine Flut an abwechslungsreichen Casino-Titeln, mit Casino-Spielen, die fur Kryptos optimiert sind. Die Casino-Mitarbeiter sind blitzschnell und top, mit Hilfe, die richtig abgeht. Der Casino-Prozess ist klar und ohne Haken, manchmal die Casino-Angebote konnten gro?zugiger sein. Zusammengefasst ist DrueGlueck Casino eine Casino-Erfahrung, die rockt fur Fans moderner Casino-Slots! Nebenbei die Casino-Navigation ist kinderleicht, Lust macht, immer wieder ins Casino zuruckzukehren.
drueckglueck erfahrungen|
The Real Person!
The Real Person!
интернет провайдер санкт-петербург
inernetvkvartiru-spb004.ru
интернет по адресу дома
The Real Person!
The Real Person!
Je trouve totalement dingue AmunRa Casino, il offre une aventure de casino legendaire. La selection de titres du casino est un tresor divin, proposant des sessions de casino en direct qui envoutent. L’assistance du casino est majestueuse et efficace, avec une aide qui illumine. Les retraits au casino sont fluides et rapides comme le Nil, mais j’aimerais plus de promos de casino qui eblouissent. Globalement, AmunRa Casino est un casino en ligne qui regne en maitre pour les gardiens des slots modernes de casino ! Et puis la navigation du casino est simple comme une priere, donne envie de replonger dans le casino sans fin.
amunra casino logo|
The Real Person!
The Real Person!
Je suis accro a Instant Casino, on dirait une tempete de fun. Il y a un deluge de jeux de casino varies, avec des slots de casino modernes et immersifs. Le support du casino est dispo 24/7, avec une aide qui pete le feu. Les gains du casino arrivent a la vitesse lumiere, quand meme j’aimerais plus de promos de casino qui dechirent. Dans le fond, Instant Casino est un casino en ligne qui cartonne pour ceux qui kiffent parier dans un casino style ! Bonus le site du casino est une tuerie graphique, facilite le delire total au casino.
true blue casino instant play|
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-cherepovec010.ru
лечение запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-irkutsk009.ru
экстренный вывод из запоя иркутск
Эндовазальная лазерная коагуляция https://evlo-phlebology.ru эффективный метод лечения варикоза. Амбулаторная процедура занимает до 40 минут, не требует госпитализации и обеспечивает быстрый косметический и медицинский результат.
Сюрвей грузов сюрвей контроль качества и количества, проверка условий перевозки, составление отчётов. Опытные эксперты обеспечивают объективную оценку для компаний и страховых организаций.
Надежная доставка из китая в Россию: от документов до контейнеров. Выбираем оптимальный маршрут, оформляем таможню, страхуем грузы. Контроль сроков и сохранность на каждом этапе.
The Real Person!
The Real Person!
подключить интернет тарифы санкт-петербург
inernetvkvartiru-spb005.ru
домашний интернет тарифы
сайт на тильде цена сайты на заказ от art-made
Надежная доставка грузов из китая: от небольших партий до контейнеров. Авиа, морем, авто и железной дорогой. Оформление, страхование и логистика в кратчайшие сроки.
Нужен автобусный билет? probilets.com онлайн просто и удобно. Поиск рейсов, сравнение цен, выбор мест и моментальная оплата. Актуальное расписание, надежные перевозчики и выгодные тарифы каждый день.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-kaluga010.ru
вывод из запоя круглосуточно калуга
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-cherepovec011.ru
вывод из запоя череповец
Ich bin total hingerissen von JackpotPiraten Casino, es fuhlt sich an wie eine wilde Piratenjagd. Es gibt eine Flut an packenden Casino-Titeln, mit Live-Casino-Sessions, die wie Kanonen donnern. Der Casino-Service ist zuverlassig und top, ist per Chat oder E-Mail erreichbar. Der Casino-Prozess ist klar und ohne Wellengang, dennoch wurde ich mir mehr Casino-Promos wunschen, die funkeln. Alles in allem ist JackpotPiraten Casino ein Casino, das man nicht verpassen darf fur Spieler, die auf krasse Casino-Kicks stehen! Extra die Casino-Plattform hat einen Look, der wie ein Schatz funkelt, was jede Casino-Session noch aufregender macht.
jackpotpiraten 5 euro|
The Real Person!
The Real Person!
Je trouve absolument epique Julius Casino, c’est un casino en ligne qui rugit comme un lion. Le repertoire du casino est une arene de divertissement, offrant des sessions de casino en direct qui en imposent. Le service client du casino est digne d’un cesar, repondant en un eclair de glaive. Les transactions du casino sont simples comme un decret, cependant j’aimerais plus de promotions de casino qui dominent. Globalement, Julius Casino promet un divertissement de casino heroique pour les amoureux des slots modernes de casino ! De surcroit la plateforme du casino brille par son style imperial, donne envie de replonger dans le casino sans fin.
julius casino mobile|
Качественный ремонт квартир https://expertremonta.kz от Компании «Эксперт ремонта» это качественный ремонт квартир под ключ в Алматы. Выбирайте Эксперт Ремонта — тут бесплатный выезд замерщика, официальные документы, гарантия документально, ремонт квартир без предоплаты.
дизтопливо с доставкой цена купить дизельное топливо евро 3 с доставкой
плодородный грунт цена грунт плодородный с доставкой цена
The Real Person!
The Real Person!
J’adore l’eclat de Bruno Casino, c’est un casino en ligne qui petille de vie. La selection du casino est une explosion de divertissement, comprenant des jeux de casino optimises pour les cryptomonnaies. Les agents du casino sont vifs comme l’eclair, repondant plus vite qu’une comete. Les retraits au casino sont rapides comme une fusee, quand meme des bonus de casino plus frequents seraient geniaux. En somme, Bruno Casino est une pepite pour les fans de casino pour les explorateurs du casino ! En plus la navigation du casino est intuitive comme une danse, ce qui rend chaque session de casino encore plus exaltante.
bruno mars mgm casino|
The Real Person!
The Real Person!
Sou viciado no role de LeaoWin Casino, da uma energia de cassino que e indomavel. A gama do cassino e simplesmente uma fera, com jogos de cassino perfeitos pra criptomoedas. O atendimento ao cliente do cassino e uma fera domada, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, mesmo assim mais bonus regulares no cassino seria top. No geral, LeaoWin Casino oferece uma experiencia de cassino que e puro rugido para os cacadores de slots modernos de cassino! De lambuja a interface do cassino e fluida e cheia de energia selvagem, faz voce querer voltar pro cassino como uma fera.
leaowin02 casino affiliates|
The Real Person!
The Real Person!
интернет провайдеры санкт-петербург
inernetvkvartiru-spb006.ru
подключить домашний интернет санкт-петербург
Мобильный выездной шиномонтаж https://master-shin.by круглосуточная помощь в дороге. Экстренная помощь в дороге может понадобиться каждому автолюбителю, и наша компания оказывает ее на высочайшем профессиональном уровне, на самых выгодных в Минске условиях. Оперативный выезд по городу и области, доступные и полностью адекватные цены, квалифицированная сервисная и консультационная поддержка, круглосуточное реагирование, вне зависимости от погодных условий.
Ремонт ноутбуков https://01km.ru телефонов, телевизоров, принтеров и компьютеров в Одинцово и Москве.
go to the website online: https://cere-india.org
The Real Person!
The Real Person!
экстренный вывод из запоя калуга
vivod-iz-zapoya-kaluga011.ru
вывод из запоя калуга
the best site for you: https://www.kapoorstudycircle.com
visit our website: https://www.acuam.com
our site is waiting for you: https://amt-games.com
The Real Person!
The Real Person!
лечение запоя череповец
vivod-iz-zapoya-cherepovec012.ru
вывод из запоя круглосуточно череповец
our official website: https://www.manaolahawaii.com
посетить сайт онлайн: https://efaflex.ru
go to our website: https://baldebranco.com.br
visit our new website: https://nature-et-avenir.org
our new official website: https://girls-media.com
I found a cool site: https://gingerparrot.co.uk
The Real Person!
The Real Person!
https://convertercase.com
The Real Person!
The Real Person!
https://usk-rus.ru/ Урал Строй Комплект – Наша компания занимается комплектацией строительных объектов, нефтегазового производства и не только. Одно из главных направлений работы – это гражданское строительство, где наши задачи – поставка металлических изделий и конструкций, труб, сыпучих строительных смесей, других стройматериалов. Также мы поставляем клиентам геосинтетические материалы для дорожного строительства, оборудование для нефтегазовой отрасли и многое другое под запрос. И все это – напрямую с заводов, с которыми у нас заключены официальные соглашения.
русское порно сиськи порно русская милфа
The Real Person!
The Real Person!
лечение запоя калуга
vivod-iz-zapoya-kaluga012.ru
вывод из запоя круглосуточно
Получите бесплатную юридическую консультацию онлайн, чтобы оперативно решить все ваши юридические вопросы!
Мы предлагаем консультации по множественным направлениям права.
Обратитесь за помощью к профессионалам на юрист бесплатно, и получите квалифицированное решение своих вопросов.
сайт yuridicheskaya-konsultaciya34.ru предлагает профессиональные юридические услуги, направленные на решение различных правовых вопросов. Наша команда готова помочь вам в самых сложных ситуациях. Мы понимаем, что правовые проблемы могут быть стрессовыми, мы предлагаем персонализированные решения к каждому клиенту.
У нас есть широкий спектр услуг, включая консультации по гражданским и уголовным делам. Мы приглашаем вас по вопросам, связанным с трудовым правом, семейными делами и другими юридическими аспектами. Мы понимаем, что каждая ситуация требует индивидуального подхода, и готовы предложить оптимальное решение.
Наша компания известна как надежный партнер в сфере юриспруденции. Наши клиенты доверяют нам за высокое качество обслуживания и результативность. Каждый специалист имеет опыт работы в различных областях права и готов поддержать вас в любое время.
Не откладывайте решение своих проблем , чтобы получить квалифицированную юридическую помощь. Мы готовы ответить на все ваши вопросы . Юридическая консультация ждет вас на yuridicheskaya-konsultaciya34.ru.
The Real Person!
The Real Person!
подключить домашний интернет екатеринбург
inernetvkvartiru-ekaterinburg004.ru
подключить проводной интернет екатеринбург
Want to have fun? hack apk Watch porn, buy heroin or ecstasy. Pick up whores or buy marijuana. Come in, we’re waiting
Mochten Sie ein hauser in Montenegro kaufen kaufen? Tolle Angebote am Meer und in den Bergen. Gro?e Auswahl an Immobilien, Unterstutzung bei der Immobilienauswahl, Transaktionsunterstutzung und Registrierung. Leben Sie in einem Land mit mildem Klima und wunderschoner Natur.
Новые актуальные промокод iherb promo kod herb для выгодных покупок! Скидки на витамины, БАДы, косметику и товары для здоровья. Экономьте до 30% на заказах, используйте проверенные купоны и наслаждайтесь выгодным шопингом.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-irkutsk007.ru
лечение запоя иркутск
best articles on the net: https://dnscompetition.in/hi/articles/the-role-of-dns-in-mobile-app-security/
The Real Person!
The Real Person!
bs2best at
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar011.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
провайдеры интернета по адресу екатеринбург
inernetvkvartiru-ekaterinburg005.ru
провайдеры интернета екатеринбург
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk008.ru
лечение запоя иркутск
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar012.ru
экстренный вывод из запоя
play puzzles online: https://optimisticmommy.com/when-the-day-drifts-slow-we-make-it-bloom/
The Real Person!
The Real Person!
лучший интернет провайдер екатеринбург
inernetvkvartiru-ekaterinburg006.ru
интернет провайдеры по адресу екатеринбург
The Real Person!
The Real Person!
J’adore la frenesie de CasinoClic, ca pulse avec une energie de casino electrisante. La collection de jeux du casino est phenomenale, incluant des jeux de table de casino d’une elegance eclatante. L’assistance du casino est chaleureuse et irreprochable, assurant un support de casino immediat et eclatant. Les retraits au casino sont rapides comme une fusee, mais j’aimerais plus de promotions de casino qui eblouissent. Pour resumer, CasinoClic c’est un casino a decouvrir en urgence pour les explorateurs du casino ! Bonus la plateforme du casino brille par son style eblouissant, amplifie l’immersion totale dans le casino.
casino clic avis|
The Real Person!
The Real Person!
Ich bin vollig geflasht von Lapalingo Casino, es pulsiert mit einer elektrisierenden Casino-Energie. Der Katalog des Casinos ist ein Kaleidoskop des Spa?es, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Die Casino-Mitarbeiter sind schnell wie ein Blitzstrahl, sorgt fur sofortigen Casino-Support, der beeindruckt. Auszahlungen im Casino sind schnell wie ein Wirbelwind, manchmal mehr Freispiele im Casino waren ein Volltreffer. Kurz gesagt ist Lapalingo Casino ein Online-Casino, das wie ein Sturm begeistert fur die, die mit Stil im Casino wetten! Und au?erdem die Casino-Navigation ist kinderleicht wie ein Windhauch, einen Hauch von Zauber ins Casino bringt.
lapalingo error 1020|
The Real Person!
The Real Person!
Ich bin suchtig nach iWild Casino, es ist ein Online-Casino, das wie ein Urwald tobt. Die Casino-Optionen sind vielfaltig und elektrisierend, inklusive stilvoller Casino-Tischspiele. Das Casino-Team bietet Unterstutzung, die wie ein Lagerfeuer gluht, sorgt fur sofortigen Casino-Support, der beeindruckt. Casino-Gewinne kommen wie ein Sturm, manchmal mehr Casino-Belohnungen waren ein wilder Gewinn. Zusammengefasst ist iWild Casino ein Casino mit einem Spielspa?, der wie ein Gewitter tobt fur Abenteurer im Casino! Und au?erdem das Casino-Design ist ein optisches Naturwunder, das Casino-Erlebnis total wild macht.
iwild casino -mobiili|
The Real Person!
The Real Person!
Je trouve absolument siderant JackpotStar Casino, ca degage une vibe de jeu celeste. Le repertoire du casino est une nebuleuse de divertissement, proposant des slots de casino a theme stellaire. Les agents du casino sont rapides comme une comete, assurant un support de casino immediat et eclatant. Les retraits au casino sont rapides comme un meteore, quand meme les offres du casino pourraient etre plus genereuses. Au final, JackpotStar Casino offre une experience de casino celeste pour ceux qui cherchent l’adrenaline lumineuse du casino ! Bonus le site du casino est une merveille graphique lumineuse, ce qui rend chaque session de casino encore plus eblouissante.
jackpotstar kasyno|
The Real Person!
The Real Person!
J’adore l’energie de LeonBet Casino, ca degage une vibe de jeu feroce. La collection de jeux du casino est titanesque, proposant des slots de casino a theme audacieux. Le support du casino est disponible 24/7, proposant des solutions claires et instantanees. Les paiements du casino sont securises et fluides, quand meme des recompenses de casino supplementaires feraient rugir. Au final, LeonBet Casino est un territoire pour les fans de casino pour ceux qui cherchent l’adrenaline sauvage du casino ! A noter la navigation du casino est intuitive comme une traque, ce qui rend chaque session de casino encore plus rugissante.
leonbet codigo promocional|
best site come on in: https://alterego.re
The most useful information: https://satapornbooks.com
Самое лучшее в сети: https://www.foto4u.su
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-irkutsk009.ru
вывод из запоя цена
Current and up-to-date information: https://clubcoma.org
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar013.ru
лечение запоя
доставка из китая доставка товаров из китая
шпонированные панели дсп шпонирование
геодезист москва геодезия
геотекстиль 600 купить геотекстиль нетканый канвалан
The Real Person!
The Real Person!
подключение интернета по адресу
inernetvkvartiru-krasnoyarsk004.ru
провайдер по адресу красноярск
Включение в реестр Минпромторга https://minprom-info.ru официальный путь для подтверждения отечественного производства. Подготовка и подача документов, юридическое сопровождение и консультации для производителей.
When we look at these issues, we know that they are the key ones for our time.
Занятия по самообороне https://safety-skills.ru практические навыки защиты в реальных ситуациях, развитие силы и выносливости. Профессиональные тренеры помогут освоить приемы борьбы, удары и тактику безопасности.
Платформа онлайн-обучения https://craftsmm.ru курсы по маркетингу, продажам и рекламе для новичков и профессионалов. Освойте современные инструменты продвижения, увеличьте продажи и развивайте карьеру в удобном формате.
https://autoevak-46.ru/
The Real Person!
The Real Person!
вывод из запоя калуга
vivod-iz-zapoya-kaluga010.ru
вывод из запоя калуга
Написание дипломов на заказ https://vasdiplom.ru помощь студентам в подготовке итоговых работ. Авторские тексты, проверка на уникальность и полное соответствие стандартам учебных заведений.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar014.ru
лечение запоя краснодар
The Real Person!
The Real Person!
Ich bin suchtig nach Lapalingo Casino, es ist ein Online-Casino, das wie ein Blitz einschlagt. Es gibt eine Welle an packenden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Kundenservice ist wie ein Leuchtfeuer, ist per Chat oder E-Mail erreichbar. Der Casino-Prozess ist klar und ohne Wellen, trotzdem mehr Casino-Belohnungen waren ein funkelnder Gewinn. Zusammengefasst ist Lapalingo Casino ein Casino, das man nicht verpassen darf fur Abenteurer im Casino! Und au?erdem die Casino-Oberflache ist flussig und strahlt wie ein Nordlicht, was jede Casino-Session noch aufregender macht.
promo code lapalingo 2022|
visit our best site online: https://intelicode.com
The Real Person!
The Real Person!
Acho simplesmente insano JabiBet Casino, e um cassino online que e uma verdadeira onda. A gama do cassino e simplesmente um maremoto, com caca-niqueis de cassino modernos e envolventes. Os agentes do cassino sao rapidos como uma onda, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, mesmo assim queria mais promocoes de cassino que arrasam. No fim das contas, JabiBet Casino e um cassino online que e uma onda gigante para os viciados em emocoes de cassino! De bonus a interface do cassino e fluida e cheia de energia oceanica, da um toque de classe aquatica ao cassino.
jabibet|
The best we have here: https://www.kapoorstudycircle.com
The Real Person!
The Real Person!
Ich bin suchtig nach JokerStar Casino, es fuhlt sich an wie ein magischer Spielrausch. Die Casino-Optionen sind bunt und mitrei?end, inklusive eleganter Casino-Tischspiele. Das Casino-Team bietet Unterstutzung, die wie ein Stern glanzt, sorgt fur sofortigen Casino-Support, der verblufft. Der Casino-Prozess ist klar und ohne Hokuspokus, ab und zu wurde ich mir mehr Casino-Promos wunschen, die funkeln. Zusammengefasst ist JokerStar Casino ein Casino, das man nicht verpassen darf fur Spieler, die auf magische Casino-Kicks stehen! Und au?erdem die Casino-Plattform hat einen Look, der wie ein Sternenstaub funkelt, das Casino-Erlebnis total verzaubert.
jokerstar no deposit bonus|
The Real Person!
The Real Person!
Je suis totalement ensorcele par LeoVegas Casino, ca pulse avec une energie de casino souveraine. La collection de jeux du casino est royale, incluant des jeux de table de casino d’une elegance royale. Le service client du casino est digne d’un monarque, repondant en un eclair imperial. Les paiements du casino sont securises et fluides, parfois j’aimerais plus de promotions de casino qui eblouissent. Pour resumer, LeoVegas Casino c’est un casino a conquerir en urgence pour les passionnes de casinos en ligne ! Par ailleurs le design du casino est une fresque visuelle royale, ce qui rend chaque session de casino encore plus majestueuse.
leovegas presenter|
The Real Person!
The Real Person!
провайдеры интернета в красноярске по адресу
inernetvkvartiru-krasnoyarsk005.ru
провайдеры интернета в красноярске по адресу проверить
The latest information is here: https://unilago.com
We have the latest information: https://fish-pet.com
visit our website: https://cere-india.org
We are waiting for you on the site: https://copychief.com
HBet – Cổng giải trí trực tuyến hàng đầu, quy tụ hàng ngàn trò chơi cá cược thể thao, casino, slot game và bắn cá đổi thưởng. Giao diện mượt mà, bảo mật tuyệt …
The Real Person!
The Real Person!
экстренный вывод из запоя калуга
vivod-iz-zapoya-kaluga011.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-krasnodar015.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
какие провайдеры интернета есть по адресу красноярск
inernetvkvartiru-krasnoyarsk006.ru
проверить интернет по адресу
Ich liebe die Strahlkraft von LuckyNiki Casino, es bietet ein Casino-Abenteuer, das wie ein Sternenstaub funkelt. Die Casino-Optionen sind vielfaltig und zauberhaft, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Das Casino-Team bietet Unterstutzung, die wie ein Stern funkelt, liefert klare und schnelle Losungen. Casino-Zahlungen sind sicher und reibungslos, dennoch mehr regelma?ige Casino-Boni waren zauberhaft. Insgesamt ist LuckyNiki Casino ein Casino mit einem Spielspa?, der wie ein Komet leuchtet fur Fans moderner Casino-Slots! Nebenbei die Casino-Navigation ist kinderleicht wie ein Zauberspruch, Lust macht, immer wieder ins Casino zuruckzukehren.
luckyniki erfahrungen|
Je suis accro a Luckster Casino, c’est un casino en ligne qui brille comme un talisman. Le repertoire du casino est une cascade de divertissement, proposant des slots de casino a theme feerique. Le personnel du casino offre un accompagnement enchante, proposant des solutions claires et instantanees. Les paiements du casino sont securises et fluides, quand meme des bonus de casino plus frequents seraient ensorcelants. Au final, Luckster Casino est un joyau pour les fans de casino pour ceux qui cherchent l’adrenaline enchantee du casino ! De surcroit le site du casino est une merveille graphique enchantee, donne envie de replonger dans le casino sans fin.
ultimate luckster|
Hi there, I would like to know more about the course, is that possible that you send to me?
Thanks.
The Real Person!
The Real Person!
Je suis fou de MonteCryptos Casino, il propose une aventure de casino qui scintille comme un glacier. Les choix de jeux au casino sont riches et vertigineux, proposant des slots de casino a theme audacieux. Le personnel du casino offre un accompagnement digne d’un sherpa, joignable par chat ou email. Les gains du casino arrivent a une vitesse alpine, parfois plus de tours gratuits au casino ce serait exaltant. Dans l’ensemble, MonteCryptos Casino promet un divertissement de casino scintillant pour les amoureux des slots modernes de casino ! A noter la plateforme du casino brille par son style audacieux, amplifie l’immersion totale dans le casino.
montecryptos bonus codes 2021|
The Real Person!
The Real Person!
вывод из запоя круглосуточно калуга
vivod-iz-zapoya-kaluga012.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-minsk007.ru
вывод из запоя цена
Want to have fun? hack apk Whores, drugs, casino. We have it all, any drugs are on sale.
цена героина на рынке купить героин
The best of our site: https://www.greenwichodeum.com
New and relevant information: https://www.colehardware.com
The Real Person!
The Real Person!
провайдеры интернета по адресу краснодар
inernetvkvartiru-krasnodar004.ru
интернет провайдеры в краснодаре по адресу дома
Обучение и семинары https://uofs-beslan.ru для профессионалов: современные программы, практические кейсы и опыт экспертов. Развивайте навыки, повышайте квалификацию и получайте новые возможности для карьерного роста.
Школа видеорекламы https://tatyanamostseeva.ru обучение созданию креативных роликов для бизнеса и брендов. Практические занятия, работа с современными инструментами и поддержка экспертов. Освойте профессию в сфере digital.
Академия парикмахерского искусства https://charm-academy.ru обучение от ведущих мастеров. Современные техники стрижек, окрашивания и укладок. Курсы для начинающих и профессионалов с практикой и дипломом по окончании.
Лицей взаимного обучения https://talgenisty.ru уникальная среда для детей и взрослых. Совместные уроки, обмен опытом, мастер-классы и творческие проекты. Образование, основанное на поддержке и сотрудничестве.
The Real Person!
The Real Person!
экстренный вывод из запоя минск
vivod-iz-zapoya-minsk008.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar011.ru
вывод из запоя круглосуточно
Je suis totalement sous le charme de Casinova, ca offre une experience inoubliable. Il y a une variete impressionnante de titres, offrant des machines a sous a theme. Le service client est irreprochable, repondant en un clin d’?il. Les gains arrivent sans delai, meme si des recompenses supplementaires seraient appreciees. En fin de compte, Casinova assure une experience de jeu memorable pour les fans de jeux modernes ! Notons aussi le design est visuellement seduisant, renforce le plaisir de jouer.
casinova sportsbook|
The Real Person!
The Real Person!
Je suis totalement envoute par LuckyBlock Casino, ca pulse avec une energie de casino ensorcelante. Le repertoire du casino est une galaxie de divertissement, offrant des sessions de casino en direct qui illuminent. Le service client du casino est une etoile porte-bonheur, assurant un support de casino immediat et eclatant. Les paiements du casino sont securises et fluides, mais des recompenses de casino supplementaires feraient rever. Au final, LuckyBlock Casino offre une experience de casino enchanteresse pour ceux qui cherchent l’adrenaline lumineuse du casino ! Bonus le site du casino est une merveille graphique lumineuse, ce qui rend chaque session de casino encore plus envoutante.
luckyblock lottery|
Great site for everyone: https://feas.ru
The best site for you: https://www.jec.qa
Приём макулатуры https://chsreda.ru в Казани работает как для частных лиц, так и для организаций. Сдают газеты, журналы, картонные коробки, офисную бумагу. Это реально удобно, потому что приезжают, забирают прямо с территории, а потом всё идёт на переработку.
Je suis accro a Lucky8 Casino, c’est un casino en ligne qui scintille comme un grigri. Les choix de jeux au casino sont riches et eclatants, comprenant des jeux de casino optimises pour les cryptomonnaies. Le support du casino est disponible 24/7, joignable par chat ou email. Les retraits au casino sont rapides comme un coup de baguette, quand meme plus de tours gratuits au casino ce serait magique. Globalement, Lucky8 Casino est un joyau pour les fans de casino pour ceux qui cherchent l’adrenaline lumineuse du casino ! De surcroit la plateforme du casino brille par son style ensorcelant, amplifie l’immersion totale dans le casino.
lucky8 code bonus|
Кухонные принадлежности https://www.kitchen-store.ru в магазине для дома: современная посуда, полезные гаджеты, текстиль и аксессуары. Стильные решения для кухни, выгодные цены и большой ассортимент.
The Real Person!
The Real Person!
À chaque aubaine son code bonus sur Lucky Wins. Le code bonus de bienvenue high roller est par exemple HR1ST. Vous y obtenez 100 % jusqu’à 2 000 $ à partir d’un dépôt de 500 $. Lucky Wins accepte les dépôts et les retraits avec six crypto-monnaies différentes : Bitcoin, Ethereum, Tether, Litecoin, Dogecoin et Bitcoin Cash. Forte volatilité Big Bass Splash avec cette application, une volatilité élevée. Oui, une dizaine de crypto-monnaies sont acceptées pour les dépôts et les retraits sur le casino Katsubet. Oui, Katsubet casino propose 2 bonus sans dépôts à ses joueurs lors de l’inscription. Nous en parlons plus haut dans cet article. Après l’ouverture de votre compte et votre premier dépôt, vous aurez alors accès au bonus de bienvenue de Bethard. Il vous offre 100 % jusqu’à 200CAD sur votre premier dépôt. Pour effectuer votre paiement de manière instantané, vous pourrez utiliser l’une des méthodes de paiement suivante : Skrill, Neteller, MiFinity, MuchBetter, Visa, Mastercard, PaySafeCard, Neosurf et Astropay. Par ailleurs, le virement bancaire est également pris en charge, mais il n’est pas instantané.
https://www.svetzacina.com/big-bass-bonanza-splash-plongee-au-coeur-de-la-nouvelle-vague-de-pragmatic-play/
Lucky Jet Classement Des Emplacements Tout ce dont vous avez besoin pour jouer dans n’importe quel casino Muchbetter est un appareil mobile avec l’application Muchbetter, vous devrez miser 30 fois ou plus les gains bonus de vos tours gratuits. Et après que le casino, Secrets of India n’est pas une machine à sous très populaire. Comme la mise de départ est si faible, licences et réglementations du casino en ligne big bass splash son intérêt consiste surtout à maintenir l’attention du joueur. Inscrivez-vous aujourd’hui et découvrez nos jeux passionnants. En 2025, les applications de casino à Kitchener redéfinissent l’expérience du jeu en ligne en offrant des plateformes plus immersives, sécurisées et accessibles que jamais. Grâce aux dernières avancées technologiques, les joueurs peuvent profiter de graphismes époustouflants, de fonctionnalités interactives et de transactions ultra-rapides, le tout depuis leur smartphone ou leur tablette. Que vous soyez amateur de machines à sous, de blackjack, de roulette ou de poker, ces applications garantissent une fluidité de jeu incomparable et des bonus exclusifs réservés aux utilisateurs mobiles.
The Real Person!
The Real Person!
Je suis totalement electrise par Madnix Casino, on dirait une tornade de plaisir. Les choix de jeux au casino sont riches et dejantes, offrant des sessions de casino en direct qui font peter les plombs. Le personnel du casino offre un accompagnement qui fait des etincelles, assurant un support de casino immediat et explosif. Les transactions du casino sont simples comme un clin d’?il, par moments des bonus de casino plus frequents seraient dements. Globalement, Madnix Casino offre une experience de casino dejantee pour les passionnes de casinos en ligne ! En plus le design du casino est une explosion visuelle delirante, facilite une experience de casino electrisante.
live chat madnix|
The Real Person!
The Real Person!
интернет провайдеры по адресу краснодар
inernetvkvartiru-krasnodar005.ru
интернет провайдеры по адресу дома
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-minsk009.ru
вывод из запоя цена
Нужен автобусный билет? билеты на автобус удобный сервис поиска и бронирования. Широкий выбор направлений, надежные перевозчики, доступные цены и моментальная отправка электронных билетов на почту.
Авто портал https://diesel.kyiv.ua все о мире автомобилей: новости, обзоры моделей, тест-драйвы, советы по выбору и уходу за авто. Каталог машин, актуальные цены, автоуслуги и полезная информация для автовладельцев.
Все для автомобилистов https://k-moto.com.ua на авто портале: новости, обзоры, статьи, каталоги и цены на автомобили. Экспертные мнения, тест-драйвы и практические советы по эксплуатации авто.
Автомобильный портал https://auto-club.pl.ua онлайн-площадка для автолюбителей. Подробные обзоры машин, тест-драйвы, свежие новости, советы по ремонту и обслуживанию. Удобный поиск и актуальные материалы.
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar012.ru
экстренный вывод из запоя краснодар
Нужна виза? цена визы в албанию Консультации, подготовка документов, сопровождение на всех этапах. Визы в Европу, США, Азию и другие страны. Доступные цены и надежная поддержка.
Женский портал https://beautyadvice.kyiv.ua все для современных женщин: красота, здоровье, семья, отношения, карьера. Полезные статьи, советы экспертов, лайфхаки и вдохновение каждый день. Онлайн-сообщество для общения и развития.
Онлайн женский портал https://elegance.kyiv.ua актуальные советы по красоте, стилю, кулинарии и семейной жизни. Разделы о здоровье, карьере и саморазвитии. Интересные статьи и общение с единомышленницами.
Портал для женщин https://fashionadvice.kyiv.ua сайт для девушек и женщин, которые ценят красоту, уют и гармонию. Советы по стилю, отношениям, материнству и здоровью. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk007.ru
лечение запоя омск
The Real Person!
The Real Person!
интернет провайдеры в краснодаре по адресу дома
inernetvkvartiru-krasnodar006.ru
провайдер интернета по адресу краснодар
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar013.ru
лечение запоя
The Real Person!
The Real Person!
Sou louco pelo role de MegaPosta Casino, da uma energia de cassino que e um vulcao. Os titulos do cassino sao um espetaculo insano, com slots de cassino unicos e contagiantes. O suporte do cassino ta sempre na ativa 24/7, garantindo suporte de cassino direto e sem enrolacao. O processo do cassino e limpo e sem turbulencia, as vezes mais bonus regulares no cassino seria brabo. Em resumo, MegaPosta Casino oferece uma experiencia de cassino que e puro fogo para os viciados em emocoes de cassino! Vale falar tambem o site do cassino e uma obra-prima de estilo, aumenta a imersao no cassino a mil.
codigo promocional megaposta|
The Real Person!
The Real Person!
Je suis totalement ensorcele par MyStake Casino, on dirait un labyrinthe de frissons. Le repertoire du casino est une toile d’enigmes ludiques, offrant des sessions de casino en direct qui hypnotisent. Le personnel du casino offre un accompagnement digne d’un sage, offrant des solutions claires et instantanees. Le processus du casino est transparent et sans ombres, parfois plus de tours gratuits au casino ce serait mystique. Pour resumer, MyStake Casino promet un divertissement de casino enigmatique pour les amoureux des slots modernes de casino ! De surcroit le design du casino est une fresque visuelle captivante, donne envie de replonger dans le casino sans fin.
impossible de parier mystake|
Estou completamente alucinado por OshCasino, e um cassino online que detona como um vulcao. A gama do cassino e simplesmente uma lava ardente, com jogos de cassino perfeitos pra criptomoedas. A equipe do cassino entrega um atendimento que e uma labareda, garantindo suporte de cassino direto e sem cinzas. Os ganhos do cassino chegam voando como um meteoro, porem mais giros gratis no cassino seria uma loucura. Em resumo, OshCasino e um cassino online que e uma erupcao de diversao para os cacadores de slots modernos de cassino! De lambuja o site do cassino e uma obra-prima de estilo, faz voce querer voltar pro cassino como uma erupcao.
avis sur osh|
The Real Person!
The Real Person!
Ich bin total begeistert von Pledoo Casino, es fuhlt sich an wie ein wilder Tanz durch die Spielwelt. Die Casino-Optionen sind vielfaltig und mitrei?end, mit modernen Casino-Slots, die einen in ihren Bann ziehen. Der Casino-Service ist zuverlassig und glanzend, ist per Chat oder E-Mail erreichbar. Casino-Zahlungen sind sicher und reibungslos, manchmal mehr regelma?ige Casino-Boni waren ein Knaller. Alles in allem ist Pledoo Casino ein Casino, das man nicht verpassen darf fur Fans moderner Casino-Slots! Extra die Casino-Seite ist ein grafisches Meisterwerk, Lust macht, immer wieder ins Casino zuruckzukehren.
pledoo casino guru|
Вы можете воспользоваться широким спектром услуг на сайте konsultaciya-advokata51.ru. Получение юридической помощи – это необходимость для многих. Мы обеспечим вас экспертными консультациями.
Получите бесплатную юридическую консультацию круглосуточно на номер бесплатного юриста.
Не забывайте, что юридическая помощь может быть востребована в самых разных ситуациях.
Важно обратить внимание на уровень профессионализма юристов. Каждый адвокат на konsultaciya-advokata51.ru прошел тщательный отбор. Что бы вы ни выбрали, мы гарантируем качественное выполнение работы.
Важно, чтобы юридические услуги были доступны каждому. Мы стараемся сделать информацию о наших услугах максимально прозрачной. Клиенты могут выбрать наиболее подходящий вариант в зависимости от своих нужд.
Кроме того, мы предлагаем услуги онлайн. Виртуальные консультации становятся всё более популярными. Вы можете задать свои вопросы в любое время.
The Real Person!
The Real Person!
лечение запоя омск
vivod-iz-zapoya-omsk008.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
какие провайдеры на адресе в москве
inernetvkvartiru-msk004.ru
домашний интернет в москве
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar014.ru
вывод из запоя цена
Авто портал https://avtoshans.in.ua для всех: свежие новости, обзоры моделей, советы по выбору и эксплуатации авто. Каталог машин, тест-драйвы и рекомендации экспертов для водителей и покупателей.
Портал про автомобили https://myauto.kyiv.ua онлайн-ресурс для автолюбителей. Обзоры, статьи, тест-драйвы, цены и полезные советы по ремонту и уходу за машиной. Всё о мире авто в одном месте.
Свежие новости авто https://orion-auto.com.ua тест-драйвы, обзоры новинок, законодательные изменения и аналитика авторынка. Подробная информация об автомобилях и автоиндустрии для водителей и экспертов.
Автомобильные новости https://reuth911.com онлайн: новые модели, отзывы, тест-драйвы, события автопрома и полезные советы. Узнайте первыми о главных новинках и трендах автомобильного мира.
The Real Person!
The Real Person!
Игровая платформа Vavada часто обсуждается в сети.
Акции и предложения делают старт комфортным.
Игровые конкурсы увеличивают азарт.
Игровой каталог обновляется постоянно.
Регистрация занимает минуты, и моментально использовать промокоды.
Все подробности смотри здесь: бездеп вавада
Been checking out Tiger787 lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more than I can say for some other places I’ve tried
The Real Person!
The Real Person!
Sou viciado no role de MonsterWin Casino, da uma energia de cassino que e indomavel. O catalogo de jogos do cassino e uma fera total, com jogos de cassino perfeitos pra criptomoedas. Os agentes do cassino sao rapidos como um predador, respondendo mais rapido que um rugido. Os ganhos do cassino chegam voando como um dragao, mesmo assim mais giros gratis no cassino seria uma loucura. No fim das contas, MonsterWin Casino e um cassino online que e uma fera total para os cacadores de slots modernos de cassino! E mais o design do cassino e uma explosao visual feroz, o que deixa cada sessao de cassino ainda mais animal.
monsterwin casino bonus|
Ich bin vollig hingerissen von PlayJango Casino, es bietet ein Casino-Abenteuer, das wie ein Regenbogen funkelt. Der Katalog des Casinos ist eine Flut voller Spa?, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Support ist rund um die Uhr verfugbar, ist per Chat oder E-Mail erreichbar. Auszahlungen im Casino sind schnell wie ein Sturm, manchmal mehr regelma?ige Casino-Boni waren ein Knaller. Kurz gesagt ist PlayJango Casino ein Casino mit einem Spielspa?, der wie ein Feuerwerk knallt fur die, die mit Stil im Casino wetten! Und au?erdem die Casino-Plattform hat einen Look, der wie ein Blitz funkelt, Lust macht, immer wieder ins Casino zuruckzukehren.
playjango casino complaints|
Estou completamente vidrado por PagolBet Casino, oferece uma aventura de cassino que faz tudo tremer. Tem uma enxurrada de jogos de cassino irados, com jogos de cassino perfeitos pra criptomoedas. O atendimento ao cliente do cassino e uma corrente de eficiencia, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, as vezes mais bonus regulares no cassino seria top. No geral, PagolBet Casino e um cassino online que e um relampago de diversao para os viciados em emocoes de cassino! E mais a plataforma do cassino detona com um visual que e puro trovao, faz voce querer voltar pro cassino como um raio.
pagolbet app|
The Real Person!
The Real Person!
Je trouve absolument enivrant PokerStars Casino, ca vibre avec une energie de casino strategique. Le repertoire du casino est un tournoi de divertissement, offrant des sessions de casino en direct qui electrisent. Le support du casino est disponible 24/7, repondant en un eclair strategique. Les paiements du casino sont securises et fluides, quand meme des bonus de casino plus frequents seraient strategiques. En somme, PokerStars Casino offre une experience de casino palpitante pour les passionnes de casinos en ligne ! En plus le design du casino est un spectacle visuel captivant, ce qui rend chaque session de casino encore plus palpitante.
bug pokerstars|
Онлайн-сайт для женщин https://musicbit.com.ua стиль, уход за собой, психология, семья, карьера и хобби. Интересные статьи, тесты и форум для общения. Пространство для вдохновения и развития.
Автомобильный сайтhttps://setbook.com.ua свежие новости, обзоры моделей, тест-драйвы и советы экспертов. Каталог авто, актуальные цены, авторынок и всё, что нужно водителям и автолюбителям в одном месте.
Онлайн-журнал для женщин https://fines.com.ua стиль, уход за собой, психология, рецепты, материнство и карьера. Актуальные материалы, тренды и экспертные рекомендации каждый день.
Женский сайт о жизни https://prettywoman.kyiv.ua секреты красоты, мода, здоровье, рецепты и отношения. Интересные статьи, советы и лайфхаки. Всё, что нужно, чтобы чувствовать себя уверенно и счастливо.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk009.ru
вывод из запоя круглосуточно омск
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar015.ru
лечение запоя
The Real Person!
The Real Person!
провайдеры по адресу москва
inernetvkvartiru-msk005.ru
подключить домашний интернет москва
Автомобильный портал https://troeshka.com.ua онлайн-ресурс для автовладельцев. Каталог машин, тест-драйвы, аналитика авторынка и советы специалистов. Будьте в курсе новинок и технологий автоиндустрии.
Женский онлайн-журнал https://feminine.kyiv.ua мода, красота, здоровье, отношения и семья. Полезные советы, вдохновляющие статьи, лайфхаки для дома и карьеры. Всё самое интересное для современных женщин.
Онлайн-сайт про автомобили https://tvregion.com.ua свежие новости, аналитика рынка, обзоры и сравнения машин. Советы по обслуживанию и выбору авто. Всё для водителей и автолюбителей в одном месте.
Сайт для женщин https://lolitaquieretemucho.com мода, красота, здоровье, отношения, семья и карьера. Полезные советы, статьи, рецепты и лайфхаки. Пространство для вдохновения и развития, созданное для современных женщин.
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-orenburg007.ru
экстренный вывод из запоя оренбург
The Real Person!
The Real Person!
https://xn--80aack7aript.xn--p1ai/%D0%BA%D0%B0%D1%80%D0%B0%D1%81%D1%83%D0%BA/
Сайт про машины https://tvk-avto.com.ua обзоры моделей, тест-драйвы, новости автопрома и советы по эксплуатации. Полезные статьи о выборе авто, уходе, ремонте и актуальные материалы для автовладельцев.
Сайт для женщин https://femaleguide.kyiv.ua гармония стиля и жизни. Уход за собой, рецепты, дом, отношения, карьера и путешествия. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
Автомобильный новостной портал https://tuning-kh.com.ua всё об авто в одном месте: новости, цены, обзоры, тест-драйвы, авторынок. Советы экспертов и полезные материалы для водителей и тех, кто планирует купить машину.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
The Real Person!
The Real Person!
экстренный вывод из запоя минск
vivod-iz-zapoya-minsk007.ru
лечение запоя
The Real Person!
The Real Person!
Je suis totalement captive par MrPlay Casino, il propose une aventure de casino qui swingue comme un orchestre. La selection de jeux du casino est une veritable parade de divertissement, proposant des slots de casino a theme vibrant. Le service client du casino est une star de la fete, garantissant un support de casino instantane et eclatant. Les paiements du casino sont securises et fluides, cependant plus de tours gratuits au casino ce serait enivrant. Globalement, MrPlay Casino promet un divertissement de casino eclatant pour ceux qui cherchent l’adrenaline festive du casino ! En plus le site du casino est une merveille graphique festive, ce qui rend chaque session de casino encore plus exuberante.
mr.play test|
The Real Person!
The Real Person!
провайдер по адресу москва
inernetvkvartiru-msk006.ru
узнать интернет по адресу
Adoro o clima explosivo de PlayUzu Casino, oferece uma aventura de cassino que faz tudo voar. A gama do cassino e simplesmente uma rajada, com jogos de cassino perfeitos pra criptomoedas. O suporte do cassino ta sempre na ativa 24/7, garantindo suporte de cassino direto e sem turbulencia. As transacoes do cassino sao simples como uma brisa, mesmo assim mais giros gratis no cassino seria uma loucura. Na real, PlayUzu Casino vale demais explorar esse cassino para os amantes de cassinos online! De bonus a interface do cassino e fluida e cheia de energia vibrante, aumenta a imersao no cassino a mil.
bono playuzu|
The Real Person!
The Real Person!
Je suis fou de Posido Casino, ca degage une ambiance de jeu aussi fluide qu’un courant oceanique. L’eventail de jeux du casino est un ocean de delices, proposant des slots de casino a theme aquatique. Le support du casino est disponible 24/7, offrant des solutions claires et instantanees. Le processus du casino est transparent et sans remous, quand meme plus de tours gratuits au casino ce serait aquatique. Pour resumer, Posido Casino offre une experience de casino fluide comme l’ocean pour ceux qui cherchent l’adrenaline fluide du casino ! Par ailleurs le design du casino est un spectacle visuel aquatique, donne envie de replonger dans le casino sans fin.
posido casino bonus|
Онлайн-сайт для женщин https://mirlady.kyiv.ua красота, стиль, здоровье, дом и семья. Практичные рекомендации, модные идеи, вдохновение и поддержка. Лучший контент для девушек и женщин любого возраста.
Сайт для женщин https://amideya.com.ua портал о красоте, стиле, здоровье, семье и саморазвитии. Ежедневные статьи, полезные рекомендации и вдохновение для современных девушек и женщин.
Женский сайт https://lubimoy.com.ua стиль, уход за собой, психология, материнство, работа и хобби. Актуальные статьи, тренды и экспертные советы. Всё самое важное для гармоничной жизни и успеха.
Женский онлайн-журнал https://gracefullady.kyiv.ua свежие статьи о моде, красоте, здоровье и саморазвитии. Практичные советы, вдохновение и позитив для девушек и женщин любого возраста.
https://peterburg2.ru/
https://autoevak-46.ru/
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-orenburg008.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно минск
vivod-iz-zapoya-minsk008.ru
лечение запоя минск
The Real Person!
The Real Person!
провайдеры интернета по адресу нижний новгород
inernetvkvartiru-nizhnij-novgorod004.ru
провайдеры по адресу
The Real Person!
The Real Person!
Игровая платформа Vavada собирает тысячи игроков.
Специальные предложения дают отличную возможность стартовать.
Регулярные соревнования увеличивают азарт.
Каталог развлечений обновляется провайдерами.
Начать игру можно за пару минут, поэтому бонусы становятся доступны сразу.
Подробности смотрите по ссылке: https://nickyboom.com
The Real Person!
The Real Person!
экстренный вывод из запоя оренбург
vivod-iz-zapoya-orenburg009.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
Je suis accro a MrXBet Casino, ca vibre avec une energie de casino enigmatique. La selection du casino est une enigme de delices, proposant des slots de casino a theme audacieux. L’assistance du casino est fiable et perspicace, repondant en un eclair mysterieux. Les gains du casino arrivent a une vitesse fulgurante, quand meme des bonus de casino plus frequents seraient captivants. Dans l’ensemble, MrXBet Casino promet un divertissement de casino enigmatique pour les joueurs qui aiment parier avec flair au casino ! Par ailleurs le design du casino est un puzzle visuel captivant, ce qui rend chaque session de casino encore plus intrigante.
mrxbet 2024|
latest information here: https://ville-barentin.fr
visit our website: https://g-r-s.fr
Here is the best on the net: https://www.manaolahawaii.com
Latest information from us: https://www.semasan.com
The Real Person!
The Real Person!
Je suis totalement envoute par ParisVegasClub, il propose une aventure de casino qui danse comme un spectacle. La selection du casino est un bouquet de plaisirs, incluant des jeux de table de casino d’une elegance theatrale. Le personnel du casino offre un accompagnement digne d’un metteur en scene, repondant en un eclair de projecteur. Les retraits au casino sont rapides comme un final de spectacle, cependant j’aimerais plus de promotions de casino qui eblouissent. Pour resumer, ParisVegasClub est un casino en ligne qui met la scene en feu pour les joueurs qui aiment parier avec panache au casino ! Bonus l’interface du casino est fluide et eclatante comme un projecteur, ce qui rend chaque session de casino encore plus spectaculaire.
paris vegas club bonus codes|
Ich finde absolut majestatisch Richard Casino, es fuhlt sich an wie ein koniglicher Triumph. Es gibt eine Flut an fesselnden Casino-Titeln, mit einzigartigen Casino-Slotmaschinen. Der Casino-Kundenservice ist wie ein koniglicher Hofstaat, antwortet blitzschnell wie ein koniglicher Erlass. Auszahlungen im Casino sind schnell wie ein koniglicher Marsch, aber mehr Freispiele im Casino waren ein Kronungsmoment. Kurz gesagt ist Richard Casino eine Casino-Erfahrung, die wie ein Kronungsjuwel glanzt fur Abenteurer im Casino! Ubrigens die Casino-Plattform hat einen Look, der wie ein Kronungsmantel glanzt, was jede Casino-Session noch prachtiger macht.
richard casino uhr|
Je suis fou de PlazaRoyal Casino, c’est un casino en ligne qui brille comme un sceptre royal. Les options de jeu au casino sont riches et somptueuses, avec des machines a sous de casino modernes et envoutantes. Le support du casino est disponible 24/7, assurant un support de casino immediat et majestueux. Les retraits au casino sont rapides comme une procession royale, quand meme des bonus de casino plus frequents seraient royaux. Dans l’ensemble, PlazaRoyal Casino promet un divertissement de casino princier pour ceux qui cherchent l’adrenaline royale du casino ! De surcroit la plateforme du casino brille par son style souverain, amplifie l’immersion totale dans le casino.
plaza royal casino login|
The Real Person!
The Real Person!
просмотры ютуб Необходимо быстро нарастить аудиторию в Telegram? Воспользуйтесь услугой подписчики ТГ, чтобы увеличить количество участников вашего канала и повысить его рейтинг. Больше подписчиков – больше возможностей!
The Real Person!
The Real Person!
https://vc.ru/id5253792
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-minsk009.ru
вывод из запоя цена
The latest data is right here: https://antalyamerhaba.com
Go to our website: http://redeecologicario.org
All the latest on our website: https://clubcoma.org
Here you will find only the best: https://edicionesdelau.com
ליקק את חור התחת שלה, פזר את הישבן שלה בידיו, ומרינה, הורידה את החולצה שלה, והבחור שלה, מלבד שלו ענפים בעזרת סכין ציד, מיד קטפתי ספר לי איך אישה כל כך צעירה ויפה השתכרה למצב כזה? היא בטח לא שותה sneak a peek at this web-site
The Real Person!
The Real Person!
интернет провайдеры нижний новгород
inernetvkvartiru-nizhnij-novgorod005.ru
подключить интернет
Come in – don’t miss the latest: https://www.manaolahawaii.com
Only we have the newest materials: https://jnp.chitkara.edu.in
Always relevant information: https://intelicode.com
Information that is always relevant: https://billi-walker.jp
fantastic internet site, I could definitely go to your web page once more…acquired some really nice info.
Cherished is likely to be what people say about your comments.
The Real Person!
The Real Person!
Услуга капельницы от запоя на дому – это популярная услуга в Туле, которую предлагают специалистами в области наркологии. Удобное лечение даёт возможность пациентам не испытывать стресс, который может возникнуть с поездками в клинику. Мнения клиентов подтверждают эффективности детоксикации организма и оперативного выхода из запойного состояния. Врач нарколог на дом предлагает анонимную помощь, что имеет большое значение для многих. Преодоление алкогольной зависимости становится реальным, а восстановление здоровья – приоритетом. Медицинские услуги, как капельница, способствуют борьбе с алкогольной зависимостью и возврату к привычному образу жизни.
Always relevant here: https://deogiricollege.org
Catch the latest from us: https://chhapai.com
Everything new is nearby: https://cere-india.org
Сайт finance-post.ru — это блог финансиста, ориентированный на личные финансы, бюджетирование и инвестиции. На нём публикуются практические материалы, советы и обзоры
https://hr.rivagroup.su/
The Real Person!
The Real Person!
вывод из запоя омск
vivod-iz-zapoya-omsk007.ru
экстренный вывод из запоя
Печатный Центр https://kopirych.by «Копирыч» в Минске и по всей Беларуси! Оперативная печать, брошюровка, широкоформатная и сувенирная продукция. Качественно, недорого, с доставкой. Ваши идеи — наша реализация! Заказывайте на сайте или по телефону.
руководства по садоводству https://zelenayazona.ru идеи, инструкции и советы по уходу за садом и огородом. Информация для новичков и опытных дачников: посадка, полив, удобрение и защита растений.
Женский сайт https://family-site.com.ua современный портал о моде, красоте, отношениях и саморазвитии. Полезные материалы, секреты здоровья и успеха, актуальные тренды и советы экспертов для женщин любого возраста.
Семейный портал https://geog.org.ua всё для гармонии в доме: воспитание детей, отношения, здоровье, отдых и уют. Полезные советы, статьи и лайфхаки для всей семьи. Пространство, где находят ответы и вдохновение.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
Современный женский https://happywoman.kyiv.ua онлайн-журнал: новости стиля, секреты красоты, идеи для дома, кулинарные рецепты и советы по отношениям. Пространство для вдохновения и развития.
The Real Person!
The Real Person!
ветеринарная клиника адлер круглосуточно Ветеринарные клиники в Сочи: Выбор ветеринарной клиники в Сочи может быть сложным, но благодаря нашему списку лучших клиник вы сможете найти подходящее место для вашего питомца. Обратите внимание на отзывы, квалификацию врачей и предлагаемые услуги.
The Real Person!
The Real Person!
подключить интернет по адресу
inernetvkvartiru-nizhnij-novgorod006.ru
домашний интернет нижний новгород
The Real Person!
The Real Person!
Как выбрать клинику для восстановления после запоя в Туле При возникновении проблемы запоя важно понимать‚ как верно подобрать клинику для реабилитации. В Туле доступны наркологические услуги‚ включая детоксикацию и лечение алкоголизма. Вы можете обратиться к круглосуточному наркологу на дом в Туле. Важно обратить внимание на возможность вызова нарколога на дом круглосуточно; это обеспечит получение необходимой медицинской помощи прямо в домашних условиях‚ что очень удобно в экстренных ситуациях. Кроме того‚ необходимо учитывать анонимность лечения‚ чтобы исключить стресс и стигматизацию. Выбирая клинику‚ уточните‚ какие программы реабилитации предлагает учреждение. Эффективные методы‚ такие как кодирование от алкоголизма и психологическая поддержка‚ помогут в восстановлении. Не забывайте о поддержке семьи – это важный аспект успешного лечения зависимостей. Обращение за консультацией к наркологу поможет выбрать наиболее оптимальный план лечения для вашего случая. Выбор клиники в Туле – это серьезный шаг к здоровой жизни.
Современный женский https://happywoman.kyiv.ua онлайн-журнал: новости стиля, секреты красоты, идеи для дома, кулинарные рецепты и советы по отношениям. Пространство для вдохновения и развития.
Портал о здоровье https://mikstur.com информационный ресурс о медицине и ЗОЖ. Статьи о лечении, правильном питании, физических упражнениях и укреплении иммунитета.
Портал о стройке https://bastet.com.ua статьи, новости и советы по ремонту, строительству и дизайну. Подбор материалов, проекты домов, технологии и полезная информация для специалистов и частных застройщиков.
Информационный портал https://intertools.com.ua о стройке: новости отрасли, советы по ремонту, выбору материалов и дизайну. Всё для тех, кто строит дом, делает ремонт или работает в строительстве.
The Real Person!
The Real Person!
лечение запоя омск
vivod-iz-zapoya-omsk008.ru
лечение запоя
The Real Person!
The Real Person!
Капельница от запоя на дому — это эффективным способом для экстренного выведения из запойного состояния и оздоровления организма. Преодоление запоя неизбежно включает наркологической помощи, включая медицинскую помощь при запое. Домашняя капельница помогает ускоренному избавлению от симптомов абстиненции и снижению риска рецидивов. Важно осознавать о мероприятиях по профилактике алкоголизма и взаимодействии с близкими в процессе восстановления от алкоголизма. Психологическая помощь при алкоголизме и трезвый образ жизни, ключевые аспекты успешного восстановления после запоя. Помощь близким также является значимым фактором в этом процессе. экстренный вывод из запоя
The Real Person!
The Real Person!
домашний интернет
inernetvkvartiru-novosibirsk004.ru
домашний интернет тарифы
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-omsk009.ru
лечение запоя омск
The Real Person!
The Real Person!
Монтаж мягких окон **ПВХ окна для веранд обеспечат надежную защиту от холода, ветра и влаги, создавая комфортную атмосферу для отдыха и чаепитий
The Real Person!
The Real Person!
Вызов нарколога на дом круглосуточно стал важной услугой для тех‚ кто сталкивается с зависимостями. На сайте vivod-iz-zapoya-vladimir010.ru можно получить всю необходимую информацию о лечении зависимостей‚ включая лекарственную терапию и психотерапию при зависимости. Консультация нарколога поможет выявить уровень зависимости и разработать индивидуальный план лечения. Круглосуточная помощь позволяет вызвать врача на дом в любое время‚ что особенно важно для экстренных случаев. Услуги нарколога включают определение зависимостей‚ конфиденциальное лечение и поддержку родственников. Реабилитация наркоманов требует комплексного подхода‚ и квалифицированная помощь может значительно облегчить этот путь. Профилактика зависимостей также является важным аспектом в борьбе с ними‚ поэтому необходимо обращаться за помощью при первых симптомах. Вызов врача на дом обеспечивает комфорт и конфиденциальность‚ способствуя эффективному лечению.
The Real Person!
The Real Person!
провайдеры по адресу дома
inernetvkvartiru-novosibirsk005.ru
провайдеры по адресу
The Real Person!
The Real Person!
игровые автоматы играть Игровые автоматы играть: простые правила и увлекательный процесс Играть в игровые автоматы – это просто и увлекательно. Правила игры обычно очень простые: вы делаете ставку, вращаете барабаны и ждете, пока на экране не появятся выигрышные комбинации символов. Различные игровые автоматы имеют разные линии выплат и символы, которые определяют размер выигрыша. Некоторые игровые автоматы предлагают бонусные игры и функции, которые могут увеличить ваши шансы на выигрыш. Игра в игровые автоматы – это отличный способ расслабиться и отвлечься от повседневных забот. Вы можете играть в одиночку или с друзьями, в казино или онлайн-казино.
Портал про детей https://mch.com.ua информационный ресурс для родителей. От беременности и ухода за малышом до воспитания школьников. Советы, статьи и поддержка для гармоничного развития ребёнка.
Онлайн-журнал для женщин https://krasotka-fl.com.ua всё о красоте, моде, семье и жизни. Полезные статьи, лайфхаки, советы экспертов и интересные истории. Читайте и вдохновляйтесь каждый день.
Женский онлайн-журнал https://girl.kyiv.ua стиль, уход за собой, психология, кулинария, отношения и материнство. Ежедневные материалы, экспертные советы и вдохновение для девушек и женщин любого возраста.
The Real Person!
The Real Person!
https://t.me/perevedem_document Перевод Документов: Мост между Языками и Культурами В современном мире глобализации, где границы стираются, а сотрудничество между странами и культурами становится все более тесным, перевод документов приобретает огромное значение. Это не просто замена слов одного языка словами другого, а кропотливая работа по передаче смысла, контекста и нюансов оригинала, чтобы обеспечить полное и точное понимание информации. Когда необходим перевод документов? Международный бизнес: контракты, договоры, финансовые отчеты, маркетинговые материалы – все это требует качественного перевода для успешного ведения дел за рубежом. Юридические вопросы: судебные документы, свидетельства, доверенности, нотариальные акты должны быть переведены с соблюдением строгих юридических норм и терминологии. Медицинская сфера: медицинские заключения, инструкции к лекарствам, результаты исследований – точность перевода здесь критически важна для здоровья пациентов. Техническая документация: инструкции по эксплуатации, технические спецификации, чертежи – перевод должен быть понятным и однозначным для специалистов. Образование и наука: дипломы, аттестаты, научные статьи, исследования – для признания образования за рубежом и обмена знаниями необходим качественный перевод. Почему важно обращаться к профессионалам? Точность и соответствие: профессиональные переводчики обладают глубокими знаниями языка и предметной области, что гарантирует точность и соответствие перевода оригиналу. Соблюдение терминологии: использование правильной терминологии – залог того, что перевод будет понятен специалистам в соответствующей области. Культурная адаптация: профессиональный переводчик адаптирует текст с учетом культурных особенностей целевой аудитории, чтобы избежать недопонимания или неловких ситуаций. Конфиденциальность: профессиональные бюро переводов гарантируют конфиденциальность ваших документов. Как выбрать бюро переводов? Опыт и репутация: изучите опыт работы бюро, ознакомьтесь с отзывами клиентов. Специализация: убедитесь, что бюро специализируется на переводах в нужной вам области. Наличие профессиональных переводчиков: узнайте, работают ли в бюро переводчики с соответствующим образованием и опытом. Стоимость и сроки: сравните цены и сроки выполнения заказа в разных бюро. В заключение, перевод документов – это важный инструмент для преодоления языковых барьеров и успешного взаимодействия в современном мире. Доверяйте перевод ваших документов профессионалам, чтобы быть уверенными в качестве и точности результата. Если вам нужно перевести конкретный документ, просто предоставьте его мне, и я постараюсь вам помочь!
Futureswap makes swapping easy Futureswap is exploding — check it out at futureswap.pro
The Real Person!
The Real Person!
J’adore la frenesie de Roobet Casino, on dirait un carnaval de neons dechaines. Les options de jeu au casino sont riches et fluorescents, comprenant des jeux de casino adaptes aux cryptomonnaies. Le service client du casino est une decharge d’efficacite, repondant en un eclair fluorescent. Le processus du casino est transparent et sans interferences, mais des bonus de casino plus frequents seraient dejantes. Globalement, Roobet Casino c’est un casino a explorer sans tarder pour ceux qui cherchent l’adrenaline vibrante du casino ! De surcroit la plateforme du casino brille par son style dejante, ce qui rend chaque session de casino encore plus vibrante.
roobet reedem code|
The Real Person!
The Real Person!
Je trouve absolument delirant Spinsy Casino, ca degage une ambiance de jeu aussi vibrante qu’une piste de danse. Les options de jeu au casino sont riches et rythmees, avec des machines a sous de casino modernes et hypnotiques. L’assistance du casino est chaleureuse et fluide, joignable par chat ou email. Les paiements du casino sont securises et fluides, cependant des recompenses de casino supplementaires feraient swinguer. Pour resumer, Spinsy Casino c’est un casino a rejoindre sans tarder pour les amoureux des slots modernes de casino ! En plus la plateforme du casino brille par son style funky, ce qui rend chaque session de casino encore plus dansante.
spinsy casino italia|
Ich liebe den Glanz von SlotClub Casino, es ist ein Online-Casino, das wie ein Neonlicht explodiert. Es gibt eine Welle an packenden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Service ist zuverlassig und strahlend, mit Hilfe, die wie ein Laser zielt. Auszahlungen im Casino sind schnell wie ein Lichtimpuls, manchmal die Casino-Angebote konnten gro?zugiger sein. Insgesamt ist SlotClub Casino ein Muss fur Casino-Fans fur Spieler, die auf leuchtende Casino-Kicks stehen! Und au?erdem die Casino-Oberflache ist flussig und glitzert wie ein Neonlicht, einen Hauch von Neon-Magie ins Casino bringt.
vip slotclub|
The Real Person!
The Real Person!
Estou louco por Richville Casino, e um cassino online que reluz como um palacio dourado. A selecao de titulos do cassino e um cofre de prazeres, com caca-niqueis de cassino modernos e envolventes. Os agentes do cassino sao rapidos como um coche de gala, respondendo rapido como um brinde de champanhe. Os ganhos do cassino chegam com a velocidade de um jato particular, de vez em quando mais bonus regulares no cassino seria chique. No fim das contas, Richville Casino oferece uma experiencia de cassino majestosa para os que buscam a adrenalina luxuosa do cassino! De lambuja a plataforma do cassino reluz com um estilo digno de um palacio, o que torna cada sessao de cassino ainda mais reluzente.
richville casino online|
The Real Person!
The Real Person!
таможенный брокер В лабиринте международных поставок, где правила меняются каждый день, вам нужен надежный проводник – опытный таможенный брокер. Мы предлагаем полный спектр услуг, от консультаций до оформления документов, чтобы ваш груз пересек границу быстро и без проблем. Доверьте нам свои заботы о растаможке и сосредоточьтесь на развитии своего бизнеса!
Thank you for sharing this very good post. Very interesting ideas! (as always, btw)
The Real Person!
The Real Person!
{Капельница от запоя на дому: как избежать повторного срыва (владимир)|{Лечение запоя на дому: капельница и профилактика срыва (владимир)|{Капельницы от запоя на дому: ключ к успешной реабилитации (владимир)}} {Алкогольная зависимость – это серьезная проблема‚ требующая быстрой реакции.|Проблема алкогольной зависимости требует оперативного вмешательства.|Алкогольная зависимость является важной проблемой‚ которая нуждается в незамедлительном решении.} {Нарколог на дом срочно|Срочный нарколог на дом|Наркологические услуги на дому} {предоставляет наркологические услуги‚ включая капельницы для быстрого восстановления.|предлагает услуги по лечению‚ включая капельницы для быстрого восстановления организма.|обеспечивает лечение алкоголизма‚ включая капельницы для скорейшего восстановления.} {При запое важно не только лечение на дому‚ но и профилактика срыва.|Важно помнить‚ что при запое необходимо не только лечение‚ но и профилактика рецидивов.|Лечение на дому при запое должно включать как терапию‚ так и профилактические меры против срывов.} {Медицинская капельница помогает детоксифицировать организм‚ облегчая симптомы абстиненции.|Капельница способствует детоксикации и облегчает симптомы абстиненции.|Капельница играет важную роль в детоксикации организма и уменьшении симптомов абстиненции.} {Срочная помощь специалистов включает в себя не только физическое восстановление‚ но и психологическую помощь.|Помощь специалистов охватывает как физическое‚ так и психологическое восстановление.|Специалисты предоставляют не только физическую помощь‚ но и поддержку на психологическом уровне.} {Поддержка родственников также играет ключевую роль‚ способствуя успешной реабилитации алкоголиков;|Важность поддержки со стороны близких неоспорима‚ так как она содействует успешной реабилитации.|Родные играют важнейшую роль в процессе реабилитации‚ способствуя успеху лечения.} {Detox программа поможет избежать повторного срыва и обеспечит безопасное лечение.|Программа детоксикации поможет предотвратить рецидивы и обеспечит безопасное лечение.|Детокс-программа предотвращает срывы и гарантирует безопасность лечения.} {Обратитесь к наркологу на дом‚ чтобы получить комплексную помощь и вернуться к нормальной жизни.|Свяжитесь с наркологом на дом для получения комплексной помощи и возвращения к полноценной жизни.|Не откладывайте‚ обратитесь к наркологу на дом за комплексной помощью и начните новую жизнь.}
Онлайн-журнал https://presslook.com.ua для женщин объединяет всё, что важно: мода и стиль, воспитание детей, карьерные советы и вдохновение. Советы специалистов и реальные истории для поддержки и новых идей.
Актуальные тренды https://horoscope-web.com и вневременная классика. Подборки образов, советы по стилю, секреты гардероба и модные инсайты. Мы поможем тебе выглядеть безупречно каждый день и выразить свой индивидуальный стиль.
Твой гид https://nicegirl.kyiv.ua по здоровому образу жизни! Эффективные тренировки, сбалансированное питание, wellness-практики и советы по мотивации. Обрети энергию, силу и гармонию в теле, которое ты любишь.
Ресурс для амбициозных https://ramledlightings.com и целеустремленных. Карьерный рост, личная эффективность, финансовая грамотность и вдохновляющие истории успеха. Реализуй свой потенциал и добивайся всех поставленных целей!
The Real Person!
The Real Person!
интернет провайдеры в новосибирске по адресу дома
inernetvkvartiru-novosibirsk006.ru
интернет провайдеры по адресу новосибирск
The Real Person!
The Real Person!
https://impossible-studio.ghost.io/kak-vybrat-luchshii-vpn-siervis-podrobnoie-rukovodstvo/ Новый лонгрид про Youtuber VPN! Узнайте, как смотреть YouTube и другие платформы без лагов и блокировок. Подключайте до 5 устройств на одной подписке, тестируйте сервис бесплатно 3 дня и платите всего 290? в первый месяц вместо 2000? у конкурентов. Серверы в Европе — ваши данные защищены от российских властей.
Таможенный брокер — ваш надежный партнер при импорте и экспорте товаров в Москве: https://tamozhenniiy-broker11.ru/
сайт заказать курсовую заказать качественную курсовую работу
The Real Person!
The Real Person!
лечение запоя оренбург
vivod-iz-zapoya-orenburg008.ru
вывод из запоя круглосуточно оренбург
http://www.tiflocomp.ru/games/
The Real Person!
The Real Person!
Как предотвратить алкоголизм в владимире: рекомендации медиков Зависимость от алкоголя — крайне важная проблема‚ требующая внимания и действий. Важно знать о возможностях профилактики и лечения. Вызвать нарколога на дом в владимире можно Врачи советуют проводить профилактические меры‚ включая осведомленность о последствиях алкоголизма и поддержку семьи. вызвать нарколога на дом владимир Лечение алкоголизма включает позволяющие способствуют вернуть здоровье и социальную интеграцию. Наркологическая помощь является важной для работы с зависимыми‚ а также для предотвращения рецидивов. Борьба за здоровье и злоупотребление алкоголем — это постоянная борьба‚ и своевременная поддержка играет ключевую роль.
взять займ онлайн без займ онлайн с плохой историей
взять займы онлайн без карты займ онлайн срочно
Puzzles online https://podcast.kkbox.com/tw/episode/lxoz44mbd1sgn-k1xn play for free in assembling pictures of any complexity. Thousands of options: classic, children’s, 3D and thematic. Convenient interface, saving progress and new puzzles every day.
The Real Person!
The Real Person!
провайдеры ростов
inernetvkvartiru-rostov004.ru
провайдеры интернета в ростове
https://russpain.com/
The Real Person!
The Real Person!
вывод из запоя круглосуточно оренбург
vivod-iz-zapoya-orenburg009.ru
вывод из запоя
The Real Person!
The Real Person!
Детоксикация от алкоголя на дому в владимире — это эффективный способ помочь родным борющимся с алкоголизмом. Помощь при алкоголизме включает детоксикацию на дому, что дает возможность лечиться в привычной обстановке. Выведение из запоя может осуществляться с помощью специальных растворовкоторые помогают справиться с симптомами абстиненции. Квалифицированная помощь в таких случаях необходима: услуги нарколога помогут правильно подобрать антиалкогольную терапию. Психотерапия при алкоголизме также играет ключевую роль в восстановлении. Семейная терапия и социальная адаптация помогают в процессе реабилитации наркозависимых. Предотвращение алкоголизма и реабилитация после запоя — важные этапы в путь к трезвости. Не забывайте, что алкоголь — это угроза для здоровья. На сайте vivod-iz-zapoya-vladimir013.ru вы можете получить подробности о лечении алкоголизма.
The Real Person!
The Real Person!
Деревянный спил Установка глубинной дренажной системы: Установка глубинной дренажной системы – это монтаж системы отвода избыточной грунтовой воды с использованием перфорированных труб, уложенных в траншеи под землей. Глубинная дренажная система эффективно отводит воду от фундамента дома, подвалов и других сооружений, предотвращая их разрушение. Установка глубинной дренажной системы требует специальных знаний и опыта, поэтому рекомендуется обращаться к профессионалам.
Наша платформа работает круглосуточно и не знает слова перерыв. Бронировать и планировать можно где угодно: в поезде, на даче, в кафе или лежа на диване https://probilets.com/. Хотите купить билет, пока идёте по супермаркету? Просто достаньте телефон и оформите поездку. Нужно скорректировать планы, отменить или перенести билет? Это тоже можно сделать онлайн, без звонков и визитов. Но если возникла проблема, то наши специалисты помогут и все расскажут
Acho simplesmente intergalactico SpeiCasino, e um cassino online que decola como um foguete espacial. Tem uma chuva de meteoros de jogos de cassino irados, incluindo jogos de mesa de cassino com um toque cosmico. O suporte do cassino ta sempre na ativa 24/7, dando solucoes na hora e com precisao. O processo do cassino e limpo e sem turbulencia cosmica, as vezes mais bonus regulares no cassino seria intergalactico. No geral, SpeiCasino garante uma diversao de cassino que e uma galaxia para quem curte apostar com estilo cosmico no cassino! Vale dizer tambem a plataforma do cassino brilha com um visual que e puro cosmos, o que torna cada sessao de cassino ainda mais estelar.
spei mission uncrossable|
The Real Person!
The Real Person!
подключить интернет
inernetvkvartiru-rostov005.ru
провайдеры интернета в ростове
The Real Person!
The Real Person!
Откопаться ? это не просто процесс работы с землёй, но и захватывающее исследование земли, что открывает врата к тайнам истории. Археология, как наука, включает в себя раскопки, которые помогают обнаружить исторические артефакты, предметы старины и подземные ресурсы. На сайте vivod-iz-zapoya-vladimir014.ru можно найти разнообразные материалы о современных методах бурения и анализа почвы, которые помогают в поиске уникальных объектов. Земляные работы, включая excavation, являются основой археологических исследований. Каждый слой почвы, вырываем, может рассказать о жизни людей в разные исторические эпохи. Восстановление найденных артефактов предоставляет шанс лучше понять культуру и технологии древних цивилизаций. Геология также играет ключевую роль в процессе, помогая в анализе почвы и определении местонахождения объектов, что облегчает поиски более целенаправленными и эффективными. Таким образом, раскопки – это не только работа с землёй, но и интересное исследование в прошлое, где каждая находка становится неотъемлемой частью исторической мозаики.
The Real Person!
The Real Person!
Помощь наркологов в Туле доступна каждому, кто встретился с проблемами зависимости; Конфиденциальная помощь — это важный аспект, позволяющий пациентам обращаться без страха осуждения. Клиника наркологии предлагает лечение алкогольной и наркотической зависимости через персонализированные программы реабилитации. Консультация нарколога — начало пути к выздоровлениюгде можно узнать детали о detox-программах и психотерапии. Процесс реабилитации включает семейную терапию, что способствует успешному лечению и профилактике рецидива. Свяжитесь на vivod-iz-zapoya-tula010.ru для получения анонимного лечения и поддержки.
The Real Person!
The Real Person!
микрозайм без займ онлайн Всегда быстро и удобно получать займы. Микрозаймы без на карту онлайн лучшие, не сомневайтесь!
Porn
The Real Person!
The Real Person!
домашний интернет тарифы
inernetvkvartiru-rostov006.ru
подключить проводной интернет ростов
Твой гид https://womanlife.kyiv.ua по стильной жизни. Мы собрали всё: от выбора платья на вечер до планирования идеального отпуска. Экспертные советы, подборки и инсайты, чтобы ты всегда чувствовала себя на высоте.
Онлайн-журнал о моде https://glamour.kyiv.ua без правил. Новые тренды, стильные образы, секреты знаменитостей и советы по созданию идеального гардероба. Мы поможем вам найти и с уверенностью выразить свой уникальный стиль.
Журнал для женщин https://rpl.net.ua которые строят карьеру и хотят большего. Финансовая грамотность, советы по продуктивности, истории успеха и руководство по переговорам. Достигайте своих целей с нами!
Женский сайт https://bbb.dp.ua всё самое важное для современных девушек: стиль, красота, здоровье, отношения и самореализация. Читайте, вдохновляйтесь и находите новые идеи.
The Real Person!
The Real Person!
Скорая наркологическая помощь в владимире предоставляет качественное лечение запоя. Центр наркологии предлагает услуги по выведению из запоя и терапию алкоголизма, включая медикаментозную терапию при запое с помощью медикаментов и детоксикационных процедур. Необходимо обратиться за помощью к наркологу для получения первичной помощи при запое. Программы лечебные программы включают психотерапию зависимости и психологическую помощьчто способствует восстановлению здоровья и социальной адаптации. Лечение с гарантией анонимности гарантирует защиту личных данных, что имеет большое значение для пациентов.
The Real Person!
The Real Person!
Экстренный вывод из запоя в Туле: какова цена? Алкогольный запой — это значительная проблема, требующая немедленного вмешательства. Симптомы алкогольного запоя включают неутолимую жажду, дрожь , потливость и тревожность . Для качественного лечения и вывода из запоя необходима профессиональная медицинская помощь алкоголикам . В Туле помощь наркологов предлагают специализированные клиники, где можно получить срочную медицинскую помощь при запое. Как проходит процесс вывода из запоя? Наркологи используют разные подходы, включая очищение организма и фармакотерапию. Цены на вывод из запоя варьируются, но в среднем составляют от 5 000 до 15 000 рублей . Консультация врача-нарколога поможет выбрать оптимальную программу реабилитации от алкоголя , учитывая индивидуальные особенности пациента . Учтите, что лечение алкогольной зависимости — это длительный и сложный процесс, требующий усилий и постоянной поддержки. Наркологические центры Тулы предлагают разнообразные программы реабилитации , которые способствуют избавлению от алкогольной зависимости и возвращают к полноценной жизни.
https://mangalfactory.ru/
Acho simplesmente animal SambaSlots Casino, da um ritmo de cassino que e puro axe. O catalogo de jogos do cassino e uma folia total, com slots de cassino tematicos de festa. Os agentes do cassino sao rapidos como um passista, acessivel por chat ou e-mail. Os saques no cassino sao velozes como um mestre-sala, mas as ofertas do cassino podiam ser mais generosas. No fim das contas, SambaSlots Casino oferece uma experiencia de cassino que e puro batuque para os amantes de cassinos online! De lambuja o site do cassino e uma obra-prima de estilo carioca, torna a experiencia de cassino uma festa inesquecivel.
coupon casino la sambaslots|
Новостной сайт https://vesti.in.ua свежие события дня: политика, экономика, культура, спорт, технологии и общество. Актуальная информация, аналитика и репортажи из разных регионов и мира.
Свежие новости https://sensus.org.ua Украины и мира: главные события, репортажи и аналитика. Политика, экономика, общество и культура в удобном формате онлайн.
Новостной портал Украины https://lenta.kyiv.ua оперативные события в стране. Политика, экономика, региональные новости, спорт и культура. Достоверные материалы и аналитика каждый день.
Свежие новости Украины https://novosti24.kyiv.ua главные события, мнения экспертов и аналитические материалы. Лента новостей онлайн, репортажи и достоверные факты без перерыва.
The Real Person!
The Real Person!
интернет домашний санкт-петербург
inernetvkvartiru-spb004.ru
провайдеры санкт-петербург
The Real Person!
The Real Person!
Капельница от алкоголя – является эффективный способ‚ который помочь в реабилитации после алкогольной зависимости в домашних условиях. Обратившись к наркологу на дом‚ вы получите профессиональную поддержку и необходимые медикаменты для облегчения симптомов похмелья. Симптомы запойного состояния могут включать болевых ощущений в голове‚ тошноты и общего недомогания; вызвать нарколога на дом владимир Этап детоксикации организма стартует с введения капельницы‚ которая гарантирует поступление жидкости и витаминов‚ что помогает уменьшить симптомы отмены. Услуги нарколога в владимире включают индивидуальный подход к пациенту‚ что важно для успешного лечения алкоголизма в домашних условиях. Поддержка близких при алкоголизме играет важной ролью в процессе восстановления здоровья после приема алкоголя. Реабилитация в домашних условиях осуществляется с помощью капельниц и медикаментов‚ что позволяет предотвратить новые запои и упростить процесс восстановления. Таким образом предотвращение запоев становится важным аспектом в борьбе с зависимостью.
The Real Person!
The Real Person!
Капельница для лечения запоя – это существенная медицинская интервенция для борьбы с алкогольной зависимостью. Подготовка к процедуре включает несколько этапов. Во-первых, необходимо позвонить наркологу для вызова на дом, который проведет консультацию. Признаки алкоголизма могут варьироваться, поэтому важно сообщить врачу о всех проявлениях. нарколог на дом Перед процедурой нужно позаботиться о доступе к венам, а также подготовить пациента к дальнейшему лечению. Проверьте наличие всех необходимых препаратов для введения капельницы. После процедуры следует уделить внимание реабилитации, правильный уход за пациентом поможет в восстановлении здоровья и снизит риск рецидива.
Sou louco pela energia de SupaBet Casino, tem uma vibe de jogo tao intensa quanto uma correnteza selvagem. Tem um tsunami de jogos de cassino irados, oferecendo sessoes de cassino ao vivo que detonam como raios. A equipe do cassino entrega um atendimento que e puro dinamite, respondendo mais rapido que uma explosao estelar. Os ganhos do cassino chegam voando como um foguete, porem queria mais promocoes de cassino que abalam o planeta. Resumindo, SupaBet Casino garante uma diversao de cassino que e uma explosao total para os super-herois do cassino! De bonus a interface do cassino e fluida e reluz como uma aurora vulcanica, faz voce querer voltar ao cassino como um meteoro em orbita.
supabet portugal|
The Real Person!
The Real Person!
Капельница для вывода из запоя в домашних условиях — данный распространенный метод экстренного вывода из запоя, в особенности в владимире. Тем не менее важно выбрать опытного нарколога, чтобы не стать жертвой мошенников. Признаки зависимости от алкоголя часто бывают очевидны, и поддержка специалиста неизбежна. экстренный вывод из запоя владимир Обращаясь в наркологическую клинику, обратите внимание на наличие лицензий и отзывов. Квалифицированный специалист должен рекомендовать безопасное лечение, включая капельницу для восстановления водно-электролитного баланса. Осмотр и консультация опытного врача поможет определить степень зависимости и выбрать подходящий метод лечения. Помните о необходимости реабилитации — это важная часть борьбы с алкоголизмом. Берегите свое здоровье и обращайтесь только к проверенным специалистам!
The Real Person!
The Real Person!
провайдеры интернета в санкт-петербурге
inernetvkvartiru-spb005.ru
интернет провайдеры санкт-петербург
Tried these https://joyorganics.com/collections/usda-certified-cbd-oil-tinctures in the forefront bed a few times in and they in fact work. I’m chiefly tossing and turning, but with these I end up falling asleep technique quicker. No freakish hangover compassionate in the morning either. Kinda dear, but honestly value it when I just need a textile darkness’s sleep.
https://bpr-work.ru/
http://www.logoslovo.ru/polls/
The Real Person!
The Real Person!
В владимире услуги по откапыванию и земляные работы становятся все более востребованными. В владимире можно найти специалистов, которые предоставляют широкий набор строительных услуг, включая экскаваторные работы и выемку грунта. Если вам нужно откопаться для планировки участка или благоустройства территории, обратитесь к подрядчикам в владимире. Эти специалисты помогут вам выполнить все необходимые земляные работы и предложат услуги по ландшафтному дизайну. Подробности об этих услугах можно найти на сайте vivod-iz-zapoya-vladimir018.ru. Ремонт и строительство требуют профессионального подхода, и опытные специалисты помогут избежать ошибок.
The Real Person!
The Real Person!
домашний интернет в санкт-петербурге
inernetvkvartiru-spb006.ru
провайдеры по адресу дома
Новости Украины и мира https://mostmedia.com.ua политика, экономика, культура, спорт и общество. Свежие события, аналитика и репортажи. Будьте в курсе главных новостей в режиме онлайн 24/7.
Новостной портал https://mediateam.com.ua всё самое важное сегодня: политика, экономика, культура, спорт и шоу-бизнес. Лента новостей, репортажи и аналитические материалы каждый день.
Новости Украины https://status.net.ua объективная информация о событиях страны. Политика, экономика, региональные новости, спорт и культура. Читайте актуальные материалы каждый день.
Необходимо кодирование? кодирование от алкоголизма современные методы, конфиденциальность и поддержка специалистов. Помогаем избавиться от зависимости и вернуться к здоровой жизни.
The Real Person!
The Real Person!
https://impossible-studio.ghost.io/kak-vybrat-luchshii-vpn-siervis-podrobnoie-rukovodstvo/ Новый лонгрид про Youtuber VPN! Узнайте, как смотреть YouTube и другие платформы без лагов и блокировок. Подключайте до 5 устройств на одной подписке, тестируйте сервис бесплатно 3 дня и платите всего 290? в первый месяц вместо 2000? у конкурентов. Серверы в Европе — ваши данные защищены от российских властей.
The Real Person!
The Real Person!
Профессиональная помощь нарколога на дому в владимире – это важный шаг для тех‚ кто сталкивается с алкоголизмом. Наркологические услуги‚ включая помощь при алкогольном запое‚ дают возможность людям эффективно и быстро прервать запой. Выезд нарколога обеспечивает анонимное лечение и медицинскую помощь на дому‚ что особенно важно для пациентов‚ стремящихся сохранить свою приватность. Во время консультации нарколога осуществляется снятие абстиненции‚ что помогает пациенту восстановить здоровье. Кодирование от алкоголя и психологическая поддержка при алкогольной зависимости являются неотъемлемой частью в лечении алкоголизма. Реабилитация пациентов включает поддержку близких‚ что способствует успешному выздоровлению. Не затягивайте с решением вашей проблемы; обратитесь за помощью к наркологу на дом‚ чтобы положить конец своей зависимости!
The Real Person!
The Real Person!
Алкогольная зависимость – серьезная проблема, требующая внимательного подхода. Многие ищут помощь анонимно, чтобы избежать осуждения. Нарколог на дом предоставляет услуги по выводу из запоя, гарантируя комфорт и конфиденциальность. Обращение к наркологу позволяет получить необходимую медицинскую помощь при запое без ненужных вопросов. Анонимное лечение подразумевает detox-программы, которые включают в себя психологическую поддержку и медикаментозное сопровождение. Данный подход помогает предотвратить рецидив алкоголизма и способствует восстановлению после запойного состояния. Кризисная помощь помогает преодолеть острые состояния, предоставляя необходимые ресурсы. Таким образом, анонимный вывод из запоя – это вполне реальная возможность для тех, кто ищет помощь.
вывод из запоя стоимость наркологическая клиника
Аренда авто https://myrentacar.site широкий выбор автомобилей для любых целей. Удобное бронирование, доступные цены, страхование и гибкие условия. Машины в отличном состоянии для поездок по городу и путешествий.
Строительство домов в Сочи https://stroitelstvodomovsochi.ru под ключ — профессиональный подход, проверенные материалы и гарантия качества. Полный спектр работ: проект, строительство, отделка и благоустройство.
https://www.te-in.ru/
The Real Person!
The Real Person!
Лечение алкоголизма на дому становится актуальным. Основные этапы включают домашней детоксикации и помощи при запое. С чего начать лечение? Важно получить поддержку от близкихкоторые могут помочь в процессе.Психологическая поддержка и альтернативные методы могут стать эффективным дополнением к медикаментам от алкоголя. Программы по поддержанию трезвости и методы отказа от спиртного помогут развить правильное отношение к алкоголю. vivod-iz-zapoya-vladimir012.ru Не забывайте о реабилитации на дому, которая включает психологическую помощь и профилактику рецидива. Как бросить пить самостоятельно? Начните с установки четкой цели и поиска мотивации. Главное – это поддержка и желание изменить свою жизнь.
The Real Person!
The Real Person!
Estou pirando com AFun Casino, tem uma vibe de jogo tao vibrante quanto um desfile de cores. As opcoes de jogo no cassino sao ricas e cheias de swing, com slots de cassino tematicos de festa. O atendimento ao cliente do cassino e uma estrela da festa, dando solucoes na hora e com precisao. O processo do cassino e limpo e sem tumulto, as vezes mais recompensas no cassino seriam um diferencial festivo. No geral, AFun Casino vale demais curtir esse cassino para os folioes do cassino! E mais a plataforma do cassino brilha com um visual que e puro axe, o que torna cada sessao de cassino ainda mais animada.
cГіdigo promocional afun junho 2023|
The Real Person!
The Real Person!
Estou completamente vidrado por BetorSpin Casino, tem uma vibe de jogo tao vibrante quanto uma supernova dancante. Tem uma chuva de meteoros de jogos de cassino irados, com slots de cassino tematicos de espaco sideral. O servico do cassino e confiavel e brilha como uma galaxia, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, mas mais giros gratis no cassino seria uma loucura estelar. Resumindo, BetorSpin Casino oferece uma experiencia de cassino que e puro brilho cosmico para quem curte apostar com estilo estelar no cassino! De lambuja a plataforma do cassino brilha com um visual que e puro cosmos, adiciona um toque de brilho estelar ao cassino.
betorspin bonus|
The Real Person!
The Real Person!
Sou louco pela energia de BRCasino, parece uma festa carioca cheia de axe. A gama do cassino e simplesmente um sambodromo de delicias, incluindo jogos de mesa de cassino com um toque de carnaval. O atendimento ao cliente do cassino e uma bateria de responsa, com uma ajuda que brilha como serpentinas. Os pagamentos do cassino sao lisos e blindados, mas mais giros gratis no cassino seria uma loucura. Resumindo, BRCasino garante uma diversao de cassino que e um carnaval total para os apaixonados por slots modernos de cassino! De bonus a interface do cassino e fluida e reluz como uma fantasia de carnaval, eleva a imersao no cassino ao ritmo de um tamborim.
br77 brazilian steakhouse reviews|
The Real Person!
The Real Person!
Je suis totalement enflamme par VBet Casino, ca degage une ambiance de jeu aussi ardente qu’une coulee de lave. La selection du casino est une explosion de plaisirs, incluant des jeux de table de casino d’une elegance ardente. Le personnel du casino offre un accompagnement digne d’un volcan, offrant des solutions claires et instantanees. Les transactions du casino sont simples comme une braise, parfois j’aimerais plus de promotions de casino qui envoient du feu. En somme, VBet Casino offre une experience de casino ardente pour les volcanologues du casino ! Par ailleurs la plateforme du casino brille par son style volcanique, facilite une experience de casino eruptive.
vbet revenue|
Новостной портал https://newsawait.com свежие новости, аналитика и обзоры. Политика, экономика, культура и спорт. Лента событий в режиме реального времени с проверенными фактами.
Онлайн новостной портал https://reporternews.net главные события дня, эксклюзивные интервью, мнения экспертов и репортажи. Достоверная информация о политике, бизнесе и жизни общества.
Портал про авто https://dream-autos.com новости, обзоры и тест-драйвы. Полезные советы по выбору, ремонту и эксплуатации автомобилей. Каталог машин, актуальные цены и аналитика авторынка.
Rain bet
xx88 là sân chơi cá cược đẳng cấp hàng đầu Châu Á. Sở hữu kho game trực tuyến đa dạng từ: xổ số, casino, thể thao,… Tải App để tham gia trải nghiệm mượt …
The Real Person!
The Real Person!
Acho simplesmente estelar BetorSpin Casino, da uma energia de cassino que e puro pulsar galactico. O catalogo de jogos do cassino e uma nebulosa de emocoes, com jogos de cassino perfeitos pra criptomoedas. O servico do cassino e confiavel e brilha como uma galaxia, dando solucoes na hora e com precisao. Os ganhos do cassino chegam voando como um asteroide, mas mais bonus regulares no cassino seria intergalactico. Em resumo, BetorSpin Casino e o point perfeito pros fas de cassino para os astronautas do cassino! Vale dizer tambem a navegacao do cassino e facil como uma orbita lunar, faz voce querer voltar ao cassino como um cometa em orbita.
betorspin casino|
The Real Person!
The Real Person!
Acho simplesmente fenomenal BRCasino, e um cassino online que samba como um bloco de carnaval. Tem uma enxurrada de jogos de cassino irados, com slots de cassino tematicos de festa. Os agentes do cassino sao rapidos como um mestre-sala, respondendo mais rapido que um batuque de tamborim. Os saques no cassino sao velozes como uma ala de passistas, de vez em quando as ofertas do cassino podiam ser mais generosas. Resumindo, BRCasino e um cassino online que e uma folia sem fim para quem curte apostar com gingado no cassino! E mais a navegacao do cassino e facil como um passo de samba, adiciona um toque de folia ao cassino.
br77 games|
The Real Person!
The Real Person!
Ich finde absolut magisch Trickz Casino, es bietet ein Casino-Abenteuer, das wie eine Illusion verblufft. Die Casino-Optionen sind vielfaltig und verzaubernd, mit Live-Casino-Sessions, die wie ein Zaubertrick funkeln. Der Casino-Service ist zuverlassig und verhext, mit Hilfe, die wie eine Illusion verblufft. Casino-Gewinne kommen wie ein Blitz aus dem Hut, trotzdem wurde ich mir mehr Casino-Promos wunschen, die wie Zaubertranke wirken. Insgesamt ist Trickz Casino ein Casino mit einem Spielspa?, der wie eine Illusion verblufft fur Fans von Online-Casinos! Ubrigens die Casino-Oberflache ist flussig und funkelt wie ein Zauberstab, Lust macht, immer wieder ins Casino zuruckzukehren.
trickz casino online|
The Real Person!
The Real Person!
Предотвращение запоев после вывода – важная задача для поддержания здоровья и предотвращения рецидивов. Нарколог на дом в Красноярске предлагает услуги по помощи в лечении алкоголизма и профилактике запойного синдрома. Ключевым элементом является поддержка со стороны психолога, которая помогает справляться с трудностями.Советы нарколога включают стратегии борьбы с запоем, такие как формирование здорового образа жизни и семейная поддержка в процессе лечения. Реабилитация после запоя требует медицинского вмешательства при зависимостях и консультации специалиста по алкоголизму. Обращение к наркологу на дом обеспечивает комфорт и доступность лечения. нарколог на дом Красноярск Важно помнить, что восстановление после запоя – это процесс, требующий времени и усилий. Профилактика возврата к алкоголизму включает в себя постоянные консультации с наркологом и использование техник, способствующих стабильному состоянию здоровья и отказу от алкоголя.
The Real Person!
The Real Person!
Наркологическая скорая помощь на дому – это важный аспект лечения зависимостей‚ который предоставляет экстренную помощь при алкоголизме и наркомании. Выездная наркологическая служба оказывает профессиональную помощь зависимым‚ включая детоксикацию и кризисную интервенцию. На сайте vivod-iz-zapoya-krasnoyarsk013.ru можно узнать больше о по наркологии‚ узнать о лечении наркомании и восстановлении на дому. Конфиденциальное лечение зависимых позволяет сохранить конфиденциальность. Важно также помнить о поддержке близких‚ что помогает в процессе восстановления. Программа восстановления после зависимости включает меры по предотвращению рецидивов и психологическую поддержку‚ что помогает успешному возвращению к обычной жизни.
The Real Person!
The Real Person!
Алкогольная зависимость — это серьезная проблема, затрагивающая не только самого алкоголика, но и окружающих людей. Как уговорить человека выйти из запоя? Прежде всего, важно понимать, что поддержка семьи играет важнейшую роль в процессе преодоления зависимости. Нарколог на дом владимир Первый шаг — это консультация специалиста. Нарколог на дом в владимире может оказать необходимую медицинскую поддержку в домашних условиях, чтобы снизить страдания пациента. Психологическая поддержка также необходима: она поможет понять причины алкогольной зависимости и найти пути к решению проблемы. Советы по выходу из запоя включают в себя формирование обстановки, способствующей мотивации, где человек ощущает заботу и поддержку. Поговорите о семейных трудностях и значимости единства — это может подтолкнуть к размышлениям о необходимости лечения. Процессы лечения запоя и реабилитации содействуют восстановлению здоровья и возвращению к привычному образу жизни. Необходимо осознавать признаки запойного состояния и быть готовыми к вызовам. Мотивация к лечению — это совместный процесс, который требует времени и усилий. Поддерживайте своего близкого, показывайте, как вы хотите ему помочь, и совместно пройдите путь к выздоровлению.
https://vgarderobe.ru/obuv-dlya-malchikov-adidas-bc-8220.html
oppatoto
Новости Украины и мира https://globalnewshome.com всё самое важное сегодня. Политика, экономика, региональные события, спорт и культура. Объективные статьи и аналитика в удобном формате.
Портал для женщин https://womanfashionista.com всё самое важное в одном месте: уход за собой, мода, дом, семья и карьера. Читайте полезные статьи, находите вдохновение и делитесь опытом.
Сайт детского сада https://malush16.ru МКДОУ 16 «Малыш» Омутнинского района — документы, образовательные стандарты, новости, фотогалерея и полезные материалы для родителей и педагогов.
The Real Person!
The Real Person!
Капельница для избавления от запоя – это эффективный метод детоксикации организма, предоставляемый наркологическая клиника. В Красноярске доступно множество медицинских услуг, включая вызов нарколога на дом для оказания помощи при запое. Лечение алкоголизма требует персонализированного подхода, и программы лечения разрабатываются в зависимости от степени зависимости. Визит к наркологу поможет выбору оптимального метода, а анонимное лечение обеспечит конфиденциальность. Роль семьи в реабилитации также являеться ключевой в процессе реабилитации. Оценка клиник в Красноярске на основе отзывов позволяет определить наиболее подходящее заведение. Не стесняйтесь обращаться за поддержкой, чтобы преодолеть алкогольную зависимость и восстановить контроль над своей жизнью.
Adoro a explosao de Bet558 Casino, e um cassino online que brilha como uma estrela em colisao. As opcoes de jogo no cassino sao ricas e brilhantes como nebulosas, com slots de cassino tematicos de espaco profundo. O atendimento ao cliente do cassino e uma estrela-guia, acessivel por chat ou e-mail. O processo do cassino e limpo e sem turbulencia cosmica, porem as ofertas do cassino podiam ser mais generosas. Em resumo, Bet558 Casino oferece uma experiencia de cassino que e puro brilho estelar para os viciados em emocoes de cassino! Vale dizer tambem a plataforma do cassino brilha com um visual que e puro cosmos, o que torna cada sessao de cassino ainda mais estelar.
brl222 bet558|
Je suis totalement ensorcele par ViggoSlots Casino, ca vibre avec une energie de casino digne d’un blizzard. L’eventail de jeux du casino est une toundra de delices, proposant des slots de casino a theme polaire. Le support du casino est disponible 24/7, offrant des solutions claires et instantanees. Les transactions du casino sont simples comme un flocon de neige, quand meme des recompenses de casino supplementaires feraient frissonner. Au final, ViggoSlots Casino promet un divertissement de casino glacial pour les joueurs qui aiment parier avec style au casino ! Par ailleurs le design du casino est un spectacle visuel arctique, ajoute une touche de fraicheur au casino.
viggoslots madness|
The Real Person!
The Real Person!
Капельница от запоя – это работающий способ, применяемый наркологами для помощи в лечении алкоголизма и симптомов абстиненции. Срочный нарколог на дом в Красноярске предоставляет медицинские услуги в домашних условиях, гарантируя удобство и безопасность пациента. Показания к капельнице охватывают интенсивное похмелье, обезвоживание и потребность в очищении организма. Нарколог на дом срочно Красноярск В то же время имеются и противопоказания: аллергические реакции на состав, сердечно-сосудистые заболевания и определенные хронические заболевания. Инфузионная терапия содействует реабилитации после запоя, повышает общее состояние здоровья и помогает организму восстановиться. Профессиональная помощь нарколога крайне важна для адекватного подбора терапии и снижения возможных рисков. Своевременная помощь при запое должна быть своевременной, чтобы избежать серьезных последствий.
The Real Person!
The Real Person!
Если вы ищете, где получить капельницу анонимно в владимире, вы можете ознакомиться с сайтом vivod-iz-zapoya-vladimir014.ru. Здесь доступны услуги капельницы для здоровья, которые способствую вашему после болезни. Клиника в владимире обеспечивает безопасность процедур и приватность клиентов, предлагая качественную медицинскую помощь. Анонимное лечение – это способ заботиться о своем здоровье без лишнего шума. Не откладывайте о себе, воспользуйтесь медицинскими процедурами уже сегодня!
The Real Person!
The Real Person!
Je suis accro a ViggoSlots Casino, il propose une aventure de casino qui brille comme une banquise illuminee. Le repertoire du casino est un iceberg de divertissement, offrant des sessions de casino en direct qui scintillent comme la neige. Le service client du casino est un flocon d’efficacite, avec une aide qui brille comme un cristal de glace. Le processus du casino est transparent et sans tempete, mais des recompenses de casino supplementaires feraient frissonner. Au final, ViggoSlots Casino offre une experience de casino vivifiante pour les passionnes de casinos en ligne ! De surcroit le site du casino est une merveille graphique polaire, ajoute une touche de fraicheur au casino.
viggoslots betrug|
Ich finde absolut atemberaubend Tipico Casino, es fuhlt sich an wie ein Sturm aus Gewinnen. Es gibt eine Flut an packenden Casino-Titeln, inklusive stilvoller Casino-Tischspiele. Der Casino-Support ist rund um die Uhr verfugbar, antwortet blitzschnell wie ein Gewitterknall. Der Casino-Prozess ist klar und ohne Turbulenzen, manchmal die Casino-Angebote konnten gro?zugiger sein. Am Ende ist Tipico Casino eine Casino-Erfahrung, die wie ein Blitz glitzert fur Fans von Online-Casinos! Ubrigens das Casino-Design ist ein optisches Gewitter, einen Hauch von Sturm-Magie ins Casino bringt.
tipico hotline|
The Real Person!
The Real Person!
Ich bin total begeistert von Turbonino Casino, es ist ein Online-Casino, das wie ein Formel-1-Wagen rast. Der Katalog des Casinos ist ein Boxenstopp voller Action, mit einzigartigen Casino-Slotmaschinen. Der Casino-Support ist rund um die Uhr verfugbar, ist per Chat oder E-Mail erreichbar. Casino-Transaktionen sind simpel wie ein Schaltvorgang, manchmal wurde ich mir mehr Casino-Promos wunschen, die wie ein Nitro-Boost knallen. Insgesamt ist Turbonino Casino ein Casino mit einem Spielspa?, der wie ein Zielsprint begeistert fur Abenteurer im Casino! Nebenbei die Casino-Navigation ist kinderleicht wie ein Kurvenmanover, was jede Casino-Session noch rasant macht.
turbonino bonus|
Статьи для садоводов https://portalteplic.ru огородников, фермеров и пчеловодов: советы по уходу за растениями, животными и пасекой. Полезные инструкции, лайфхаки и сезонные рекомендации.
Портал о ремонте https://studio-nd.ru статьи, инструкции и советы для дома и квартиры. От выбора материалов до дизайна интерьеров. Полезные рекомендации для мастеров, новичков и частных застройщиков.
Сайт про металлопрокат https://the-master.ru каталог продукции, характеристики и сферы применения. Арматура, балки, трубы, листы и профили. Актуальные цены, советы специалистов и полезные статьи.
The Real Person!
The Real Person!
Если вы или человек из вашего окружения испытываете с трудностями алкогольной зависимости, медицинская капельница может стать начальным этапом к восстановлению. В Красноярске услуги по детоксикации тела оказывают различные клиники для людей с зависимостью от алкоголя. Специалист-нарколог в Красноярске проведет консультацию и подберет медикаментозное лечение алкогольной зависимости. Капельницы для облегчения похмелья способствуют оперативному улучшению состояния пациента. Восстановление после алкогольной зависимости включает в себя реабилитацию от алкоголя и поддержку профессионалов. Закажите медицинские услуги на сайте vivod-iz-zapoya-krasnoyarsk015.ru для получения профессиональной помощи.
The Real Person!
The Real Person!
Если вы ищете, где прокапаться анонимно в Красноярске, вы можете ознакомиться с сайтом vivod-iz-zapoya-krasnoyarsk015.ru. Здесь вы можете получить услуги капельницы для здоровья, которые помогут вам после болезни. Клиника в Красноярске обеспечивает безопасность процедур и конфиденциальность клиентов, предлагая качественную медицинскую помощь. Анонимное лечение – это шанс заботиться о своем здоровье в спокойной обстановке. Не упустите возможность позаботиться о себе, воспользуйтесь медицинскими процедурами в ближайшее время!
Строительный портал https://krovlyaikrysha.ru база знаний и идей. Статьи о строительстве, ремонте и благоустройстве, инструкции, подбор материалов и советы специалистов для качественного результата.
Всё про ремонт https://gbu-so-svo.ru и строительство — статьи, инструкции и советы для мастеров и новичков. Обзоры материалов, проекты домов, дизайн интерьеров и современные технологии.
Автомобильный портал https://ivanmotors.ru всё о машинах в одном месте. Тест-драйвы, обзоры, аналитика авторынка и советы специалистов. Актуальные события мира авто для водителей и экспертов.
The Real Person!
The Real Person!
Проблема алкогольной зависимости: важность внимательного подхода. Множество людей предпочитают анонимный поиск помощи, чтобы не столкнуться с осуждением. Нарколог на дом предоставляет услуги по выводу из запоя, гарантируя комфорт и конфиденциальность. Вызов нарколога помогает получить медицинскую помощь при запое без лишних вопросов. Анонимное лечение подразумевает detox-программы, которые включают в себя психологическую поддержку и медикаментозное сопровождение. Данный подход помогает предотвратить рецидив алкоголизма и способствует восстановлению после запойного состояния. Кризисная помощь предоставляет поддержку для преодоления острых состояний, предлагая все необходимые ресурсы. Таким образом, анонимный вывод из запоя – это реальность, доступная каждому, кто нуждается в помощи.
Estou completamente alucinado por BetPrimeiro Casino, tem uma vibe de jogo tao ardente quanto lava derretida. As opcoes de jogo no cassino sao ricas e escaldantes, oferecendo sessoes de cassino ao vivo que brilham como brasas. O suporte do cassino ta sempre na ativa 24/7, com uma ajuda que incendeia como uma tocha. As transacoes do cassino sao simples como uma brasa, porem queria mais promocoes de cassino que explodem como vulcoes. No geral, BetPrimeiro Casino vale demais explorar esse cassino para os apaixonados por slots modernos de cassino! Vale dizer tambem o site do cassino e uma obra-prima de estilo vulcanico, eleva a imersao no cassino ao calor de um vulcao.
suporte betprimeiro|
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-smolensk016.ru
лечение запоя смоленск
The Real Person!
The Real Person!
I’m crazy about Wazamba Casino, it pulses with a casino energy as fierce as a tribal drum. There’s a stampede of captivating casino games, supporting casino games optimized for cryptocurrencies. The casino support is available 24/7, with help that shines like a tribal torch. Casino payments are secure and smooth, however extra casino rewards would make hearts pound. In the end, Wazamba Casino is an online casino that thrives like a jungle oasis for players who love betting with jungle flair! As a bonus the casino interface is smooth and vibrant like a tropical sunrise, amplifying total immersion in the casino.
wazamba casino my|
Сайт о ремонте https://e-proficom.ru полезные статьи, пошаговые инструкции и советы экспертов. От выбора материалов до дизайна интерьеров. Всё, что нужно для ремонта квартир и домов.
Сайт для женщин https://devchenky.ru всё самое важное в одном месте: семья, дети, красота, здоровье, дом и работа. Советы специалистов, лайфхаки и вдохновение на каждый день.
Блог о ремонте https://ivinstrument.ru полезные статьи, пошаговые инструкции и советы экспертов. Всё о ремонте квартир и домов: выбор материалов, дизайн интерьеров и современные технологии.
The Real Person!
The Real Person!
Sou louco pela energia de BetPrimeiro Casino, tem uma vibe de jogo tao ardente quanto lava derretida. A gama do cassino e simplesmente uma explosao de delicias, com slots de cassino tematicos de erupcao. O servico do cassino e confiavel e ardente, respondendo mais rapido que uma explosao vulcanica. As transacoes do cassino sao simples como uma brasa, as vezes queria mais promocoes de cassino que explodem como vulcoes. Em resumo, BetPrimeiro Casino oferece uma experiencia de cassino que e puro magma para os amantes de cassinos online! De bonus a interface do cassino e fluida e reluz como lava derretida, adiciona um toque de fogo ao cassino.
betprimeiro com|
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-smolensk016.ru
вывод из запоя круглосуточно смоленск
The Real Person!
The Real Person!
I’m obsessed with the vibe of Wazamba Casino, it radiates a gaming vibe as untamed as a tropical storm. Its collection is a canopy of thrilling entertainment, with modern casino slots that mesmerize like a shaman’s chant. The casino team delivers support worthy of a jungle king, reachable via chat or email. Casino withdrawals are as fast as a jungle sprint, at times extra casino rewards would make hearts pound. All in all, Wazamba Casino is a gem for casino fans for those chasing the adrenaline rush of the casino! As a bonus casino navigation is intuitive like a tribal path, adding a touch of jungle magic to the casino.
wazamba 1003|
The Real Person!
The Real Person!
Стоимость капельницы от запоя на дому в владимире могут варьироваться в зависимости от ряда факторов. Первым делом, стоимость услуг нарколога может различаться в зависимости от репутации клиники и опыта врача. Нарколог на выезде обеспечивает комфорт, но это также влияет на цену.Кроме того, состав капельницы и её ингредиенты (такие как глюкоза и витамины) также влияют на цену. Обычно, стоимость капельницы включает в себя услуги медицинской помощи на дому, что добавляет к общим расходам.Услуги наркологии, такие как лечение алкогольной зависимости и реабилитация при запое, могут различаться по стоимости в зависимости от длительности и сложности лечения. Необходимо учитывать, что первый шаг в борьбе с алкогольной зависимостью требует квалифицированной поддержки и комплексного подхода. нарколог на дом Если вы ищете помощь при запое, целесообразно обратиться к профессионалам, которые предоставят вам информацию о ценах и услугах. В владимире услуги наркологии предлагают разнообразные решения для восстановления здоровья и улучшения качества жизни.
Городской портал Москвы https://moscowfy.ru свежие новости столицы, афиша мероприятий, транспорт, жильё, работа и сервисы для жителей. Полезная информация для москвичей и гостей города на одном сайте.
Современные наливные полы https://proffi-floor.ru это не только идеально ровная поверхность, но и долговечность покрытия. Если вы планируете ремонт в квартире или офисе, узнайте подробнее об услугах по устройству наливных полов
перевод документов организации бюро переводов апостиль
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk017.ru
лечение запоя
The Real Person!
The Real Person!
https://reccagniangelo-msk.ru/ru-ru/
The Real Person!
The Real Person!
mostbet casino Neue Online Casinos
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk017.ru
вывод из запоя цена
The Real Person!
The Real Person!
Обращение к наркологу анонимно — это значимый этап на пути к избавлению от зависимости. На сайте vivod-iz-zapoya-vladimir017.ru вы можете получить профессиональной наркологической помощьюне опасаясь за свою конфиденциальность. Встреча с врачом поможет определить оптимальные варианты терапии. Анонимное лечение и реабилитационные программы предусматривают психотерапию при алкогольной зависимостичто значительно повышает шансы на успех. Также доступны медицинские услуги для пациентов с зависимостями и горячая линия для помощи людям в трудной ситуации. Профилактика наркомании и участие в группах поддержки являются ключевыми аспектами в восстановлении социальной адаптации и возвращении к нормальной жизни.
Je suis totalement submerge par Winamax Casino, ca vibre avec une energie de casino aquatique. Il y a une maree de jeux de casino captivants, comprenant des jeux de casino adaptes aux cryptomonnaies. Le support du casino est disponible 24/7, offrant des solutions claires et instantanees. Le processus du casino est transparent et sans remous, quand meme plus de tours gratuits au casino ce serait aquatique. Globalement, Winamax Casino c’est un casino a explorer sans tarder pour les amoureux des slots modernes de casino ! De surcroit la navigation du casino est intuitive comme un courant marin, ce qui rend chaque session de casino encore plus fluide.
wams winamax|
Adoro o trovao de VikingLuck Casino, tem uma vibe de jogo tao feroz quanto uma tempestade nordica. A gama do cassino e simplesmente um salao de Valhalla, com jogos de cassino perfeitos pra criptomoedas. O servico do cassino e confiavel e forte como um escudo, com uma ajuda que reluz como uma runa encantada. Os pagamentos do cassino sao lisos e blindados, de vez em quando queria mais promocoes de cassino que rugem como guerreiros. Resumindo, VikingLuck Casino oferece uma experiencia de cassino que e puro trovao para os apaixonados por slots modernos de cassino! De bonus o site do cassino e uma obra-prima de estilo nordico, torna a experiencia de cassino uma batalha inesquecivel.
viking rune for good luck|
The Real Person!
The Real Person!
вывод из запоя смоленск
vivod-iz-zapoya-smolensk018.ru
вывод из запоя цена
The Real Person!
The Real Person!
Ich bin suchtig nach Wheelz Casino, es ist ein Online-Casino, das wie eine Achterbahn durch die Lufte rast. Es gibt eine Flut an mitrei?enden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Die Casino-Mitarbeiter sind schnell wie ein Loopingstart, ist per Chat oder E-Mail erreichbar. Casino-Gewinne kommen wie ein Geschwindigkeitsrausch, ab und zu mehr regelma?ige Casino-Boni waren ein Volltreffer. Alles in allem ist Wheelz Casino ein Casino mit einem Spielspa?, der wie ein Turbo-Ride fesselt fur Adrenalinjunkies im Casino! Und au?erdem die Casino-Plattform hat einen Look, der wie ein Looping strahlt, Lust macht, immer wieder ins Casino zuruckzukehren.
2 wheelz|
Hello friends!
I came across a 133 very cool page that I think you should visit.
This platform is packed with a lot of useful information that you might find valuable.
It has everything you could possibly need, so be sure to give it a visit!
https://augsburg-entwickeln.de/casinospiele-online/tom-dwan-das-internet-poker-phanomen/
Подборка статей https://yandex-direct-info.ru про Яндекс Директ: пошаговые инструкции, советы по таргетингу, ретаргетингу и аналитике. Всё о рекламе в Яндексе в одном месте для вашего бизнеса.
Яндекс Бизнес https://business-yandex3.ru описание сервиса, его инструменты и функции. Как компаниям привлекать клиентов, управлять рекламой и повышать эффективность онлайн-продвижения.
Just what I needed to know thank you for this.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-smolensk018.ru
вывод из запоя цена
https://vgarderobe.ru/zhenskie-svitshoty-roxy-bc-1171.html
The Real Person!
The Real Person!
Детоксикация от алкоголя в владимире: профессиональная помощь Проблема алкогольной зависимости требует опытного подхода и квалифицированной помощи. В владимире предлагается широкий спектр услуг по детоксикации и лечению зависимости. Наркологическая помощь в владимире включает медицинскую детоксикацию, что позволяет безопасно вывести токсины из организма. помощь нарколога владимир Специалисты предлагают программы реабилитации, где осуществляется поддержка нарколога, психологическая поддержка при детоксикации и консультации по алкоголизму. Необходимо понимать, что успешное восстановление после алкоголизма требует комплексного подхода, включая лечение зависимости от алкоголя. Если вам нужна профессиональная помощь при алкоголизме, в владимире есть клиники, предоставляющие эффективное лечение и поддержку. Важно не затягивать с обращением за помощью; сделайте первый шаг к здоровой жизни уже сегодня.
Игра в города https://studentopedia.ru/populyarnaya-igra-v-goroda.html для всех возрастов. Проверьте знания географии, вспоминайте названия городов и соревнуйтесь с друзьями. Увлекательная логическая игра для тренировки памяти и эрудиции.
All the most important here: https://possible11.com
Open the latest data here: https://www.greenwichodeum.com
Details are waiting for you here: https://manorhousedentalpractice.co.uk
The most relevant place is here: https://koreatesol.org
Only verified facts are here: https://www.levelupacademy.in
В поисках самых свежих трейлеров фильмов 2026 и трейлеров 2026 на русском? Наш портал — это место, где собираются лучшие трейлеры сериалов 2026. Здесь вы можете смотреть трейлер бесплатно в хорошем качестве, будь то громкая премьера лорд трейлер или долгожданный трейлер 3 сезона вашего любимого сериала. Мы тщательно отбираем видео, чтобы вы могли смотреть трейлеры онлайн без спойлеров и в отличном разрешении. Всю коллекцию вы найдете по ссылке ниже: лучшие трейлеры смотреть онлайн бесплатно
Everything in one place: https://www.lnrprecision.com
Everything you need: https://heatherparker.com
Read the best here: https://www.sportsoddshistory.com/
The Real Person!
The Real Person!
Обращение к наркологу анонимно — это важный шаг для людей, столкнувшихся с зависимостями при лечении алкогольной и наркотической зависимости. На сайте vivod-iz-zapoya-vladimir019.ru вы можете обратиться за помощью и поискать наркологической помощью. Это особенно актуально для людей, страдающих от алкоголем и наркотиками. Профессиональная помощь нарколога включают диагностику зависимостей и медицинскую помощь при наркотической зависимости. Анонимный нарколог может приехать на дом, что гарантирует приватность и удобство. Выездные медработники предоставляют поддержку психолога и проводят реабилитацию наркозависимых. Виртуальная встреча с врачом позволяет незамедлительно узнать нужные советы. Лечение наркотиков анонимно — это возможность начать новую жизнь без опасений о предвзятости. Не упустите шанс изменить свою судьбу с помощью анонимных услуг здоровья!
Hot news is already here: https://sernexuss.com
News for you – right here: https://fish-pet.com
Everything the brightest here: https://intelicode.com
Bk8
Galera, vim dividir minhas impressoes no 4PlayBet Casino porque me pegou de surpresa. A variedade de jogos e simplesmente incrivel: slots modernos, todos funcionando perfeito. O suporte foi bem prestativo, responderam em minutos pelo chat, algo que vale elogio. Fiz saque em Bitcoin e o dinheiro entrou muito rapido, ponto fortissimo. Se tivesse que criticar, diria que senti falta de ofertas recorrentes, mas isso nao estraga a experiencia. Pra concluir, o 4PlayBet Casino me conquistou. Recomendo sem medo.
office 4play intern edition|
The Real Person!
The Real Person!
Je suis totalement anime par Simsinos Casino, on dirait un scenario plein de bulles de fun. L’assortiment de jeux du casino est un storyboard de delices. proposant des slots de casino a theme anime. Le service client du casino est un realisateur fidele. repondant en un flash colore. fluisent comme une sonate animee. cependant plus de bonus pour une harmonie dessinee. Dans l’ensemble, Simsinos Casino est un joyau pour les fans de casino pour les animateurs du casino! De surcroit la plateforme du casino brille par son style anime. enchante chaque partie avec une symphonie de toons.
simsinos casino online|
The Real Person!
The Real Person!
Sou louco pela torcida de MarjoSports Casino, pulsa com uma forca de cassino digna de um craque. A colecao e um gol de entretenimento. incluindo jogos de mesa com um toque de estrategia. O atendimento e solido como um campo. oferecendo respostas claras como uma quadra. Os ganhos chegam rapido como um gol. de vez em quando mais bonus seriam um diferencial de campo. Para encurtar, MarjoSports Casino e um cassino online que e um estadio de diversao para os amantes de cassinos online! Adicionalmente a plataforma marca gol com um visual vibrante. transformando cada aposta em uma aventura de gol.
baixa aplicativo marjosports|
Sou louco pela vibracao de BR4Bet Casino, vibra como um farol em alto-mar. A selecao de titulos e uma chama de emocoes. incluindo jogos de mesa com um toque de brilho. Os agentes sao rapidos como um raio de farol. disponivel por chat ou e-mail. Os pagamentos sao seguros e fluidos. mesmo assim queria mais promocoes que brilham como farois. Resumindo, BR4Bet Casino e uma fogueira de adrenalina para os viciados em emocoes de cassino! Vale dizer o layout e vibrante como uma tocha. fazendo o cassino brilhar como um farol.
br4bet paga bem|
The Real Person!
The Real Person!
Je suis accro a Simsinos Casino, on dirait un scenario plein de bulles de fun. Il y a une cascade de jeux de casino captivants. incluant des tables qui vibrent comme un storyboard. est un virtuose de l’animation. joignable par chat ou email. Les gains du casino arrivent a une vitesse cartoon. mais les offres du casino pourraient etre plus genereuses. Globalement, Simsinos Casino cadence comme une sonate de victoires pour les fans de symphonies colorees! A noter la navigation du casino est intuitive comme une bulle. amplifie l’immersion totale dans le casino.
simsinos 7|
The Real Person!
The Real Person!
Acho simplesmente esportivo MarjoSports Casino, pulsa com uma forca de cassino digna de um craque. A selecao de titulos e uma rede de prazeres. com caca-niqueis modernos que driblam como craques. O atendimento e solido como um campo. disponivel por chat ou e-mail. As transacoes sao simples como um passe. ocasionalmente mais bonus regulares seriam esportivos. Resumindo, MarjoSports Casino promete uma diversao que e um gol para os viciados em emocoes de cassino! De lambuja o design e um espetaculo visual de torcida. adicionando um toque de drible ao cassino.
suporte marjosports|
Futureswap is made for efficiency Discover the power of future swap now on futureswap.pro
Rainbet Casino
Топливные карты https://alcomauto.ru для юридических лиц помогут сократить затраты компании и обеспечить полный контроль за автопарком.
Топливные карты https://karta-vezdehod.ru удобный способ экономить на заправках, контролировать расходы и получать прозрачную отчетность.
Топливные карты https://auto-nv.ru для юр лиц — это надежный инструмент учета топлива, скидки на АЗС и удобная онлайн-отчетность.
The Real Person!
The Real Person!
हाँ, बिल्कुल। लेकिन ध्यान दें कि ऐप स्टोर या प्ले स्टोर से जो डाउनलोडेबल वर्जन उपलब्ध है, वह केवल डेमो या ट्रेनिंग के लिए होता है — इसमें पैसे नहीं जीते जा सकते। एविएटर एक सरल खेल है जो इसे आजमाने वाले सभी लोगों के लिए मनोरंजक है। डेमो संस्करण असली पैसे के दांव के लिए खेला जाता है या नहीं, वही बहुत मज़ा प्रदान करता है।
https://eduproof.io/aviator-%e0%a4%97%e0%a5%87%e0%a4%ae-%e0%a4%b8%e0%a4%ae%e0%a5%80%e0%a4%95%e0%a5%8d%e0%a4%b7%e0%a4%be-%e0%a4%ad%e0%a4%be%e0%a4%b0%e0%a4%a4-%e0%a4%95%e0%a5%87-%e0%a4%96%e0%a4%bf%e0%a4%b2%e0%a4%be/
हेलो दोस्तों आज मार्केट में नया एप आया ही। जिससे आप बहुत सारा पैसा कमा सकते हो। आप जोखिम तय करते हैं – हमारे Chicken Road गेम में आप खुद चुनते हैं कि हर राउंड कितना जोखिम भरा हो। कठिनाई का स्तर बदलते ही मल्टीप्लायर रेंज बदल जाती है, जिससे संभावित जीत या हार पर असर पड़ता है। यह क्रैश गेम हर राउंड से पहले वोलैटिलिटी को समायोजित करने की सुविधा देता है।
The Real Person!
The Real Person!
Estou alucinado com Fogo777 Casino, tem um ritmo de jogo que danca como labaredas. A selecao de titulos e uma pira de emocoes. com caca-niqueis modernos que hipnotizam como fogos. O servico e confiavel como uma fogueira. assegurando apoio sem fumaca. Os pagamentos sao seguros e fluidos. ocasionalmente mais bonus seriam um diferencial ardente. Em resumo, Fogo777 Casino vale explorar esse cassino ja para os xamas do cassino! Como extra o design e um espetaculo visual flamejante. dando vontade de voltar como uma labareda.
fogo777 app download|
The Real Person!
The Real Person!
Estou alucinado com Verabet Casino, explode com uma vibe de cassino flamejante. O catalogo de jogos e um altar de prazeres. com caca-niqueis que reluzem como brasas. Os agentes sao rapidos como uma faisca. com ajuda que ilumina como uma pira. Os ganhos chegam rapido como uma labareda. entretanto mais bonus regulares seriam flamejantes. Em resumo, Verabet Casino oferece uma experiencia que e puro ritual para os apaixonados por slots modernos! De bonus a plataforma reluz com um visual ritualistico. adicionando um toque de fogo ao cassino.
plataforma vera bet|
Топливная карта https://autobsn.ru для юридических лиц дает доступ к выгодным тарифам, снижает расходы и делает контроль проще.
Что такое Agile https://agile-metod.ru и как его внедрить? Подробные статьи о гибких методологиях, инструментах и практиках. Scrum, Kanban и Lean — всё о современном управлении проектами.
Что такое CPI https://cost-per-install.ru в маркетинге? Полное объяснение показателя Cost Per Install: как он работает, зачем нужен бизнесу, примеры расчётов и советы по использованию метрики в рекламе приложений.
The Real Person!
The Real Person!
Sou viciado no calor de Fogo777 Casino, tem um ritmo de jogo que danca como labaredas. A selecao de titulos e uma pira de emocoes. com caca-niqueis modernos que hipnotizam como fogos. Os agentes sao rapidos como uma faisca. respondendo veloz como uma faisca. Os saques sao velozes como um ritual de fogo. em alguns momentos mais giros gratis seriam incendiarios. No fim das contas, Fogo777 Casino e um cassino online que e uma fogueira de diversao para os apaixonados por slots modernos! Como extra o design e um espetaculo visual flamejante. amplificando o jogo com vibracao ardente.
fogo777.com Г© confiГЎvel|
buy cocaine in telegram weed in prague
united statesn online casinos real money, new zealandn online casino sign up no deposit bonus and free sign up bonus usa bingo, or how to get vault contents casino heist [https://Goplayslots.net/] in australia near
detroit
смм накрутка подписчиков тг
Je suis charme par Grandz Casino, il propose une aventure de casino qui tournoie comme un voile mystique. Les options de jeu au casino sont riches et ephemeres. incluant des jeux de table de casino d’une elegance spectrale. offre un soutien qui effleure tout. assurant un support de casino immediat et voile. arrivent comme un concerto voile. neanmoins plus de tours gratuits au casino ce serait theatral. En somme, Grandz Casino est un casino en ligne qui joue une danse d’ombres pour les joueurs qui aiment parier avec style au casino! Bonus le site du casino est une merveille graphique ephemere. cadence l’aventure avec une rhapsodie spectrale.
grandz bet casino avis|
Je suis totalement envoute par Casinia Casino, ca degage une ambiance de jeu aussi noble qu’un tournoi de chevaliers. La selection du casino est une cour de plaisirs. offrant des lives qui pulsent comme un tournoi. est un virtuose de la noblesse. joignable par chat ou email. Le processus du casino est transparent et sans trahison. parfois des recompenses de casino supplementaires feraient vibrer. Globalement, Casinia Casino est un joyau pour les fans de casino pour les joueurs qui aiment parier avec panache au casino! A noter la plateforme du casino brille par son style epique. amplifie l’immersion totale dans le casino.
casinia espaГ±a|
накрутка подписчиков тг
buy cocaine prague pure cocaine in prague
The Real Person!
The Real Person!
J’adore le voile de Grandz Casino, offre un spectacle de plaisir qui s’evapore. La selection du casino est une danse de plaisirs. offrant des lives qui pulsent comme un theatre. Le service client du casino est un maitre des ombres. proposant un appui qui enchante. se deroulent comme une rhapsodie d’ombres. cependant des offres qui vibrent comme une cadence spectrale. En somme, Grandz Casino enchante avec une rhapsodie de jeux pour les virtuoses des jeux! A noter le design du casino est une fresque visuelle spectrale. fait vibrer le jeu comme un concerto ephemere.
grandz bet casino|
The Real Person!
The Real Person!
Galera, preciso contar o que achei sobre o Bingoemcasa porque me ganhou de verdade. O site tem um ar amigavel que lembra um bate-papo animado. As salas de bingo sao super animadas, e ainda testei blackjack e poker tambem, todos rodaram sem travar. O atendimento no chat foi respondeu em segundos, o que ja me deixou satisfeito. As retiradas foram mais velozes que imaginei, inclusive testei PIX e caiu em minutos. Se pudesse apontar algo, diria que senti falta de ofertas extras, mas nada que estrague a experiencia. Na minha visao, o Bingoemcasa vale demais a pena. Eu mesmo ja voltei varias vezes
https m bingoemcasa net|
The Real Person!
The Real Person!
Je trouve absolument legendaire Casinia Casino, c’est un casino en ligne qui s’eleve comme un chateau medieval. Il y a une cascade de jeux de casino captivants. proposant des slots de casino a theme medieval. repond comme un bouclier. proposant un appui qui enchante. se deroulent comme une quete. par moments j’aimerais plus de promotions de casino qui brillent comme des joyaux royaux. En somme, Casinia Casino promet un divertissement de casino legendaire pour les explorateurs de melodies en ligne! Bonus le design du casino est une fresque visuelle chevaleresque. cadence l’aventure avec une rhapsodie epique.
casinia fr|
https://www.wildberries.ru/catalog/171056105/detail.aspx
https://mp-digital.ru/
The Real Person!
The Real Person!
code promo 1xbet 2026 vietnam code promo 1xbet cote d’ivoire Le monde des paris sportifs en ligne est en constante evolution, et 1xbet s’est etabli comme un leader mondial, offrant une plateforme complete et diversifiee aux parieurs du monde entier. En Cote d’Ivoire, l’enthousiasme pour les paris sportifs est palpable, et 1xbet Cote d’Ivoire propose une gamme allechante de promotions pour ameliorer l’experience de pari de ses utilisateurs. Au c?ur de ces promotions se trouvent les codes promotionnels, des cles magiques qui debloquent des bonus exclusifs et des opportunites de pari ameliorees. Qu’est-ce qu’un code promo 1xbet Cote d’Ivoire? Un code promo 1xbet Cote d’Ivoire est une combinaison unique de lettres et de chiffres qui peuvent etre entres lors de l’inscription ou du depot pour activer un bonus specifique. Ces bonus peuvent inclure: Bonus de bienvenue: Un bonus offert aux nouveaux utilisateurs lors de leur premier depot. Bonus de depot: Un bonus accorde sur les depots ulterieurs. Paris gratuits: La possibilite de placer un pari sans risquer son propre argent. Cotes ameliorees: Des cotes plus elevees sur certains evenements sportifs. Ou trouver les codes promo 1xbet Cote d’Ivoire? Les codes promotionnels 1xbet Cote d’Ivoire sont disponibles via plusieurs canaux, notamment: Le site Web officiel de 1xbet: Consultez regulierement le site Web de 1xbet pour les dernieres offres. Les sites Web affilies: De nombreux sites Web affilies proposent des codes promotionnels exclusifs pour les joueurs ivoiriens. Les reseaux sociaux: Suivez les comptes de medias sociaux officiels de 1xbet pour rester informe des nouvelles versions de code promotionnel. Les newsletters par e-mail: Abonnez-vous aux newsletters par e-mail de 1xbet pour recevoir les codes promotionnels directement dans votre boite de reception. L’utilisation efficace des codes promotionnels 1xbet Cote d’Ivoire peut considerablement augmenter vos chances de gagner et rendre votre experience de pari plus agreable. Gardez un ?il sur les derniers codes et profitez des avantages qu’ils offrent.
Графитовые и угольные щетки для электроинструмента. Большой выбор, надёжность и долговечность. Подходят для дрелей, болгарок, перфораторов и другого оборудования.
Нужны двери? межкомнатные двери спб недорого Широкий ассортимент межкомнатных дверей от Вектордорс. У нас вы найдете модели на любой вкус: от классических до современных дизайнерских решений. Выбор межкомнатных дверей — важный этап обустройства помещения. Правильно подобранные двери не только украсят интерьер, но и обеспечат комфорт и функциональность на долгие годы.
Je suis envoute par RollBit Casino, est un tourbillon de divertissement qui pixelise. La selection du casino est une chaine de plaisirs. offrant des lives qui pulsent comme un defilement. L’assistance du casino est chaleureuse et precise. offrant des solutions claires et instantanees. Le processus du casino est transparent et sans glitch. par moments les offres du casino pourraient etre plus genereuses. A la fin, RollBit Casino offre une experience de casino pixelisee pour les fans de symphonies de bits! Ajoutons la plateforme du casino brille par son style bit. fait vibrer le jeu comme un concerto cubique.
rollbit.|
Sou viciado no fluxo de XPBet Casino, pulsa com uma forca de cassino digna de uma roda. Os jogos formam um redemoinho de diversao. incluindo mesas com charme de roda. O suporte e um loop preciso. garantindo suporte direto e sem pausas. As transacoes sao simples como um giro. as vezes as ofertas podiam ser mais generosas. No fim das contas, XPBet Casino promete uma diversao que e um espiral para os apaixonados por slots modernos! Vale dizer a plataforma reluz com um visual ciclico. elevando a imersao ao nivel de um vortice.
44 xp bet|
The Real Person!
The Real Person!
Curto demais a vibracao de DonaldBet Casino, oferece uma aventura que brilha como uma lona de circo. Tem um turbilhao de jogos de cassino irados. com caca-niqueis que reluzem como malabares. O suporte e um mestre de cerimonias. com ajuda que ilumina como um refletor. Os saques voam como um acrobata. porem queria mais promocoes que brilham como holofotes. No fim das contas, DonaldBet Casino oferece uma experiencia que e puro espetaculo para os apaixonados por slots modernos! E mais o design e um espetaculo visual de circo. transformando cada aposta em uma aventura de picadeiro.
donaldbet.|
The Real Person!
The Real Person!
J’adore le flux de RollBit Casino, ca vibre avec une energie de casino digne d’un defilement. est une structure de sensations qui enchante. incluant des tables qui structurent comme un cube. Les agents du casino sont rapides comme un flux de donnees. joignable par chat ou email. se deroulent comme une rhapsodie de bits. neanmoins des offres qui vibrent comme une cadence pixelisee. Dans l’ensemble, RollBit Casino est un casino en ligne qui deroule une symphonie de bits pour les explorateurs de melodies en ligne! Ajoutons la plateforme du casino brille par son style bit. donne envie de replonger dans le casino sans fin.
rollbit bronze 1 reward|
The Real Person!
The Real Person!
Acho simplesmente ciclico XPBet Casino, explode com uma vibe de cassino ciclica. Os jogos formam um redemoinho de diversao. com caca-niqueis que reluzem como espirais. O servico e confiavel como um ciclo. respondendo veloz como um redemoinho. Os pagamentos sao seguros e fluidos. de vez em quando mais giros gratis seriam uma loucura de loop. Na real, XPBet Casino e um cassino online que e um vortice de diversao para os viciados em emocoes de cassino! Por sinal a plataforma reluz com um visual ciclico. criando uma experiencia de cassino ciclica.
44 xp bet|
The Real Person!
The Real Person!
Sou viciado no brilho de DonaldBet Casino, tem uma energia de jogo tao vibrante quanto um malabarista em acao. O leque do cassino e um circo de delicias. incluindo jogos de mesa com um toque de circo. O time do cassino e digno de um diretor de circo. com ajuda que ilumina como um refletor. Os saques sao velozes como um trapezio. mesmo assim queria mais promocoes que brilham como holofotes. Em resumo, DonaldBet Casino e o point perfeito pros fas de cassino para os apaixonados por slots modernos! Vale dizer o site e uma obra-prima de estilo vibrante. transformando cada aposta em uma aventura de picadeiro.
donaldbet cnpj|
купить щебень https://pesko.ru
CPL (Cost Per Lead) https://cost-per-lead1.ru ключевая метрика рекламы. Узнайте, что это, как правильно рассчитывать стоимость лида, где применяется и как помогает оценить эффективность кампаний.
Интернет-маркетинг https://internet-marketing1.ru SEO, контекстная реклама, SMM, email-рассылки и аналитика. Статьи, советы и инструменты для бизнеса, которые помогают привлекать клиентов и увеличивать продажи онлайн.
The Real Person!
The Real Person!
психическое здоровье Психотерапевт онлайн – это проводник в глубины подсознания, предлагающий безопасное пространство для исцеления душевных ран. Это не просто беседа, а целенаправленный процесс, направленный на выявление глубинных причин психологических проблем и разработку эффективных стратегий их преодоления. Онлайн-терапия открывает двери к личностному росту и трансформации, позволяя изменить не только свое состояние, но и восприятие мира. Это шанс взглянуть на жизнь под новым углом, обрести внутреннюю свободу и построить более счастливое будущее.
Интернет-маркетинг https://yandex-reklama2.ru для компаний и специалистов: SEO, SMM, контекстная реклама и email. Советы по выбору стратегий, разбор ошибок и методы повышения эффективности.
You appear to know so much about this, and I see you’re a published author. Thanks
как накрутить подписчиков в телеграм
The Real Person!
The Real Person!
Je suis enthousiaste a propos de 7BitCasino, ca procure une experience de jeu electrisante. Il y a une profusion de titres varies, proposant des jeux de table elegants et classiques. Le personnel offre un accompagnement irreprochable, garantissant une aide immediate via chat en direct ou email. Les retraits sont ultra-rapides, par moments les bonus pourraient etre plus reguliers, comme des offres de cashback plus avantageuses. Globalement, 7BitCasino est un incontournable pour les amateurs de casino en ligne ! En bonus la navigation est intuitive et rapide, facilite chaque session de jeu.
7bitcasino online|
The Real Person!
The Real Person!
Ich bin suchtig nach King Billy Casino, es ist ein Online-Casino, das wie ein Konig regiert. Die Casino-Optionen sind vielfaltig und prachtig, mit modernen Casino-Slots, die verzaubern. Die Casino-Mitarbeiter sind schnell wie ein koniglicher Bote, ist per Chat oder E-Mail erreichbar. Auszahlungen im Casino sind schnell wie ein koniglicher Marsch, trotzdem mehr Freispiele im Casino waren ein Kronungsmoment. Am Ende ist King Billy Casino ein Casino, das man nicht verpassen darf fur Fans moderner Casino-Slots! Extra die Casino-Navigation ist kinderleicht wie ein koniglicher Marsch, Lust macht, immer wieder ins Casino zuruckzukehren.
king billy casino promo code 2022|
The Real Person!
The Real Person!
Je trouve absolument sensationnel BetFury Casino, il offre une aventure pleine de frissons. Les options de jeu sont riches et diversifiees, incluant des slots de derniere generation. Le personnel offre un accompagnement de qualite, avec un suivi exemplaire. Les gains arrivent en un temps record, de temps en temps ??? les bonus pourraient etre plus reguliers. Dans l’ensemble, BetFury Casino est une plateforme revolutionnaire pour les joueurs en quete de sensations fortes ! Par ailleurs le site est concu avec modernite et elegance, ce qui renforce l’immersion.
betfury mines game|
The Real Person!
The Real Person!
Je suis carrement scotche par Gamdom, il propose une aventure qui dechire. Le catalogue de jeux est juste enorme, avec des slots qui claquent grave. Le crew assure un suivi de malade, avec une aide qui dechire tout. Les transactions sont simples comme un clin d’?il, mais bon des bonus plus reguliers ce serait la classe. Dans le fond, Gamdom est un spot a ne pas louper pour les accros aux sensations extremes ! En prime le site est une tuerie graphique, facilite le delire total.
rain notifier gamdom|
The Real Person!
The Real Person!
Je suis totalement seduit par 7BitCasino, c’est une veritable experience de jeu electrisante. Les options de jeu sont riches et diversifiees, offrant des sessions de casino en direct immersives. Le service client est remarquable, offrant des reponses rapides et precises. Les retraits sont ultra-rapides, cependant plus de tours gratuits seraient un atout, afin de maximiser l’experience. En resume, 7BitCasino est une plateforme d’exception pour les amateurs de casino en ligne ! Ajoutons que la navigation est intuitive et rapide, ajoute une touche de raffinement a l’experience.
7bitcasino bonuses|
J’adore l’energie de DBosses, il propose une experience de jeu intense. Les options sont riches et dynamiques, proposant des sessions live vibrantes. Le support est disponible 24/7, avec une aide personnalisee. Les transactions sont simples et efficaces, parfois plus de tours gratuits seraient top. Dans l’ensemble, DBosses est un choix incontournable pour les joueurs pour les passionnes de sensations fortes ! De surcroit la navigation est intuitive et rapide, renforce l’envie de revenir.
dbosses casino|
The Real Person!
The Real Person!
Je suis enthousiaste a propos de BetFury Casino, c’est une veritable immersion dans un univers vibrant. Les options de jeu sont riches et diversifiees, avec des machines a sous modernes et captivantes. Le service client est exceptionnel, offrant des reponses claires et rapides. Le processus de retrait est simple et fiable, bien que j’aimerais plus de promotions frequentes. Dans l’ensemble, BetFury Casino ne decoit jamais pour les joueurs en quete de sensations fortes ! Ajoutons que l’interface est fluide et intuitive, ajoute une touche de sophistication a l’experience.
betfury legit or scam|
The Real Person!
The Real Person!
J’adore le delire total de Gamdom, ca balance une vibe de folie. Il y a un tsunami de titres varies, comprenant des jeux parfaits pour les cryptos. Le crew assure un suivi de malade, avec une aide qui dechire tout. Les transactions sont simples comme un clin d’?il, par moments j’aimerais plus de promos qui defoncent. Au final, Gamdom offre une experience de ouf pour les accros aux sensations extremes ! Bonus le site est une tuerie graphique, donne envie de replonger non-stop.
gamdom crash predictor|
Памятники на заказ https://top-memorial.ru широкий выбор форм и материалов, профессиональное изготовление и монтаж. Индивидуальный подход, гарантия качества и доступные цены.
Генеральная уборка https://cleaningplus.ru/services/uborka-kvartiry в Москве: квартиры, дома, офисы. Полный комплекс услуг — мытьё окон, чистка мебели, удаление пыли и грязи. Профессиональные клинеры, безопасные средства и гарантия качества.
накрутка подписчиков тг
The Real Person!
The Real Person!
Je suis accro au style de FatPirate, il offre une aventure totalement barge. La selection est carrement dingue, avec des slots qui dechirent. Le support est dispo 24/7, joignable par chat ou email. Les transactions sont simples comme bonjour, des fois les offres pourraient etre plus genereuses. Pour resumer, FatPirate garantit un fun total pour les fans de casinos en ligne ! Et puis le design est style et accrocheur, booste l’immersion a fond.
fatpirate 1|
The Real Person!
The Real Person!
Je trouve incroyable 1win Casino, on dirait une experience de jeu captivante. La selection est incroyablement riche, incluant des slots de derniere generation. Le support est ultra-reactif et disponible, repondant rapidement. Le processus de retrait est simple et efficace, parfois j’aimerais plus de promotions regulieres. Pour conclure, 1win Casino offre une experience de jeu memorable pour les fans de divertissement numerique ! En bonus la navigation est simple et rapide, ajoute une touche d’elegance a l’experience.
1win bet apk|
The Real Person!
The Real Person!
J’adore passionnement Betify Casino, ca ressemble a une sensation de casino unique. Il y a une profusion de titres varies, incluant des slots de derniere generation. Le personnel offre un accompagnement de qualite, avec un suivi de qualite. Les transactions sont parfaitement protegees, bien que davantage de recompenses seraient appreciees. En resume, Betify Casino est une plateforme d’exception pour ceux qui aiment parier ! Notons egalement que le site est concu avec elegance, facilite chaque session de jeu.
betify espana|
We are a group of volunteers and starting a new initiative in our community. Your blog provided us with valuable information to work on|.You have done a marvellous job!
На сайте Детский Класс нашим посетителям в любое время доступны материалы для приятного совместного досуга детей и их родителей: детские песни на разные тематики, которые можно разучивать и распевать в будни и праздники, интересные и познавательные легенды и мифы, раскраски различной сложности, а также волшебные и поучительные сказки.
https://www.spyuganda.com/this-is-for-sheebah-nwagi-seek-approval-from-media-council-before-you-release-nudes-mistaken-as-music-videos-ucc-finally-bites/#comment-1583176
подписчики в тг канал
The Real Person!
The Real Person!
Ich bin suchtig nach DrueGlueck Casino, es bietet eine krasse Spielerfahrung. Der Katalog des Casinos ist der Wahnsinn, inklusive stylischer Casino-Tischspiele. Das Casino-Team bietet erstklassige Unterstutzung, mit Hilfe, die richtig abgeht. Casino-Transaktionen sind simpel und zuverlassig, trotzdem mehr Freispiele im Casino waren top. Kurz gesagt ist DrueGlueck Casino eine Casino-Erfahrung, die rockt fur Fans von Online-Casinos! Nebenbei das Casino-Design ist ein optischer Volltreffer, Lust macht, immer wieder ins Casino zuruckzukehren.
gutscheincode drueckglueck|
The Real Person!
The Real Person!
J’adore sans reserve 1xbet Casino, ca ressemble a une sensation de casino unique. Les options de jeu sont riches et diversifiees, comprenant des titres innovants et engageants. Le personnel offre un accompagnement irreprochable, avec un suivi de qualite. Le processus de retrait est simple et fiable, bien que les promotions pourraient etre plus genereuses. En fin de compte, 1xbet Casino est une plateforme d’exception pour ceux qui aiment parier ! De plus l’interface est fluide et moderne, ajoute une touche de raffinement a l’experience.
1xbet register|
The Real Person!
The Real Person!
Estou pirando total com Flabet Casino, da uma energia de cassino que e um espetaculo. O catalogo de jogos do cassino e uma loucura, com jogos de cassino perfeitos pra criptomoedas. O suporte do cassino ta sempre na area 24/7, acessivel por chat ou e-mail. Os saques no cassino sao velozes como um raio, porem mais bonus regulares no cassino seria brabo. Resumindo, Flabet Casino e um cassino online que e um vulcao para quem curte apostar com estilo no cassino! Alem disso a interface do cassino e fluida e cheia de vibe, aumenta a imersao no cassino a mil.
como usar a freebet da flabet|
The Real Person!
The Real Person!
J’adore a fond Instant Casino, on dirait une tempete de fun. La gamme de casino est un veritable feu d’artifice, incluant des jeux de table de casino styles. Les agents du casino sont rapides comme l’eclair, offrant des reponses qui claquent. Les paiements du casino sont fluides et securises, par contre des recompenses de casino en plus ca ferait kiffer. Dans le fond, Instant Casino offre une experience de casino inoubliable pour les aventuriers du casino ! A noter aussi le design du casino est une explosion visuelle, booste l’immersion dans le casino a fond.
betfair instant play casino|
Утка принт футболка и печать на футболки в Казани в Нижневартовске. Вязаный шарф женский и шарик на шапке 6 букв в Сыктывкаре. Футболки с надписью черный и футболка руки вверх в Ижевске. Для одежды несмывающиеся и We Don T Care одежда в Нальчике. Футболки оптом из 90 х мужские и печать логотипа на футболке: https://futbolki-s-printom.ru/
подписчики в тг канал без отписок
pure cocaine in prague coke in prague
columbian cocain in prague plug in prague
The Real Person!
The Real Person!
Ich bin total begeistert von DrueGlueck Casino, es ist ein Casino, das richtig abgeht. Der Katalog des Casinos ist der Wahnsinn, mit einzigartigen Casino-Slotmaschinen. Der Casino-Support ist rund um die Uhr da, sorgt fur sofortigen Casino-Support. Casino-Zahlungen sind sicher und reibungslos, dennoch wurde ich mir mehr Casino-Promos wunschen. Kurz gesagt ist DrueGlueck Casino ein Muss fur Casino-Fans fur Fans moderner Casino-Slots! Und au?erdem das Casino-Design ist ein optischer Volltreffer, einen Hauch von Wahnsinn ins Casino bringt.
drueckglueck spil|
The Real Person!
The Real Person!
Je trouve absolument epoustouflant 1xbet Casino, on dirait une experience de jeu exaltante. Le catalogue est incroyablement vaste, offrant des sessions de casino en direct immersives. Le service d’assistance est de premier ordre, joignable 24/7. Les retraits sont ultra-rapides, occasionnellement les promotions pourraient etre plus genereuses. En fin de compte, 1xbet Casino est une plateforme d’exception pour ceux qui aiment parier ! De plus l’interface est fluide et moderne, ce qui intensifie le plaisir de jouer.
1xbet РїСЂРѕРјРѕРєРѕРґ|
The Real Person!
The Real Person!
Acho simplesmente fantastico o 888 Casino, e como se fosse sensacao unica de cassino. A selecao de jogos e impressionante, com caca-niqueis modernos e envolventes. O suporte e super responsivo e profissional, com acompanhamento de excelencia. As transacoes sao totalmente protegidas, as vezes os bonus poderiam ser mais frequentes. Em ultima analise, o 888 Casino e indispensavel para os amantes de cassino online! Como bonus a interface e fluida e intuitiva, adiciona um toque de sofisticacao a experiencia.
888 casino paypal|
The Real Person!
The Real Person!
Acho completamente fora da curva Flabet Casino, da uma energia de cassino que e um espetaculo. As opcoes de jogo no cassino sao ricas e alucinantes, oferecendo sessoes de cassino ao vivo que sao um fogo. O servico do cassino e confiavel e brabo, acessivel por chat ou e-mail. As transacoes do cassino sao simples como um estalo, mesmo assim mais bonus regulares no cassino seria brabo. No fim das contas, Flabet Casino oferece uma experiencia de cassino que e puro gas para os aventureiros do cassino! De bonus a navegacao do cassino e facil como brincadeira, torna o cassino uma curticao total.
flabet app download|
The Real Person!
The Real Person!
Je suis accro a Instant Casino, il propose une experience de casino explosive. Les options de jeu en casino sont ultra-riches, avec des slots de casino modernes et immersifs. Le service client du casino est une bombe, repondant en un flash. Les gains du casino arrivent a la vitesse lumiere, quand meme des recompenses de casino en plus ca ferait kiffer. Pour resumer, Instant Casino c’est un casino de ouf a tester direct pour les accros aux sensations de casino ! Cote plus la navigation du casino est simple comme un jeu d’enfant, booste l’immersion dans le casino a fond.
instant bank transfer casino usa|
как безопасно накрутить подписчиков в телеграм
The Real Person!
The Real Person!
J’apprecie beaucoup le casino TonyBet, ca ressemble a un univers de jeu unique. La selection de machines est vaste, offrant des options de casino en direct. Le personnel est tres competent, repondant rapidement. Les paiements sont fluides, neanmoins il pourrait y avoir plus de promos. En resume, TonyBet est une plateforme fiable pour les joueurs passionnes ! Ajoutons que, l’interface est fluide, renforcant le plaisir de jouer.
tonybet kazino|
The Real Person!
The Real Person!
J’adore a fond le casino AllySpin, ca donne une sensation de casino unique. Les titres proposes sont nombreux, avec des machines a sous captivantes. Le support est ultra-reactif, avec des reponses precises et utiles. Les gains arrivent vite, mais parfois plus de tours gratuits seraient top. En resume, AllySpin est une plateforme de choix pour les fans de divertissement numerique ! Ajoutons que la navigation est fluide, facilitant chaque session.
allyspin casino en ligne|
The Real Person!
The Real Person!
Estou pirando total com LeaoWin Casino, tem uma vibe de jogo que e pura selva. Tem uma enxurrada de jogos de cassino irados, com slots de cassino unicos e eletrizantes. O servico do cassino e confiavel e brabo, dando solucoes na hora e com precisao. O processo do cassino e limpo e sem emboscada, mas mais recompensas no cassino seriam um diferencial brabo. Em resumo, LeaoWin Casino e um cassino online que e uma fera total para quem curte apostar com garra no cassino! E mais a interface do cassino e fluida e cheia de energia selvagem, da um toque de ferocidade braba ao cassino.
casino val andre – partouche|
The Real Person!
The Real Person!
Je suis fou de Julius Casino, c’est un casino en ligne qui rugit comme un lion. La collection de jeux du casino est colossale, incluant des jeux de table de casino d’une noblesse rare. Le personnel du casino offre un accompagnement imperial, avec une aide qui commande le respect. Les gains du casino arrivent a une vitesse triomphale, parfois des bonus de casino plus frequents seraient glorieux. Dans l’ensemble, Julius Casino offre une experience de casino legendaire pour les passionnes de casinos en ligne ! A noter l’interface du casino est fluide et majestueuse comme un palais, donne envie de replonger dans le casino sans fin.
photos de julius casino|
The Real Person!
The Real Person!
Je suis totalement conquis par le casino AllySpin, ca offre une energie de jeu incroyable. Il y a une quantite impressionnante de jeux, offrant des jeux de table authentiques. Les agents sont toujours disponibles, offrant des solutions rapides. Les retraits sont super rapides, mais parfois plus de tours gratuits seraient top. En resume, AllySpin est un incontournable pour les joueurs en quete d’adrenaline ! Par ailleurs le style visuel est dynamique, renforcant l’immersion.
allyspin casino|
The Real Person!
The Real Person!
Estou completamente enlouquecido por LeaoWin Casino, da uma energia de cassino que e indomavel. O catalogo de jogos do cassino e uma selva braba, com caca-niqueis de cassino modernos e instintivos. O atendimento ao cliente do cassino e uma fera domada, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, de vez em quando mais giros gratis no cassino seria uma loucura. Em resumo, LeaoWin Casino e um cassino online que e uma fera total para os aventureiros do cassino! De bonus o design do cassino e uma explosao visual feroz, aumenta a imersao no cassino a mil.
leaowin02 casino betrug|
накрутка подписчиков тг канале бесплатно без отписок
cocaine prague telegram cocaine prague
vhq cocaine in prague columbian cocain in prague
накрутка премиум подписчиков тг
онлайн накрутка подписчиков в тг канал
The Real Person!
The Real Person!
J’adore a fond 7BitCasino, il offre une sensation de casino unique. La gamme de jeux est tout simplement impressionnante, avec des machines a sous modernes et captivantes. Le service d’assistance est de premier ordre, offrant des reponses rapides et precises. Les retraits sont ultra-rapides, neanmoins plus de tours gratuits seraient un atout, comme des offres de cashback plus avantageuses. Pour conclure, 7BitCasino offre une experience de jeu securisee et equitable pour les amateurs de casino en ligne ! Notons egalement que le site est concu avec style et modernite, renforce l’immersion totale.
promo code 7bitcasino|
The Real Person!
The Real Person!
J’apprecie enormement BetFury Casino, c’est une veritable energie de jeu irresistible. Les options de jeu sont riches et diversifiees, offrant des sessions de casino en direct immersives. Le support est ultra-reactif et disponible 24/7, offrant des reponses claires et rapides. Le processus de retrait est simple et fiable, de temps en temps ??? j’aimerais plus de promotions frequentes. En fin de compte, BetFury Casino vaut pleinement le detour pour les adeptes de divertissement innovant ! En bonus l’interface est fluide et intuitive, amplifie le plaisir de jouer.
betfury giris|
The Real Person!
The Real Person!
Je kiffe grave Gamdom, on dirait une explosion de fun. La gamme est une vraie pepite, proposant des sessions live qui tabassent. L’assistance est au top du top, joignable par chat ou email. Les paiements sont fluides et blindes, quand meme des bonus plus reguliers ce serait la classe. En gros, Gamdom offre une experience de ouf pour les fans de casinos en ligne ! Bonus le site est une tuerie graphique, facilite le delire total.
bonus gamdom|
The Real Person!
The Real Person!
tripscan зеркало Трип скан: “Трип скан” – это общее понятие, которое может относиться к процессу сканирования или анализа информации, связанной с путешествиями (трипами). В контексте туристических сервисов, “трип скан” может означать поиск и сравнение цен на авиабилеты, отели, туры и другие услуги для путешественников. Эта фраза также может использоваться для обозначения инструментов и технологий, которые помогают пользователям планировать и организовывать свои поездки, например, онлайн-калькуляторы маршрутов, сервисы для бронирования билетов и отелей, а также приложения для составления списков необходимых вещей. В некоторых случаях, “трип скан” может также относиться к процессу проверки документов и виз перед поездкой.
The Real Person!
The Real Person!
Ich liebe die Pracht von King Billy Casino, es fuhlt sich an wie ein koniglicher Triumph. Die Auswahl im Casino ist ein wahres Kronungsjuwel, mit modernen Casino-Slots, die verzaubern. Der Casino-Support ist rund um die Uhr verfugbar, mit Hilfe, die wie ein Thron majestatisch ist. Casino-Transaktionen sind simpel wie ein koniglicher Befehl, trotzdem die Casino-Angebote konnten gro?zugiger sein. Am Ende ist King Billy Casino ein Online-Casino, das wie ein Konigreich strahlt fur die, die mit Stil im Casino wetten! Ubrigens die Casino-Plattform hat einen Look, der wie ein Kronungsmantel glanzt, das Casino-Erlebnis total veredelt.
king billy casino review australia|
The Real Person!
The Real Person!
Je suis absolument conquis par DBosses, il propose une experience de jeu intense. Il y a une multitude de titres captivants, incluant des jeux de table sophistiques. Le personnel offre un suivi irreprochable, garantissant un support immediat. Les paiements sont securises et fiables, bien que j’aimerais plus de promotions variees. Pour conclure, DBosses garantit un divertissement de haut niveau pour les passionnes de sensations fortes ! Notons aussi l’interface est fluide et moderne, renforce l’envie de revenir.
dbosses casino bonus|
The Real Person!
The Real Person!
подшипники оптом от производителей Купить подшипники оптом: Этот запрос подразумевает намерение приобрести подшипники в больших количествах, обычно с целью получения скидок и более выгодных условий поставки. Оптовые закупки подшипников обычно осуществляются производственными предприятиями, сервисными центрами, ремонтными мастерскими или крупными дистрибьюторами. При оптовой закупке подшипников необходимо учитывать не только цену за единицу продукции, но и такие факторы, как условия оплаты, сроки поставки, гарантии качества, возможность возврата или обмена бракованной продукции, а также репутацию поставщика. Целесообразно заключать долгосрочные договоры с надежными поставщиками, чтобы обеспечить стабильные поставки качественных подшипников по конкурентоспособным ценам. Также важно учитывать возможность предоставления технической поддержки и консультаций со стороны поставщика.
щетки
Услуги таможенного брокера в Москве — это удобный и надежный способ оформить груз без лишних проблем: https://tamozhenniiy-broker11.ru/
The Real Person!
The Real Person!
перманентные брови севастополь Оформление бровей Севастополь – это искусство, которое требует знаний и опыта! Наши мастера создадут для вас уникальный образ, учитывая все ваши пожелания и особенности лица. Мы используем различные техники оформления бровей: архитектура бровей, микроблейдинг, пудровое напыление. Запишитесь на консультацию, и мы поможем вам выбрать идеальный вариант!
The Real Person!
The Real Person!
Je suis accro a CasinoClic, il propose une aventure de casino qui fait vibrer. La selection du casino est une explosion de plaisirs, proposant des slots de casino a theme audacieux. Les agents du casino sont rapides comme un eclair, avec une aide qui fait des etincelles. Les transactions du casino sont simples comme une etincelle, cependant des recompenses de casino supplementaires feraient vibrer. Globalement, CasinoClic promet un divertissement de casino electrisant pour les joueurs qui aiment parier avec panache au casino ! De surcroit le site du casino est une merveille graphique brillante, facilite une experience de casino vibrante.
avis casino clic|
https://www.restate.ru/
The Real Person!
The Real Person!
Je suis fou de CasinoClic, c’est un casino en ligne qui petille d’energie. Les choix de jeux au casino sont riches et scintillants, proposant des slots de casino a theme audacieux. Le personnel du casino offre un accompagnement scintillant, assurant un support de casino immediat et eclatant. Les retraits au casino sont rapides comme une fusee, cependant des bonus de casino plus frequents seraient explosifs. Globalement, CasinoClic c’est un casino a decouvrir en urgence pour les joueurs qui aiment parier avec panache au casino ! Bonus la plateforme du casino brille par son style eblouissant, amplifie l’immersion totale dans le casino.
casino clic en ligne|
сколько стоит плоская крыша https://ploskaya-krovlya-pod-klyuch.ru
I imagine so. Very good stuff, I agree totally.
накрутка подписчиков в телеграм канал
накрутка подписчиков в тг бесплатно за задания
The Real Person!
The Real Person!
Acho simplesmente insano JabiBet Casino, da uma energia de cassino que e uma mare alta. A gama do cassino e simplesmente um maremoto, com slots de cassino unicos e empolgantes. A equipe do cassino entrega um atendimento que e uma perola, respondendo mais rapido que um maremoto. As transacoes do cassino sao simples como uma brisa, mas as ofertas do cassino podiam ser mais generosas. No fim das contas, JabiBet Casino oferece uma experiencia de cassino que e puro fluxo para os aventureiros do cassino! Alem disso a interface do cassino e fluida e cheia de energia oceanica, aumenta a imersao no cassino como uma onda gigante.
jabibet bangladesh|
Ich bin total begeistert von Platin Casino, es bietet ein Casino-Abenteuer, das wie ein Schatz funkelt. Es gibt eine Flut an packenden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Service ist zuverlassig und prazise, antwortet blitzschnell wie ein geschliffener Diamant. Casino-Transaktionen sind simpel wie ein Edelstein, dennoch wurde ich mir mehr Casino-Promos wunschen, die wie Gold glanzen. Zusammengefasst ist Platin Casino ein Casino mit einem Spielspa?, der wie ein Edelstein funkelt fur die, die mit Stil im Casino wetten! Nebenbei die Casino-Navigation ist kinderleicht wie ein Funkeln, was jede Casino-Session noch glanzvoller macht.
platin casino bonusbedingungen|
The Real Person!
The Real Person!
Estou completamente alucinado por JabiBet Casino, tem uma vibe de jogo que e puro tsunami. Os titulos do cassino sao um espetaculo a parte, oferecendo sessoes de cassino ao vivo que sao uma explosao. A equipe do cassino entrega um atendimento que e uma perola, acessivel por chat ou e-mail. As transacoes do cassino sao simples como uma brisa, mesmo assim as ofertas do cassino podiam ser mais generosas. Em resumo, JabiBet Casino e o point perfeito pros fas de cassino para os cacadores de slots modernos de cassino! E mais a plataforma do cassino detona com um visual que e puro mar, o que deixa cada sessao de cassino ainda mais alucinante.
jabibet app download|
The Real Person!
The Real Person!
Je trouve absolument grandiose LeoVegas Casino, on dirait une tempete de fun majestueux. Le repertoire du casino est un palais de divertissement, comprenant des jeux de casino optimises pour les cryptomonnaies. Les agents du casino sont rapides comme un decret royal, proposant des solutions claires et immediates. Les paiements du casino sont securises et fluides, mais des bonus de casino plus frequents seraient royaux. En somme, LeoVegas Casino promet un divertissement de casino royal pour les joueurs qui aiment parier avec panache au casino ! A noter le design du casino est une fresque visuelle royale, ce qui rend chaque session de casino encore plus majestueuse.
leovegas sports review|
Портал про авто https://ivanmotors.ru обзоры автомобилей, новости автопрома, советы по ремонту и обслуживанию. Тест-драйвы, автообзоры и полезная информация для автолюбителей и профессионалов.
Всё о Москве https://moscowfy.ru в одном месте: городской портал с новостями, афишей, расписанием транспорта, объявлениями и услугами. Полезные материалы для москвичей и туристов.
накрутка подписчиков в телеграм купить
Been 18+ xxx checking out keyword lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more than I can say for some other places I’ve tried.
Been checking out keyword lately and it’s actually not bad. Their game selection 18+ xxx is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more than I can say for some other places I’ve tried.
Портал о строительстве https://e-proficom.ru и ремонте: полезные статьи, советы специалистов, обзоры материалов и технологий. Всё для тех, кто планирует ремонт квартиры, дома или дачи.
Женский портал https://devchenky.ru секреты красоты, модные тенденции, здоровье, любовь и кулинария. Актуальные статьи, тесты и советы для женщин, которые ценят себя и своё время.
The Real Person!
The Real Person!
J’adore la feerie de Luckland Casino, ca pulse avec une energie de casino enchanteresse. Le repertoire du casino est une constellation de divertissement, comprenant des jeux de casino optimises pour les cryptomonnaies. Le personnel du casino offre un accompagnement enchante, repondant en un eclair magique. Les transactions du casino sont simples comme un charme, par moments des recompenses de casino supplementaires feraient rever. Dans l’ensemble, Luckland Casino offre une experience de casino enchantee pour ceux qui cherchent l’adrenaline chanceuse du casino ! De surcroit la plateforme du casino brille par son style ensorcelant, ce qui rend chaque session de casino encore plus magique.
luckland free bets|
Your thing regarding creating will be practically nothing in short supply of awesome. This informative article is incredibly useful and contains offered myself a better solution to be able to my own issues. Which can be the specific purpose MY PARTNER AND I has been doing a search online. I am advocating this informative article with a good friend. I know they are going to get the write-up since beneficial as i would. Yet again many thanks.
The Real Person!
The Real Person!
Je suis fou de MonteCryptos Casino, c’est un casino en ligne qui grimpe comme un sommet enneige. La selection du casino est une ascension de plaisirs, comprenant des jeux de casino optimises pour les cryptomonnaies. Le personnel du casino offre un accompagnement digne d’un sherpa, repondant en un eclair glacial. Les paiements du casino sont securises et fluides, quand meme plus de tours gratuits au casino ce serait exaltant. En somme, MonteCryptos Casino c’est un casino a conquerir en urgence pour les passionnes de casinos en ligne ! De surcroit la navigation du casino est intuitive comme un sentier de montagne, facilite une experience de casino vertigineuse.
montecryptos contact|
The Real Person!
The Real Person!
Je suis accro a Luckland Casino, c’est un casino en ligne qui scintille comme un trefle a quatre feuilles. Le repertoire du casino est une constellation de divertissement, avec des machines a sous de casino modernes et envoutantes. Le support du casino est disponible 24/7, joignable par chat ou email. Les retraits au casino sont rapides comme un coup de chance, parfois plus de tours gratuits au casino ce serait ensorcelant. En somme, Luckland Casino c’est un casino a decouvrir en urgence pour ceux qui cherchent l’adrenaline chanceuse du casino ! De surcroit le site du casino est une merveille graphique eclatante, amplifie l’immersion totale dans le casino.
luckland casino kokemuksia|
The Real Person!
The Real Person!
J’adore l’eclat de Lucky8 Casino, on dirait une fontaine de chance. Les choix de jeux au casino sont riches et eclatants, proposant des slots de casino a theme feerique. Le service client du casino est une etoile porte-bonheur, proposant des solutions claires et instantanees. Le processus du casino est transparent et sans malefice, quand meme j’aimerais plus de promotions de casino qui eblouissent. Globalement, Lucky8 Casino est un joyau pour les fans de casino pour les passionnes de casinos en ligne ! Par ailleurs le design du casino est une explosion visuelle feerique, ajoute une touche de magie au casino.
lucky8 canada|
Weboldalunk, a joszaki.hu buszken tamogatja a kormanypartot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
Строительные материалы https://stroy-marketplace.ru в Серпухове: кирпич, цемент, сухие смеси, пиломатериалы и утеплители. Большой выбор для ремонта и строительства, доставка по городу и району.
The Real Person!
The Real Person!
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Shining crown lines müxtəlif kombinasiyalar təqdim edir.
Shining crown free slot risksiz məşq üçün möhtəşəmdir. Shining crown demo superbet variantı real casino təcrübəsinə yaxındır. Shining crown online casino real pulla oynamaq imkanı verir.
Shining crown pacanele Rumıniya bazarında liderdir.
Shining crown free play risksiz məşq şansı yaradır.
Daha ətraflı buradan baxın shining crown demo.
Shining crown jackpot həyəcan dolu anlar yaşadır.
Shining crown casino hər kəs üçün etibarlı platformadır.
Компания Единство https://edinvent.ru производитель и комплексный поставщик приточно-вытяжных установок, вентиляционных установок, а также систем автоматического управления климатическим оборудованием.
Женский блог https://www.evasolar.ru о здоровье и красоте: советы по уходу за собой, правильному питанию, фитнесу и косметике. Полезные статьи, лайфхаки и вдохновение для гармоничной и счастливой жизни.
The Real Person!
The Real Person!
J’adore l’univers de Casinova, ca offre une experience inoubliable. Il y a une variete impressionnante de titres, comprenant des options pour les cryptomonnaies. Le personnel assure un suivi exemplaire, garantissant une aide instantanee. Les transactions sont simples et efficaces, par moments des recompenses supplementaires seraient appreciees. Pour conclure, Casinova assure une experience de jeu memorable pour les fans de jeux modernes ! Par ailleurs l’interface est fluide et moderne, amplifie l’experience de jeu.
casinova casino login|
The Real Person!
The Real Person!
Je suis fou de Madnix Casino, ca degage une vibe de jeu completement demente. La selection du casino est une veritable explosion de fun, offrant des sessions de casino en direct qui font peter les plombs. Le service client du casino est une decharge electrique, joignable par chat ou email. Les retraits au casino sont rapides comme une fusee, parfois des bonus de casino plus frequents seraient dements. Globalement, Madnix Casino est une pepite pour les fans de casino pour les passionnes de casinos en ligne ! De surcroit la plateforme du casino brille par son style completement fou, ajoute une touche de folie au casino.
madnix casino roulette|
The Real Person!
The Real Person!
Je suis accro a LuckyBlock Casino, il propose une aventure de casino qui explose de chance. Le repertoire du casino est une galaxie de divertissement, proposant des slots de casino a theme chanceux. Le service client du casino est une etoile porte-bonheur, joignable par chat ou email. Les retraits au casino sont rapides comme un coup de chance, mais des recompenses de casino supplementaires feraient rever. Dans l’ensemble, LuckyBlock Casino promet un divertissement de casino scintillant pour les amoureux des slots modernes de casino ! A noter la navigation du casino est intuitive comme un sortilege, amplifie l’immersion totale dans le casino.
luckyblock 1.7.10|
Je suis totalement envoute par Lucky31 Casino, c’est un casino en ligne qui brille comme un porte-bonheur. Le repertoire du casino est un tourbillon de divertissement, offrant des sessions de casino en direct qui eblouissent. L’assistance du casino est chaleureuse et irreprochable, assurant un support de casino immediat et eclatant. Les transactions du casino sont simples comme un charme, mais j’aimerais plus de promotions de casino qui eblouissent. Globalement, Lucky31 Casino promet un divertissement de casino scintillant pour ceux qui cherchent l’adrenaline lumineuse du casino ! En plus le site du casino est une merveille graphique eclatante, facilite une experience de casino feerique.
lucky31 bonus sans dГ©pГґt|
The Real Person!
The Real Person!
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Shining crown slot game yüksək keyfiyyətli qrafika ilə hazırlanıb.
EGT digital shining crown müasir interfeys ilə seçilir. Shining crown casino etibarlı oyun mühitinə zəmanət verir. Pinco casino shining crown üçün ən yaxşı platformadır.
Shining crown jackpot oyunçuların əsas hədəfidir.
Shining crown demo superbet sürətli işləyir.
Burada pulsuz sınaqdan keçin play shining crown.
Shining crown slot game yüksək qrafika və səs effektləri ilə zəngindir.
Shining crown casino hər kəs üçün etibarlı platformadır.
Курсы по заработку онлайн
накрутка подписчиков в тг канал без отписок
The Real Person!
The Real Person!
терміновий ремонт холодильників вдома
The Real Person!
The Real Person!
Play shining crown oyunu ilə böyük həyəcan yaşamaq mümkündür.
Shining crown buy bonus funksiyası əlavə qazanclar verir.
Shining crown 40 slotları klassik kombinasiya təklif edir. Shining crown free tamamilə risksizdir. Shining crown online casino real pulla oynamaq imkanı verir.
Shining crown joc gratis təcrübə qazandırır.
Shining crown bell link demo tez-tez istifadə olunur.
Tam məlumat əldə et ətraflı bax.
40 shining crown klassik və sadə oyun mexanikası təqdim edir.
Shining crown pacanele oyunçular arasında çox populyardır.
The Real Person!
The Real Person!
продвижение в социальных сетях Услуги PR (Public Relations) Услуги PR (Public Relations) – это комплекс мероприятий, направленных на формирование и поддержание позитивного имиджа компании, продукта или личности в глазах общественности. PR – это не только работа со СМИ, но и взаимодействие с целевой аудиторией, инвесторами, партнерами и другими заинтересованными сторонами. Основные задачи PR: Формирование позитивного имиджа: Создание благоприятного впечатления о компании, продукте или личности. Повышение узнаваемости бренда: Увеличение осведомленности о компании и ее продуктах. Управление репутацией: Реагирование на негативные отзывы и предотвращение кризисных ситуаций. Установление доверительных отношений: Создание прочных связей с целевой аудиторией, инвесторами и партнерами. Поддержка маркетинговых кампаний: Усиление эффекта от рекламных кампаний за счет PR-активностей. Основные инструменты PR: Пресс-релизы: Распространение информации о компании, продуктах или событиях в СМИ. Пресс-конференции: Организация мероприятий для общения с журналистами и ответов на их вопросы. Интервью: Предоставление интервью представителям СМИ для распространения информации о компании. Публикации: Размещение статей и других материалов о компании в СМИ. Мероприятия: Организация и участие в мероприятиях для повышения узнаваемости бренда и установления контакта с целевой аудиторией. Социальные сети: Ведение страниц в социальных сетях для общения с целевой аудиторией и распространения информации о компании. Работа с блогерами и лидерами мнений: Привлечение блогеров и лидеров мнений для продвижения компании и ее продуктов. Инвестиции в PR – это инвестиции в репутацию вашей ко
Arcade website https://baji-bj.com
The Real Person!
The Real Person!
https://konselgroup.kz/ Модуль газового пожаротушения V Не существует общепринятого стандарта или типа модулей газового пожаротушения, обозначаемых просто как “Модуль газового пожаротушения V”. Вероятнее всего, “V” является частью маркировки конкретного производителя, модели или серии оборудования. Чтобы получить точную информацию о характеристиках и применении такого модуля, необходимо знать его производителя и полную маркировку. Однако, в общем смысле, модуль газового пожаротушения (МГП) – это автономное устройство, предназначенное для хранения и автоматического выпуска газового огнетушащего вещества (ГОТВ) при возникновении пожара. Основные характеристики, которые следует учитывать при выборе МГП (независимо от наличия буквы “V” в обозначении): Тип ГОТВ: Инертные газы (азот, аргон, углекислый газ) или химические газы (хладоны). Выбор зависит от типа защищаемого оборудования, наличия людей в помещении и экологических требований. Объем и давление: Определяют площадь и объем помещения, которое может быть защищено данным модулем. Тип запорно-пускового устройства (ЗПУ): Электромагнитное, пневматическое или механическое. Определяет скорость срабатывания и надежность модуля. Тип распылителя: Обеспечивает равномерное распределение ГОТВ по защищаемому объему. Условия эксплуатации: Температурный диапазон, влажность и другие факторы, влияющие на работоспособность модуля. Сертификация: Наличие сертификатов соответствия требованиям пожарной безопасности. Рекомендации: Уточните полную маркировку требуемого модуля газового пожаротушения, включая производителя и модель. Обратитесь к документации производителя для получения подробной информации о технических характеристиках, области применения и правилах эксплуатации данного модуля. Проконсультируйтесь со специалистами в области пожарной безопасности для подбора оптимального решения, соответствующего требованиям вашего объекта.
Монтаж освітлення виконується спеціалістами remontuem.te.ua
The Real Person!
The Real Person!
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Shining crown joc gratis pulsuz təcrübə üçün əladır.
Superbet demo shining crown ilə oyun pulsuzdur. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown apk mobil cihazlarda problemsiz işləyir.
Play shining crown asan interfeys ilə təklif olunur.
Shining crown demo superbet sürətli işləyir.
Bu linkdən oyuna keçid edin shining crown slot game.
Pinco casino shining crown üçün çox seçim təqdim edir.
Shining crown lines qazanma yollarını artırır.
The Real Person!
The Real Person!
40 shining crown bell link çox sevilən versiyadır.
Shining crown lines müxtəlif kombinasiyalar təqdim edir.
Shining crown nasıl oynanır sualına cavab çox sadədir. Shining crown amusnet çoxlu oyun variantı ilə tanınır. Shining crown online casino real pulla oynamaq imkanı verir.
Shining crown pacanele Rumıniya bazarında liderdir.
Shining crown free slot real uduş öncəsi sınamağa imkan verir.
Tam məlumat əldə et ətraflı bax.
Shining crown jackpot həyəcan dolu anlar yaşadır.
Shining crown pacanele oyunçular arasında çox populyardır.
как безопасно накрутить подписчиков в телеграм
сколько стоит подписчик в тг канал
Descopera joaca World of Tanks – simulator de razboi cu tancuri istorice. Lupte dinamice, har?i variate ?i batalii tactice. Creeaza-?i strategia ?i domina impreuna cu prietenii tai.
Joaca World of Warships gratuit! Exploreaza marile, folose?te-?i strategia ?i condu nave de razboi celebre. Batalii realiste ?i echipe interna?ionale te a?teapta.
The Real Person!
The Real Person!
Mobil cihazlar üçün shining crown apk yükləmək çox rahatdır.
Shining crown pacanele Rumıniyada məşhurdur, Azərbaycanda da sevilir.
Shining crown online oyunu sürətli yüklənir. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown rtp göstəricisi qazanma şansını artırır.
Shining crown demo oyna pulsuz və sürətlidir.
Shining crown bell link demo tez-tez istifadə olunur.
Casino üçün keçid online casino link.
Shining crown jackpot həyəcan dolu anlar yaşadır.
Shining crown pacanele oyunçular arasında çox populyardır.
Descubra o caca-niqueis 9masksoffire.club – um jogo cheio de adrenalina, simbolos especiais e rodadas bonus. Experimente graficos intensos e altas chances de ganhar em um caca-niqueis classico moderno.
Jogue big bamboo e descubra o charme do Oriente! Rolos coloridos, recursos bonus, rodadas gratis e surpresas que trazem ganhos espetaculares.
The Real Person!
The Real Person!
Ich finde absolut uberwaltigend Platin Casino, es pulsiert mit einer luxuriosen Casino-Energie. Die Casino-Optionen sind vielfaltig und glanzvoll, inklusive eleganter Casino-Tischspiele. Die Casino-Mitarbeiter sind schnell wie ein Lichtstrahl, liefert klare und schnelle Losungen. Auszahlungen im Casino sind schnell wie ein Funkeln, aber die Casino-Angebote konnten gro?zugiger sein. Kurz gesagt ist Platin Casino eine Casino-Erfahrung, die wie Platin glanzt fur die, die mit Stil im Casino wetten! Extra das Casino-Design ist ein optischer Schatz, was jede Casino-Session noch glanzvoller macht.
platin casino angebote|
The Real Person!
The Real Person!
Acho simplesmente estelar BetorSpin Casino, da uma energia de cassino que e puro pulsar galactico. Tem uma chuva de meteoros de jogos de cassino irados, com jogos de cassino perfeitos pra criptomoedas. A equipe do cassino entrega um atendimento que e puro cometa, com uma ajuda que reluz como uma aurora boreal. O processo do cassino e limpo e sem turbulencia cosmica, as vezes mais giros gratis no cassino seria uma loucura estelar. No fim das contas, BetorSpin Casino vale demais explorar esse cassino para os astronautas do cassino! Alem disso o site do cassino e uma obra-prima de estilo estelar, eleva a imersao no cassino a um nivel cosmico.
betorspin casino reseГ±a|
Kingpin Crown in Australia is a premium entertainment venue offering bowling, laser tag, arcade games, karaoke, and dining: Kingpin Crown support and help
The Real Person!
The Real Person!
Fresh’s Sugar Lip Treatment Sugar Citrus Rush is an innovative lip balm that offers up to 24 hours of moisture. The revitalising formula aims to deeply nourish and soften the lips, while working to provide a long-lasting rush of freshness. The Fresh Sugar Mint Rush Freshening Lip Treatment is an ultra-moisturising way to reenergise your pout. *95% felt an immediate burst of minty freshness. *95% felt an immediate burst of minty freshness. Enriched with innovative time-release technologies, the balm is designed to offer a revitalising minty freshness that can last up to 24 hours.* The rush of mint is activated when lips are pressed together, helping to release the expert cooling action. RRP: 24.45€ RRP: 24.45€ Enriched with innovative time-release technologies, the balm is designed to offer a revitalising minty freshness that can last up to 24 hours.* The rush of mint is activated when lips are pressed together, helping to release the expert cooling action.
https://trisvetiteli.com/%ce%b7-%ce%b1%cf%80%cf%8c%ce%bb%cf%85%cf%84%ce%b7-%ce%b5%cf%80%ce%b9%cf%83%ce%ba%cf%8c%cf%80%ce%b7%cf%83%ce%b7-%cf%84%ce%b7%cf%82-ivibet-%ce%b3%ce%b9%ce%b1-%cf%80%ce%b1%ce%af%ce%ba%cf%84%ce%b5%cf%82/
Η μουσική υπόκρουση είναι χαρούμενη και ταιριάζει απόλυτα με το γλυκό θέμα του παιχνιδιού. Οι ήχοι των κερδών και των ειδικών λειτουργιών ενισχύουν την ένταση και την αίσθηση επιβράβευσης. Η συνολική ηχητική εμπειρία είναι ευχάριστη και δεν κουράζει, διατηρώντας το ενδιαφέρον του παίκτη. Αν θέλετε να δοκιμάσετε το Sugar Rush 1000 χωρίς ρίσκο, τότε η Sugar Rush 1000 Demo έκδοση είναι η ιδανική επιλογή. Σας επιτρέπει να εξερευνήσετε το παιχνίδι, να εξοικειωθείτε με τα σύμβολα, τις λειτουργίες και τις δωρεάν περιστροφές, χωρίς να ξοδέψετε ούτε ένα ευρώ.
The Real Person!
The Real Person!
Ich bin suchtig nach Platin Casino, es fuhlt sich an wie ein glitzernder Gewinnrausch. Die Auswahl im Casino ist ein echtes Meisterwerk, mit modernen Casino-Slots, die einen in ihren Bann ziehen. Der Casino-Support ist rund um die Uhr verfugbar, liefert klare und schnelle Losungen. Auszahlungen im Casino sind schnell wie ein Funkeln, ab und zu die Casino-Angebote konnten gro?zugiger sein. Alles in allem ist Platin Casino ein Casino, das man nicht verpassen darf fur Fans von Online-Casinos! Und au?erdem das Casino-Design ist ein optischer Schatz, das Casino-Erlebnis total luxurios macht.
platin casino gratis|
The Real Person!
The Real Person!
Sou louco pelo role de MegaPosta Casino, da uma energia de cassino que e um vulcao. A gama do cassino e simplesmente um estouro, com slots de cassino unicos e contagiantes. O suporte do cassino ta sempre na ativa 24/7, respondendo mais rapido que um raio. Os saques no cassino sao velozes como uma tempestade, mesmo assim queria mais promocoes de cassino que explodem. No geral, MegaPosta Casino e o point perfeito pros fas de cassino para os viciados em emocoes de cassino! Alem disso a interface do cassino e fluida e cheia de energia explosiva, aumenta a imersao no cassino a mil.
plataforma megaposta|
ваш провідник у житті Львова https://79000.com.ua актуальні новини, культурні та громадські події міста, урбаністика, інтерв’ю з цікавими людьми, фотоогляди локальних заходів. Все про те, що формує атмосферу Львова сьогодні — оновлення, проекти, історії.
інформаційний портал https://21000.com.ua Вінниці і області: місцеві новини, анонси культурних, спортивних та громадських подій, репортажі з місця подій, інтерв’ю з вінничанами. Все про те, що відбувається у Вінниці — ближче, живіше, щодня.
ООО “Мир ремней” https://yandex.ru/profile/147633783627 Производство приводных ремней, тефлоновых сеток и лент – телефон +7 (936) 333-93-03
Пиломатериалы в Минске https://farbwood.by оптом и в розницу. Доска обрезная и строганая, брус, лаги, террасная доска. Качественная древесина для строительства и ремонта. Быстрая доставка.
The Real Person!
The Real Person!
контент план для бизнеса Услуги интернет-маркетолога В современном цифровом мире, где онлайн-присутствие играет ключевую роль в успехе любого бизнеса, услуги опытного интернет-маркетолога становятся необходимостью. Я предлагаю полный спектр услуг, направленных на увеличение видимости вашего бренда, привлечение целевой аудитории и, как следствие, повышение продаж. Мои услуги включают: Анализ рынка и конкурентов: Тщательный анализ позволит определить оптимальные стратегии продвижения, выявить сильные и слабые стороны конкурентов и определить целевую аудиторию. Разработка маркетинговой стратегии: Создание индивидуальной стратегии, учитывающей особенности вашего бизнеса, цели и бюджет. SEO-оптимизация: Повышение позиций вашего сайта в поисковых системах, что обеспечит приток органического трафика. Контекстная реклама: Настройка и ведение рекламных кампаний в Яндекс.Директ и Google Ads для быстрого привлечения целевых клиентов. SMM-продвижение: Разработка и реализация стратегии продвижения в социальных сетях, создание контента, привлечение подписчиков и повышение вовлеченности аудитории. Контент-маркетинг: Создание полезного и интересного контента для привлечения и удержания аудитории, повышения лояльности к бренду. Email-маркетинг: Разработка стратегии email-рассылок, создание писем и автоматизация процессов для поддержания связи с клиентами и стимулирования продаж. Аналитика и отчетность: Регулярный анализ результатов и предоставление отчетов о проделанной работе, что позволит оценить эффективность стратегии и внести необходимые корректировки. Я готов предложить вам комплексные решения, которые помогут вашему бизнесу достичь новых высот в онлайн-пространстве. Свяжитесь со мной, чтобы обсудить ваши задачи и разработать индивидуальный план продвижения.
The Real Person!
The Real Person!
Ich finde absolut verruckt PlayJango Casino, es bietet ein Casino-Abenteuer, das wie ein Regenbogen funkelt. Die Spielauswahl im Casino ist wie ein funkelnder Ozean, mit Live-Casino-Sessions, die wie ein Gewitter knistern. Die Casino-Mitarbeiter sind schnell wie ein Blitzstrahl, ist per Chat oder E-Mail erreichbar. Auszahlungen im Casino sind schnell wie ein Sturm, dennoch mehr Freispiele im Casino waren ein Volltreffer. Alles in allem ist PlayJango Casino ein Online-Casino, das wie ein Orkan begeistert fur die, die mit Stil im Casino wetten! Ubrigens das Casino-Design ist ein optisches Spektakel, was jede Casino-Session noch aufregender macht.
playjango casino bonus ohne einzahlung|
https://3dlx.ru/
The Real Person!
The Real Person!
Sou viciado no role de MonsterWin Casino, da uma energia de cassino que e indomavel. Os titulos do cassino sao um espetaculo selvagem, incluindo jogos de mesa de cassino cheios de garra. O suporte do cassino ta sempre na ativa 24/7, acessivel por chat ou e-mail. O processo do cassino e limpo e sem emboscada, mas mais giros gratis no cassino seria uma loucura. No geral, MonsterWin Casino e um cassino online que e uma fera total para quem curte apostar com garra no cassino! E mais a interface do cassino e fluida e cheia de energia selvagem, aumenta a imersao no cassino a mil.
casino monsterwin|
The Real Person!
The Real Person!
Je suis fou de PokerStars Casino, il propose une aventure de casino qui scintille comme un tapis vert. La selection du casino est une constellation de delices, comprenant des jeux de casino adaptes aux cryptomonnaies. Le service client du casino est une carte maitresse, avec une aide qui mise juste. Les paiements du casino sont securises et fluides, mais les offres du casino pourraient etre plus genereuses. Au final, PokerStars Casino c’est un casino a rejoindre sans hesiter pour les joueurs qui aiment parier avec flair au casino ! Par ailleurs l’interface du casino est fluide et eclatante comme un tapis de jeu, ce qui rend chaque session de casino encore plus palpitante.
pokerstars romania|
I like the helpful information you provide in your articles. I’ll bookmark your blog and check again here frequently. I am quite certain I’ll learn many new stuff right here! Best of luck for the next!
The Real Person!
The Real Person!
Ich bin suchtig nach PlayJango Casino, es bietet ein Casino-Abenteuer, das wie ein Regenbogen funkelt. Es gibt eine Woge an packenden Casino-Titeln, mit modernen Casino-Slots, die einen in ihren Bann ziehen. Das Casino-Team bietet Unterstutzung, die wie ein Stern glanzt, antwortet blitzschnell wie ein Donnerschlag. Casino-Gewinne kommen wie ein Komet, ab und zu wurde ich mir mehr Casino-Promos wunschen, die wie ein Vulkan ausbrechen. Insgesamt ist PlayJango Casino ein Casino, das man nicht verpassen darf fur Fans moderner Casino-Slots! Nebenbei die Casino-Seite ist ein grafisches Meisterwerk, Lust macht, immer wieder ins Casino zuruckzukehren.
playjango casino kokemuksia|
The Real Person!
The Real Person!
Shining crown online casino əyləncəsi 24/7 mövcuddur.
Shining crown slot game yüksək keyfiyyətli qrafika ilə hazırlanıb.
Shining crown bell link demo yeni oyunçular üçün idealdır. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown apk mobil cihazlarda problemsiz işləyir.
EGT digital shining crown yüksək keyfiyyətə malikdir.
Shining crown rtp oyunun ədalətini təsdiqləyir.
Sayta birbaşa keçid https://shining-crown.com.az/.
40 shining crown klassik və sadə oyun mexanikası təqdim edir.
Shining crown casino hər kəs üçün etibarlı platformadır.
The Real Person!
The Real Person!
Estou pirando com MonsterWin Casino, da uma energia de cassino que e indomavel. A gama do cassino e simplesmente uma besta, com jogos de cassino perfeitos pra criptomoedas. A equipe do cassino entrega um atendimento que e uma fera, acessivel por chat ou e-mail. O processo do cassino e limpo e sem emboscada, mesmo assim as ofertas do cassino podiam ser mais generosas. No fim das contas, MonsterWin Casino e um cassino online que e uma fera total para os viciados em emocoes de cassino! Vale falar tambem o design do cassino e uma explosao visual feroz, faz voce querer voltar pro cassino como uma fera.
monsterwin casino|
The Real Person!
The Real Person!
J’adore la ferveur de PokerStars Casino, ca vibre avec une energie de casino strategique. La selection du casino est une constellation de delices, incluant des jeux de table de casino d’une elegance tactique. Le service client du casino est une carte maitresse, offrant des solutions claires et immediates. Le processus du casino est transparent et sans mauvaise donne, par moments plus de tours gratuits au casino ce serait un full house. Dans l’ensemble, PokerStars Casino promet un divertissement de casino strategique pour les strateges du casino ! De surcroit le site du casino est une merveille graphique etoilee, ce qui rend chaque session de casino encore plus palpitante.
pokerstars codes|
Crown Metropol Perth is a luxury hotel located near the Swan River. It offers modern rooms, a stunning pool area, fine dining, a casino, and entertainment options: Crown Metropol Perth booking
сайт для накрутки подписчиков в тг
Been checking out keyword lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more than I can aoprojects gambling say for some other places I’ve tried.
Been checking out keyword lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The aoprojects slots site runs smoothly, which is more than I can say for some other places I’ve tried.
накрутка живых подписчиков в тг дешево
займ деньги взять займ
микрозаймы взять микрозайм
Acho simplesmente brabissimo ParamigoBet Casino, oferece uma aventura de cassino que faz tudo voar. A gama do cassino e simplesmente uma ventania, incluindo jogos de mesa de cassino cheios de energia. Os agentes do cassino sao rapidos como um raio, dando solucoes na hora e com precisao. O processo do cassino e limpo e sem tempestades, as vezes queria mais promocoes de cassino que arrasam. Em resumo, ParamigoBet Casino garante uma diversao de cassino que e uma tempestade para os viciados em emocoes de cassino! E mais a navegacao do cassino e facil como uma rajada, da um toque de forca braba ao cassino.
paramigobet petos|
The Real Person!
The Real Person!
Sou louco pela energia de BetPrimeiro Casino, e um cassino online que explode como um vulcao em furia. A selecao de titulos do cassino e um fluxo de lava de prazeres, com caca-niqueis de cassino modernos e inflamados. O suporte do cassino ta sempre na ativa 24/7, garantindo suporte de cassino direto e sem cinzas. Os pagamentos do cassino sao lisos e blindados, porem queria mais promocoes de cassino que explodem como vulcoes. No fim das contas, BetPrimeiro Casino oferece uma experiencia de cassino que e puro magma para os apaixonados por slots modernos de cassino! De bonus o design do cassino e um espetaculo visual escaldante, faz voce querer voltar ao cassino como uma lava sem fim.
betprimeiro sports betting|
The Real Person!
The Real Person!
Je suis accro a Posido Casino, ca vibre avec une energie de casino aquatique. L’eventail de jeux du casino est un ocean de delices, offrant des sessions de casino en direct qui eclaboussent. L’assistance du casino est chaleureuse et limpide, repondant en un eclat d’ecume. Le processus du casino est transparent et sans remous, par moments des bonus de casino plus frequents seraient marins. Pour resumer, Posido Casino est un tresor pour les fans de casino pour ceux qui cherchent l’adrenaline fluide du casino ! Bonus la plateforme du casino brille par son style oceanique, donne envie de replonger dans le casino sans fin.
posido casino logo|
получить микрозайм микрозайм взять
The Real Person!
The Real Person!
Estou completamente alucinado por ParamigoBet Casino, tem uma vibe de jogo que e pura adrenalina. Os titulos do cassino sao um espetaculo avassalador, com jogos de cassino perfeitos pra criptomoedas. O servico do cassino e confiavel e brabo, dando solucoes na hora e com precisao. As transacoes do cassino sao simples como uma brisa, mesmo assim mais bonus regulares no cassino seria top. Na real, ParamigoBet Casino e o point perfeito pros fas de cassino para os cacadores de slots modernos de cassino! De bonus a navegacao do cassino e facil como uma rajada, torna o cassino uma curticao total.
paramigobet erfahrung|
The Real Person!
The Real Person!
Je suis fou de MrPlay Casino, c’est un casino en ligne qui deborde de panache comme un festival. Le catalogue du casino est une explosion de couleurs ludiques, proposant des slots de casino a theme vibrant. Les agents du casino sont rapides comme un numero de cirque, garantissant un support de casino instantane et eclatant. Les paiements du casino sont securises et fluides, parfois des recompenses de casino supplementaires feraient danser. Dans l’ensemble, MrPlay Casino est un casino en ligne qui met la fete dans les jeux pour les amoureux des slots modernes de casino ! De surcroit l’interface du casino est fluide et eclatante comme une parade, ajoute une touche de panache au casino.
mr.play arvostelu|
The Real Person!
The Real Person!
Je suis totalement emporte par Posido Casino, ca vibre avec une energie de casino aquatique. Il y a une maree de jeux de casino captivants, offrant des sessions de casino en direct qui eclaboussent. Les agents du casino sont rapides comme une vague deferlante, assurant un support de casino immediat et fluide. Les paiements du casino sont securises et fluides, parfois des bonus de casino plus frequents seraient marins. Au final, Posido Casino promet un divertissement de casino aquatique pour les amoureux des slots modernes de casino ! A noter l’interface du casino est fluide et eclatante comme une mer turquoise, amplifie l’immersion totale dans le casino.
posido kasiino|
Нужны автостекла? автостекла ремонт трещин лобового стекла Khan Auto Glass — это профессиональная компания, которая специализируется на продаже и установке автомобильных стёкол в Санкт-Петербурге. Мы гордимся тем, что стали надёжным партнёром для множества автовладельцев, которые ценят качество и профессионализм.
https://labrezerv.ru/
Получите скидку – центр переводчиков. Перевод документов для международных молодежных форумов. Программы, заявки, резолюции. Для молодежных организаций.
зварювальні роботи https://remontuem.te.ua
Нужна лабораторная? https://lab-ucheb.ru Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
Нужна презентация? сколько стоит заказать презентацию Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
Нужен чертеж? стоимость чертежей на заказ выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
The Real Person!
The Real Person!
J’adore la houle de Winamax Casino, c’est un casino en ligne qui ondule comme une mer dechainee. Les options de jeu au casino sont riches et fluides, avec des machines a sous de casino modernes et immersives. Le service client du casino est une perle rare, assurant un support de casino immediat et fluide. Les gains du casino arrivent a une vitesse de maree, mais les offres du casino pourraient etre plus genereuses. En somme, Winamax Casino est un casino en ligne qui fait des vagues pour les passionnes de casinos en ligne ! En plus l’interface du casino est fluide et eclatante comme une mer turquoise, donne envie de replonger dans le casino sans fin.
winamax retrait instantanГ©|
The Real Person!
The Real Person!
Ich bin suchtig nach Richard Casino, es ist ein Online-Casino, das wie ein koniglicher Palast strahlt. Es gibt eine Flut an fesselnden Casino-Titeln, mit einzigartigen Casino-Slotmaschinen. Die Casino-Mitarbeiter sind schnell wie ein kaiserlicher Bote, ist per Chat oder E-Mail erreichbar. Der Casino-Prozess ist klar und ohne Intrigen, trotzdem mehr Freispiele im Casino waren ein Kronungsmoment. Am Ende ist Richard Casino eine Casino-Erfahrung, die wie ein Kronungsjuwel glanzt fur Spieler, die auf majestatische Casino-Kicks stehen! Nebenbei das Casino-Design ist ein optisches Kronungsjuwel, einen Hauch von Majestat ins Casino bringt.
richard marcus casino film|
The Real Person!
The Real Person!
Je suis fou de PlazaRoyal Casino, il propose une aventure de casino qui scintille comme un trone. Le repertoire du casino est une salle de bal ludique, incluant des jeux de table de casino d’une elegance princiere. Le personnel du casino offre un accompagnement digne d’une cour, avec une aide qui rayonne comme une couronne. Les paiements du casino sont securises et fluides, cependant des bonus de casino plus frequents seraient royaux. Globalement, PlazaRoyal Casino c’est un casino a decouvrir sans tarder pour les amoureux des slots modernes de casino ! A noter le design du casino est un spectacle visuel royal, facilite une experience de casino somptueuse.
plaza royal casino no deposit bonus|
The Real Person!
The Real Person!
Je trouve absolument captivant MrXBet Casino, ca degage une ambiance de jeu aussi envoutante qu’un secret. Le repertoire du casino est un dedale de divertissement, proposant des slots de casino a theme audacieux. Le service client du casino est un detective hors pair, repondant en un eclair mysterieux. Les gains du casino arrivent a une vitesse fulgurante, parfois des bonus de casino plus frequents seraient captivants. Dans l’ensemble, MrXBet Casino est un joyau pour les fans de casino pour ceux qui cherchent l’adrenaline secrete du casino ! De surcroit le site du casino est une merveille graphique enigmatique, ajoute une touche de suspense au casino.
casino mrxbet|
The Real Person!
The Real Person!
Ich liebe die Pracht von Richard Casino, es ist ein Online-Casino, das wie ein koniglicher Palast strahlt. Es gibt eine Flut an fesselnden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Service ist zuverlassig und furstlich, sorgt fur sofortigen Casino-Support, der beeindruckt. Casino-Gewinne kommen wie ein Triumphzug, ab und zu mehr Freispiele im Casino waren ein Kronungsmoment. Am Ende ist Richard Casino ein Muss fur Casino-Fans fur Spieler, die auf majestatische Casino-Kicks stehen! Und au?erdem die Casino-Seite ist ein grafisches Meisterwerk, einen Hauch von Majestat ins Casino bringt.
richard casino no deposit bonus for existing players|
Нужна лабораторная? заказ лабораторный работы Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
накрутка подписчиков в тг чат
Нужен чертеж? чертежи закажи выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
Нужна презентация? презентация заказать цена Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
Алкоголь ночью с доставкой на дом в Москве. Работаем 24/7, когда обычные магазины закрыты. Широкий ассортимент: пиво, сидр, вино, шампанское, крепкие напитки. Быстрая доставка в пределах МКАД за 30-60 минут: алкоголь домой доставка москва
Нужна лабораторная? https://lab-ucheb.ru Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
накрутка подписчиков в тг канал живые бесплатно
Нужен чертеж? https://chertezhi-kurs.ru выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
Нужна презентация? заказать сделать презентацию Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
https://2572.ru/
The Real Person!
The Real Person!
Shining Crown demo oyunu ilə klassik meyvə slotunu risksiz sına.
Shining crown big win qazanmaq motivasiya yaradır.
Superbet demo shining crown ilə oyun pulsuzdur. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. 40 shining crown bell link ən məşhur slotlardan biridir.
Shining crown 77777 böyük qrafika ilə hazırlanıb.
Shining crown free play risksiz məşq şansı yaradır.
Sadə kliklə açılır bu link.
Shining crown slot game yüksək qrafika və səs effektləri ilə zəngindir.
Shining crown slot online tez yüklənir.
The Real Person!
The Real Person!
https://kitehurghada.ru/
You need to really control the comments listed here
buy coke in prague buy cocaine prague
prague drugs buy drugs in prague
Консультации на дому врачей https://www.docplus24.ru
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
бот для накрутки подписчиков в телеграм канале
The Real Person!
The Real Person!
Je trouve absolument delirant Spinsy Casino, c’est un casino en ligne qui scintille comme une boule a facettes. Il y a une vague de jeux de casino captivants, comprenant des jeux de casino adaptes aux cryptomonnaies. Les agents du casino sont rapides comme un pas de danse, repondant en un eclair rythmique. Le processus du casino est transparent et sans fausse note, par moments plus de tours gratuits au casino ce serait dansant. Au final, Spinsy Casino promet un divertissement de casino electrisant pour ceux qui cherchent l’adrenaline rythmee du casino ! A noter la plateforme du casino brille par son style funky, donne envie de replonger dans le casino sans fin.
spinsy casino spielen|
The Real Person!
The Real Person!
Sou viciado no glamour de Richville Casino, parece um banquete de opulencia e diversao. As opcoes de jogo no cassino sao ricas e reluzentes, incluindo jogos de mesa de cassino com um toque de sofisticacao. O servico do cassino e confiavel e majestoso, respondendo rapido como um brinde de champanhe. As transacoes do cassino sao simples como abrir um cofre, mas mais recompensas no cassino fariam qualquer um se sentir rei. Na real, Richville Casino vale a pena explorar esse cassino com urgencia para os amantes de cassinos online! De lambuja a navegacao do cassino e facil como um passeio de carruagem, torna a experiencia de cassino um evento de gala.
new home construction richville|
The Real Person!
The Real Person!
Ich finde absolut elektrisierend SlotClub Casino, es pulsiert mit einer schillernden Casino-Energie. Es gibt eine Welle an packenden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Kundenservice ist wie ein leuchtender Funke, liefert klare und schnelle Losungen. Der Casino-Prozess ist klar und ohne Flackern, aber wurde ich mir mehr Casino-Promos wunschen, die wie ein Feuerwerk knallen. Alles in allem ist SlotClub Casino ein Casino mit einem Spielspa?, der wie ein Blitz einschlagt fur Fans von Online-Casinos! Und au?erdem die Casino-Oberflache ist flussig und glitzert wie ein Neonlicht, das Casino-Erlebnis total elektrisiert.
slotclub promo code|
The Real Person!
The Real Person!
Je trouve absolument delirant Spinsy Casino, c’est un casino en ligne qui scintille comme une boule a facettes. Il y a une vague de jeux de casino captivants, comprenant des jeux de casino adaptes aux cryptomonnaies. Les agents du casino sont rapides comme un pas de danse, avec une aide qui fait vibrer la piste. Le processus du casino est transparent et sans fausse note, quand meme plus de tours gratuits au casino ce serait dansant. Pour resumer, Spinsy Casino est une pepite pour les fans de casino pour les danseurs du casino ! De surcroit le site du casino est une merveille graphique rythmee, amplifie l’immersion totale dans le casino.
spinsy login|
The Real Person!
The Real Person!
J’adore la frenesie de Spinanga Casino, ca degage une ambiance de jeu aussi virevoltante qu’un cyclone. Le repertoire du casino est un tourbillon de divertissement, offrant des sessions de casino en direct qui tournoient. Le support du casino est disponible 24/7, repondant en un eclair tumultueux. Les transactions du casino sont simples comme une brise, cependant les offres du casino pourraient etre plus genereuses. Pour resumer, Spinanga Casino c’est un casino a decouvrir sans tarder pour les passionnes de casinos en ligne ! A noter la navigation du casino est intuitive comme une bourrasque, ce qui rend chaque session de casino encore plus virevoltante.
spinanga apk|
The Real Person!
The Real Person!
Sou viciado no glamour de Richville Casino, e um cassino online que reluz como um palacio dourado. A gama do cassino e um verdadeiro palacio de delicias, incluindo jogos de mesa de cassino com um toque de sofisticacao. A equipe do cassino oferece um atendimento digno de realeza, acessivel por chat ou e-mail. Os ganhos do cassino chegam com a velocidade de um jato particular, porem queria mais promocoes de cassino que brilhem como diamantes. No fim das contas, Richville Casino e um cassino online que exsuda riqueza para os apaixonados por slots modernos de cassino! Alem disso a interface do cassino e fluida e brilha como um salao de baile, o que torna cada sessao de cassino ainda mais reluzente.
richville play|
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
накрутка подписчиков тг
The Real Person!
The Real Person!
Ich bin total hingerissen von SlotClub Casino, es verstromt eine Spielstimmung, die wie ein Laserstrahl funkelt. Der Katalog des Casinos ist ein Kaleidoskop voller Spa?, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Die Casino-Mitarbeiter sind schnell wie ein Blitzstrahl, ist per Chat oder E-Mail erreichbar. Casino-Transaktionen sind simpel wie ein Schalter, dennoch die Casino-Angebote konnten gro?zugiger sein. Am Ende ist SlotClub Casino eine Casino-Erfahrung, die wie ein Lichtspiel glitzert fur Fans von Online-Casinos! Und au?erdem die Casino-Seite ist ein grafisches Meisterwerk, einen Hauch von Neon-Magie ins Casino bringt.
slotclub casino отзывы|
накрутка подписчиков телеграм телеграмм
The Real Person!
The Real Person!
https://t.me/bonus_dragon_money
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
живые подписчики в тг
Very nice post. I just stumbled upon your blog and wanted to say that I’ve really enjoyed browsing your blog posts. In any case I’ll be subscribing to your rss feed and I hope you write again soon!
The Real Person!
The Real Person!
Estou completamente vidrado por BetorSpin Casino, e um cassino online que gira como um asteroide em chamas. A selecao de titulos do cassino e um buraco negro de diversao, com slots de cassino tematicos de espaco sideral. O suporte do cassino ta sempre na ativa 24/7, acessivel por chat ou e-mail. Os ganhos do cassino chegam voando como um asteroide, mas queria mais promocoes de cassino que explodem como supernovas. Na real, BetorSpin Casino e o point perfeito pros fas de cassino para os viciados em emocoes de cassino! Vale dizer tambem o site do cassino e uma obra-prima de estilo estelar, adiciona um toque de brilho estelar ao cassino.
betorspin bГґnus|
The Real Person!
The Real Person!
Estou completamente vidrado por SpinFest Casino, tem uma vibe de jogo que explode como um festival de cores. Tem uma enxurrada de jogos de cassino irados, oferecendo sessoes de cassino ao vivo que vibram como tambores. O atendimento ao cliente do cassino e uma bateria de eficiencia, com uma ajuda que samba com estilo. Os pagamentos do cassino sao lisos e blindados, as vezes mais recompensas no cassino seriam um diferencial festivo. Em resumo, SpinFest Casino oferece uma experiencia de cassino que e puro batuque para quem curte apostar com gingado no cassino! Vale dizer tambem a plataforma do cassino brilha com um visual que e puro axe, adiciona um toque de folia ao cassino.
spinfest casino no deposit bonus|
The Real Person!
The Real Person!
Acho simplesmente intergalactico SpeiCasino, tem uma vibe de jogo tao reluzente quanto uma supernova. Tem uma chuva de meteoros de jogos de cassino irados, com jogos de cassino perfeitos pra criptomoedas. Os agentes do cassino sao rapidos como um cometa, garantindo suporte de cassino direto e sem buracos negros. O processo do cassino e limpo e sem turbulencia cosmica, mesmo assim queria mais promocoes de cassino que explodem como estrelas. No geral, SpeiCasino vale demais explorar esse cassino para os amantes de cassinos online! E mais o site do cassino e uma obra-prima de estilo estelar, o que torna cada sessao de cassino ainda mais estelar.
spei wagering requirements|
The Real Person!
The Real Person!
akun demo slot demo slot pg Pemain sering mencoba berbagai jenis slot Glucofort Blood Sugar Support is an all-natural dietary formula that works to support healthy blood sugar levels. It also supports glucose metabolism. According to the manufacturer, this supplement can help users keep their blood sugar levels healthy and within a normal range with herbs, vitamins, plant extracts, and other natural ingredients. glucofortbuynow.us €19,95 gates of olympus hilesi: gates of olympus 1000 demo – gates of olympus demo free spin glycofortin is a uniquely crafted liquid dietary supplement designed to aid in maintaining balanced blood sugar levels. gates of olympus hilesi: gates of olympus 1000 demo – gates of olympus demo free spin slot demo slotdemo Mesin slot dapat dimainkan dalam berbagai bahasa
https://badulaquemix.com.br/book-of-dead-spelen-zo-werkt-het-spel-stap-voor-stap/
De lay-out en interface van de Michigan BetMGM casino-app zijn bijna identiek aan wat u op uw laptop of desktop vindt, de bonus functies van de Joker Hot Reels pokies spel zijn vrij eenvoudig. Regle black jack 21 nou als dat is wat je verwachtte je zal niet teleurgesteld worden – de makers zijn naar de stad gegaan met deze, is het krediet alleen beschikbaar voor de leasemaatschappij. Je hoeft geen moeilijke beslissingen te nemen, evenals elk ander casino dat samenwerkt met NetEnt-producten. Alle gellakproducten van Pink Gellac, inclusief onze base- en topcoats, zijn HEMA-vrij en 100% vegan, en bieden een hoogwaardige manicure-ervaring. Met een uitgebreid assortiment van meer dan 300 kleuren willen we kleur in jouw leven brengen. Geniet thuis van prachtige, lang houdende salonkwaliteit gellak, wetende dat elk product in ons assortiment met zorg en kwaliteit is gemaakt.
The Real Person!
The Real Person!
Adoro o swing de RioPlay Casino, parece uma festa carioca de diversao. As opcoes de jogo no cassino sao ricas e dancantes, com caca-niqueis de cassino modernos e cheios de ritmo. O atendimento ao cliente do cassino e uma bateria de escola de samba, garantindo suporte de cassino direto e sem perder o ritmo. As transacoes do cassino sao simples como um passo de samba, mas mais bonus regulares no cassino seria um arraso. Resumindo, RioPlay Casino vale demais sambar nesse cassino para os viciados em emocoes de cassino! De bonus o design do cassino e um desfile visual colorido, adiciona um toque de axe ao cassino.
roblox lite rioplay atualizado wix|
The Real Person!
The Real Person!
Sou louco pela vibe de SpinWiz Casino, oferece uma aventura de cassino que reluz como um portal magico. As opcoes de jogo no cassino sao ricas e magicas, incluindo jogos de mesa de cassino com um toque de magia. A equipe do cassino entrega um atendimento que e puro genio, dando solucoes na hora e com precisao. Os pagamentos do cassino sao lisos e blindados, mas mais recompensas no cassino seriam um diferencial encantado. Na real, SpinWiz Casino garante uma diversao de cassino que e magica para quem curte apostar com estilo mistico no cassino! Alem disso a navegacao do cassino e facil como um passe de magica, o que torna cada sessao de cassino ainda mais encantadora.
spinwiz bet|
пароізоляція для даху https://remontuem.te.ua
Авто в ОАЭ https://auto.ae покупка, продажа и аренда новых и б/у машин. Популярные марки, выгодные условия, помощь в оформлении документов и доступные цены.
The Real Person!
The Real Person!
Sou viciado na vibe de SpeiCasino, e um cassino online que decola como um foguete espacial. As opcoes de jogo no cassino sao ricas e brilhantes como estrelas, com slots de cassino tematicos de espaco. Os agentes do cassino sao rapidos como um cometa, dando solucoes na hora e com precisao. Os ganhos do cassino chegam voando como um meteoro, de vez em quando mais giros gratis no cassino seria uma loucura estelar. Em resumo, SpeiCasino oferece uma experiencia de cassino que e puro brilho estelar para os viciados em emocoes de cassino! De bonus a plataforma do cassino brilha com um visual que e puro cosmos, faz voce querer voltar ao cassino como um cometa em orbita.
spei wagering requirements|
https://mama-creative.com/razrabotka-po-pod-zakaz-ot-idei-do-gotovogo-produkta/
как взять займ займ кредит
Заметки практикующего врача https://phlebolog-blog.ru флеболога. Профессиональное лечение варикоза ног. Склеротерапия, ЭВЛО, УЗИ вен и точная диагностика. Современные безболезненные методики, быстрый результат и забота о вашем здоровье!
purebred kittens for sale in NY https://catsdogs.us
подписчики в тг канал боты
Нужна презентация? https://generator-prezentaciy.ru Создавайте убедительные презентации за минуты. Умный генератор формирует структуру, дизайн и иллюстрации из вашего текста. Библиотека шаблонов, фирстиль, графики, экспорт PPTX/PDF, совместная работа и комментарии — всё в одном сервисе.
Проблемы с откачкой? насос помпа для откачки воды сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
buy drugs in prague weed in prague
The Real Person!
The Real Person!
Adoro o brilho de BetorSpin Casino, tem uma vibe de jogo tao vibrante quanto uma supernova dancante. O catalogo de jogos do cassino e uma nebulosa de emocoes, com slots de cassino tematicos de espaco sideral. A equipe do cassino entrega um atendimento que e puro cometa, acessivel por chat ou e-mail. As transacoes do cassino sao simples como uma orbita lunar, as vezes queria mais promocoes de cassino que explodem como supernovas. Resumindo, BetorSpin Casino e o point perfeito pros fas de cassino para os amantes de cassinos online! E mais a navegacao do cassino e facil como uma orbita lunar, adiciona um toque de brilho estelar ao cassino.
betorspin registo|
The Real Person!
The Real Person!
Estou completamente encantado por SpinGenei Casino, tem uma vibe de jogo tao magica quanto um tapete voador. O catalogo de jogos do cassino e um bau de tesouros misticos, incluindo jogos de mesa de cassino com um toque de magia. O servico do cassino e confiavel e encantador, acessivel por chat ou e-mail. As transacoes do cassino sao simples como um feitico, de vez em quando mais recompensas no cassino seriam um diferencial encantado. No geral, SpinGenei Casino e o point perfeito pros fas de cassino para os viciados em emocoes de cassino! De lambuja a plataforma do cassino brilha com um visual que e puro feitico, eleva a imersao no cassino a um nivel magico.
spingenie bonus code|
Сначала немного стеснялся, но девушка быстро сняла все напряжение. Массаж плавный, чувственный, каждое движение доставляло удовольствие. Хочется вернуться снова. Обязательно попробуйте, шлюхи заказать Новосибирск – https://sibirki3.vip/. Массаж был волшебный, эмоции зашкаливали.
The Real Person!
The Real Person!
Acho simplesmente magico SpellWin Casino, parece um portal mistico cheio de adrenalina. A gama do cassino e simplesmente um feitico de prazeres, oferecendo sessoes de cassino ao vivo que brilham como runas. O suporte do cassino ta sempre na ativa 24/7, acessivel por chat ou e-mail. O processo do cassino e limpo e sem truques, mas mais bonus regulares no cassino seria brabo. Na real, SpellWin Casino e um cassino online que e um portal de diversao para quem curte apostar com estilo mistico no cassino! Alem disso o site do cassino e uma obra-prima de estilo mistico, eleva a imersao no cassino a um nivel magico.
spellwin de|
The Real Person!
The Real Person!
Je suis fou de Stake Casino, il propose une aventure de casino qui pulse comme un geyser. L’eventail de jeux du casino est une eruption de delices, incluant des jeux de table de casino d’une elegance ardente. Le personnel du casino offre un accompagnement digne d’un pyromane, avec une aide qui fait jaillir des flammes. Les retraits au casino sont rapides comme une coulee de lave, par moments les offres du casino pourraient etre plus genereuses. En somme, Stake Casino promet un divertissement de casino brulant pour ceux qui cherchent l’adrenaline enflammee du casino ! Par ailleurs la navigation du casino est intuitive comme une flamme, donne envie de replonger dans le casino sans fin.
stake us|
The Real Person!
The Real Person!
Estou completamente encantado por SpinGenei Casino, da uma energia de cassino que e pura magia. Tem uma enxurrada de jogos de cassino fascinantes, oferecendo sessoes de cassino ao vivo que reluzem como pocoes. A equipe do cassino entrega um atendimento que e puro genio, respondendo mais rapido que um estalar de dedos. Os pagamentos do cassino sao lisos e blindados, as vezes mais giros gratis no cassino seria um feitico. Em resumo, SpinGenei Casino e um cassino online que e um portal de diversao para quem curte apostar com estilo mistico no cassino! Vale dizer tambem o design do cassino e um espetaculo visual encantado, o que torna cada sessao de cassino ainda mais encantadora.
spingenie recension|
The Real Person!
The Real Person!
Acho simplesmente magico SpellWin Casino, e um cassino online que brilha como uma pocao encantada. A selecao de titulos do cassino e um caldeirao de emocoes, oferecendo sessoes de cassino ao vivo que brilham como runas. O servico do cassino e confiavel e encantador, respondendo mais rapido que um estalo magico. Os ganhos do cassino chegam voando como uma vassoura encantada, porem as ofertas do cassino podiam ser mais generosas. No geral, SpellWin Casino garante uma diversao de cassino que e magica para quem curte apostar com estilo mistico no cassino! Alem disso a interface do cassino e fluida e brilha como uma pocao reluzente, faz voce querer voltar ao cassino como num desejo concedido.
george spellwin|
The Real Person!
The Real Person!
https://finance-info.ru/
buy cocaine prague cocaine prague telegram
cocaine in prague cocain in prague from dominican republic
The Real Person!
The Real Person!
Аренда авто аэропорт Краснодар
накрутка подписчиков в тг навсегда
plug in prague buy weed prague
buy coke in prague prague plug
The Real Person!
The Real Person!
Ən yaxşı seçimlərdən biri də Sunny Coin Hold The Spin onlayn versiyasıdır. Sunny Coin 2: hold the spin demo rahat və əlçatandır.
Sunny Coin Hold The Spin pulsuz demo yeni başlayanlar üçün əladır. Sunny coin 2 hold the spin slot oynamadan əvvəl demo sınayın.
Bütün detallar üçün baxın sunny-coin.com.az/.
Sunny coin: hold the spin slot çox əyləncəli seçimdir. Sunny coin hold the spin slot bonus raundları çox cəlbedicidir. Sunny Coin Hold The Spin demo versiyası sürətlidir.
Sunny Coin 2 hold the spin slot yeni oyunçular üçün uyğundur. Sunny Coin Hold The Spin casino slotları içində fərqlənir.
как добавить подписчиков в тг канал
The Real Person!
The Real Person!
Estou alucinado com BetorSpin Casino, da uma energia de cassino que e puro pulsar galactico. O catalogo de jogos do cassino e uma nebulosa de emocoes, oferecendo sessoes de cassino ao vivo que reluzem como supernovas. A equipe do cassino entrega um atendimento que e puro cometa, garantindo suporte de cassino direto e sem buracos negros. Os saques no cassino sao velozes como uma viagem interestelar, mas mais giros gratis no cassino seria uma loucura estelar. Na real, BetorSpin Casino vale demais explorar esse cassino para os apaixonados por slots modernos de cassino! De bonus a interface do cassino e fluida e reluz como uma constelacao, torna a experiencia de cassino uma viagem espacial.
betorspin giris|
The Real Person!
The Real Person!
Sou louco pela vibe de SpinSala Casino, tem uma vibe de jogo tao vibrante quanto um show de luzes. As opcoes de jogo no cassino sao ricas e cheias de luz, com jogos de cassino perfeitos pra criptomoedas. O atendimento ao cliente do cassino e uma estrela de neon, respondendo mais rapido que um estalo de luzes. Os ganhos do cassino chegam voando como um foguete, mesmo assim mais bonus regulares no cassino seria brabo. Em resumo, SpinSala Casino oferece uma experiencia de cassino que e puro brilho para os apaixonados por slots modernos de cassino! E mais a plataforma do cassino reluz com um visual que e puro show, faz voce querer voltar ao cassino como uma estrela em um palco.
spinsala casino review|
The Real Person!
The Real Person!
Bu gün sunny coin hold the spin oynadım və xoş təəssürat qaldı. Sunny Coin 2 hold the spin slot real pul ilə daha həyəcanlıdır.
Sunny coin hold the spin slot online casino üçün ideal seçimdir. Sunny coin: hold the spin slot oyun təcrübəsi mükəmməldir.
Sürətli keçid üçün klik edin sunny coin com.
Sunny Coin Hold The Spin oyunu həm pulsuz, həm də real pul üçündür. Sunny coin hold the spin slot bonus raundları çox cəlbedicidir. Sunny Coin Hold The Spin slot oyunu çoxlu bonus təklif edir.
Sunny coin hold the spin slot dizaynı çox rəngarəngdir. Sunny Coin Hold The Spin oyunu hər büdcəyə uyğundur.
The Real Person!
The Real Person!
Sou louco pela energia de SupremaBet Casino, e um cassino online que ressoa como um trovao epico. A selecao de titulos do cassino e um trono de prazeres, oferecendo sessoes de cassino ao vivo que reluzem como cetros. A equipe do cassino entrega um atendimento que e puro reinado, garantindo suporte de cassino direto e sem revoltas. As transacoes do cassino sao simples como um mandato, mas mais giros gratis no cassino seria uma loucura. Em resumo, SupremaBet Casino oferece uma experiencia de cassino que e puro cetro para os viciados em emocoes de cassino! De lambuja a plataforma do cassino brilha com um visual que e puro trono, eleva a imersao no cassino a um nivel imperial.
supremabet pb|
The Real Person!
The Real Person!
Maraqlı dizayn və yüksək RTP ilə Sunny Coin Hold The Spin fərqlənir. Sunny Coin 2: hold the spin demo rahat və əlçatandır.
Sunny coin 2 hold the spin slot strategiyaları öyrənməyə dəyər. Sunny coin: hold the spin slot oyun təcrübəsi mükəmməldir.
Bütün detallar üçün baxın sunny-coin.com.az/.
Sunny coin: hold the spin slot maraqlı mexanikaya malikdir. Sunny Coin 2: Hold The Spin slotu çox realistik görünür. Sunny coin 2 hold the spin slot real həyəcan bəxş edir.
Sunny Coin Hold The Spin slot oyunu sürprizlərlə doludur. Sunny coin 2 hold the spin slot oyununda böyük qaliblər var.
The Real Person!
The Real Person!
Ich liebe den Nervenkitzel von SportingBet Casino, es verstromt eine Spielstimmung, die wie ein Stadion tobt. Die Auswahl im Casino ist ein echter Torerfolg, mit modernen Casino-Slots, die einen mitrei?en. Der Casino-Service ist zuverlassig und prazise, ist per Chat oder E-Mail erreichbar. Casino-Gewinne kommen wie ein Torjubel, dennoch mehr Freispiele im Casino waren ein Torerfolg. Insgesamt ist SportingBet Casino ein Casino, das man nicht verpassen darf fur Abenteurer im Casino! Und au?erdem die Casino-Plattform hat einen Look, der wie ein Torjubel strahlt, Lust macht, immer wieder ins Casino zuruckzukehren.
sportingbet opinie|
The Real Person!
The Real Person!
Acho simplesmente fantastico SpinSala Casino, oferece uma aventura de cassino que reluz como um holofote. Os titulos do cassino sao uma explosao de cores dancantes, incluindo jogos de mesa de cassino com um toque de glamour. O servico do cassino e confiavel e cheio de brilho, com uma ajuda que reluz como um show. As transacoes do cassino sao simples como um pisca-pisca, de vez em quando mais giros gratis no cassino seria uma loucura. Em resumo, SpinSala Casino vale demais girar nesse cassino para os dancarinos do cassino! Alem disso a plataforma do cassino reluz com um visual que e puro show, adiciona um toque de glamour reluzente ao cassino.
spinsala no deposit bonus|
The Real Person!
The Real Person!
Трансы Екатеринбург Трансы Екатеринбурга
The Real Person!
The Real Person!
Estou alucinado com SupremaBet Casino, tem uma vibe de jogo tao poderosa quanto um raio majestoso. As opcoes de jogo no cassino sao ricas e soberanas, com caca-niqueis de cassino modernos e eletrizantes. O servico do cassino e confiavel e majestoso, garantindo suporte de cassino direto e sem revoltas. Os pagamentos do cassino sao lisos e blindados, as vezes mais bonus regulares no cassino seria supremo. Na real, SupremaBet Casino vale demais conquistar esse cassino para os apaixonados por slots modernos de cassino! De lambuja a navegacao do cassino e facil como um decreto real, faz voce querer voltar ao cassino como um rei ao seu trono.
supremabet Г© confiГЎvel|
Проблемы с откачкой? помпа для откачки воды из подвала сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
coke in prague cocain in prague fishscale
The Real Person!
The Real Person!
Ən yaxşı seçimlərdən biri də Sunny Coin Hold The Spin onlayn versiyasıdır. Sunny Coin 2: Hold The Spin slot dizaynı diqqəti cəlb edir.
Sunny Coin Hold The Spin 2 yeni versiyası çox maraqlıdır. Sunny Coin 2: Hold The Spin slotunda bonus raundları maraqlıdır.
Slotu sınamaq üçün rəsmi link sunny-coin.com.az.
Sunny Coin Hold The Spin slot böyük qaliblərə şans verir. Sunny coin hold the spin oyna və fərqli təcrübə qazan. Sunny coin 2 hold the spin slot istifadəçilərin sevimlisidir.
Sunny Coin Hold The Spin slot online hər kəs üçün əlçatan olur. Sunny Coin Hold The Spin real pul üçün əlverişlidir.
The Real Person!
The Real Person!
Sunny Coin Hold The Spin slotu haqqında çox müsbət rəylər eşitmişəm. Sunny Coin Hold The Spin casino oyunları arasında məşhurdur.
Sunny coin 2 hold the spin slot free play rahat seçimdir. Sunny coin 2 hold the spin slot qazancları maraqlı edir.
Rəsmi səhifə http://www.sunny-coin.com.az/.
Sunny Coin 2 hold the spin slot bonus imkanları çox genişdir. Sunny coin 2 hold the spin slot oyunçu rəyləri çox müsbətdir. Sunny coin 2 hold the spin slot real həyəcan bəxş edir.
Sunny Coin Hold The Spin pulsuz demo çox faydalıdır. Sunny coin 2 hold the spin slot oyununda böyük qaliblər var.
buy xtc prague weed in prague
weed in prague cocaine prague telegram
The Real Person!
The Real Person!
Sunny Coin Hold The Spin slotu haqqında çox müsbət rəylər eşitmişəm. Sunny Coin Hold The Spin onlayn casino seçimləri arasında öndədir.
Sunny Coin Hold The Spin pulsuz demo yeni başlayanlar üçün əladır. Sunny coin 2 hold the spin slot qazancları maraqlı edir.
Oyuna giriş ünvanı http://www.sunny-coin.com.az.
Sunny coin: hold the spin slot maraqlı mexanikaya malikdir. Sunny coin 2 hold the spin slot demo rahat giriş imkanı yaradır. Sunny coin hold the spin slot online rahat oynanır.
Sunny coin hold the spin demo sınamağa dəyər. Sunny Coin Hold The Spin oyunu hər büdcəyə uyğundur.
buy mdma prague pure cocaine in prague
Zivjeti u Crnoj Gori? https://www.placevi-zabljak-nekretnine.com Novi apartmani, gotove kuce, zemljisne parcele. Bez skrivenih provizija, trzisna procjena, pregovori sa vlasnikom. Pomoci cemo da otvorite racun, zakljucite kupoprodaju i aktivirate servis izdavanja. Pisite — poslacemo vam varijante.
Смотрите онлайн мультфильмы смотреть онлайн лучшие детские мультфильмы, сказки и мульсериалы. Добрые истории, веселые приключения и любимые герои для малышей и школьников. Удобный поиск, качественное видео и круглосуточный доступ без ограничений.
Портал о строительстве https://gidfundament.ru и ремонте: обзоры материалов, сравнение цен, рейтинг подрядчиков, тендерная площадка, сметные калькуляторы, образцы договоров и акты. Актуальные ГОСТ/СП, инструкции, лайфхаки и готовые решения для дома и бизнеса.
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: https://phlebology-blog.ru/
Всё о ремонте https://remontkit.ru и строительстве: технологии, нормы, сметы, каталоги материалов и инструментов. Дизайн-идеи для квартиры и дома, цветовые схемы, 3D-планы, кейсы и ошибки. Подрядчики, прайсы, калькуляторы и советы экспертов для экономии бюджета.
Мир гаджетов https://indevices.ru новости, обзоры и тесты смартфонов, ноутбуков, наушников и умного дома. Сравнения, рейтинги автономности, фото/видео-примеры, цены и акции. Поможем выбрать устройство под задачи и бюджет. Подписка на новые релизы.
Все автоновинки https://myrexton.ru премьеры, тест-драйвы, характеристики, цены и даты продаж. Электромобили, гибриды, кроссоверы и спорткары. Фото, видео, сравнения с конкурентами, конфигуратор и уведомления о старте приема заказов.
Женский портал https://art-matita.ru о жизни и балансе: модные идеи, уход за кожей и волосами, здоровье, йога и фитнес, отношения и семья. Рецепты, чек-листы, антистресс-практики, полезные сервисы и календарь событий.
The Real Person!
The Real Person!
Sunny coin 2 hold the spin slot demo versiyası çox rahatdır. Sunny coin hold the spin oyna və pulsuz fırlanmaları yoxla.
Sunny coin 2 hold the spin ilə uğurlu nəticələr mümkündür. Sunny coin: hold the spin slot oyun təcrübəsi mükəmməldir.
Sürətli keçid üçün klik edin sunny coin com.
Sunny Coin Hold The Spin oyunu həm pulsuz, həm də real pul üçündür. Sunny coin: hold the spin slot şanslı oyunçular üçün əladır. Sunny coin hold the spin oyna və yeni imkanlar kəşf et.
Sunny coin hold the spin demo sınamağa dəyər. Sunny Coin Hold The Spin oyunu hər büdcəyə uyğundur.
The Real Person!
The Real Person!
Acho simplesmente alucinante AFun Casino, parece uma rave de diversao sem fim. As opcoes de jogo no cassino sao ricas e cheias de swing, com jogos de cassino perfeitos pra criptomoedas. O suporte do cassino ta sempre na ativa 24/7, garantindo suporte de cassino direto e sem pausa. Os ganhos do cassino chegam voando como serpentinas, de vez em quando queria mais promocoes de cassino que botam pra quebrar. No fim das contas, AFun Casino garante uma diversao de cassino que e uma folia total para os apaixonados por slots modernos de cassino! Vale dizer tambem a plataforma do cassino brilha com um visual que e puro axe, torna a experiencia de cassino uma folia inesquecivel.
codigo indicacao afun|
The Real Person!
The Real Person!
Sunny coin 2 hold the spin slot demo versiyası çox rahatdır. Sunny Coin 2: hold the spin demo rahat və əlçatandır.
Sunny Coin Hold The Spin 2 yeni versiyası çox maraqlıdır. Sunny coin 2 hold the spin slot oynamadan əvvəl demo sınayın.
Demo versiya üçün keçid http://sunny-coin.com.az.
Sunny coin: hold the spin slot maraqlı mexanikaya malikdir. Sunny Coin Hold The Spin oyunu sürətli və maraqlıdır. Sunny coin 2 hold the spin slot real həyəcan bəxş edir.
Sunny Coin 2 hold the spin slot yeni oyunçular üçün uyğundur. Sunny Coin Hold The Spin slotu yeni istifadəçiləri cəlb edir.
The Real Person!
The Real Person!
Sou louco pela energia de BetorSpin Casino, tem uma vibe de jogo tao vibrante quanto uma supernova dancante. A gama do cassino e simplesmente uma constelacao de prazeres, incluindo jogos de mesa de cassino com um toque intergalactico. Os agentes do cassino sao rapidos como um foguete estelar, garantindo suporte de cassino direto e sem buracos negros. As transacoes do cassino sao simples como uma orbita lunar, mas queria mais promocoes de cassino que explodem como supernovas. No fim das contas, BetorSpin Casino e o point perfeito pros fas de cassino para os apaixonados por slots modernos de cassino! De lambuja a interface do cassino e fluida e reluz como uma constelacao, torna a experiencia de cassino uma viagem espacial.
betorspin portugal|
https://device-rf.ru/catalog/iphone/
Only a smiling visitor here to share the love (:, btw outstanding style and design .
подписчики в тг канал без отписок
Новостной портал https://daily-inform.ru главные события дня, репортажи, аналитика, интервью и мнения экспертов. Лента 24/7, проверка фактов, региональные и мировые темы, экономика, технологии, спорт и культура.
Всё о стройке https://lesnayaskazka74.ru и ремонте: технологии, нормы, сметы и планирование. Каталог компаний, аренда техники, тендерная площадка, прайс-мониторинг. Калькуляторы, чек-листы, инструкции и видеоуроки для застройщиков, подрядчиков и частных мастеров.
Актуальные новости https://pr-planet.ru без лишнего шума: политика, экономика, общество, наука, культура и спорт. Оперативная лента 24/7, инфографика,подборки дня, мнения экспертов и расследования.
Строительный портал https://nastil69.ru новости, аналитика, обзоры материалов и техники, каталог поставщиков и подрядчиков, тендеры и прайсы. Сметные калькуляторы, ГОСТ/СП, шаблоны договоров, кейсы и лайфхаки.
сайт для накрутки подписчиков в тг
Ремонт и стройка https://stroimsami.online без лишних затрат: гайды, сметы, план-графики, выбор подрядчика и инструмента. Честные обзоры, сравнения, лайфхаки и чек-листы. От отделки до инженерии — поможем спланировать, рассчитать и довести проект до результата.
ціни електрика https://remontuem.te.ua
https://plenka-okna.ru/
изготовление значков в москве значок изготовить на заказ
значки из металла на заказ значок индивидуальный
The Real Person!
The Real Person!
Acho simplesmente sensacional BacanaPlay Casino, oferece uma aventura de cassino que pulsa como um tamborim. A gama do cassino e simplesmente um sambodromo de prazeres, oferecendo sessoes de cassino ao vivo que sambam com energia. O atendimento ao cliente do cassino e uma rainha de bateria, respondendo mais rapido que um batuque de pandeiro. O processo do cassino e limpo e sem tumulto, de vez em quando queria mais promocoes de cassino que botam pra quebrar. No geral, BacanaPlay Casino e um cassino online que e uma folia sem fim para os amantes de cassinos online! De lambuja a interface do cassino e fluida e reluz como uma fantasia de carnaval, adiciona um toque de folia ao cassino.
bacanaplay disponГvel appstore|
значки эмаль на заказ изготовление значков с логотипом на заказ
The Real Person!
The Real Person!
Estou alucinado com BetorSpin Casino, e um cassino online que gira como um asteroide em chamas. O catalogo de jogos do cassino e uma nebulosa de emocoes, com jogos de cassino perfeitos pra criptomoedas. A equipe do cassino entrega um atendimento que e puro cometa, com uma ajuda que reluz como uma aurora boreal. Os ganhos do cassino chegam voando como um asteroide, mas as ofertas do cassino podiam ser mais generosas. Em resumo, BetorSpin Casino e um cassino online que e uma galaxia de diversao para os apaixonados por slots modernos de cassino! De lambuja o design do cassino e um espetaculo visual intergalactico, adiciona um toque de brilho estelar ao cassino.
betorspin legit|
What’s up to every one, the contents present at this web site are in fact awesome for people knowledge, well, keep up the nice work fellows.
официальный сайт kra40.at
The Real Person!
The Real Person!
Ich bin vollig aufgeheizt von Turbonino Casino, es verstromt eine Spielstimmung, die wie ein Turboboost explodiert. Die Auswahl im Casino ist ein echtes Geschwindigkeitsspektakel, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Der Casino-Service ist zuverlassig und prazise, sorgt fur sofortigen Casino-Support, der die Konkurrenz uberholt. Casino-Zahlungen sind sicher und reibungslos, aber mehr Freispiele im Casino waren ein Geschwindigkeitsrausch. Am Ende ist Turbonino Casino ein Casino, das man nicht verpassen darf fur Spieler, die auf rasante Casino-Kicks stehen! Und au?erdem die Casino-Seite ist ein grafisches Meisterwerk, was jede Casino-Session noch rasant macht.
turbonino casino.|
Ich bin vollig hingerissen von Tipico Casino, es verstromt eine Spielstimmung, die wie ein Blitz einschlagt. Die Casino-Optionen sind vielfaltig und elektrisierend, mit Live-Casino-Sessions, die wie ein Sturm toben. Der Casino-Kundenservice ist wie ein Leuchtfeuer im Sturm, ist per Chat oder E-Mail erreichbar. Casino-Zahlungen sind sicher und reibungslos, ab und zu mehr regelma?ige Casino-Boni waren ein Volltreffer. Am Ende ist Tipico Casino ein Online-Casino, das wie ein Sturm begeistert fur die, die mit Stil im Casino wetten! Ubrigens das Casino-Design ist ein optisches Gewitter, einen Hauch von Sturm-Magie ins Casino bringt.
tipico leipzig|
The Real Person!
The Real Person!
You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Sim, dá para jogar de graça em cassinos online. É possível fazer isso através das versões demo gratuitas dos melhores jogos, que estão disponíveis na maioria dos cassinos online. Sim, os cassinos criptomoedas, geralmente, oferecem uma ampla variedade de jogos, incluindo slots, jogos de mesa, apostas esportivas e jogos exclusivos específicos de criptomoedas. Além disso, normalmente não é preciso concluir a verificação de identidade. Outro ponto é que esses operadores não estão sujeitos a regulamentações rigorosas. Isso, por sua vez, lhes permite oferecer ofertas de bônus mais generosas e uma seleção mais ampla de jogos de cassino.
https://rdsthe30thparallel.com/plataformas-do-aviator-qual-escolher-para-jogar-online/
Leia a resenha Sim, dá para jogar de graça em cassinos online. É possível fazer isso através das versões demo gratuitas dos melhores jogos, que estão disponíveis na maioria dos cassinos online. Assim sendo, a ferramenta também se tornou uma das principais opções de pagamento em plataformas de cassinos online. No entanto, é essencial adotar boas práticas para garantir uma experiência segura. Dito isso, veja a seguir o que observar ao escolher um cassino que aceita Pix. Em geral, cassinos internacionais tendem a oferecer uma gama de jogos maior, além de diversas opções de pagamento. Entre outras vantagens adicionais, os cassinos internacionais offshore disponibilizam atendimento multilíngue e suporte a várias moedas. Os melhores slots são aqueles que possuem RTP mais alto e boa jogabilidade. Os slots mais famosos são: Fortune Tiger, Starburst, Big Bass Bonanza, Dead or Alive 2, Sweet Bonanza, Book of Dead e Mega Moolah.
Do you have a spam issue on this site; I also am a blogger, and I was wanting to know your situation; many of us have created some nice procedures and we are looking to trade techniques with others, please shoot me an e-mail if interested.
официальный маркетплейс kra40.cc
живые активные подписчики в тг
The Real Person!
The Real Person!
Je suis totalement captive par Unibet Casino, on dirait une melodie envoutante de gains. Le repertoire du casino est une partition de divertissement, offrant des sessions de casino en direct qui chantent comme un ch?ur. Le service client du casino est un maestro virtuose, assurant un support de casino immediat et vibrant. Les gains du casino arrivent a une vitesse virtuose, quand meme les offres du casino pourraient etre plus genereuses. Au final, Unibet Casino c’est un casino a rejoindre sans tarder pour les passionnes de casinos en ligne ! A noter la navigation du casino est intuitive comme une partition, amplifie l’immersion totale dans le casino.
unibet 100 euros offert|
The Real Person!
The Real Person!
Estou alucinado com BetorSpin Casino, da uma energia de cassino que e puro pulsar galactico. As opcoes de jogo no cassino sao ricas e brilhantes como estrelas, oferecendo sessoes de cassino ao vivo que reluzem como supernovas. A equipe do cassino entrega um atendimento que e puro cometa, com uma ajuda que reluz como uma aurora boreal. Os ganhos do cassino chegam voando como um asteroide, porem queria mais promocoes de cassino que explodem como supernovas. Na real, BetorSpin Casino e um cassino online que e uma galaxia de diversao para os astronautas do cassino! Vale dizer tambem o site do cassino e uma obra-prima de estilo estelar, torna a experiencia de cassino uma viagem espacial.
betorspin bonus registo|
Je suis accro a Tortuga Casino, ca degage une ambiance de jeu aussi intrepide qu’un equipage pirate. Les options de jeu au casino sont riches et aventureuses, offrant des sessions de casino en direct qui rugissent comme la mer. L’assistance du casino est chaleureuse et fiable, avec une aide qui hisse les voiles. Le processus du casino est transparent et sans ecueils, quand meme les offres du casino pourraient etre plus genereuses. Dans l’ensemble, Tortuga Casino est un casino en ligne qui navigue comme un vaisseau pirate pour les joueurs qui aiment parier avec panache au casino ! En plus la navigation du casino est intuitive comme une boussole, facilite une experience de casino epique.
tortuga casino kode|
The Real Person!
The Real Person!
World SettingOne day, money starts raining from the sky. O 1xSlot é um cassino on-line moderno que combina bônus generosos, uma ampla seleção de jogos e uma interface fácil de usar. Se quiser tentar a sorte no Money Coming e em outros caça-níqueis populares, esta é a plataforma ideal para você. Jornalista e fã de videogames desde criança. Já teve Mega Drive, Game Boy Color, PS1, PS2, PS3, PS4, PSVR, PS Vita, Nintendo 3DS e agora tem “só” um PS5, um Nintendo Switch e um PC Gamer. Para ele, o melhor jogo da história é Chrono Trigger, mas Metal Gear Solid 3, Final Fantasy X, The Last of Us Part II e Red Dead Redemption 2 completam o Top-5. X-Kira apresenta uma variação do papel clássico de Kira, com a capacidade de recrutar secretamente um Investigador durante as fases de reunião. No entanto, personagens-chave como L, Watari e Mello permanecem imunes. Além disso, o personagem conta com Command Cards exclusivas, como “Fake Surveillance Tasks”, que permitem gerar desconfiança entre os membros da Equipe L.
https://contasaestrada.es/index.php/2025/09/22/analise-completa-da-plataforma-3355bet-o-destino-do-jogo-online-no-brasil/
Assim como em outros caça-níqueis, muitos cassinos oferecem bônus de recarga de saldo para jogar Money Coming. Por exemplo, rodadas grátis ou bônus de depósito de até 100%. Para não perder essas ofertas, recomendamos que fique de olho nas promoções no site do cassino. Além disso, a estratégia adequada de gerenciamento de bankroll também desempenha um papel fundamental. Recomendamos começar com apostas pequenas e aumentá-las gradualmente à medida que se sentir confortável com a mecânica do jogo e ativar os recursos de bônus. Lembre-se de que apostas altas podem levar a grandes riscos, mas a chance de uma grande vitória também aumenta. Assim como em outros caça-níqueis, muitos cassinos oferecem bônus de recarga de saldo para jogar Money Coming. Por exemplo, rodadas grátis ou bônus de depósito de até 100%. Para não perder essas ofertas, recomendamos que fique de olho nas promoções no site do cassino.
бот для накрутки подписчиков в тг бесплатно
https://www.wildberries.ru/catalog/183263596/detail.aspx
The Real Person!
The Real Person!
Acho simplesmente alucinante Bet4Slot Casino, tem uma vibe de jogo tao vibrante quanto uma roda-gigante iluminada. Tem uma enxurrada de jogos de cassino irados, oferecendo sessoes de cassino ao vivo que giram como um tornado. O suporte do cassino ta sempre na ativa 24/7, dando solucoes na hora e com precisao. Os pagamentos do cassino sao lisos e blindados, de vez em quando mais giros gratis no cassino seria uma loucura. Em resumo, Bet4Slot Casino vale demais girar nesse cassino para quem curte apostar com estilo giratorio no cassino! Alem disso a plataforma do cassino brilha com um visual que e puro redemoinho, adiciona um toque de adrenalina giratoria ao cassino.
bet4slot mx|
The Real Person!
The Real Person!
I’m obsessed with the vibe of Wazamba Casino, it offers a casino adventure that swings like a vine in the wild. There’s a stampede of captivating casino games, including classy casino table games with a tribal flair. The casino’s customer service is a tribal chief of efficiency, ensuring immediate casino support as bold as a warrior. Casino payments are secure and smooth, occasionally extra casino rewards would make hearts pound. All in all, Wazamba Casino is an online casino that thrives like a jungle oasis for casino adventurers! Plus the casino site is a graphical wonder, making you want to dive back into the casino endlessly.
wazamba download app|
The Real Person!
The Real Person!
Sou louco pela energia de BetorSpin Casino, e um cassino online que gira como um asteroide em chamas. As opcoes de jogo no cassino sao ricas e brilhantes como estrelas, com jogos de cassino perfeitos pra criptomoedas. O suporte do cassino ta sempre na ativa 24/7, dando solucoes na hora e com precisao. As transacoes do cassino sao simples como uma orbita lunar, porem mais giros gratis no cassino seria uma loucura estelar. No geral, BetorSpin Casino garante uma diversao de cassino que e uma supernova para os astronautas do cassino! Alem disso o design do cassino e um espetaculo visual intergalactico, o que torna cada sessao de cassino ainda mais estelar.
betorspin cassino|
The Real Person!
The Real Person!
Je suis totalement envoute par Tortuga Casino, on dirait une ile au tresor pleine de frissons. La selection du casino est une vague de plaisirs pirates, offrant des sessions de casino en direct qui rugissent comme la mer. Les agents du casino sont rapides comme un vent de tempete, assurant un support de casino immediat et audacieux. Le processus du casino est transparent et sans ecueils, par moments plus de tours gratuits au casino ce serait corsaire. Pour resumer, Tortuga Casino offre une experience de casino audacieuse pour les passionnes de casinos en ligne ! En plus le site du casino est une merveille graphique pirate, facilite une experience de casino epique.
jeux casino tortuga|
The Real Person!
The Real Person!
габапентин для чего назначают Габапентин: Мнение экспертов Врачи, словно мудрые советники, подчеркивают эффективность габапентина в лечении нейропатической боли и эпилепсии. Однако, они неустанно напоминают о необходимости строгого соблюдения дозировки и учета возможных побочных эффектов. Самолечение недопустимо.
joszaki regisztracio http://joszaki.hu
joszaki regisztracio joszaki.hu
joszaki regisztracio http://joszaki.hu
The Real Person!
The Real Person!
What’s up colleagues, how is the whole thing, and what you wish for to say about this piece of writing, in my view its genuinely awesome in support of me.
маркетплейс kraken
Today, while I was at work, my sister stole my iphone and tested to see if it can survive a twenty five foot drop, just so she can be a youtube sensation. My iPad is now destroyed and she has 83 views. I know this is entirely off topic but I had to share it with someone!
https://http-kra40.cc
joszaki regisztracio joszaki.hu/
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar016.ru
вывод из запоя
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-krasnodar016.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar016.ru
вывод из запоя цена
The Real Person!
The Real Person!
Acho simplesmente esportivo MarjoSports Casino, tem um ritmo de jogo que vibra como um apito. Os jogos formam uma quadra de diversao. com slots tematicos de esportes. O atendimento esta sempre ativo 24/7. com solucoes precisas e instantaneas. Os ganhos chegam rapido como um gol. porem mais giros gratis seriam uma loucura de torcida. Ao final, MarjoSports Casino e o point perfeito pros fas de cassino para quem curte apostar com estilo esportivo! Vale dizer o design e fluido como um contra-ataque. elevando a imersao ao nivel de um gol.
cГіdigo promocional marjosports depГіsito|
The Real Person!
The Real Person!
Curto demais a energia de BR4Bet Casino, pulsa com uma forca de cassino digna de um holofote. A selecao de titulos e uma chama de emocoes. com slots tematicos de aventuras radiantes. Os agentes voam como claroes. respondendo rapido como um brilho na noite. Os saques voam como um facho de luz. mesmo assim as ofertas podiam ser mais generosas. Ao final, BR4Bet Casino promete uma diversao que e uma chama para os cacadores de vitorias reluzentes! De bonus o design e um espetaculo visual iluminado. fazendo o cassino brilhar como um farol.
br4bet br4bet login|
The Real Person!
The Real Person!
Curto demais o asfalto de F12.Bet Casino, oferece uma aventura que ruge como um escapamento. O catalogo de jogos e uma pista de emocoes. incluindo jogos de mesa com um toque de velocidade. O atendimento esta sempre ativo 24/7. respondendo rapido como uma largada. As transacoes sao faceis como um drift. de vez em quando as ofertas podiam ser mais generosas. Na real, F12.Bet Casino e um cassino online que e uma pista de diversao para os pilotos do cassino! Alem disso o design e fluido como um pit stop. fazendo o cassino roncar como um motor.
plataforma f12 bet|
The Real Person!
The Real Person!
Sou viciado no brilho de BR4Bet Casino, tem um ritmo de jogo que acende como uma tocha. As opcoes sao ricas e reluzem como luzes. com jogos adaptados para criptomoedas. O atendimento e solido como um farol. disponivel por chat ou e-mail. Os pagamentos sao seguros e fluidos. porem queria mais promocoes que brilham como farois. Em sintese, BR4Bet Casino e uma fogueira de adrenalina para os amantes de cassinos online! Como extra a navegacao e facil como um facho de luz. transformando cada aposta em uma aventura iluminada.
codigo br4bet|
http://tourout.ru/
The Real Person!
The Real Person!
экстренный вывод из запоя краснодар
vivod-iz-zapoya-krasnodar017.ru
вывод из запоя цена
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar017.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar017.ru
вывод из запоя цена
The Real Person!
The Real Person!
https://dzen.ru/110km
The Real Person!
The Real Person!
вывод из запоя краснодар
vivod-iz-zapoya-krasnodar018.ru
лечение запоя
The Real Person!
The Real Person!
Acho simplesmente ardente Verabet Casino, oferece uma aventura que reluz como brasas vivas. Tem uma enxurrada de jogos de cassino irados. com slots tematicos de cerimonias antigas. O time do cassino e digno de um guardiao do fogo. com ajuda que ilumina como uma pira. As transacoes sao simples como uma tocha. mesmo assim queria promocoes que explodem como labaredas. Ao final, Verabet Casino vale explorar esse cassino ja para os amantes de cassinos online! Alem disso o design e fluido como uma pira. tornando cada sessao ainda mais flamejante.
vera bet saque minimo|
Je suis obsede par BankOnBet Casino, c’est un casino en ligne qui brille comme un coffre-fort de lingots. La selection du casino est une pile de plaisirs. incluant des jeux de table de casino d’une elegance bancaire. Le personnel du casino offre un accompagnement digne d’un gestionnaire. joignable par chat ou email. Les gains du casino arrivent a une vitesse bancaire. mais I’d want more promos that compound like interest. Au final, BankOnBet Casino is a casino that banks on fun pour those chasing smart stakes! A noter the interface is smooth as a deposit. facilite une experience de casino rentable.
bankonbet affiliates|
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar018.ru
вывод из запоя цена
motocitee – Their layout feels modern without being cluttered—nice balance.
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar018.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
Sou louco pela fornalha de Fogo777 Casino, e um cassino online que queima como uma fogueira ancestral. A selecao de titulos e uma pira de emocoes. com caca-niqueis que reluzem como brasas. O time do cassino e digno de um guardiao do fogo. com ajuda que ilumina como uma pira. Os ganhos chegam rapido como uma labareda. de vez em quando mais bonus regulares seriam flamejantes. Resumindo, Fogo777 Casino e uma pira de adrenalina para os amantes de cassinos online! Por sinal o site e uma obra-prima de estilo ardente. transformando cada aposta em uma aventura flamejante.
fogo777 com|
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-krasnodar019.ru
лечение запоя краснодар
The Real Person!
The Real Person!
Fluffy Favourites is a highly popular online slot game developed by Eyecon, widely available at many UK-licensed online casinos. Known for its charming fairground theme and cuddly toy symbols, the game has maintained its status as a fan-favourite since its release in 2006. Fluffy Spins is giving new players a shot at up to 500 Free Spins! Daily Free Spins Casino, spin the Mega Reel for a chance to win on Starburst and Fluffy Favourites. Ever since the launch of the Starburst Slot Machine in 2013, it has risen to the top as the most popular of the UK and European Starburst free play online. So what makes Starburst as popular today as when it launched in 2013? Cryptoleo Casino No Deposit Bonus 100 Free Spins Ivibet casino review and free chips bonus The answer to this question depends on the online poker provider that you are using, and this is a high variance game which often rewards.
https://gbm.ltd/bet-aviator-game-strategies-for-pakistani-players/
Roulette Sign Up Bonus Ireland Mr Green is a well-established online casino that offers a no deposit free spins bonus of up to 25 spins, which means that you can play for free every day. By far one of the most popular games in the UK, a safe and secure betting platform. Online slots and casino. Where you should play the casino games presented here. But the all have one thing in common as they wont reveal any sensitive information to the merchant, the first deposit bonus is only valid once. All you need is a decent smartphone and a reasonable internet connection, video slots United Kingdom the bottom menu bar is your main menu. If you are new to the online casino world, then NetBet Casino may be the perfect site for you due to its expert site design, which makes the site nice to look at and easy to navigate. All games are organised neatly into categories, and support, promotions, and payment methods can all be found at the top of the home screen. The excellent site design carries over to the mobile versions of NetBet Casino.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-krasnodar019.ru
экстренный вывод из запоя краснодар
The Real Person!
The Real Person!
вывод из запоя круглосуточно краснодар
vivod-iz-zapoya-krasnodar019.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
Estou completamente hipnotizado por IJogo Casino, tem um ritmo de jogo que se enrosca como uma cobra. A colecao e uma teia de entretenimento. incluindo jogos de mesa com um toque de selva. O atendimento esta sempre ativo 24/7. respondendo veloz como uma rede. Os pagamentos sao lisos como uma raiz. mas mais recompensas fariam o coracao se enrolar. No geral, IJogo Casino e o point perfeito pros fas de cassino para os apaixonados por slots modernos! Vale dizer a plataforma reluz com um visual labirintico. elevando a imersao ao nivel de uma selva.
aplicativo ijogo|
The Real Person!
The Real Person!
Je suis accro a Grandz Casino, il propose une aventure de casino qui tournoie comme un voile mystique. vibre avec un voile de jeux varies. offrant des lives qui pulsent comme un theatre. Le support du casino est disponible 24/7. resonnant comme une illusion parfaite. arrivent comme un concerto voile. cependant des recompenses de casino supplementaires feraient danser. A la fin, Grandz Casino promet un divertissement de casino spectral pour les passionnes de casinos en ligne! En plus la plateforme du casino brille par son style theatral. fait vibrer le jeu comme un concerto ephemere.
la grandz bouffe|
The Real Person!
The Real Person!
Je suis captive par Casinia Casino, ca vibre avec une energie de casino digne d’un roi. vibre avec un rempart de jeux varies. proposant des slots de casino a theme medieval. Le service client du casino est un chevalier fidele. repondant en un eclat de lance. arrivent comme un tournoi. tout de meme plus de tours gratuits au casino ce serait legendaire. A la fin, Casinia Casino promet un divertissement de casino legendaire pour les joueurs qui aiment parier avec panache au casino! En bonus le design du casino est une fresque visuelle chevaleresque. ajoute une touche de grandeur au casino.
casinia casino betrug|
The Real Person!
The Real Person!
Je trouve absolument spectral Grandz Casino, c’est un casino en ligne qui danse comme une ombre fugace. propose un ballet de divertissement qui seduit. incluant des jeux de table de casino d’une elegance spectrale. Le support du casino est disponible 24/7. joignable par chat ou email. Les retraits au casino sont rapides comme un ballet fugace. neanmoins j’aimerais plus de promotions de casino qui flottent comme des ombres. Pour resumer, Grandz Casino cadence comme une sonate de victoires pour les passionnes de casinos en ligne! En bonus offre un orchestre de couleurs fugaces. cadence l’aventure avec une rhapsodie spectrale.
grandz-casino|
The Real Person!
The Real Person!
Galera, resolvi compartilhar minha experiencia sobre o Bingoemcasa porque foi melhor do que pensei. O site tem um jeito leve que lembra um barzinho cheio de risadas. As salas de bingo sao cheias de energia, e ainda testei uns joguinhos extras, todos foram bem estaveis. O atendimento no chat foi rapido como nunca vi, o que ja me deixou tranquilo. As retiradas foram instantaneas, inclusive testei cripto e caiu em minutos. Se pudesse apontar algo, diria que podiam ter bonus semanais, mas nada que estrague a experiencia. Resumindo, o Bingoemcasa me conquistou. Vale testar sem medo
bingoemcasa net|
Slot games https://betvisabengal.com
Coin Strike: Hold and Win https://lemon-cazino-pl.com
The Real Person!
The Real Person!
лечение запоя краснодар
vivod-iz-zapoya-krasnodar020.ru
вывод из запоя краснодар
The Real Person!
The Real Person!
Bakı Formula 1 biletləri ilə bağlı ən sərfəli təklifləri araşdırın. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Bakı mərhələsi barədə bütün məlumatlar ➡ formula 1 baku.
Formula 1 haqqinda melumat axtaranlar üçün faydalı mənbə. Formula 1 volunteer təcrübəsi ilə bağlı məsləhətlər.
Formula 1 canlı izləmə üçün şifresiz linklər. Formula 1 2025 Baku mərhələsi barədə ən son xəbərlər. Formula 1 Bakı 2024 nə zaman keçiriləcək. Formula 1 logo simvolikasının mənası barədə məlumat.
Cleaning is needed cleaning toronto eco-friendly supplies, vetted cleaners, flat pricing, online booking, same-day options. Bonded & insured crews, flexible scheduling. Book in 60 seconds—no hidden fees.
Портал Чернівців https://58000.com.ua оперативні новини, анонси культурних, громадських та спортивних подій, репортажі з міста, інтерв’ю з чернівчанами та цікаві історії. Все про життя Чернівців — щодня, просто й доступно
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-krasnodar020.ru
вывод из запоя цена
The Real Person!
The Real Person!
2024 və 2025 Formula 1 təqvimlərinin tam siyahısını əldə edin. Formula 1 azerbaycan mərhələsi barədə dəqiq xəbərlər.
Könüllü proqramı ilə bağlı ətraflı məlumat ➡ formula 1 volunteer 2025.
Formula 1 canli yayım üçün yeni platformalar. Formula 1 Baku 2025 üçün ən maraqlı məlumatlar.
Formula 1 izləmək üçün ən yaxşı saytlar. Formula 1 haqqında ən çox verilən suallar. Formula 1 volunteer 2025 qeydiyyatı başladı. Formula 1 logo simvolikasının mənası barədə məlumat.
The Real Person!
The Real Person!
Formula 1 canlı izləmə imkanı ilə yarışları qaçırmayın. Formula 1 sürücüləri haqqında maraqlı faktlar.
Könüllü proqramı ilə bağlı ətraflı məlumat ➡ formula 1 volunteer 2025.
Formula 1 schedule daim yenilənir və asan izlənilir. Formula 1 2024 Bakı yarışlarının tarixi artıq məlumdur.
Formula 1 Baku track barədə dəqiq xəritə məlumatı. Formula 1 tickets almaq üçün sərfəli variantlar. Formula 1 cars dizaynı və yenilikləri. Formula 1 logo simvolikasının mənası barədə məlumat.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-krasnodar020.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
2024 və 2025 Formula 1 təqvimlərinin tam siyahısını əldə edin. Formula 1 avtomobillərinin texniki göstəricilərini müqayisə edin.
Ən yaxşı nəticələr və statistikalar üçün baxın ➡ formula 1 standings.
Formula 1 pilotları haqqında ətraflı bioqrafiyalar. Formula 1 puan cədvəli ilə favoritlərinizi izləyin.
Formula 1 drivers siyahısı daim dəqiqləşdirilir. Formula 1 Azərbaycan mərhələsinin atmosferi barədə şərhlər. Formula 1 2024 takvimi ilə bağlı maraqlı dəyişikliklər. Formula 1 canlı izləmə platformaları haqqında təcrübələr.
The Real Person!
The Real Person!
Formula 1 izləmək üçün hansı kanalları seçmək barədə tövsiyələr. Formula 1 logo və brendləşmə detallarını kəşf edin.
Ətraflı təqvim və nəticələr üçün baxın ➡ http://www.formula-1.com.az.
Formula 1 volunteer 2025 proqramına qoşulmaq üçün şərtlər. Formula 1 Bakı 2024 biletləri onlayn əldə oluna bilər.
Formula 1 canlı izləmə üçün şifresiz linklər. Formula 1 haqqında ən çox verilən suallar. Formula 1 izləmək üçün ən yaxşı kanalı seçin. Formula 1 baku 2024 tickets artıq satışdadır.
Good day! This is my first comment here so I just wanted to give a quick shout out and say I really enjoy reading through your articles. Can you recommend any other blogs/websites/forums that cover the same subjects? Thanks a lot!
Do you offer workshops?
The Real Person!
The Real Person!
Formula 1 puan durumu daim yenilənir və oyunçular üçün əlçatandır. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Yarışları canlı izləmək istəyənlər üçün keçid ➡ formula 1 canlı izle.
Formula 1 Türkiye mərhələsi haqqında ətraflı yazılar. Formula 1 təqvimində dəyişikliklər barədə xəbərlər.
Formula 1 Baku track barədə dəqiq xəritə məlumatı. Formula 1 2025 Baku mərhələsi barədə ən son xəbərlər. Formula 1 izləmə yolları haqqında faydalı təlimatlar. Formula 1 canlı izləmə platformaları haqqında təcrübələr.
The Real Person!
The Real Person!
Капельница от запоя в Красноярске — прекрасный способ вернуть здоровье, который помогает быстро восстановить здоровье пациента. При употреблении алкоголя в больших количествах наблюдаются тяжелые симптомы, такие как тошнота, головные боли и нарушения эмоционального фона. В клиниках по лечению зависимостей в Красноярске доступны профессиональные услуги, включая инфузионную терапию для детоксикации организма. vivod-iz-zapoya-krasnoyarsk016.ru Капельницы — это только часть лечения алкоголизма, но и поддержку при отказе от алкоголя. Консультация нарколога поможет определить индивидуальный подход к восстановлению после запоя. Могут быть предложены антидепрессанты и уколы для улучшения состояния для улучшения состояния пациента. vivod-iz-zapoya-krasnoyarsk016.ru предлагает профессиональное лечение алкогольной зависимости, где опытные врачи гарантируют профессиональный подход к вашему лечению. Не откладывайте на завтра, начните путь к здоровой жизни уже сегодня!
The Real Person!
The Real Person!
Je suis accro a RollBit Casino, il propose une aventure de casino qui deroule comme un bit en chaine. Il y a une cascade de jeux de casino captivants. comprenant des jeux de casino adaptes aux cryptomonnaies. offre un soutien qui deroule tout. offrant des solutions claires et instantanees. Les retraits au casino sont rapides comme un deroulement. quand meme des bonus de casino plus frequents seraient numeriques. A la fin, RollBit Casino enchante avec une rhapsodie de jeux pour les passionnes de casinos en ligne! Ajoutons resonne avec une melodie graphique pixelisee. ce qui rend chaque session de casino encore plus pixelisee.
rollbit careers|
The Real Person!
The Real Person!
Помощь при запое в Красноярске – это недостаток времени, где важно не упустить момент. На сайте vivod-iz-zapoya-krasnoyarsk016.ru вы можете найти информацию о медицинских центрах, где доступны услуги по лечению зависимостей. Следует учитывать, что борьба с алкоголизмом требует не только медикаментозное лечение, но и психологическую помощь, программы восстановления. Консультация врача поможет определить оптимальный план лечения зависимости. Выход из запоя может потребовать экстренной помощи при алкогольной интоксикации, что также доступно в специализированных учреждениях. Процедуры кодирования – одна из способствующих методик, которая способствует отказу от алкоголя. Семейная поддержка играет ключевую роль в процессе реабилитации. После лечения необходима помощь в социальной реинтеграции. Заботьтесь о своем здоровье и ищите помощь у специалистов!
The Real Person!
The Real Person!
Estou alucinado com JonBet Casino, e um cassino online que ressoa como um sino ancestral. Tem uma enxurrada de jogos de cassino irados. oferecendo sessoes ao vivo que ressoam como corais. Os agentes sao rapidos como uma onda sonora. assegurando apoio sem dissonancias. Os pagamentos sao seguros e fluidos. entretanto mais giros gratis seriam uma loucura sonora. Na real, JonBet Casino e uma vibracao de adrenalina para os amantes de cassinos online! De lambuja o design e fluido como uma onda sonora. fazendo o cassino ecoar como um sino.
jonbet cnpj|
The Real Person!
The Real Person!
Je suis charme par Boomerang Casino, il propose une aventure de casino qui ricoche comme un projectile. La selection du casino est une boucle de plaisirs. avec des slots qui bouclent comme des arcs. offre un soutien qui boucle tout. repondant en un ricochet circulaire. Les retraits au casino sont rapides comme un retour boomerang. tout de meme plus de tours gratuits au casino ce serait arque. En somme, Boomerang Casino resonne comme un concerto de plaisir pour les fans de symphonies circulaires! De surcroit resonne avec une melodie graphique arque. ce qui rend chaque session de casino encore plus circulaire.
boomerang casino opinie|
The Real Person!
The Real Person!
Je suis envoute par RollBit Casino, on dirait un labyrinthe de frissons numeriques. L’assortiment de jeux du casino est une matrice de delices. avec des slots qui pixelisent comme des bits. L’assistance du casino est chaleureuse et precise. assurant un support de casino immediat et structure. Les transactions du casino sont simples comme un pixel. parfois plus de tours gratuits au casino ce serait cubique. Au final, RollBit Casino est un casino en ligne qui deroule une symphonie de bits pour les codeurs du casino! Par ailleurs le design du casino est une fresque visuelle numerique. ajoute une touche de rythme numerique au casino.
rollbit airdrop|
The Real Person!
The Real Person!
Sou viciado no reverb de JonBet Casino, pulsa com uma forca de cassino digna de um sino. As opcoes sao ricas e vibram como cordas. com jogos adaptados para criptomoedas. O suporte e um reverb preciso. disponivel por chat ou e-mail. As transacoes sao simples como um reverb. mesmo assim queria promocoes que vibram como sinos. Na real, JonBet Casino vale explorar esse cassino ja para os maestros do cassino! Vale dizer o layout e vibrante como uma corda. tornando cada sessao ainda mais ressonante.
jonbet com Г© confiГЎvel|
The Real Person!
The Real Person!
Прокапка после запоя — это важная медицинская процедура , предназначенная для очищения организма и восстановление состояния здоровья пациента . Во время обращения к наркологу для оказания помощи на дому осуществляется введение лекарственных средств через капельницу, что способствует очищению крови от токсичных веществ, которые накапливаются при злоупотреблении алкоголем. Нарколог на дом срочно В процессе обычно используются солевые растворы, витамины и минералы, что способствует улучшению самочувствия и уменьшает проявления похмелья. Уход на дому позволяет пациенту находиться в привычной обстановке , что важно для успешной реабилитации . Наркологическая помощь включает в себя психологическую и медицинскую поддержку, что значительно повышает шансы на восстановление организма после алкоголя . Необходимо соблюдать рекомендации по лечению и проводить прокапку регулярно для достижения наилучших результатов .
The Real Person!
The Real Person!
Je suis totalement captive par Boomerang Casino, est un retour de divertissement qui boucle. Le repertoire du casino est un arc de divertissement. incluant des tables qui ricochet comme un projectile. Le support du casino est disponible 24/7. proposant un appui qui enchante. Les paiements du casino sont securises et fluides. cependant des recompenses de casino supplementaires feraient revenir. En fin de compte, Boomerang Casino promet un divertissement de casino arque pour ceux qui cherchent l’adrenaline boomerang du casino! Par ailleurs la navigation du casino est intuitive comme une boucle. enchante chaque partie avec une symphonie de retours.
boomerang casino codice bonus|
The Real Person!
The Real Person!
Актуальные методы лечение запойного состояния в Красноярске основываются на интегрированном подходе, который гарантирует эффективное лечение алкоголизма и поддерживающие мероприятия. Основная цель состоит в том, чтобы очистка организма, что способствует устранить телесную зависимость. Наркологическая помощь предлагает медицинскую помощь и психологическую поддержку, направленную на восстановление психологического состояния. помощь нарколога Неотложная психотерапия часто требуется для минимизации негативных эффектов. Крайне важно обеспечить поддержку семьи, так как это способствует успешной реабилитации. Индивидуальный подход к каждому случаю дает возможность разработать программы лечения алкоголизма, учитывающие особенности клиента. Консультации врачей-наркологов способствуют лучше осознать путь к выздоровлению после запоя, а психологическая поддержка играет ключевую роль в поддержании трезвости.
The Real Person!
The Real Person!
трип скан TripSkan – это ваш надежный путеводитель в мире путешествий. Мы предлагаем удобный поиск и сравнение цен на авиабилеты, отели, автомобили и другие услуги, необходимые для вашего идеального отдыха. Наша цель – сделать процесс планирования путешествия максимально простым и приятным, чтобы вы могли сосредоточиться на самом главном – получении незабываемых впечатлений. С TripSkan вы всегда найдете лучшие предложения и сможете сэкономить время и деньги. Присоединяйтесь к нашему сообществу путешественников и откройте для себя новые горизонты! tripskan
The Real Person!
The Real Person!
Formula 1 izləmək üçün hansı kanalları seçmək barədə tövsiyələr. Formula 1 avtomobillərinin texniki göstəricilərini müqayisə edin.
Pilotlar haqqında geniş məlumat əldə edin ➡ formula 1 drivers.
Formula 1 schedule daim yenilənir və asan izlənilir. Formula 1 Baku 2025 üçün ən maraqlı məlumatlar.
Formula 1 schedule izləyicilər üçün rahat seçimdir. Formula 1 puan durumu ilə komandaları izləyin. Formula 1 movie izləyicilər üçün əyləncəli seçimdir. Formula 1 2025 Baku üçün təqvim yenilənib.
The Real Person!
The Real Person!
Использование капельниц при запое – это незаменимый метод при интоксикации алкоголем. Нарколог на дом круглосуточно может оказать экстренную помощь‚ обеспечивая дезинтоксикацию и восстановление после запоя. Симптомы алкоголизма‚ такие как тревожные симптомы‚ головная боль и слабость‚ требуют незамедлительных действий. Устранение запойного состояния включает инфузии‚ которые помогают бороться с абстиненцией и детоксифицировать организм из организма. Обращение к наркологу важна для установления последующей терапии: лечение в стационаре или амбулаторная помощь. Наркологическая клиника предлагает квалифицированную поддержку‚ чтобы исключить возможность рецидива и заботиться о здоровье пациента.
The Real Person!
The Real Person!
школа английского в москве
The Real Person!
The Real Person!
Mentre si accende la slot, si vede un vecchio veliero di legno in mare guidato da una luna piena. Questa introduzione aumenta l’emozione nell’attesa di vedere cosa porterà la Quest di Gonzo. Il primo ad arrivare è il piccolo eroe in persona, raffigurato come un tizio barbuto seduto alla sinistra dei rulli. Le immagini in 3D sono molto impressionanti, in quanto sembra arrotondato e realistico, soprattutto quando continua a grattarsi la barba. Sta fissando intensamente i rulli, che sono composti da simboli inca. Gli effetti sonori portano dritti nella giungla con il suono degli uccelli, degli altri animali, dei battiti e dei tintinnii. Potrebbe essere il tintinnio delle monete d’oro che sentiamo? Anche se sembra che la funzione Avalanche rallenti il gioco, in realtà i simboli cadono molto velocemente. L’indicatore del moltiplicatore in alto a sinistra dello schermo aiuterà a tenere il passo con le vincite moltiplicate effettuate. Le impostazioni avanzate di Autoplay sono altresì disponibili e permettono di programmare a che punto si desidera che la slot Gonzo’s Quest smetta di puntare automaticamente.
https://www.alifnagri.net/recensione-pirots-la-nostra-opinione-sul-gioco-di-elk-studios/
Giocando alla slot Gonzo’s Quest da smartphone o tablet troverete un layout più essenziale: informazioni e impostazioni di puntata sono accessibili dalle tre lineette in alto a destra; è stato inoltre eliminato il personaggio di Gonzo. © 2021 Jannìa – Tutti i diritti riservati | Privacy Policy | Termini e Condizioni Gonzo’s Quest Megaways Come hai appena visto, il gioco bonus della slot Gonzo’s Quest di NetEnt si basa sulla funzionalità “Free Fall” che, durante una “Avalanche”, dà il meglio di sé. È giunto il momento di scoprirne le ragioni. Se sei alla ricerca di un’esperienza di scommessa diversa, puoi provare le scommesse virtuali, dove puoi puntare su eventi simulati in tempo reale. Per attivare i bonus sui depositi, inserisci il codice BLBET50 al primo deposito e in automatico avrai attivi i successivi tre bonus di benvenuto. Effettua un deposito minimo di 10€ per ogni bonus e sblocca progressivamente i bonus depositati. Il primo deposito è del 50% fino a 100€, il secondo del 75% fino a 200€, il terzo dell’80% fino a 300€ e il quarto del 100% fino a 400€. Ogni bonus deve essere rigiocato 35 volte per essere convertito in bonus reale, che deve essere rigiocato una volta entro 7 giorni per essere prelevabile.
Для склада мебели мы приобрели оборудование Labelaire, а также беспроводные сканеры штрих-кодов. Теперь сотрудники могут быстро находить и маркировать товары. Вся информация фиксируется в системе автоматически. Это решение помогло нам ускорить инвентаризацию и сократить количество ошибок: https://labelaire.ru/
The Real Person!
The Real Person!
Алкогольный запой – это состояние‚ характеризующееся продолжительным приемом алкоголя‚ и это может вызвать опасные последствия. Признаки алкогольной зависимости в себя необходимость в спиртном‚ поэтому незамедлительное лечение запоя необходимым. В Красноярске медицинская помощь при запое осуществляется в специальных медицинских учреждениях‚ где предлагаются услуги нарколога; последствия запоя могут варьироваться от физического ухудшения здоровья до психологических проблем. экстренный вывод из запоя Красноярск Экстренный вывод из запоя предполагает detox-процедуры‚ которые помогают организму избавиться от токсинов. Психологическая поддержка при алкоголизме также играет ключевой ролью в восстановлении. Реабилитация алкоголиков в Красноярске предлагает комплексный подход к лечению‚ включая профилактику запоев и поддержку в период восстановления. Важно помнить‚ что лечение алкоголизма нуждается в профессиональной помощи для снижения рисков и повышения качества жизни.
Сколько стоит монтаж видеонаблюдения https://vcctv.ru
Use Marinade Solana for fast staking execution
Marinade Finance is gaining momentum — check it out at MarinadeFinance.at
The Real Person!
The Real Person!
Je suis hante par Casombie, c’est une experience qui reveille les sens. Les options sont vastes comme un cimetiere, offrant des sessions live dignes d’un film d’horreur. Le service client est d’une efficacite surnaturelle, offrant des solutions claires comme un clair de lune. Les transactions sont fluides et fiables, neanmoins des recompenses en plus seraient diaboliques. Pour resumer, Casombie est un must pour les amateurs de sensations fortes pour les joueurs en quete de frissons ! De plus l’interface est fluide comme un fantome, donne envie de revenir hanter le site.
casombie jГЎtГ©kok|
The Real Person!
The Real Person!
J’ai une obsession totale pour Freespin Casino, ca propulse dans un vortex de plaisir. Il y a une cascade de jeux envoutants, avec des slots aux visuels electrisants. Les agents repondent plus vite qu’un eclair, offrant des solutions nettes comme un cristal. Les paiements sont securises comme une voute celeste, cependant des recompenses supplementaires seraient stellaires. En somme, Freespin Casino est une plateforme qui illumine l’esprit pour les amateurs de sensations eclatantes ! A noter la plateforme brille comme une constellation, facilite une experience fluide et radieuse.
free spin casino coupons|
The Real Person!
The Real Person!
Je suis circuite par Robocat Casino, on detecte un blueprint de strategies innovantes. Le kit est un hub de diversite high-tech, avec des slots aux circuits thematiques qui font clignoter les gains. Les techs interviennent avec une precision laser, declenchant des protocoles multiples pour une resolution instantanee. Le pipeline est code pour une fluidite exemplaire, toutefois des spins gratuits supplementaires boosteraient les modules. En compilant le code, Robocat Casino se pose comme un core pour les innovateurs pour les hackers de casinos virtuels ! En plus la circulation est instinctive comme un script, simplifie la traversee des labs ludiques.
robocat 270 hobbygaga|
The Real Person!
The Real Person!
Стоимость капельницы от запоя на дому в Красноярске могут изменяться в зависимости от нескольких условий. Во-первых, стоимость услуг нарколога может различаться в зависимости от опыта врача и репутации медицинского учреждения. Выездной нарколог предлагает удобство, но это также влияет на цену.Кроме того, тип капельницы и её компоненты (например, глюкоза, витамины) также определяют стоимость. Обычно, стоимость капельницы включает в себя услуги медицинской помощи на дому, что добавляет к общим расходам.Наркологические услуги, такие как лечение алкоголизма и реабилитация от запоя, могут различаться по стоимости в зависимости от длительности и сложности лечения. Необходимо учитывать, что борьба с зависимостью от алкоголя требует комплексного подхода и профессиональной помощи. нарколог на дом Если вы ищете помощь при запое, рекомендуется обратиться к специалистам, которые могут предоставить актуальную информацию о ценах и услугах. В Красноярске услуги наркологии предлагают разнообразные решения для восстановления здоровья и улучшения качества жизни.
The Real Person!
The Real Person!
Je suis irremediablement appate par MrPacho Casino, c’est un festin ou chaque tour deploie des parfums de victoire. Les plats forment un tableau de textures innovantes, incluant des crash games comme JetX pour des pics de saveur. Les hotes interviennent avec une delicatesse remarquable, avec une maestria qui anticipe les appetits. Les retraits glissent avec une souplesse remarquable, toutefois plus de hors-d’?uvre bonus journaliers agrementeraient le festin. Dans l’ensemble du menu, MrPacho Casino devoile un itineraire de triomphes succulents pour les architectes de victoires savoureuses ! En sus le portail est une salle a manger visuelle imprenable, instille une essence de mystere culinaire.
mrpacho code|
інформаційний портал https://01001.com.ua Києва: актуальні новини, політика, культура, життя міста. Анонси подій, репортажі з вулиць, інтерв’ю з киянами, аналітика та гід по місту. Все, що треба знати про Київ — щодня, просто й цікаво.
інформаційний портал https://65000.com.ua Одеси та регіону: свіжі новини, культурні, громадські та спортивні події, репортажі з вулиць, інтерв’ю з одеситами. Всі важливі зміни та цікаві історії про життя міста — у зручному форматі щодня
The Real Person!
The Real Person!
Алкоголизм — это острое заболевание, требующее своевременного вмешательства и профессиональной помощи. В Красноярске доступен услуга вызова нарколога на дом, что позволяет получить необходимую медицинскую помощь в случае алкогольной зависимости в комфортной обстановке. Признаки алкоголизма состоят в частом употреблении алкоголя, потере контроля над количеством выпиваемого и возникновении признаков зависимости от алкоголя. вызов нарколога на дом Красноярск Борьба с алкоголизмом можно начать с диагностики зависимости, которую проведет опытный специалист в области наркологии. Консультация нарколога поможет выявить степень зависимости и определить оптимальную программу реабилитации. Важно помнить о роли поддержки семьи, которая является важным фактором в процессе выздоровления. Последствия алкоголизма могут быть очень серьезными, включая проблемы со здоровьем и психикой. Наркологическая помощь в Красноярске предлагает как фармакотерапию, так и реабилитационные программы, направленные на восстановление пациента. Не затягивайте с решением проблемы, обратитесь за помощью!
The Real Person!
The Real Person!
Je suis totalement envoute par Casombie, c’est une experience qui reveille les sens. La selection de jeux est terrifiante de richesse, avec des slots au design effrayant. Les agents repondent plus vite qu’un zombie affame, avec une aide aussi fluide qu’un spectre. Les gains arrivent a une vitesse demoniaque, de temps a autre plus de promos macabres seraient un plus. En somme, Casombie vaut chaque frisson qu’il procure pour ceux qui aiment l’adrenaline sombre ! A noter l’interface est fluide comme un fantome, facilite une experience aussi fluide qu’un spectre.
casombie code promo|
The Real Person!
The Real Person!
J’ai une obsession totale pour Freespin Casino, ca propulse dans un vortex de plaisir. Il y a une cascade de jeux envoutants, offrant des sessions live qui captivent l’ame. L’assistance est precise comme une etoile filante, joignable a tout moment. Les gains atterrissent a la vitesse lumiere, cependant des tours gratuits en plus feraient scintiller. En conclusion, Freespin Casino offre une experience aussi brillante qu’une comete pour les fans de casinos en ligne ! En bonus le site est un chef-d’?uvre visuel scintillant, donne envie de replonger dans l’univers du jeu.
energy casino free spin|
The Real Person!
The Real Person!
Je suis circuite par Robocat Casino, ca assemble un reseau de quetes ludiques. Les composants tracent un schema de gadgets avant-gardistes, incluant des blackjacks pour des reboots tactiques. Le suivi optimise avec une efficacite absolue, declenchant des protocoles multiples pour une resolution instantanee. Les flux sont securises par des firewalls crypto, toutefois des updates promotionnels plus frequents upgradieraient le kit. Pour clore le boot, Robocat Casino forge une saga de jeu futuriste pour les builders de victoires high-tech ! A logger la structure vibre comme un processeur ancestral, infuse une essence de mystere algorithmique.
robocat manual|
The Real Person!
The Real Person!
Je suis emoustille par MrPacho Casino, il orchestre une symphonie de gains succulents. La carte est un grimoire de divertissements savoureux, proposant des blackjack revisites pour des bouffees d’adrenaline. Le support client est un sommelier attentif et omnipresent, accessible par messagerie ou appel instantane. Les butins affluent via USDT ou fiat fluide, bien qu’ des amuse-bouches gratuits supplementaires rehausseraient les plats. En bouclant le repas, MrPacho Casino tisse une tapisserie de divertissement gustatif pour ceux qui cuisinent leur fortune en ligne ! En cerise sur le gateau le portail est une salle a manger visuelle imprenable, accroit l’envoutement dans le royaume du gout.
mrpacho promotion|
Я читаю theHold, чтобы следить за прогнозами и курсами криптовалют. Материалы независимые и всегда в тему. Калькулятор доходности и обзоры бирж делают работу удобной. Очень доволен этим ресурсом https://thehold.ru/
The Real Person!
The Real Person!
Капельница при похмелье – данный метод является действительным методом лечения, который помогает быстро справиться с симптомами алкогольной интоксикации. Похмелье сопровождается обезвоживанием, что проявляется головной болью, тошнотой и общей слабостью. Капельница включает глюкозу и электролиты, которые возвращают баланс жидкости в организме. Использование капельницы для лечения похмелья позволяет быстро устранить симптомы, обеспечивая детоксикацию. Внутривенное введение жидкости ускоряет процесс восстановления. Капельница в домашних условиях может оказаться полезной, но требуется медицинская помощь для правильного подбора компонентов. Существуют и альтернативные методы: различные травы, например, мяту или зверобой, которые помогают улучшить состояние. Однако капельница остается наиболее быстрым и надежным способом облегчить страдания после пьянки. На сайте vivod-iz-zapoya-krasnoyarsk018.ru можно найти информацию о различных вариантах капельниц и их воздействии на организм.
The Real Person!
The Real Person!
вывод из запоя круглосуточно смоленск
vivod-iz-zapoya-smolensk019.ru
лечение запоя смоленск
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-smolensk019.ru
вывод из запоя
J’ai un veritable coup de c?ur pour Cheri Casino, on dirait un festival de sensations. La gamme de jeux est un veritable arc-en-ciel, offrant des sessions live qui hypnotisent. Le suivi est d’une precision eclatante, garantissant un support d’une clarte cristalline. Les paiements sont securises comme un coffre-fort, parfois des bonus plus petillants seraient un regal. Au final, Cheri Casino merite d’etre explore sans tarder pour les fans de casinos en ligne ! Cerise sur le gateau le site est un chef-d’?uvre visuel, ce qui rend chaque session absolument feerique.
cheri casino français|
Je suis facette par RubySlots Casino, ca expose un musee de defis cristallins. Le portfolio est un salon de variete lapidaire, integrant des progressifs comme Aztec’s Millions pour des mines de millions. Le service brille en permanence 24/7, avec une expertise qui prevoit les inclusions. Les retraits s’extraient avec une purete remarquable, occasionnellement des inclusions promotionnelles plus frequentes rehausseraient le portfolio. En sertissant le lot, RubySlots Casino se pose comme un pilier pour les joailliers pour les gardiens des voutes numeriques ! En carat supplementaire la structure rayonne comme un rubis ancestral, simplifie la traversee des galeries ludiques.
ruby slots $30 free chips|
The Real Person!
The Real Person!
Je suis irresistiblement bande par WildRobin Casino, ca eleve le jeu a une quete heroique. La clairiere de jeux est un carquois debordant de plus de 9000 fleches, integrant des lives comme Blackjack pour des duels d’arcs. Le service guette en continu 24/7, bandant des cordes multiples pour une frappe immediate. Les retraits filent avec une velocite remarquable, malgre cela des embuscades promotionnelles plus frequentes dynamiseraient le repaire. A la fin de cette quete, WildRobin Casino devoile un sentier de triomphes inattendus pour les tireurs des paris crypto ! En fleche supplementaire le portail est une clairiere visuelle imprenable, incite a prolonger la quete infinie.
wild robin casino avis|
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk019.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-smolensk020.ru
экстренный вывод из запоя
J’ai une passion debordante pour Frumzi Casino, ca pulse comme une tempete tropicale. Les options forment un ocean de surprises, offrant des sessions live qui hypnotisent. Le service client est d’une efficacite fulgurante, offrant des solutions limpides et rapides. Les retraits sont rapides comme une bourrasque, de temps a autre des recompenses supplementaires seraient torrentielles. Pour resumer, Frumzi Casino est une plateforme qui secoue les sens pour les amateurs de sensations tumultueuses ! En bonus la navigation est intuitive comme une brise oceane, ce qui rend chaque session absolument electrisante.
frumzi casino free spins|
Been checking out keyword lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more kittivit gambling than I can say for some other places I’ve tried.
Je suis aromatise par PepperMill Casino, ca exhale un jardin de defis parfumes. Il regorge d’une abondance de melanges interactifs, incluant des jackpots progressifs pour des pics d’essence. Le support client est un maitre herboriste vigilant et persistant, infusant des remedes limpides et immediats. Les recoltes affluent via USDT ou canaux fiat, bien qu’ des herbes de recompense additionnelles epiceraient les alliances. A la fin de cette degustation, PepperMill Casino tisse une tapisserie de divertissement olfactif pour ceux qui cultivent leur fortune en ligne ! Par surcroit le portail est une serre visuelle imprenable, pousse a prolonger le festin infini.
the peppermill resort spa casino|
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: узи вен ног
The Real Person!
The Real Person!
Je suis vise par WildRobin Casino, il decoche une salve de gains inattendus. Le feuillage est un parchemin de divertissements forestiers, offrant des cashbacks et VIP 5 tiers des maitres comme Pragmatic Play et Evolution Gaming. Le suivi protege avec une vigilance absolue, assurant une garde fidele dans la foret. Les retraits filent avec une velocite remarquable, toutefois davantage de fleches bonus quotidiennes affuteraient le carquois. En apotheose legendaire, WildRobin Casino invite a une traque sans fin pour les bandits de casinos virtuels ! Par surcroit le portail est une clairiere visuelle imprenable, amplifie l’engagement dans le repaire du jeu.
wild blossoms by robin pickens|
The Real Person!
The Real Person!
экстренный вывод из запоя смоленск
vivod-iz-zapoya-smolensk020.ru
экстренный вывод из запоя смоленск
Top topics and trends: https://clubcoma.org
Smart crypto trading https://terionbot.com with auto-following and DCA: bots, rebalancing, stop-losses, and take-profits. Portfolio tailored to your risk profile, backtesting, exchange APIs, and cold storage. Transparent analytics and notifications.
Everything you need is here: https://lesma-shop.de
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-smolensk021.ru
вывод из запоя смоленск
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-smolensk020.ru
лечение запоя
Thanks for your patience and sorry for the inconvenience!
The Real Person!
The Real Person!
Soccer tətbiqi futbol həvəskarları üçün idealdır.
Mini soccer star mod apk futbol oynamaq üçün əla seçimdir. Əlavə məlumat üçün http://soccer.com.az/ səhifəsinə baxmaq olar.
Bet365 soccer ilə idman mərclərinə qoşulun. Rocket soccer derby fərqli futbol janrını təqdim edir. World soccer champs apk para hilesi ilə əlavə resurs əldə edin.
Soccer game klassik futbol təcrübəsi təqdim edir. Soccer field dizaynı oyunda çox real görsənir. Spbo live score soccer ilə hər dəqiqə nəticələri görün. Soccer champs para hilesi ilə oyunda üstünlük qazanın.
Je suis totalement electrifie par Donbet Casino, c’est une deflagration de fun absolu. Il y a une avalanche de jeux captivants, offrant des sessions live qui electrisent. Le service client est d’une efficacite foudroyante, joignable a tout moment. Les transactions sont fiables et fluides, cependant les offres pourraient etre plus volcaniques. Pour resumer, Donbet Casino offre une experience aussi puissante qu’une eruption pour les joueurs en quete d’intensite ! En bonus la navigation est intuitive comme un eclair, ajoute une touche de magie volcanique.
donbet casino promo codes|
J’eprouve un vertige total pour Shuffle Casino, c’est une pioche ou chaque clic melange les destinees. Il foisonne d’une ribambelle de tirages interactifs, offrant des loteries SHFL avec des pots jusqu’a 2 millions des maitres comme Pragmatic Play. Les dealers reagissent avec une vivacite remarquable, accessible par bluff ou appel direct. Les flux financiers sont verrouilles par des jokers crypto, malgre cela des tirages gratuits supplementaires brasseraient les mains. Dans l’ensemble du jeu, Shuffle Casino forge une partie de jeu aleatoire pour les baroudeurs de casinos virtuels ! En plus la structure tourne comme un sablier ancestral, amplifie l’engagement dans le casino du hasard.
shuffle casino codes|
The Real Person!
The Real Person!
вывод из запоя круглосуточно смоленск
vivod-iz-zapoya-smolensk021.ru
вывод из запоя смоленск
Our local network of agencies has found your research so helpful.
I concur with your conclusions and will eagerly look forward to your future updates. The usefulness and significance is overwhelming and has been invaluable to me!
The Real Person!
The Real Person!
Je suis enrobe par Sugar Casino, il petrit une pate de recompenses fondantes. Il deborde d’une cascade de gourmandises interactives, incluant des crash pour des montees de miel. Le service mijote en continu 24/7, assurant une attention fidele dans la patisserie. Les transferts glissent stables et acceleres, occasionnellement des sucreries gratuites supplementaires rehausseraient les saveurs. A la fin de cette degustation, Sugar Casino forge une recette de jeu savoureuse pour ceux qui melangent leur destin en ligne ! De surcroit la circulation est instinctive comme un coulis, incite a prolonger la degustation infinie.
sugar casino no deposit bonus codes|
That’s some inspirational stuff. Never knew that opinions might be this varied. Thanks for all the enthusiasm to supply such helpful information here.
The Real Person!
The Real Person!
Soccer tətbiqi futbol həvəskarları üçün idealdır.
Soccer skills euro cup ilə bacarıqlarınızı artırın. Rəsmi mənbə kimi soccer.com.az istifadə edin.
Soccer random oyunu sadə və əyləncəlidir. Soccer streams ilə matçları online izləyin. Soccer guru tətbiqi ilə futbol biliklərinizi artırın.
Soccer game klassik futbol təcrübəsi təqdim edir. World soccer champs android oyun club versiyası çox populyardır. Spbo live score soccer ilə hər dəqiqə nəticələri görün. Dream league soccer 2017 sevilən versiyadır.
I would share your post with my sis.
The Real Person!
The Real Person!
Je suis subjugue par Donbet Casino, ca pulse comme un eclair foudroyant. Il y a une avalanche de jeux captivants, avec des slots au design audacieux. Le service client est d’une efficacite foudroyante, garantissant un support d’une puissance rare. Les transactions sont fiables et fluides, neanmoins plus de promos percutantes seraient un plus. Dans l’ensemble, Donbet Casino promet une aventure incandescente pour ceux qui cherchent l’adrenaline pure ! En bonus l’interface est fluide comme un torrent, amplifie l’immersion dans un tourbillon de fun.
donbet promo code 2025|
The Real Person!
The Real Person!
Je suis irresistiblement epice par PepperMill Casino, ca transfigure le jeu en une infusion eternelle. Le bouquet est un potager de diversite exuberante, proposant des blackjacks revisites pour des bouffees d’excitation. Le support client est un maitre herboriste vigilant et persistant, accessible par infusion ou missive instantanee. Les flux coulent stables et acceleres, toutefois plus d’infusions bonus quotidiennes parfumeraient l’atelier. Pour sceller l’arome, PepperMill Casino emerge comme un pilier pour les epicuriens pour ceux qui cultivent leur fortune en ligne ! En primeur le visuel est une mosaique dynamique et odorante, allege la traversee des vergers ludiques.
peppermill las vegas casino|
The Real Person!
The Real Person!
Mobil cihazlarda soccer oyunu yüksək keyfiyyətlə işləyir.
Flashscore mobile livescore today soccer ilə ani nəticələr alın. Real futbol təcrübəsi üçün ən doğru seçim http://soccer.com.az/ linkidir.
Super liquid soccer fərqli futbol təcrübəsi bəxş edir. Soccer star oyunçular üçün motivasiyaedici seçimdir. World soccer champs apk para hilesi ilə əlavə resurs əldə edin.
Soccer skills champions league ilə yeni bacarıqlar öyrənin. Fifa soccer mod apk team2earn futbolu daha real edir. Soccer live score ilə canlı nəticələri izləyin. Soccer games müxtəlif janrlarda futbol təqdim edir.
The Real Person!
The Real Person!
Je suis irresistiblement sucre par Sugar Casino, on savoure un comptoir de tactiques allechantes. La collection est un sirop de divertissements delicieux, proposant des roulettes pour des tours de sucre. Le service mijote en continu 24/7, assurant une attention fidele dans la patisserie. Le processus est lisse comme du caramel, bien que des friandises de recompense additionnelles glaceraient les alliances. Pour clore le sirop, Sugar Casino invite a une exploration sans indigestion pour les gardiens des bonbonnieres numeriques ! Par surcroit le portail est une vitrine visuelle imprenable, infuse une essence de mystere sucre.
sugar creek casino in hinton oklahoma|
The Real Person!
The Real Person!
iOS üçün soccer yükləyib pulsuz oynayın.
Dream league soccer oyunu gənclər arasında çox məşhurdur. Real futbol təcrübəsi üçün ən doğru seçim http://soccer.com.az/ linkidir.
Super liquid soccer fərqli futbol təcrübəsi bəxş edir. Dream league soccer 2019 hələ də sevilən versiyalardandır. Soccer bros multiplayer futbol üçün əla seçimdir.
Pro direct soccer məhsulları keyfiyyətli seçimdir. Fifa soccer mod apk team2earn futbolu daha real edir. Dream league soccer 2023 yeni komanda imkanları təqdim edir. World soccer champs hile son sürüm oyunçuların seçimi olub.
The Real Person!
The Real Person!
Ən son versiya ilə soccer daha real görünür.
Flashscore mobile livescore today soccer ilə ani nəticələr alın. Real futbol təcrübəsi üçün ən doğru seçim http://soccer.com.az/ linkidir.
Soccer live tətbiqi ilə canlı izləmə daha rahatdır. Dream league soccer 2019 hələ də sevilən versiyalardandır. Soccer guru tətbiqi ilə futbol biliklərinizi artırın.
Globe soccer awards beynəlxalq səviyyədə tanınır. Fifa soccer mod apk team2earn futbolu daha real edir. Sure soccer predictions mərc üçün yaxşı ipucları verir. Mini soccer star apk sadə və əyləncəlidir.
The Real Person!
The Real Person!
Эффективная капельница от алкогольной зависимости – это весьма проверенный метод лечения зависимости от алкоголя, который можно использовать в городе владимир с помощью врачей-наркологов, работающих на выезде. Процедура включает инъекции специальных растворов, которые помогают организму скорее справится с эффектами алкогольного опьянения. При обращении за помощью при запое, вы можете получить не лишь капельницу для снятия запоя, но и советы нарколога. врач нарколог на дом владимир Выведение из запоя гарантирует быстрое восстановление состояния пациента, а безопасность процедуры капельницы гарантируется опытными специалистами. Наркологическая помощь включает детокс-программу, которая направлена на восстановление организма. Этап реабилитации зависимых – это ключевой этап после терапии, который способствует избежать возврат к употреблению. В владимире предоставляются услуги врача нарколога на дом, что дает возможность получить терапию в комфортной обстановке. Не откладывайте, позвоните за экстренной помощью врача-нарколога прямо сейчас!
Сломалась машина? выездная техпомощь на дороге мы создали профессиональную службу автопомощи, которая неустанно следит за безопасностью автомобилистов в Санкт-Петербурге и Ленинградской области. Наши специалисты всегда на страже вашего спокойствия. В случае любой нештатной ситуации — от банальной разрядки аккумулятора до серьёзных технических неисправностей — мы незамедлительно выезжаем на место.
The Real Person!
The Real Person!
вывод из запоя круглосуточно смоленск
vivod-iz-zapoya-smolensk021.ru
лечение запоя смоленск
Долго пытались самостоятельно продвигать сайт, но результатов не было. В середине пути решили обратиться в Mihaylov Digital, и это было правильное решение. Команда сделала аудит, исправила ошибки и запустила комплексное SEO. Теперь наш сайт в топе и приносит стабильные заявки – https://mihaylov.digital/
The Real Person!
The Real Person!
Soccer tətbiqi futbol həvəskarları üçün idealdır.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Mobil oyunlar barədə öyrənmək üçün soccer com az linkinə keçid edin.
Soccer live tətbiqi ilə canlı izləmə daha rahatdır. Pro soccer online multiplayer həvəskarları üçün uyğundur. Soccer manager 2025 mod apk ilə komandanızı idarə edin.
Soccer game klassik futbol təcrübəsi təqdim edir. Fifa soccer mod apk team2earn futbolu daha real edir. Livescore.com.tr live soccer scores azarkeşlər üçün əhəmiyyətlidir. World soccer futbol sevərlərin əvəzolunmaz seçimidir.
Мы давно хотели дом в скандинавском стиле и нашли решение у СтройСинтез. Проект получился стильный, с большими окнами и просторными комнатами. Дом идеально вписался в участок и радует нас каждый день. Другие проекты можно найти по ссылке – https://stroysyntez.com/
The Real Person!
The Real Person!
Врач нарколог на дом: помощь в лечении зависимости Зависимость от наркотиков – это серьезная проблема, для решения которой необходима квалифицированная помощь. Первый шаг к выздоровлению – это консультация с наркологом. Вы можете узнать больше о том, как вызвать нарколога на дом, на сайте vivod-iz-zapoya-vladimir021.ru. Медицинская помощь включает детоксикацию организма и психологическую поддержку. Часто требуется реабилитация зависимых, где применяется программа реабилитации. Анонимное лечение обеспечивает комфорт и безопасность для пациента. Терапия на дому позволяет обеспечить необходимую помощь на дому, что особенно важно для тех, кто стесняется обращаться в клиники. Лечение алкогольной зависимости также доступно на дому, что способствует быстрейшему восстановлению. Обращение к квалифицированному специалисту – это залог успешной борьбы с зависимостями.
The Real Person!
The Real Person!
Mobil cihazlarda soccer oyunu yüksək keyfiyyətlə işləyir.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Futbol xəbərlərinin toplanıldığı əsas yer http://www.soccer.com.az sayılır.
Dream league soccer 2024 versiyası daha müasir imkanlar verir. Soccer streams ilə matçları online izləyin. Real soccer 2012 nostalji sevənlər üçün uyğundur.
Soccer super star mod apk ilə ulduz olun. World soccer champs android oyun club versiyası çox populyardır. Livescore.com.tr live soccer scores azarkeşlər üçün əhəmiyyətlidir. World soccer champs apk 8.3.2 para hilesi ilə resurs artırın.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
The Real Person!
The Real Person!
J’eprouve une gourmandise infinie pour MrPacho Casino, il orchestre une symphonie de gains succulents. Les plats forment un tableau de textures innovantes, offrant des jackpots progressifs des fournisseurs renommes comme Pragmatic Play. Les hotes interviennent avec une delicatesse remarquable, distillant des remedes clairs et prompts. Les retraits glissent avec une souplesse remarquable, bien qu’ des garnitures de recompense additionnelles epiceraient les alliances. Pour couronner le plat, MrPacho Casino emerge comme un pilier pour les epicuriens pour les maitres des paris crypto ! En primeur le visuel est une mosaique dynamique et appetissante, accroit l’envoutement dans le royaume du gout.
mrpacho casino en ligne|
Je suis anobli par SlotsPalace Casino, ca erige un empire de defis somptueux. La galerie de jeux est un trone abondant de plus de 6 000 sceptres, avec des slots aux themes grandioses qui font briller les lignees. L’assistance proclame des edits nets, declarant des solutions claires et promptes. Les flux tresoriers sont gardes par des remparts crypto, a l’occasion les edits d’offres pourraient s’etendre en opulence. Pour clore le trone, SlotsPalace Casino devoile un arbre de triomphes opulents pour les seigneurs des paris crypto ! En sus la circulation est instinctive comme un decret, simplifie la traversee des halls ludiques.
slots palace casino slots|
The Real Person!
The Real Person!
Если вы или ваши родные столкнулись с проблемой алкогольной зависимости, важно знать о возможностях вызова капельницы от запоя в владимире. Услуги медиков, предлагающие медицинскую помощь, включают эффективное лечение алкоголизма и детоксикацию организма. Капельница от алкоголя способствует выведению токсинов и восстановлению после запоя. Клиника в владимире предлагает экстренную помощь при запое и обсуждения нарколога. Вызов капельницы — это не только комфорт, но и важный шаг к здоровью и реабилитации. Мы понимаем, что вызов капельницы может стать первым шагом к избавлению от алкогольной зависимости. Свяжитесь за наркологической помощью, чтобы обрести профессиональную поддержку и начать путь к восстановлению. Не откладывайте свое здоровье на потом — приступайте к действию!} vivod-iz-zapoya-vladimir020.ru
The Real Person!
The Real Person!
Je suis pimente par PepperMill Casino, il concocte une alchimie de gains epoustouflants. Il regorge d’une abondance de melanges interactifs, proposant des blackjacks revisites pour des bouffees d’excitation. Le suivi cultive avec une constance impenetrable, avec une expertise qui presage les appetits. Les recoltes affluent via USDT ou canaux fiat, bien qu’ des herbes de recompense additionnelles epiceraient les alliances. A la fin de cette degustation, PepperMill Casino tisse une tapisserie de divertissement olfactif pour les alchimistes des enjeux crypto ! En sus la trame irradie comme un elixir ancestral, pousse a prolonger le festin infini.
comfort inn peppermill shepparton vic|
The Real Person!
The Real Person!
J’eprouve une energie surhumaine pour Super Casino, il deploie une campagne de gains extraordinaires. Il vrombit d’une flotte de quetes interactives, offrant des super spins et cash drops des heros comme Evolution et Pragmatic Play. Le service patrouille en continu 24/7, avec une intelligence qui anticipe les menaces. Les trophees atterrissent via Bitcoin ou Ethereum, bien que des missions promotionnelles plus intenses dynamisent l’arsenal. Dans la globalite du QG, Super Casino se pose comme un phare pour les vigilantes pour les sentinelles des bases numeriques ! Par surcroit le graphisme est un scan dynamique et immersif, incite a prolonger l’assaut infini.
super slots casino welcome offer|
The Real Person!
The Real Person!
Mobil cihazlarda soccer oyunu yüksək keyfiyyətlə işləyir.
Live soccer izləmək üçün ən rahat tətbiqlərdən biridir. Rəsmi mənbə kimi soccer.com.az istifadə edin.
Bet365 soccer ilə idman mərclərinə qoşulun. Soccer star oyunçular üçün motivasiyaedici seçimdir. Bubble soccer dostlarla əyləncəli vaxt keçirməyə imkan verir.
World soccer champs hile apk oyunçular arasında məşhurdur. World soccer champs maç atlama hilesi ilə oyun sürətlənir. Spbo live score soccer ilə hər dəqiqə nəticələri görün. World soccer champs hile son sürüm oyunçuların seçimi olub.
https://www.wildberries.ru/catalog/514992745/detail.aspx
The Real Person!
The Real Person!
Je suis irremediablement appate par MrPacho Casino, on discerne un jardin de defis appetissants. Il deborde d’une plethore de mets interactifs, integrant des live roulettes pour des tourbillons de suspense. Les hotes interviennent avec une delicatesse remarquable, assurant un accompagnement fidele au banquet. Les retraits glissent avec une souplesse remarquable, par intermittence des amuse-bouches gratuits supplementaires rehausseraient les plats. En bouclant le repas, MrPacho Casino tisse une tapisserie de divertissement gustatif pour les maitres des paris crypto ! Par surcroit le visuel est une mosaique dynamique et appetissante, allege la traversee des menus ludiques.
mrpacho code|
Промышленное оборудование и станки под ключ
The Real Person!
The Real Person!
Je suis anobli par SlotsPalace Casino, on percoit un cortege de man?uvres opulentes. La collection est un decret de divertissements imperiaux, offrant des cashbacks VIP et free spins des seigneurs comme Evolution et Pragmatic Play. Le support client est un chambellan vigilant et perpetuel, accessible par messager ou appel royal. Le ceremonial est sculpte pour une fluidite imperiale, a l’occasion des sceaux de recompense additionnels forgeraient des dynasties. En apotheose royale, SlotsPalace Casino sculpte une lignee de jeu grandiose pour les architectes de victoires imperiales ! A proclamer l’interface est un couloir navigable avec splendeur, amplifie l’immersion dans le royaume du jeu.
best spin palace slots|
The Real Person!
The Real Person!
Je suis irresistiblement epice par PepperMill Casino, ca exhale un jardin de defis parfumes. Il regorge d’une abondance de melanges interactifs, avec des dice slots aux motifs piquants qui titillent les sens. Les artisans repondent avec une acuite remarquable, mobilisant des sentiers multiples pour une extraction fulgurante. Les recoltes affluent via USDT ou canaux fiat, nonobstant les bouquets d’offres pourraient s’epanouir en generosite. En concluant l’infusion, PepperMill Casino convie a une exploration sans satiete pour ceux qui cultivent leur fortune en ligne ! En piment sur le gateau le parcours est instinctif comme un parfum familier, instille une quintessence de mystere epice.
peppermill empress inn centralia|
The Real Person!
The Real Person!
J’eprouve une energie surhumaine pour Super Casino, ca galvanise un arsenal de missions exaltantes. La panoplie de jeux est un arsenal surequipe de plus de 4 000 gadgets, incluant des blackjacks pour des parades defensives. Les sentinelles reagissent avec une alerte fulgurante, activant des signaux multiples pour une intervention immediate. Les trophees atterrissent via Bitcoin ou Ethereum, bien que des modules de recompense additionnels fortifient les escadrons. Dans la globalite du QG, Super Casino forge une legende de jeu supersonique pour les champions de victoires explosives ! En plus l’interface est un cockpit navigable avec precision, amplifie l’engagement dans l’arsenal du jeu.
super duper cherry casino|
Мир гаджетов без воды https://indevices.ru честные обзоры, реальные замеры, фото/видео-примеры. Смартфоны, планшеты, аудио, гейминг, аксессуары. Сравнения моделей, советы по апгрейду, трекер цен и уведомления о скидках. Помогаем выбрать устройство под задачи.
Ремонт и стройка https://remontkit.ru без лишних затрат: инструкции, таблицы расхода, сравнение цен, контроль скрытых работ. База подрядчиков, отзывы, чек-листы, калькуляторы. Тренды дизайна, 3D-планировки, лайфхаки по хранению и зонированию. Практика и цифры.
Ваш портал о стройке https://gidfundament.ru и ремонте: материалы, инструменты, сметы и бюджеты. Готовые решения для кухни, ванной, спальни и террасы. Нормы, чертежи, контроль качества, приёмка работ. Подбор подрядчика, прайсы, акции и полезные образцы документов.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
The Real Person!
The Real Person!
Капельница от запоя – это результативный способ терапии алкогольной зависимости‚ который реализует врач-нарколог на дом. Подготовка к процедуре включает важные моменты. Первым делом‚ важно выявить симптомы запоя: боли в голове‚ повышенная тревожность‚ потливость. Затем пациент должен организовать комфортное место для процедуры‚ подготовив место для установки капельницы. Наркологические услуги на дому позволяют снизить уровень стресса‚ который может возникнуть из-за поездки в медицинское учреждение. Медик проведет процедуру детоксикации‚ вводя растворы для восстановления‚ которые нормализуют состояние организма и повышают общее самочувствие. Преимущества капельницы заключается в оперативном удалении токсинов и улучшении состояния. В дополнение‚ инъекции для снятия запойного синдрома могут дополнительно помочь в стабилизации состояния. После процедуры начинается программа реабилитации‚ направленная на предотвращение рецидивов. врач нарколог на дом
The Real Person!
The Real Person!
лечение тревожных расстройств в Москве Лечение генерализованного тревожного расстройства (ГТР) требует комплексного подхода, поскольку это хроническое состояние характеризуется постоянным и чрезмерным беспокойством по поводу различных аспектов жизни. Психотерапия, в частности когнитивно-поведенческая терапия (КПТ), является важной частью лечения. КПТ помогает пациентам осознавать и изменять негативные мысли и поведение, которые способствуют тревоге. Медикаментозное лечение включает антидепрессанты (например, СИОЗС, СИОЗСН), которые помогают регулировать уровень серотонина и норадреналина в мозге, а также анксиолитики (например, буспирон), которые снижают тревожность без выраженного седативного эффекта. Важно отметить, что бензодиазепины, хотя и эффективны для быстрого снятия тревоги, не рекомендуются для длительного использования из-за риска развития зависимости. Дополнительные методы лечения включают техники релаксации, такие как диафрагмальное дыхание и прогрессивная мышечная релаксация, а также изменение образа жизни, включающее регулярные физические упражнения, здоровое питание и достаточный сон. В некоторых случаях может быть полезна групповая терапия или поддержка со стороны семьи и друзей. Индивидуальный план лечения, разработанный врачом, может значительно улучшить качество жизни людей с ГТР.
The Real Person!
The Real Person!
Детоксикация от алкоголя на дому в владимире — это эффективный способ помочь близким борющимся с алкоголизмом. Помощь при алкоголизме включает детоксикацию на дому, что дает возможность лечиться в привычной обстановке. Выведение из запоя может осуществляться с помощью медикаментовкоторые помогают справиться с симптомами абстиненции. Квалифицированная помощь в таких случаях важна: услуги нарколога помогут правильно подобрать антиалкогольную терапию. Психотерапия при алкоголизме также нужна для успешного восстановления в реабилитации. Поддержка семьи и возвращение к нормальной жизни помогают в процессе возвращения к трезвой жизни. Профилактические меры и реабилитация после запоя — ключевые моменты в возвращении к здоровой жизни. Не забывайте, что здоровый образ жизни без алкоголя. На сайте vivod-iz-zapoya-vladimir021.ru вы можете получить информацию о доступных услугах и методах лечения.
The Real Person!
The Real Person!
кайт египет Кайт школа – это учебное заведение, специализирующееся на обучении кайтсерфингу как начинающих, так и опытных райдеров. В кайт школах работают профессиональные инструкторы, которые обучают основам управления кайтом, правилам безопасности, нормам поведения на воде и базовым принципам кайтсерфинга. Кайт школы предоставляют все необходимое оборудование для обучения, в том числе кайты, доски, гидрокостюмы и спасательные жилеты. Обучение в кайт школе обеспечивает безопасное и эффективное освоение кайтсерфинга.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
Ремонт и строительство https://nastil69.ru от А до Я: планирование, закупка, логистика, контроль и приёмка. Калькуляторы смет, типовые договора, инструкции по инженерным сетям. Каталог подрядчиков, отзывы, фото-примеры и советы по снижению бюджета проекта.
The Real Person!
The Real Person!
кайт школа в хургаде Detivetra: “Detivetra” – это бренд, ассоциирующийся с высоким качеством обучения, профессиональным сервисом и дружной атмосферой в мире кайтсерфинга. Это синоним русскоязычного кайтсерфинга в Египте и за его пределами. “Detivetra” предлагает широкий спектр услуг, от обучения кайтсерфингу до организации кайт-сафари и кайт-кемпов. Бренд “Detivetra” известен своим профессиональным подходом к обучению, вниманием к безопасности и индивидуальным подходом к каждому клиенту. “Detivetra” – это ваш надежный партнер в мире кайтсерфинга, который поможет вам осуществить вашу мечту о покорении волн.
Нужен аккумулятор? https://dostavka-akb-spb.ru/ в наличии: топ-бренды, все размеры, правый/левый токовывод. Бесплатная проверка генератора при установке, trade-in старого АКБ. Гарантия до 3 лет, честные цены, быстрый самовывоз и курьер. Поможем выбрать за 3 минуты.
Хочешь сдать акб? сдать аккумулятор автомобильный в спб цена честная цена за кг, моментальная выплата, официальная утилизация. Самовывоз от 1 шт. или приём на пункте, акт/квитанция. Безопасно и законно. Узнайте текущий тариф и ближайший адрес.
Thanks so much for this, keep up the good work 🙂
The Real Person!
The Real Person!
Прокапывание на дому — это комфортное и результативное лечение, которое позволяет пациентам проходить инфузионную терапию в комфортной обстановке домашней обстановке. Услуги медсестры обеспечивают квалифицированное введение внутривенных инъекций, что помогает быстрому восстановлению и укреплению здоровья на дому. vivod-iz-zapoya-vladimir023.ru Уход на дому включает в себя не лишь саму процедуру, но и мониторинг здоровья пациента. Это особенно важно для людей с хроническими заболеваниями или тем, кто нуждается в профилактике заболеваний. При организации домашнего лечения можно быть уверенным в высоком качестве медицинских услуг, что существенно облегчает процесс реабилитации.
The Real Person!
The Real Person!
кайт сафари Кайт школа – это учебное заведение, специализирующееся на обучении кайтсёрфингу и другим видам спорта с кайтом. В кайт школах работают сертифицированные инструкторы, которые обучают основам управления кайтом, правилам безопасности и продвинутым техникам катания. Кайт школы предоставляют необходимое оборудование, включая кайты, доски, гидрокостюмы и шлемы. Обучение в кайт школе позволяет безопасно и эффективно освоить кайтсёрфинг и получить удовольствие от этого экстремального вида спорта.
Ищешь аккумулятор? купить аккумулятор для авто с обменом старого AKB SHOP занимает лидирующие позиции среди интернет-магазинов автомобильных аккумуляторов в Санкт-Петербурге. Наш ассортимент охватывает все категории транспортных средств. Независимо от того, ищете ли вы надёжный аккумулятор для легкового автомобиля, мощного грузовика, комфортного катера, компактного скутера, современного погрузчика или специализированного штабелёра
Нужен надежный акб? где купить аккумулятор для авто AKB STORE — ведущий интернет-магазин автомобильных аккумуляторов в Санкт-Петербурге! Мы специализируемся на продаже качественных аккумуляторных батарей для самой разнообразной техники. В нашем каталоге вы найдёте идеальные решения для любого транспортного средства: будь то легковой или грузовой автомобиль, катер или лодка, скутер или мопед, погрузчик или штабелер.
The Real Person!
The Real Person!
Je trouve completement incroyable Betsson Casino, ca ressemble a une energie de jeu irresistible. La gamme de jeux est tout simplement impressionnante, offrant des sessions de casino en direct immersives par Evolution Gaming. Le support est ultra-reactif avec une reponse en 30 secondes via chat, joignable a toute heure. Les gains sont verses en 24 heures pour les e-wallets, bien que les bonus pourraient etre plus frequents. En resume, Betsson Casino vaut pleinement le detour pour ceux qui aiment parier ! Par ailleurs l’interface est fluide et intuitive avec un theme orange vif, renforce l’immersion totale.
code betsson|
The Real Person!
The Real Person!
Je kiffe grave Gamdom, ca balance une vibe de folie. Les options sont ultra-riches et captivantes, avec des slots qui claquent grave. L’assistance est au top du top, avec une aide qui dechire tout. Les transactions sont simples comme un clin d’?il, quand meme j’aimerais plus de promos qui defoncent. Bref, Gamdom est une plateforme qui dechire tout pour les accros aux sensations extremes ! Et puis la navigation est simple comme un jeu d’enfant, ajoute un max de swag.
refferal code gamdom|
The Real Person!
The Real Person!
J’adore l’incandescence de Celsius Casino, on dirait une eruption de fun. La collection de jeux du casino est incandescente, proposant des slots de casino a theme volcanique. Le personnel du casino offre un accompagnement incandescent, avec une aide qui fait des etincelles. Les transactions du casino sont simples comme une etincelle, parfois des recompenses de casino supplementaires feraient monter la chaleur. En somme, Celsius Casino promet un divertissement de casino brulant pour les joueurs qui aiment parier avec panache au casino ! Par ailleurs la navigation du casino est intuitive comme un feu de camp, ce qui rend chaque session de casino encore plus enflammee.
celsius casino review|
The Real Person!
The Real Person!
Лечение зависимостей в владимире – это неотъемлемая часть борьбы с различными зависимостями. Если ваши знакомые столкнулись с трудностями, связанными с алкогольной зависимостью, реабилитационные центры предлагают эффективные программы лечения зависимостей. В наркологической клинике владимир предоставляется всесторонняя поддержка, включая выведение токсинов и психотерапию. Консультации нарколога помогут определить уровень зависимости и разработать индивидуальный план лечения. Психологическая помощь при зависимости и работа с семьями зависимых играют важное значение в процессе лечения. Также важно учитывать профилактику зависимостей, чтобы не допустить ухудшения ситуации. Анонимная помощь доступна всем, кто нуждается в ней. Способы борьбы с алкогольной зависимостью включают различные методы, подходящие для всех нуждающихся. Обратитесь на vivod-iz-zapoya-vladimir022.ru для получения консультации и записи на консультацию.
Все этапы лицензирования медицинской деятельности проходили под строгим контролем центра, что позволило получить лицензию без ошибок и задержек, https://licenz.pro/
The Real Person!
The Real Person!
J’apprecie enormement Betsson Casino, il offre une experience de jeu electrisante. La bibliotheque de jeux est phenomenale, incluant des slots de derniere generation comme Starburst. Les agents sont disponibles 24/7 et professionnels, offrant des solutions rapides et precises. Les paiements sont fluides et securises avec un maximum de 50 000 € par jour, cependant davantage de recompenses via le programme VIP seraient appreciees. Dans l’ensemble, Betsson Casino est un incontournable pour les amateurs de casino en ligne ! Ajoutons que l’interface est fluide et intuitive avec un theme orange vif, ce qui amplifie le plaisir de jouer.
avis betsson|
The Real Person!
The Real Person!
Je suis accro a Celsius Casino, c’est un casino en ligne qui fait jaillir des etincelles. La selection du casino est une explosion de plaisirs, proposant des slots de casino a theme volcanique. L’assistance du casino est chaleureuse et efficace, joignable par chat ou email. Le processus du casino est transparent et sans combustion, cependant des bonus de casino plus frequents seraient torrides. Globalement, Celsius Casino c’est un casino a decouvrir en urgence pour les amoureux des slots modernes de casino ! Par ailleurs la plateforme du casino brille par son style flamboyant, facilite une experience de casino torride.
celsius casino bonus|
The Real Person!
The Real Person!
Je trouve absolument fantastique 7BitCasino, ca ressemble a une sensation de casino unique. Il y a une profusion de titres varies, avec des machines a sous modernes et captivantes. Le service d’assistance est de premier ordre, joignable a toute heure. Les paiements sont fluides et securises, bien que les bonus pourraient etre plus reguliers, ou des promotions hebdomadaires plus frequentes. Pour conclure, 7BitCasino offre une experience de jeu securisee et equitable pour les adeptes de sensations fortes ! Notons egalement que le site est concu avec style et modernite, facilite chaque session de jeu.
7bitcasino no deposit bonus codes|
The Real Person!
The Real Person!
Срочная помощь при запое: капельница на дому в владимире Алкогольная зависимость – серьезная проблема‚ требующая профессионального вмешательства. Симптомы запойного состояния могут проявляться в тяжелых формах‚ такими как тремор‚ потливость‚ тревога и даже галлюцинации. В таких ситуациях необходима помощь нарколога для получения грамотной медицинской помощи. Домашняя терапия имеет свои преимущества: пациент находится в привычной обстановке‚ что ускоряет восстановление после запоя. Кроме того‚ стоит обратить внимание на профилактику рецидивов‚ чтобы избежать новых запоев. Обращаясь за помощью к специалистам‚ вы получите не только капельницы для детоксикации‚ но и комплексное лечение алкоголизма‚ направленное на полное восстановление.
The Real Person!
The Real Person!
Частный нарколог на дом – это важный шаг в борьбе с зависимостями. Выездной нарколог предоставляет анонимное лечение и профессиональные консультации‚ что особенно актуально для людей‚ ценящих комфорт и безопасность. Лечение наркотической зависимости и помощь при алкоголизме могут проводиться в домашней обстановке‚ что способствует более глубокому восприятию терапии. site;com Лечение алкоголизма включает детоксикацию на дому и реабилитационные программы‚ адаптированные под индивидуальные нужды пациента . Семейная терапия и психотерапевтическая помощь играют значительную роль в процессе выздоровления. Нарколог на дому обеспечит индивидуальный подход к каждому пациенту ‚ что повышает вероятность успешного выздоровления.
The Real Person!
The Real Person!
1хБет сегодня Ищете 1xBet официальный сайт? Он может быть заблокирован, но у 1хБет есть решения. 1xbet зеркало на сегодня — ваш главный инструмент. Это 1xbet зеркало рабочее всегда актуально. Также вы можете скачать 1xbet приложение для iOS и Android — это надежная альтернатива. Неважно, используете ли вы 1xbet сайт или 1хБет зеркало, вас ждет полный функционал: ставки на спорт и захватывающее 1xbet casino. 1хБет сегодня — это тысячи возможностей. Начните прямо сейчас!
The Real Person!
The Real Person!
Поддержка психолога при выходе из запоя в владимире: как преодолеть абстиненцию При абстинентном синдроме пациент часто испытывает физическими и психологическими страданиями. В таких случаях консультации психолога и кризисная интервенция могут значительно облегчить состояние. Центры психотерапии в владимире предлагают различные программы восстановления и методы борьбы с зависимостями, что помогает справиться с трудностями. вывод из запоя цена Советы по выходу из запоя включают использование психологической помощи и программы реабилитации наркозависимых, что значительно увеличивает вероятность успешного выздоровления. Необходимо учитывать, что процесс достижения трезвости требует времени, настойчивости и надежной поддержки.
Lucky Mate is an online casino for Australian players, offering pokies, table games, and live dealer options. It provides a welcome bonus up to AUD 1,000, accepts Visa, PayID, and crypto with AUD 20 minimum deposit, and has withdrawal limits of AUD 5,000 weekly. Licensed, it promotes safe play: Luckymate casino
Когда мы покупали кухню в Кухни в Дом, особенно понравился комплексный подход. Замер, проект, доставка и сборка — всё организовано чётко и без лишних хлопот. Дизайнер помог выбрать цвета и материалы, которые идеально подошли к интерьеру нашей квартиры. Кухня получилась очень функциональной: удобные шкафы, просторная рабочая зона, продуманное расположение техники. Доставка пришла вовремя, а сборщики сработали быстро и чисто. Теперь готовка стала настоящим удовольствием, а кухня радует глаз каждый день. Мы смело рекомендуем Кухни в Дом тем, кто ищет надёжную компанию https://kuhni-v-dom.ru/
The Real Person!
The Real Person!
Подбор клиники для вывода из запоя в владимире – значимый этап‚ который требует внимания к нескольким аспектам‚ включая цену предоставляемых услуг и качество медицинской помощи. При выборе клиники необходимо обратить внимание на следующие моменты. Во-первых‚ обратите внимание на наркологическими услугами‚ которые имеет учреждение. Многие коммерческие медицинские центры владимира имеют опытных профессиональных наркологов‚ которые могут обеспечить срочную помощь нарколога на дом. Это особенно актуально‚ если состояние пациента нуждается в оперативном решении.Во-вторых‚ ознакомьтесь с отзывами о клиниках владимира. Это даст возможность оценить‚ как другие пациенты отметили качество оказанной помощи и эффективность терапии. Не забудьте узнать о ценах на услуги вывода из запоя – цены могут варьироваться в зависимости от уровня сервиса и используемых методов. Также стоит рассмотреть программы реабилитации от алкоголизма и другие предложения‚ такие как терапия алкогольной зависимости. Некоторые клиники используют интегрированные методы‚ что может быть более эффективным. Важно помнить‚ что доступные цены на лечение не всегда гарантируют хорошее качество. Выбирая клинику‚ ищите оптимальное соотношение цены и качества при лечении алкоголизма. Не стесняйтесь информировать себя о методах лечения и возможностях‚ которые предлагает клиника. нарколог на дом срочно
The Real Person!
The Real Person!
Je suis scotche par Impressario, ca donne une energie de star absolue. Il y a un deluge de jeux captivants, proposant des sessions live qui en mettent plein la vue. Le service client est digne d’un gala, joignable par chat ou email. Le processus est limpide et sans fausse note, mais bon des recompenses en plus ca serait le feu. Bref, Impressario est une plateforme qui vole la vedette pour ceux qui kiffent parier avec style ! Et puis l’interface est fluide et glamour, facilite un show total.
impressario casino no deposit bonus code|
The Real Person!
The Real Person!
J’adore a fond 1win Casino, on dirait une aventure palpitante. La selection est incroyablement riche, proposant des jeux de table classiques et raffines. Les agents sont toujours prets a aider, garantissant une assistance de qualite. Les gains arrivent en un temps record, neanmoins plus de tours gratuits seraient un plus. Pour conclure, 1win Casino ne decoit jamais pour les fans de divertissement numerique ! De plus le design est visuellement attrayant, ajoute une touche d’elegance a l’experience.
1win betting|
The Real Person!
The Real Person!
Je suis totalement envoute par FatPirate, c’est une plateforme qui envoie du lourd. La selection est carrement dingue, offrant des machines a sous qui claquent. Le crew assure un suivi de ouf, repondant en deux secondes chrono. Les retraits sont rapides comme une tempete, mais bon des recompenses en plus ca serait la cerise. Pour resumer, FatPirate est un spot incontournable pour les joueurs pour les pirates des slots modernes ! Cote plus le design est style et accrocheur, booste l’immersion a fond.
fatpirate nl|
The Real Person!
The Real Person!
Капельница от запоя — это необходимая мера в помощи при алкогольной зависимости. Капельница способствует детоксикации детоксикации организма‚ выводя токсины и нормализуя водно-электролитный баланс. После ее применения пациенты испытывают положительные изменения в психоэмоциональном состоянии.Поддержка нарколога в этом процессе играет важную роль. Он проводит оценку симптомов абстиненции и разрабатывает план лечения запоя. Реабилитация после запоя включает обе стороны: физическую и психологическую. Поддержка близких играет ключевую роль‚ позволяя преодолеть последствия запойного состояния. помощь нарколога Реабилитация является следующим шагом в лечении‚ где пациент проходит лечение и обучение для предотвращения рецидивов. Анонимная помощь предоставляет анонимность и защиту. Стоит помнить‚ что лечение алкоголизма — это долгий путь‚ который требует как усилий‚ так и поддержки.
Получение лицензии на медицинскую деятельность прошло быстро и удобно, специалисты центра сопровождали весь процесс, проверяли документы и консультировали по нюансам https://licenz.pro/
The Real Person!
The Real Person!
Алкоголизм у женщин является серьезной проблемой, требующей особого подхода к лечению. В владимире специализированная клиника предлагает экстренный вывод из запоя, что особенно важно для женщин, которые нередко сталкиваются с обществом, осуждающим их проблему. Зависимость от алкоголя у женщин может развиваться быстрее, чем у мужчин, и проявляется особенными признаками, такими как подавленное настроение и тревожность.Лечение алкоголизма включает detox программу и медицинские методы лечения, а также психотерапию при алкоголизме. Важно позаботиться о поддержке со стороны близких и включение в группы взаимопомощи, что позволяет создать комфортные условия для выздоровления. Процесс реабилитации зависимых в владимире акцентирует внимание на восстановлении социальной активности, что особенно важно для женщин, оказавшихся в такой ситуации. экстренный вывод из запоя владимир Предотвращение зависимости и обращение за экстренной помощью — основные элементы в борьбе с алкоголизмом. Комплексное лечение к лечению обеспечит успешное восстановление и возвращение к нормальной жизни.
The Real Person!
The Real Person!
Je suis accro a Instant Casino, ca balance une vibe de jeu dementielle. La selection de titres de casino est dingue, offrant des machines a sous de casino uniques. Le support du casino est dispo 24/7, avec une aide qui pete le feu. Le processus du casino est clean et sans galere, quand meme j’aimerais plus de promos de casino qui dechirent. Pour resumer, Instant Casino est un casino en ligne qui cartonne pour ceux qui kiffent parier dans un casino style ! Et puis la plateforme du casino claque avec son look electrisant, facilite le delire total au casino.
golden tiger casino instant play|
Je suis totalement envoute par Cresus, il propose une aventure scintillante. La selection est tout simplement majestueuse, comprenant des jeux compatibles avec les cryptos. Le support est disponible 24/7, garantissant un support instantane. Les retraits sont fluides et rapides, de temps en temps des recompenses supplementaires seraient appreciees. Pour conclure, Cresus vaut largement le detour pour les joueurs en quete de magie ! De surcroit le site est elegant et bien concu, ajoute une touche de luxe.
service client cresus casino|
The Real Person!
The Real Person!
Je suis completement conquis par 1xbet Casino, il offre une aventure pleine de frissons. La selection de jeux est monumentale, incluant des slots de pointe. Le personnel offre un accompagnement irreprochable, offrant des reponses rapides et precises. Le processus de retrait est simple et fiable, bien que j’aimerais plus d’offres promotionnelles. Dans l’ensemble, 1xbet Casino ne decoit jamais pour les joueurs en quete d’adrenaline ! En bonus la navigation est intuitive et rapide, ajoute une touche de raffinement a l’experience.
telecharger 1xbet pour android|
The Real Person!
The Real Person!
Как предотвратить алкоголизм в владимире: рекомендации медиков Алкогольная зависимость — это сложная проблема‚ требующая внимания и мер. Необходимо быть в курсе способов профилактики и лечения. Можно обратиться к наркологу на дом в владимире можно Врачи рекомендуют проводить профилактические меры‚ включая информирование о последствиях алкоголизма и поддержку семьи. вызвать нарколога на дом владимир В процесс лечения алкоголизма входят реабилитационные программы‚ которые помогают вернуть здоровье и социальную интеграцию. Наркологическая помощь необходима для работы с зависимыми‚ а также для предотвращения рецидивов. Здоровье и алкоголь — это вечная борьба‚ и своевременная поддержка играет ключевую роль.
Marinade Finance brings you real-time staking rewards
The fastest way to stake? Marinade Finance at MarinadeFinance.at
The Real Person!
The Real Person!
Je kiffe grave Instant Casino, c’est un casino en ligne qui envoie du lourd. Le catalogue de jeux de casino est colossal, comprenant des jeux de casino tailles pour les cryptos. Le support du casino est dispo 24/7, joignable par chat ou email. Les transactions de casino sont simples comme un neon, des fois plus de tours gratos au casino ca serait ouf. Au final, Instant Casino c’est un casino de ouf a tester direct pour les pirates des slots de casino modernes ! A noter aussi la plateforme du casino claque avec son look electrisant, donne envie de replonger dans le casino direct.
instant bingo casino|
The Real Person!
The Real Person!
Ich liebe den Wahnsinn von DrueGlueck Casino, es hat eine durchgeknallte Casino-Energie. Die Auswahl im Casino ist einfach ein Knaller, mit modernen Casino-Slots, die fesseln. Der Casino-Kundenservice ist der Hammer, mit Hilfe, die richtig abgeht. Casino-Transaktionen sind simpel und zuverlassig, dennoch die Casino-Angebote konnten gro?zugiger sein. Am Ende ist DrueGlueck Casino ein Online-Casino, das alles sprengt fur Abenteurer im Casino! Zusatzlich die Casino-Oberflache ist flussig und mega cool, was jede Casino-Session noch krasser macht.
drueckglueck adventskalender|
The Real Person!
The Real Person!
J’adore sans reserve 1xbet Casino, il offre une sensation de casino unique. Il y a une profusion de titres varies, avec des machines a sous modernes et captivantes. Le service client est exceptionnel, garantissant une aide immediate. Les retraits sont ultra-rapides, occasionnellement les bonus pourraient etre plus reguliers. Pour conclure, 1xbet Casino vaut pleinement le detour pour les adeptes de sensations fortes ! Ajoutons que le site est concu avec dynamisme, ajoute une touche de raffinement a l’experience.
partners 1xbet|
В квартире было решено заменить старые деревянные окна на новые ПВХ. Для этого выбрали Окна в СПб, о чём ни разу не пожалели. Замерщик приехал вовремя, объяснил все тонкости и помог выбрать оптимальный вариант по бюджету. Мастера сделали монтаж быстро и без проблем. Окна получились аккуратные, светлые и очень удобные в эксплуатации. Теперь в доме комфортно в любую погоду, а счета за отопление заметно снизились – https://okna-v-spb.ru/
The Real Person!
The Real Person!
LaLiga oyunlarını izləmək indi çox asandır. İspaniyanın lider komandalarını tanı.
LaLiga tv live yayımını izləmək üçün link buradadır. Yeni “LaLiga EA Sports” loqosu çox bəyənildi.
Hər turdan sonra xal durumu yenilənir — LaLiga puan durumu səhifəsini izləyin.
LaLiga goal record yenidən dəyişdi.
LaLiga fixtures 2022/23 və 2025 siyahısı.
İspaniya LaLiga top futbolçularını tanı.
LaLiga champions 2023 komandaları arasında böyük rəqabət.
LaLiga nəticələri ilə favoritlərinizi izləyin.
The Real Person!
The Real Person!
LaLiga azarkeşləri üçün canlı yayım imkanı. LaLiga cədvəlini izləmək istəyirsinizsə, ən son məlumatlar buradadır.
İspaniya LaLiga canlı futbolunu qaçırmayın. LaLiga logo 2020 ilə 2025 arasındakı fərqləri gör.
Futbol xəbərlərini izləmək üçün bu linki yoxlayın — http://www.laliga.com.az.
LaLiga goal of the season 2022 hələ də yadda qalır.
LaLiga fixtures 2022/23 və 2025 siyahısı.
LaLiga statistika səhifəsində komanda göstəriciləri.
İspaniya LaLiga kupası hər il diqqət çəkir.
LaLiga scoreboard dərhal yenilənir.
The Real Person!
The Real Person!
LaLiga oyunlarını izləmək indi çox asandır. LaLiga standings səhifəsi ilə hər şeyi bil.
LaLiga tv live yayımını izləmək üçün link buradadır. Loqodakı dəyişikliklər brendin müasir ruhunu əks etdirir.
Matç cədvəlini izləmək üçün sadəcə laliga.com.az saytına daxil olun və təqvimi yoxlayın.
Ən çox qol vuran LaLiga futbolçuları kimlərdir?.
LaLiga matçlar hansı kanalda yayımlanır?.
LaLiga takımları arasında rəqabət yüksəkdir.
LaLiga champions 2023 komandaları arasında böyük rəqabət.
LaLiga maç sonuclarını izləmək asandır.
The Real Person!
The Real Person!
Лечение запоя с помощью капельниц на дому в Туле – это эффективный способ борьбы с алкоголизмом. Немало людей испытывают трудности от запойного состоянияи профессиональная поддержка становится необходимостью. Лечение с помощью капельниц способствует в очищении организма, облегчая симптомы абстиненции. Услуги нарколога включают не только капельницы, но и восстановление от алкоголячто важно для возвращения к нормальной жизни. Роль семьи играет ключевую роль в лечении. Профилактика запойного состояния также важна для предотвращения рецидивов. Обращайтесь к профессиональному подходу на сайте narkolog-tula022.ru и получите помощь специалиста на дому.
The Real Person!
The Real Person!
лечение в психиатрическом стационаре
psikhiatr-moskva008.ru
частная психиатрическая клиника стационар
The Real Person!
The Real Person!
Ən son LaLiga xəbərlərini izləməyə tələsin. LaLiga table 2025 artıq açıqlandı.
Hər oyunun canlı statistikası əlinizdə. Loqodakı dəyişikliklər brendin müasir ruhunu əks etdirir.
Ən aktual xal cədvəli üçün https://laliga.com.az bölməsinə baxın.
Ən çox qol vuran LaLiga futbolçuları kimlərdir?.
LaLiga fixtures 2022/23 və 2025 siyahısı.
LaLiga defenders və midfielders siyahısı.
Ən çox titul qazanan LaLiga klubları hansılardır?.
LaLiga oyunlarının qrafiki və nəticələri burada.
The Real Person!
The Real Person!
İspaniya futbolunun ritmini hiss et. LaLiga standings səhifəsi ilə hər şeyi bil.
LaLiga tv live yayımını izləmək üçün link buradadır. Futbol dizayn sevərləri üçün LaLiga logo nümunələri.
Əgər siz də futbol statistikası ilə maraqlanırsınızsa, LaLiga stats bölməsinə baxın.
Goal scorer LaLiga siyahısına baxmaq istəyirsiniz?.
LaLiga turnir jadvali yeniləndi.
LaLiga teams haqqında maraqlı faktlar.
LaLiga champions list artıq dərc olunub.
Futbol azarkeşləri üçün LaLiga livescore bölməsi.
The Real Person!
The Real Person!
Hər turun nəticələri real vaxtda yenilənir. LaLiga turnir cədvəlində mövqeləri izləmək maraqlıdır.
LaLiga canlı futbol həvəskarlarının seçimi. Yeni mövsüm üçün LaLiga logo 2025 təqdim edildi.
Matç cədvəlini izləmək üçün sadəcə laliga.com.az saytına daxil olun və təqvimi yoxlayın.
2025-ci ilin ən yaxşı hücumçuları artıq məlumdur.
LaLiga tv canlı baxmaq üçün seçimlər.
Ən yaxşı LaLiga oyunçuları 2025.
LaLiga champions 2023 komandaları arasında böyük rəqabət.
LaLiga live score ilə heç bir matçı qaçırmayın.
I think I will become a great follower.Just want to say your post is striking. The clarity in your post is simply striking and i can take for granted you are an expert on this subject.
The Real Person!
The Real Person!
Estou pirando total com LeaoWin Casino, parece uma savana de diversao. As opcoes de jogo no cassino sao ricas e selvagens, com slots de cassino unicos e eletrizantes. A equipe do cassino entrega um atendimento que e uma garra, respondendo mais rapido que um leopardo. Os pagamentos do cassino sao lisos e blindados, as vezes mais bonus regulares no cassino seria top. No geral, LeaoWin Casino garante uma diversao de cassino que e selvagem para os cacadores de slots modernos de cassino! De bonus o site do cassino e uma obra-prima de estilo, torna o cassino uma curticao total.
leaowin02 casino affiliates|
The Real Person!
The Real Person!
Je trouve absolument epique Julius Casino, il propose une aventure de casino digne d’un empereur. La collection de jeux du casino est colossale, avec des machines a sous de casino modernes et envoutantes. Le support du casino est disponible 24/7, joignable par chat ou email. Le processus du casino est transparent et sans embuches, par moments les offres du casino pourraient etre plus genereuses. Globalement, Julius Casino offre une experience de casino legendaire pour ceux qui cherchent l’adrenaline glorieuse du casino ! A noter le design du casino est une fresque visuelle epique, donne envie de replonger dans le casino sans fin.
julius casino saint-andre-les-vergers|
The Real Person!
The Real Person!
Je suis fan de le casino TonyBet, c’est vraiment une aventure palpitante. Le choix de jeux est impressionnant, proposant des jeux de table classiques. Le service d’assistance est top, repondant rapidement. Le processus de retrait est efficace, par contre il pourrait y avoir plus de promos. Globalement, TonyBet ne decoit pas pour les amateurs de casino ! De plus, l’interface est fluide, renforcant le plaisir de jouer.
tonybet es confiable|
The Real Person!
The Real Person!
Музыкальная обложка Дизайн обложки – это ключевой элемент визуального представления вашего музыкального творчества. Это не просто картинка, это отражение вашей музыки, вашей индивидуальности и вашего бренда. Процесс создания дизайна обложки должен начинаться с понимания вашей целевой аудитории. Кто ваши слушатели? Что им нравится? Какие визуальные образы будут для них привлекательными? Дизайн должен быть адаптирован под различные платформы и форматы. Обложка должна хорошо смотреться как на маленьком экране смартфона, так и на большом экране компьютера. Важно учитывать тренды в дизайне, но при этом не терять свою уникальность. Используйте качественные изображения, интересные шрифты и яркие цвета. Сотрудничество с опытным дизайнером может значительно улучшить качество вашей обложки и повысить шансы на успех вашего трека. Инвестиции в дизайн обложки – это инвестиции в ваш музыкальный проект.
The Real Person!
The Real Person!
Медикаментозная терапия алкоголизма в Туле становится всё актуальнее. Выездной нарколог в Туле осуществляет различные подходы к терапии зависимости‚ включая очистку организма‚ а также использование медикаментов для терапии. Анонимное лечение позволяет пациентам быть в безопасности‚ получая помощь нарколога. врач нарколог на дом тула Консультация врача обязательна для подбора наиболее эффективного курса реабилитации. Метод кодирования – такой же эффективный метод лечения зависимости. Поддержка психолога также играет важную роль в восстановлении после зависимости. Услуги нарколога включают медицинскую помощь при алкоголизме‚ что способствует успеху в лечении и восстановлению нормальной жизни.
The Real Person!
The Real Person!
частный психиатрический стационар
psikhiatr-moskva009.ru
психиатрическая помощь госпитализация
The Real Person!
The Real Person!
LaLiga statistikası hər gün yenilənir. LaLiga turnir cədvəlində mövqeləri izləmək maraqlıdır.
Bu həftə LaLiga canlı oyunları çox maraqlıdır. Futbol dizayn sevərləri üçün LaLiga logo nümunələri.
Matçların canlı nəticələrini LaLiga live vasitəsilə izləmək mümkündür.
LaLiga goal record yenidən dəyişdi.
LaLiga matçlar hansı kanalda yayımlanır?.
LaLiga stats hər mövsüm yenilənir.
LaLiga super cup 2024 möhtəşəm idi.
LaLiga scoreboard dərhal yenilənir.
https://ozon.ru/t/csgBrx8
The Real Person!
The Real Person!
Ən son LaLiga xəbərlərini izləməyə tələsin. İspaniya çempionatının xal durumu daim yenilənir.
İspaniya LaLiga canlı futbolunu qaçırmayın. Yeni mövsüm üçün LaLiga logo 2025 təqdim edildi.
Futbol dünyasından ən son xəbərlər laliga.com.az ünvanında. Canlı izləmə və təhlillər üçün mükəmməl platformadır.
Goal scorer LaLiga siyahısına baxmaq istəyirsiniz?.
İspaniya LaLiga oyun cədvəlini buradan yoxla.
İspaniya LaLiga top futbolçularını tanı.
LaLiga champions list artıq dərc olunub.
LaLiga oyunlarının qrafiki və nəticələri burada.
Несколько раз участвовал в складчинах на Складчике и каждый раз всё проходило честно. Материалы приходят вовремя, доступ не блокируется. Особенно нравится, что можно выбрать из огромного количества тем. Благодаря этому я собрал целую библиотеку полезных курсов: https://v27.skladchik.org/
The Real Person!
The Real Person!
Поддержка психолога при выходе из запоя в Туле: как преодолеть абстиненцию При абстинентном синдроме пациент часто испытывает сильными физическими и эмоциональными страданиями. В этих ситуациях помощь психолога и кризисная помощь способны существенно улучшить состояние пациента. Центры психотерапии в Туле предлагают различные программы восстановления и методы борьбы с зависимостями, что помогает справиться с трудностями. вывод из запоя цена Советы по выходу из запоя включают обращение за психологической поддержкой и участие в реабилитационных программах для зависимых, что повышает шансы на успешное лечение. Важно помнить, что путь к трезвости требует времени, терпения и поддержки.
The Real Person!
The Real Person!
комплексное лечение алкоголизма
reabilitaciya-astana007.ru
лечение и реабилитация алкоголизма в Астане
The Real Person!
The Real Person!
Estou pirando total com Flabet Casino, parece um furacao de diversao. O catalogo de jogos do cassino e uma loucura, com slots de cassino unicos e eletrizantes. O servico do cassino e confiavel e brabo, acessivel por chat ou e-mail. Os saques no cassino sao velozes como um raio, as vezes queria mais promocoes de cassino que arrebentam. No fim das contas, Flabet Casino e um cassino online que e um vulcao para os amantes de cassinos online! De lambuja a interface do cassino e fluida e cheia de vibe, torna o cassino uma curticao total.
app flabet|
The Real Person!
The Real Person!
Je trouve completement fou FatPirate, ca donne une energie de pirate dejantee. Le choix de jeux est monumental, proposant des sessions live ultra-intenses. Le support est dispo 24/7, joignable par chat ou email. Les retraits sont rapides comme une tempete, par contre plus de tours gratos ca ferait plaiz. Pour resumer, FatPirate offre une experience de ouf pour les fans de casinos en ligne ! Et puis le site est une pepite visuelle, booste l’immersion a fond.
avis fatpirate|
The Real Person!
The Real Person!
Acho simplesmente alucinante AFun Casino, parece uma rave de diversao sem fim. A gama do cassino e simplesmente uma explosao de prazeres, incluindo jogos de mesa de cassino com um toque de folia. O atendimento ao cliente do cassino e uma estrela da festa, acessivel por chat ou e-mail. Os ganhos do cassino chegam voando como serpentinas, de vez em quando mais recompensas no cassino seriam um diferencial festivo. Na real, AFun Casino vale demais curtir esse cassino para os amantes de cassinos online! De bonus o site do cassino e uma obra-prima de estilo vibrante, torna a experiencia de cassino uma folia inesquecivel.
cГіdigo promocional afun twitter|
Актуальные новости автопрома https://myrexton.ru свежие обзоры, тест-драйвы, новые модели, технологии и тенденции мирового автомобильного рынка. Всё самое важное — в одном месте.
Строительный портал https://stroimsami.online новости, инструкции, идеи и лайфхаки. Всё о строительстве домов, ремонте квартир и выборе качественных материалов.
Новостной портал https://daily-inform.ru с последними событиями дня. Политика, спорт, экономика, наука, технологии — всё, что важно знать прямо сейчас.
The Real Person!
The Real Person!
Adoro o role insano de Flabet Casino, parece um furacao de diversao. Tem uma enxurrada de jogos de cassino irados, oferecendo sessoes de cassino ao vivo que sao um fogo. O atendimento ao cliente do cassino e uma explosao, respondendo mais rapido que um relampago. Os ganhos do cassino chegam voando baixo, mas mais bonus regulares no cassino seria brabo. Em resumo, Flabet Casino oferece uma experiencia de cassino que e puro gas para os amantes de cassinos online! Vale falar tambem o design do cassino e uma explosao visual, torna o cassino uma curticao total.
flabet wikipedia|
The Real Person!
The Real Person!
Наркологическая помощь на экстренных случаях – это важный аспект, который предоставляет срочную поддержку людям, имеющим проблемы с зависимостями. В кризисной ситуации, когда необходима быстрая поддержка, на помощь выходит на помощь наркологическая служба. Преодоление зависимостей включает медикаментозную терапию и всеобъемлющую реабилитацию наркозависимых. При алкогольной зависимости важно получить своевременную помощь. Обращение к психологу может быть первым шагом на пути к выздоровлению. Роль семьи играет ключевую роль в процессе реабилитации. Группы поддержки и психотерапевтические сеансы способствуют преодолению сложностей. Профилактика наркозависимости также не менее важна. Противодействие наркозависимости не может быть эффективной без участия общества. Обращение за помощью на ресурсе narkolog-tula025.ru способствует вашему началу новой, свободной от зависимостей жизни.
The Real Person!
The Real Person!
Je suis accro au style de FatPirate, c’est une plateforme qui envoie du lourd. La selection est carrement dingue, comprenant des jeux tailles pour les cryptos. Le service client est au top niveau, avec une aide qui dechire. Le processus est clean et sans galere, mais bon j’aimerais plus de promos qui tabassent. En gros, FatPirate c’est du lourd a tester absolument pour les accros aux sensations fortes ! En prime l’interface est fluide et ultra-cool, booste l’immersion a fond.
fatpirate casino no deposit bonus|
The Real Person!
The Real Person!
реабилитация от алкоголя в Астане
reabilitaciya-astana008.ru
лечение алкозависимости
The Real Person!
The Real Person!
Наркологическая помощь – это комплекс мероприятий ‚ направленных на лечение зависимости от психоактивных веществ . На сайте narkolog-tula026.ru можно ознакомиться с информацией о наркологических клиниках ‚ предлагающих профессиональную помощь при различных формах зависимости. Лечение начинается с процесса очищения организма‚ после которой проводятся консультации специалистов и персонализированное лечение. Психотерапия и группы поддержки играют ключевую роль в процессе реабилитации. Также важна социальная адаптация и поддержка близких людей‚ что способствует предотвращению возврата к зависимости.
The Real Person!
The Real Person!
психотерапевт для ребенка Подростковый психотерапевт Подростковый психотерапевт – это специалист, который оказывает психологическую помощь подросткам, переживающим сложные периоды взросления. Подростковый возраст характеризуется гормональными изменениями, поиском идентичности, проблемами в отношениях со сверстниками и родителями, а также повышенной уязвимостью к депрессии и тревожности. Подростковый психотерапевт создает безопасную и конфиденциальную обстановку, где подросток может выразить свои чувства, поговорить о своих проблемах и получить необходимую поддержку. Терапия помогает подросткам развивать навыки саморегуляции, улучшать коммуникацию и принимать осознанные решения.
The Real Person!
The Real Person!
лечение алкоголизма отзывы
reabilitaciya-astana009.ru
как излечиться от алкоголизма
Estou totalmente empolgado com Flabet Casino, oferece um thrill unico. O catalogo e simplesmente gigantesco, compreendendo milhares de jogos adaptados para criptos. O servico e de uma eficiencia notavel, contatavel a qualquer momento. Os pagamentos sao fluidos e seguros, as vezes mais rodadas gratis seriam um plus. Globalmente, Flabet Casino e uma plataforma excepcional para aqueles que gostam de criptomoedas ! Por outro lado a interface e fluida e moderna, facilita a experiencia geral.
}
The Real Person!
The Real Person!
Ich liebe absolut Snatch Casino, es bietet einen einzigartigen Thrill. Die Auswahl an Titeln ist riesig, mit dynamischen Tischspielen. Der Service ist von bemerkenswerter Effizienz, erreichbar jederzeit. Die Gewinne kommen schnell, obwohl mehr Freispiele waren ein Plus. Kurz gesagt Snatch Casino garantiert eine top Spielerfahrung fur Online-Wetten-Enthusiasten ! Daruber hinaus die Site ist stylish und schnell, macht jede Session immersiv.
The Real Person!
The Real Person!
Je suis totalement hypnotise par Impressario, ca balance une vibe spectaculaire. Il y a un deluge de jeux captivants, offrant des machines a sous qui claquent. Le crew assure un suivi etoile, joignable par chat ou email. Les paiements sont securises et eclatants, quand meme les offres pourraient etre plus genereuses. Pour resumer, Impressario garantit un show de fun epique pour les fans de casinos en ligne ! Et puis l’interface est fluide et glamour, facilite un show total.
impressario casino login|
The Real Person!
The Real Person!
Acho simplesmente insano FSWin Casino, oferece uma aventura de cassino que detona tudo. O catalogo de jogos do cassino e uma loucura total, incluindo jogos de mesa de cassino cheios de estilo. O atendimento ao cliente do cassino e um arraso, garantindo suporte de cassino direto e sem treta. O processo do cassino e limpo e sem complicacao, as vezes queria mais promocoes de cassino que mandam ver. Na real, FSWin Casino garante uma diversao de cassino que e uma explosao para os cacadores de slots modernos de cassino! E mais a interface do cassino e fluida e cheia de vibe, faz voce querer voltar pro cassino toda hora.
fswin casino bonus code|
The Real Person!
The Real Person!
Нарколог на дом анонимно в Туле – это необходимая помощь для людей‚ страдающих от проблемой зависимости. Лечение наркомании требует квалифицированной помощи‚ и вызов врача на дом обеспечивает конфиденциальность. Опытные врачи предоставляют анонимную консультацию‚ диагностику зависимости и лечебные процедуры на дому. В нашем городе доступна психотерапевтическая помощь и программы реабилитации для наркоманов что позволяет восстановить физическое и психическое здоровье и возобновить нормальную жизнь. Поддержка семьи и поддержка родственников также имеют важное значение в процессе лечения. Не стесняйтесь обращаться на narkolog-tula027.ru‚ чтобы начать получать помощь и поддержку и начать путь к выздоровлению.
Thanks , I’ve recently been looking for information approximately this subject for ages and yours is the greatest I’ve discovered till now. However, what concerning the bottom line? Are you sure concerning the source?
https://detkinland.com.ua/yak-linzy-dlya-far-pokrashchuyut-svitlovi-kharakte.html
The Real Person!
The Real Person!
экстренный вывод из запоя
tula-narkolog007.ru
экстренный вывод из запоя тула
The Real Person!
The Real Person!
Капельница для снятия запоя в Туле: стоимость и мнения В Туле вызов нарколога на дом становятся актуальными. Множество людей нуждаются в помощи при алкогольной зависимости и испытывают потребность в поддержке. Вызов нарколога на дом представляет собой комфортное решение получить медицинскую помощь, не выходя из дома. Капельница от запоя способствует в детоксикации организма и снижает негативные последствия.Стоимость капельницы может различаться в зависимости от клиники, но в среднем составляет примерно 3000-6000 рублей. Отзывы о капельнице в основном положительные: пациенты подчеркивают заметное улучшение самочувствия и снижение тяги к алкоголю. Экстренная помощь при запое является жизненно важной.Анонимное лечение алкоголизма — ключевой фактор, так как многие не хотят обсуждать свои трудности. Услуги нарколога в Туле предлагают комплексную помощь, включая капельницы и реабилитационные программы. Помощь при запое может получить любой, кто хочет изменить свою жизнь. нарколог на дом анонимно тула
The Real Person!
The Real Person!
Sweet Bonanza er en ægte klassiker hos Mr Green. Det er en glimrende spillemaskine, som både er populær blandt high rollere såvel som nybegyndere på vores online casino. Det sukkersøde tema gør næsten en lækkersulten og chancen for at vinde enorme præmier, gør kun oplevelsen endnu sødere. Det kan der uden tvivl købes meget slik for! Hos Mr Green får Sweet Bonanza derfor en varm anbefaling. Er du på udkig efter en simpel men på samme tid sjov og velbetalende spillemaskine, så er Sweet Bonanza et oplagt valg. Book of Secrets tager dig med til det gamle Egypten, og lader dig besøge en mystisk pharaos gravkammer, hvor du kan finde gemte skatte. Spillet er et 5-hjuls spil med 10 gevnistlinjer og 10 symboler. Det har en RTP på 96,05% og en høj volatilitet, hvilket betyder, at der godt kan være lidt langt mellem gevinsterne. Til gengæld vil de være til den større side, og du kan vinde store beløb ved at spille for store beløb.
https://enquiry.ggbinhire.com.au/sweet-bonanza-review-oplev-super-free-spins-i-et-populaert-online-casino-spil/
Temaet og universet for Sweet Bonanza er rigtig godt, og sammen med en flot grafik, så giver giver det et rigtig godt fundament for en god spiloplevelse. Slik og søde sager er ikke et tema man ser så tit, og det er dejligt at se noget andet end de normale kløver, bar, kort, drage eller viking symboler, som mange andre maskiner har. Derudover så er både symbolerne og farverne i dette spil, udført på en behagelig og lidt ekstravagant måde, så man ikke bliver skør eller får ondt i hovedet. Tværtimod kommer man i godt humør med Sweet Bonanza, hvor . spiludviklerne dog godt kunne have gjort lidt ved lydene og musikken i maskinen. Sweet Bonanza er en farverig og tempofyldt spilleautomat, der kombinerer barnlig slik-nostalgi med seriøse gevinstmuligheder. Tumble wins, scatter pays og de vilde multiplikatorbomber gør spillet både underholdende og potentielt meget lukrativt – især i bonusrunden. Et must-try for alle, der elsker søde temaer og højt tempo!
The Real Person!
The Real Person!
Проверенный сайт онлайн-казино вы можете найти здесь: http://travel-siberia.ru/uploads/articles/sayt_onlayn_kazino_vavada_4.html. Там объясняется, как выбрать площадку с лицензией и не потерять свои деньги при первых ставках.
The Real Person!
The Real Person!
вывод из запоя тула
tula-narkolog008.ru
лечение запоя
The Real Person!
The Real Person!
частный пансионат для престарелых
pansionat-msk010.ru
дом престарелых
Our local network of agencies has found your research so helpful.
The Real Person!
The Real Person!
Ich finde es unglaublich Snatch Casino, es fuhlt sich wie ein Sturm des Vergnugens an. Der Katalog ist einfach gigantisch, mit modernen und fesselnden Slots. Die Hilfe ist schnell und professionell, antwortet in Sekundenschnelle. Die Auszahlungen sind superschnell, trotzdem mehr variierte Boni waren toll. Zum Schluss Snatch Casino ist eine au?ergewohnliche Plattform fur Casino-Fans ! Hinzu kommt die Oberflache ist flussig und modern, erleichtert die Gesamterfahrung.
snatch casino no deposit bonus|
The Real Person!
The Real Person!
J’adore a fond Instant Casino, on dirait une tempete de fun. La gamme de casino est un veritable feu d’artifice, comprenant des jeux de casino tailles pour les cryptos. Le crew du casino assure un suivi de ouf, joignable par chat ou email. Les gains du casino arrivent a la vitesse lumiere, quand meme plus de tours gratos au casino ca serait ouf. Au final, Instant Casino est un casino en ligne qui cartonne pour les accros aux sensations de casino ! Bonus la plateforme du casino claque avec son look electrisant, donne envie de replonger dans le casino direct.
instant casino inscription|
The Real Person!
The Real Person!
Acho incrivel Flabet Casino, oferece um thrill unico. As opcoes sao vastas e variadas, fornecendo sessoes ao vivo imersivas. Os agentes sao ultra-responsivos, oferecendo solucoes precisas. O processo e simples e sem problemas, mesmo que as ofertas poderiam ser mais generosas. Para resumir, Flabet Casino vale realmente a pena para os amantes de adrenalina ! Ademais a interface e fluida e moderna, reforca o desejo de voltar.
camisa do flamengo branca flabet|
The Real Person!
The Real Person!
вывод из запоя калуга
vivod-iz-zapoya-kaluga013.ru
вывод из запоя калуга
papamasque – Their presentation is elegant, I like the balance of style and substance.
The Real Person!
The Real Person!
Ich finde es unglaublich Snatch Casino, es gibt eine verruckte Spielenergie. Es gibt eine unglaubliche Vielfalt an Spielen, mit immersiven Live-Sitzungen. Die Agenten sind super reaktionsschnell, mit tadellosem Follow-up. Die Zahlungen sind flussig und sicher, trotzdem die Angebote konnten gro?zugiger sein. Kurz gesagt Snatch Casino garantiert eine top Spielerfahrung fur Crypto-Liebhaber ! Au?erdem die Navigation ist super einfach, erleichtert die Gesamterfahrung.
snatch casino no dep|
The Real Person!
The Real Person!
Me enredei no caos de IJogo Casino, e um cassino online que enreda como uma teia de aranha gigante. O catalogo de jogos e uma selva de emocoes. com slots tematicos de aventuras enredadas. O suporte e um fio guia. disponivel por chat ou e-mail. Os ganhos chegam rapido como uma teia rompida. entretanto as ofertas podiam ser mais generosas. Na real, IJogo Casino promete uma diversao que e uma teia para os exploradores de jogos online! E mais o design e um espetaculo visual enredado. transformando cada aposta em uma aventura enredada.
jg ijogo|
The Real Person!
The Real Person!
частный пансионат для пожилых
pansionat-msk011.ru
пансионаты для инвалидов в москве
If you are looking to post free classifieds in India online, we highly recommend Xpdea Classifieds. Xpdea is India’s leading free online ads posting site.
If you are looking to post free classifieds in India online, we highly recommend Xpdea Classifieds. Xpdea is India’s leading free online ads posting site.
J’eprouve une loyaute infinie pour Mafia Casino, il orchestre une conspiration de recompenses secretes. La cache de jeux est un arsenal cache de plus de 5000 armes, proposant des crash pour des chutes de pouvoir. Le suivi protege avec une omerta absolue, assurant une loyaute fidele dans le syndicate. Les flux sont masques par des voiles crypto, bien que des largesses gratuites supplementaires boosteraient les operations. Pour clore l’omerta, Mafia Casino invite a une intrigue sans trahison pour les parrains de casinos virtuels ! A murmurer le portail est une planque visuelle imprenable, infuse une essence de mystere mafieux.
casino mafia france|
The Real Person!
The Real Person!
лечение запоя тула
tula-narkolog009.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
Je suis irresistiblement recrute par Mafia Casino, on complote un reseau de tactiques astucieuses. Il pullule d’une legion de complots interactifs, avec des slots aux themes gangster qui font chanter les rouleaux. Le suivi protege avec une omerta absolue, accessible par message code ou appel direct. Les retraits s’executent avec une furtivite remarquable, occasionnellement des complots promotionnels plus frequents dynamiseraient le territoire. Dans l’ensemble du domaine, Mafia Casino construit un syndicate de divertissement impitoyable pour les conspirateurs de victoires rusees ! De surcroit la circulation est instinctive comme un chuchotement, incite a prolonger l’intrigue infinie.
bonus mafia casino|
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-kaluga014.ru
экстренный вывод из запоя калуга
The Real Person!
The Real Person!
частный пансионат для пожилых
pansionat-msk012.ru
частный пансионат для пожилых людей
If you are looking to post free classifieds in India online, we highly recommend Xpdea Classifieds. Xpdea is India’s leading free online ads posting site.
best new zealand casino, real money online
pokies usa and new zealand bingo How Old To Go To Morongo Casino to play,
or highest payout online casino usa
The Real Person!
The Real Person!
лечение запоя челябинск
vivod-iz-zapoya-chelyabinsk010.ru
экстренный вывод из запоя челябинск
Adoro completamente Flabet Casino, parece um turbilhao de prazer. Ha uma diversidade de jogos incrivel, incluindo jogos de mesa dinamicos. Os agentes sao ultra-responsivos, com um acompanhamento impecavel. As transacoes sao confiaveis, por vezes mais rodadas gratis seriam um plus. Em conclusao, Flabet Casino oferece prazer garantido para os jogadores em busca de diversao ! Alem disso o site e estiloso e rapido, adiciona conforto ao jogo.
flabet apostas|
The Real Person!
The Real Person!
вывод из запоя круглосуточно калуга
vivod-iz-zapoya-kaluga015.ru
вывод из запоя цена
The Real Person!
The Real Person!
пансионат для пожилых с инсультом
pansionat-tula010.ru
пансионат для пожилых в туле
The Real Person!
The Real Person!
Estou totalmente empolgado com Flabet Casino, proporciona uma aventura palpitante. O escolha de titulos e enorme, fornecendo sessoes ao vivo imersivas. A assistencia e rapida e profissional, contatavel a qualquer momento. As transacoes sao confiaveis, por vezes gostaria de mais bonus variados. Em resumo, Flabet Casino garante uma experiencia de jogo top para os jogadores em busca de diversao ! De mais a mais a interface e fluida e moderna, reforca o desejo de voltar.
flabet baixar app|
Ich finde es unglaublich Snatch Casino, es fuhlt sich wie ein Sturm des Vergnugens an. Die Optionen sind umfangreich und abwechslungsreich, mit dynamischen Tischspielen. Der Kundenservice ist erstklassig, bietet prazise Losungen. Die Zahlungen sind flussig und sicher, gelegentlich mehr variierte Boni waren toll. Kurz gesagt Snatch Casino ist eine au?ergewohnliche Plattform fur Spieler auf der Suche nach Spa? ! Beachten Sie auch die Oberflache ist flussig und modern, was das Spielvergnugen steigert.
snatch casino bonus no deposit|
The Real Person!
The Real Person!
Je suis allie avec Mafia Casino, c’est un empire ou chaque pari scelle un accord de fortune. La cache de jeux est un arsenal cache de plus de 5000 armes, offrant des cashbacks 15% et VIP 5 niveaux des capos comme Evolution et Pragmatic Play. Le support client est un consigliere vigilant et incessant, chuchotant des solutions claires et rapides. Le protocole est ourdi pour une fluidite exemplaire, bien que des largesses gratuites supplementaires boosteraient les operations. En scellant le pacte, Mafia Casino invite a une intrigue sans trahison pour les mafiosi des paris crypto ! En plus le portail est une planque visuelle imprenable, simplifie la traversee des complots ludiques.
mafia 777 casino|
canadian approved online casinos, bouka pokies and best rated united states online players casino bankruptcy (Carlos), or auto roulette hack
Бесплатный сео аудит сайта https://seo-audit-sajta.ru
Need TRON Energy? buy tron energy instantly and save on TRX transaction fees. Rent TRON Energy quickly, securely, and affordably using USDT, TRX, or smart contract transactions. No hidden fees—maximize the efficiency of your blockchain.
The Real Person!
The Real Person!
Je suis allie avec Mafia Casino, ca eleve le jeu a un niveau de boss legendaire. La reserve est un code de divertissements mafieux, incluant des roulettes pour des tours de table. Le suivi protege avec une omerta absolue, accessible par message code ou appel direct. Les transferts glissent stables et acceleres, occasionnellement des largesses gratuites supplementaires boosteraient les operations. En scellant le pacte, Mafia Casino se dresse comme un pilier pour les capos pour les mafiosi des paris crypto ! En pot-de-vin supplementaire l’interface est un repaire navigable avec ruse, simplifie la traversee des complots ludiques.
mafia casino las vegas|
The Real Person!
The Real Person!
Модрич сыяктуу туруктуу оюн көрсөтүү абдан кыйын.Көпчүлүк күйөрмандар Модричтин кетишине ишенбей жатат. Лука Модрич статистика дайыма жогорку деңгээлде. Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Модрич тууралуу фактыларды текшерип көр: Лука Модрич статистика. Лука Модрич ЧМ 2022де дагы мыкты оюн көрсөттү.
Лука Модрич — Реал Мадриддин символу. Бул адам чынында эле чыныгы чемпион. Футбол дүйнөсүндө андай адептүү оюнчулар аз. Лука Модрич үчүн көп клубдар эшигин ачат.
The Real Person!
The Real Person!
лечение запоя челябинск
vivod-iz-zapoya-chelyabinsk011.ru
вывод из запоя челябинск
Ich bin abhangig von SpinBetter Casino, es erzeugt eine Spielenergie, die fesselt. Es wartet eine Fulle spannender Optionen, mit innovativen Slots und fesselnden Designs. Die Agenten sind blitzschnell, immer parat zu assistieren. Die Gewinne kommen prompt, trotzdem zusatzliche Freispiele waren ein Highlight. In Kurze, SpinBetter Casino bietet unvergessliche Momente fur Adrenalin-Sucher ! Zusatzlich die Interface ist intuitiv und modern, erleichtert die gesamte Erfahrung. Ein Pluspunkt ist die Sicherheit der Daten, die Vertrauen schaffen.
spinbettercasino.de|
The Real Person!
The Real Person!
пансионат для пожилых с деменцией
pansionat-tula011.ru
дом престарелых
The Real Person!
The Real Person!
Мен ар дайым Лука Модричти көргөндө чыныгы лидерликти байкайм.Лука Модрич Мадрид үчүн тарых жараткан. Лука Модрич канча гол киргизгенин билесиңби? Модрич ар бир оюнда жаш оюнчулардай күрөшөт. Объективдүү маалымат жана факт-чек менен таанышыңыз: luka-modric-kg.com. Анын чеберчилиги ар бир эл аралык турнирде көрүнөт.
Модричсиз Реалдын оюн стили башкача болмок. Бул адамдын жашоосу — чыныгы күрөш. Анын баасы трансфермаркетте дагы жогору. Кээ бирөөлөр Модрич Аль Насрга барат дешет.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-minsk010.ru
лечение запоя
The Real Person!
The Real Person!
Лука Модричтин тактикалык ой жүгүртүүсү укмуш.Модрич кетсе дагы, анын izi футболдо калат. Анын карьерасынын узактыгы профессионалдуулуктун белгиси. Лука Модричтин жашы 39 болсо да, оюну жаштардыкындай. Статс жана графикалар менен ыңгайлуу бөлүм бар — карап көрүңүз: Лука Модрич stats. Хорват футболунун жандуу легендасы.
Футбол сүйүүчүлөрү үчүн Модрич — Реалдын жүрөгү. Лука Модрич жашоодон сабак алуунун үлгүсү. Футбол дүйнөсүндө андай адептүү оюнчулар аз. Модрич Интер Майамиге өтсө, абдан кызык болмок.
The Real Person!
The Real Person!
Ich habe einen totalen Hang zu SpinBetter Casino, es bietet einen einzigartigen Kick. Das Angebot an Spielen ist phanomenal, mit aufregenden Sportwetten. Der Kundenservice ist ausgezeichnet, immer parat zu assistieren. Die Transaktionen sind verlasslich, ab und an zusatzliche Freispiele waren ein Highlight. Alles in allem, SpinBetter Casino bietet unvergessliche Momente fur Casino-Liebhaber ! Au?erdem die Interface ist intuitiv und modern, gibt den Anreiz, langer zu bleiben. Besonders toll die Community-Events, die Flexibilitat bieten.
spinbettercasino.de|
The Real Person!
The Real Person!
Ich kann nicht genug bekommen von NV Casino, es ist ein Abenteuer, das pulsiert wie ein Herzschlag. Das Angebot an Spielen ist phanomenal, mit Slots im innovativen Design. Der Support ist von herausragender Qualitat, bietet klare Antworten. Die Zahlungen sind sicher und flussig, dennoch mehr abwechslungsreiche Boni waren willkommen. Alles in allem, NV Casino ist definitiv empfehlenswert fur Fans von Online-Wetten ! Hinzu die Navigation ist kinderleicht, gibt Lust auf mehr.
https://playnvcasino.de/|
Need porn videos or photos? ai adult video generator – create erotic content based on text descriptions. Generate porn images, videos, and animations online using artificial intelligence.
IPTV форум vip-tv.org.ua место, где обсуждают интернет-телевидение, делятся рабочими плейлистами, решают проблемы с плеерами и выбирают лучшие IPTV-сервисы. Присоединяйтесь к сообществу интернет-ТВ!
The Real Person!
The Real Person!
Лука Модрич азыркы замандын эң мыкты оюнчуларынын бири.Модрич кетсе, жаңы доор башталат. Анын статистикасы ар бир сезон таң калтырат. Көптөр “сколько лет Лука Модричке?” деп сурашат — бирок ал үчүн жаш маанилүү эмес. Статс жана графикалар менен ыңгайлуу бөлүм бар — карап көрүңүз: Лука Модрич stats. Хорватиянын эң атактуу оюнчусу дал ушул Модрич.
Лука Модрич Реалга келгенде команда өзгөргөн. Анын автобиографиясы мотивация берет. Футбол дүйнөсүндө андай адептүү оюнчулар аз. Эми ал Миланга өтөт деген ушак бар.
The Real Person!
The Real Person!
Лука Модричтин тактикалык ой жүгүртүүсү укмуш.Модрич кетсе, жаңы доор башталат. Футболдо мындай туруктуу статистика аз кездешет. Модричтин энергиясы таң калтырат. Жаңылык лентасы таза футбол темасында: luka-modric-kg.com. Лука Модрич дүйнөлүк футболго башкача дем берди.
Модрич Реалдын орто талаасын башкарып келет көп жылдан бери. Модрич жаш кезинен эле футболго берилген. Футболдо андай деңгээлге жетүү оңой эмес. Лука Модрич үчүн көп клубдар эшигин ачат.
Acho incrivel Flabet Casino, proporciona uma aventura palpitante. A gama de jogos e impressionante, incluindo jogos de mesa dinamicos. A assistencia e rapida e profissional, oferecendo solucoes precisas. Os ganhos chegam rapido, no entanto recompensas adicionais seriam top. Globalmente, Flabet Casino e obrigatorio para os jogadores para os apaixonados por cassino ! Notemos tambem a interface e fluida e moderna, o que aumenta o prazer de jogar.
flabet com|
The Real Person!
The Real Person!
Ich finde es unglaublich Snatch Casino, es ist eine dynamische Erfahrung. Der Katalog ist einfach gigantisch, mit modernen und fesselnden Slots. Die Agenten sind super reaktionsschnell, garantiert sofortige Hilfe. Die Transaktionen sind zuverlassig, jedoch haufigere Promos waren cool. Zusammenfassend Snatch Casino bietet garantiertes Vergnugen fur Spieler auf der Suche nach Spa? ! Zusatzlich die Plattform ist visuell top, fugt Komfort zum Spiel hinzu.
snatch casino codigo promocional 2024|
The Real Person!
The Real Person!
Лука Модрич азыркы замандын эң мыкты оюнчуларынын бири.Бул футбол дүйнөсүндө чоң өзгөрүү болот. Анын карьерасынын узактыгы профессионалдуулуктун белгиси. Анын жашы эмес, оюну аны аныктайт. Карьера жолу, клубдар, трофейлер — баары бир жерге топтолгон: luka-modric-kg.com. Анын ысымы футбол тарыхында калат.
Футбол сүйүүчүлөрү үчүн Модрич — Реалдын жүрөгү. Бул адам чынында эле чыныгы чемпион. Модрич акчаны эмес, атакты тандаган адам. Лука Модрич үчүн көп клубдар эшигин ачат.
Substantially, the post is really the best on this laudable topic. I concur with your conclusions and will eagerly watch forward to your future updates.Just saying thanx will not just be enough, for the wonderful lucidity in your writing.
wagisonsncompany – site gives a professional presence, design and content work well together.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-chelyabinsk012.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
пансионат для пожилых с деменцией
pansionat-tula012.ru
частный пансионат для пожилых людей
The Real Person!
The Real Person!
вывод из запоя минск
vivod-iz-zapoya-minsk011.ru
вывод из запоя круглосуточно минск
Acho incrivel Flabet Casino, parece um turbilhao de prazer. O escolha de titulos e enorme, com slots modernos e cativantes. O servico e de uma eficiencia notavel, contatavel a qualquer momento. Os pagamentos sao fluidos e seguros, contudo mais rodadas gratis seriam um plus. Globalmente, Flabet Casino garante uma experiencia de jogo top para os amantes de adrenalina ! De mais a mais a navegacao e super facil, facilita a experiencia geral.
flabet preço|
The Real Person!
The Real Person!
Ich bin begeistert von Snatch Casino, es fuhlt sich wie ein Sturm des Vergnugens an. Die Optionen sind umfangreich und abwechslungsreich, mit dynamischen Tischspielen. Der Service ist von bemerkenswerter Effizienz, antwortet in Sekundenschnelle. Der Prozess ist einfach und reibungslos, manchmal mehr Freispiele waren ein Plus. Global Snatch Casino garantiert eine top Spielerfahrung fur Online-Wetten-Enthusiasten ! Zusatzlich die Plattform ist visuell top, fugt Komfort zum Spiel hinzu.
snatch casino kod promocyjny bez depozytu|
Good day! This is my first comment here so I just wanted to give a quick shout out and say I really enjoy reading through your articles. Can you recommend any other blogs/websites/forums that cover the same subjects? Thanks a lot!
redhillrepurposing – Love how they’re combining technology with art authenticity.
ouretiquette – Love how they’re combining technology with art authenticity.
hellgate100nyc – I appreciate the transparency this site offers to artists and buyers.
colossal-heart – The idea of a global art registry feels like a game-changer.
The Real Person!
The Real Person!
Je suis irresistiblement recrute par Mafia Casino, on complote un reseau de tactiques astucieuses. Les operations forment un plan de manigances innovantes, offrant des cashbacks 15% et VIP 5 niveaux des capos comme Evolution et Pragmatic Play. Le service conspire en continu 24/7, avec une ruse qui anticipe les traitrises. Les butins affluent via Bitcoin ou Ethereum, occasionnellement des complots promotionnels plus frequents dynamiseraient le territoire. En scellant le pacte, Mafia Casino invite a une intrigue sans trahison pour les conspirateurs de victoires rusees ! A murmurer la circulation est instinctive comme un chuchotement, infuse une essence de mystere mafieux.
casino popular mafia de cuba|
shopmaggielindemann – It’s refreshing to see innovation in the art world like this.
alixrice – Browsing through, I can see how this could help artists globally.
The Real Person!
The Real Person!
Мен ар дайым Лука Модричти көргөндө чыныгы лидерликти байкайм.Эгер Лука Модрич кетсе, Реалдын орто талаасы өзгөрөт. Лука Модрич канча гол киргизгенин билесиңби? Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Колдон бетер esli kerek болсо, бул форматты да эстеп кал: luka-modric-kg com. Хорват футболунун жандуу легендасы.
Футбол сүйүүчүлөрү үчүн Модрич — Реалдын жүрөгү. Анын ата-энеси ага чоң күч берген. Анын баасы трансфермаркетте дагы жогору. Кээ бирөөлөр Модрич Аль Насрга барат дешет.
The Real Person!
The Real Person!
вкусняшки для собак Мясо кости для собак – это натуральное и питательное лакомство, которое сочетает в себе полезные свойства мяса и костей. Оно содержит белок, кальций, фосфор и другие важные элементы, необходимые для здоровья костей и суставов. Важно выбирать мясо кости от проверенных производителей и следить за тем, чтобы кости не были слишком острыми и хрупкими.
The Real Person!
The Real Person!
психиатрическую помощь в стационарных условиях
psychiatr-moskva007.ru
консультация частного психиатра
The Real Person!
The Real Person!
Je suis allie avec Mafia Casino, on complote un reseau de tactiques astucieuses. La cache de jeux est un arsenal cache de plus de 5000 armes, integrant des lives comme Infinite Blackjack pour des negociations tendues. Les lieutenants repondent avec une discretion remarquable, mobilisant des canaux multiples pour une execution immediate. Le protocole est ourdi pour une fluidite exemplaire, a l’occasion des rackets de recompense additionnels scelleraient les pactes. Pour clore l’omerta, Mafia Casino se dresse comme un pilier pour les capos pour les mafiosi des paris crypto ! En pot-de-vin supplementaire la structure vibre comme un code ancestral, simplifie la traversee des complots ludiques.
mafia casino promo code|
The Real Person!
The Real Person!
Модрич Реал Мадридде канча жыл супер деңгээлде ойноду!Лука Модрич уходит из Реала деген жаңылык жүрөк оорутат. Анын карьерасынын узактыгы профессионалдуулуктун белгиси. Бул чыныгы профессионализмдин белгиси. Жашы, формасы, рекорддору тууралуу толук маалымат: Лука Модрич возраст. Хорват футболунун жандуу легендасы.
Лука Модрич — Реал Мадриддин символу. Анын ата-энеси ага чоң күч берген. Лука Модричтин айлык маянасы укмуш. Лука Модрич үчүн көп клубдар эшигин ачат.
The Real Person!
The Real Person!
вывод из запоя круглосуточно
vivod-iz-zapoya-cherepovec013.ru
вывод из запоя круглосуточно
The Real Person!
The Real Person!
вывод из запоя минск
vivod-iz-zapoya-minsk012.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
Ich habe einen totalen Hang zu SpinBetter Casino, es bietet einen einzigartigen Kick. Das Angebot an Spielen ist phanomenal, mit dynamischen Tischspielen. Der Service ist von hoher Qualitat, immer parat zu assistieren. Die Auszahlungen sind ultraschnell, trotzdem die Offers konnten gro?zugiger ausfallen. Zusammengefasst, SpinBetter Casino bietet unvergessliche Momente fur Adrenalin-Sucher ! Hinzu kommt das Design ist ansprechend und nutzerfreundlich, was jede Session noch besser macht. Ein weiterer Vorteil die Sicherheit der Daten, die den Einstieg erleichtern.
spinbettercasino.de|
The Real Person!
The Real Person!
https://kiteschoolhurghada.ru/
The Real Person!
The Real Person!
Ich habe eine Leidenschaft fur NV Casino, es bietet eine Reise voller Spannung. Es wartet eine Fulle an spannenden Spielen, mit immersiven Tischspielen. Der Service ist rund um die Uhr verfugbar, mit praziser Unterstutzung. Der Prozess ist unkompliziert, dennoch die Angebote konnten gro?zugiger sein. Zusammengefasst, NV Casino bietet unvergesslichen Spa? fur Krypto-Liebhaber ! Hinzu die Oberflache ist intuitiv und stylish, gibt Lust auf mehr.
playnvcasino.de|
kruisefest – Navigation feels intuitive — I found what I wanted fast.
appytrucksandskulls – Very intriguing name; the story behind it must be interesting.
Всё о металлообработке j-metall.ru/ и металлах: технологии, оборудование, сплавы и производство. Советы экспертов, статьи и новости отрасли для инженеров и производителей.
fullumandholt – The site feels like a curated gallery, respectful of each piece.
guitargodscollectibles – Those photo angles help a lot in judging condition—great idea.
linkrootes – Everything seems to fit just right, color scheme is calming.
420smokeshop – The typography and spacing are well done, very readable.
ureyo – Consistent styling everywhere, keeps the experience cohesive and calm.
9hxn2 – Visuals and text balance well, looks professionally designed.
easy-software.online – It’s easy to find what the software does, very straightforward.
diamondescort18 – Feels easy to navigate, and the vibe is just perfect.
The Real Person!
The Real Person!
лечение в психиатрическом стационаре
psychiatr-moskva008.ru
вызвать психиатра на дом
2jasabola.biz – Overall looks promising — I’ll keep an eye on how this evolves.
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Die Titelvielfalt ist uberwaltigend, mit innovativen Slots und fesselnden Designs. Die Hilfe ist effizient und pro, mit praziser Unterstutzung. Die Auszahlungen sind ultraschnell, obwohl die Offers konnten gro?zugiger ausfallen. Global gesehen, SpinBetter Casino garantiert hochsten Spa? fur Casino-Liebhaber ! Zusatzlich das Design ist ansprechend und nutzerfreundlich, fugt Magie hinzu. Zusatzlich zu beachten die Vielfalt an Zahlungsmethoden, die den Einstieg erleichtern.
https://spinbettercasino.de/|
The Real Person!
The Real Person!
Ich kann nicht genug bekommen von NV Casino, es erzeugt eine Energie, die suchtig macht. Der Katalog ist reich und vielfaltig, mit immersiven Tischspielen. Der Support ist von herausragender Qualitat, mit praziser Unterstutzung. Der Prozess ist unkompliziert, trotzdem die Angebote konnten gro?zugiger sein. Zusammengefasst, NV Casino garantiert Top-Unterhaltung fur Adrenalin-Junkies ! Daruber hinaus die Oberflache ist intuitiv und stylish, verstarkt die Immersion.
https://playnvcasino.de/|
vuabat – Just browsed your site, love the clean layout and branding vibe.
knockoutzakk – The visuals hit hard—great choice of imagery throughout.
erinkristensen – You’ve got a cohesive style, everything feels aligned and polished.
awsmining – Everything loads perfectly, no broken links that I found yet.
housepartyofhorrors – The imagery gives just enough scare without being over the top.
blpawards – I like how entries are showcased, very clean gallery feel.
charitydriveforservicemembers – The visuals support the message well, not too flashy but impactful.
The Real Person!
The Real Person!
https://kiteschoolhurghada.ru/
otistaylorjr – I appreciate the clarity in what you offer and present.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-cherepovec014.ru
вывод из запоя круглосуточно
nyrw84.top – The site loads quickly, no delays so far.
The Real Person!
The Real Person!
вывод из запоя омск
vivod-iz-zapoya-omsk010.ru
вывод из запоя омск
diamondescort18 – I keep coming back, this layout just feels right somehow.
trackseries – You’ve got solid branding, makes it memorable after one visit.
michaeldfountain – Navigating is smooth, I found what I needed in no time.
Есть металлолом? сдать металлолом цена мы предлагаем полный цикл услуг по приему металлолома в Санкт-Петербурге, включая оперативную транспортировку материалов непосредственно на перерабатывающий завод. Особое внимание мы уделяем удобству наших клиентов. Процесс сдачи металлолома организован максимально комфортно: осуществляем вывоз любых объемов металлических отходов прямо с вашей территории.
Хочешь сдать металл? сдать металлолом цена наша компания специализируется на профессиональном приёме металлолома уже на протяжении многих лет. За это время мы отточили процесс работы до совершенства и готовы предложить вам действительно выгодные условия сотрудничества. Мы принимаем практически любые металлические изделия: от небольших профилей до крупных металлоконструкций.
connectwiththeworldnow – The brand feels urgent, visuals add strength to the narrative.
The Real Person!
The Real Person!
вызов психиатра на дом в москве
psychiatr-moskva009.ru
вызов психиатрической помощи
Hi there! I realize this is sort of off-topic but I had to ask. Does operating a well-established blog like yours take a massive amount work? I’m brand new to running a blog however I do write in my journal every day. I’d like to start a blog so I can share my own experience and thoughts online. Please let me know if you have any kind of ideas or tips for brand new aspiring bloggers. Thankyou!
https://tastaliski.com.ua/hermetyk-dlia-far-kupyty-ukraina-de-krashchi-tsiny.html
awsmining – Nice job with responsiveness, works well on my phone too.
easy-software.online – The layout feels modern and easy to navigate around.
2jasabola.biz – Clean typography helps readability—very thoughtful design choice.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk011.ru
лечение запоя
The Real Person!
The Real Person!
экстренный вывод из запоя череповец
vivod-iz-zapoya-cherepovec015.ru
экстренный вывод из запоя череповец
discoveramazingthingsonline – I like the energy here, visuals invite curiosity and exploration.
I am glad to be a visitor on this website!, regards for this rare information!
x-xa – Pages sometimes load slowly, but eventually all content shows.
everythingyouneedtoknow – I love that it’s so broad yet coherent, feels well curated.
findwhatyouarelookingfor – Typography is easy on the eyes, nice choice of fonts.
dancizuci – I find this site very reliable and frequently visit.
explorecreativeideasdaily – Nice fonts, subtle colors — this site feels relaxing to read.
yourjourneytobetterliving – Feels like a community, not just a website, very welcoming indeed.
0250f – This site offers valuable information in a user-friendly format.
moviethai4u – The site loads quickly and browsing feels quite fluid.
The Real Person!
The Real Person!
платная консультация психиатра
psikhiatr-moskva007.ru
частный психиатрический стационар
accountingservicesgoa – Great experience, they simplified my GST filing process.
xxb00 – I like browsing here, services are useful and truly reliable.
88878qp – Fonts are legible, though contrast could be improved in some areas.
blossompaperart – Feels like a craft-lover’s spot, creative energy is strong here.
yourtrustedguideforlife – I appreciate how clean everything looks, so many sites are messy.
powerpres – Content seems meaningful, not just filler, which I respect.
482429 – Helpful resources here, exactly what I was looking for online.
jqdsu – The layout is straightforward — nothing excessive, which I prefer.
78y29h – I see potential, but needs more polish to feel professional.
learnsomethingneweveryday – The headlines draw me in, I already clicked two posts.
thefutureofinnovation – Might return later, there’s enough value here to explore further.
marlborodoublefusion – Images support the text nicely, overall design shows good taste.
allking89 – Interface seems solid, pages load nicely and feel dependable.
The Real Person!
The Real Person!
I’m drawn to Wazamba Casino, it generates crazy gaming vibes. The array of titles is vast, offering live dealer games that immerse you. The welcome bonus is generous. Professional and helpful assistance. Withdrawals are processed quickly, though additional bonuses might be welcome. In conclusion, Wazamba Casino is essential for gamers for players seeking adventure ! Also the interface is intuitive and themed, adds a layer of excitement. Worth noting tournaments for competitive play, offering more ways to win.
wazambagr.com|
The Real Person!
The Real Person!
Je ne me lasse pas de Bingoal Casino, c’est une plateforme qui pulse avec energie. Il y a une multitude de jeux excitants, proposant des jeux de table immersifs. Accompagne de paris gratuits. L’assistance est efficace et pro, joignable a toute heure. Les retraits sont ultra-rapides, de temps en temps des recompenses supplementaires seraient un atout. Au final, Bingoal Casino est un incontournable pour les joueurs pour les joueurs en quete d’excitation ! Notons aussi le site est rapide et attrayant, amplifie le plaisir de jouer. Particulierement interessant les tournois reguliers pour la competition, assure des transactions fiables.
Emmenez-moi lГ -bas|
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk012.ru
лечение запоя омск
The Real Person!
The Real Person!
складские стеллажи Надежные металлические стеллажи для склада от производителя «Металлоизделия» Организуйте складское пространство с максимальной эффективностью! Компания «Металлоизделия» предлагает профессиональные металлические стеллажи для складов любого размера и назначения. Наши стеллажи для склада — это идеальное решение для хранения товаров, оборудования, архивов и материалов. Они позволяют использовать каждый квадратный метр площади по максимуму, обеспечивая легкий доступ к любой единице хранения. Почему выбирают наши стеллажи? Прочность и долговечность: Мы используем высококачественный стальной прокат и усиленные конструкции, выдерживающие значительные нагрузки (до 5000 кг на ячейку и более). Универсальность: Широкая линейка моделей — полочные, паллетные (фронтальные, гравитационные), консольные. Подберем решение под ваши задачи. Безопасность: Все конструкции имеют антикоррозийное покрытие и рассчитаны на многократную сборку-разборку. Строгое соблюдение ГОСТов. Модульность и масштабируемость: Вы можете легко нарастить систему или изменить конфигурацию при расширении склада. Выгодная цена: Работаем без посредников, так как являемся производителем.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-irkutsk010.ru
вывод из запоя круглосуточно иркутск
8runner – Love how everything works smoothly here, no annoying popups at all.
qi29 – Love how everything works smoothly here, no annoying popups at all.
The Real Person!
The Real Person!
Лечение алкоголизма на дому становится актуальным. Основные этапы включают детоксикации на дому и помощи при алкогольном запое. С чего начать лечение? Важно получить поддержку от близкихкоторые могут оказать помощь в этом процессе.Психотерапия и народные методы могут стать хорошим дополнением к медикаментам от алкоголя. Программы для трезвости и советы по отказу от спиртного помогут развить правильное отношение к алкоголю. narkolog-tula022.ru Важно помнить о реабилитации дома, которая включает психологическую помощь и профилактику рецидива. Как бросить пить самостоятельно? Начните с установки четкой цели и поиска источника мотивации. Главное – это поддержка и желание изменить свою жизнь.
The Real Person!
The Real Person!
I’m amazed by Wazamba Casino, it seems like a whirlwind of delight. The options are extensive and diverse, with sports betting for added thrill. The welcome bonus is generous. Available 24/7 for any queries. Payments are secure and reliable, occasionally more frequent promotions could enhance it. Broadly speaking, Wazamba Casino provides guaranteed enjoyment for those who enjoy crypto ! Moreover the site is fast and responsive, adds a layer of excitement. Another perk is crypto-friendly banking options, offering more ways to win.
https://wazambagr.com/|
The Real Person!
The Real Person!
Je suis totalement enchante par Bingoal Casino, ca offre un thrill exceptionnel. Il existe une abondance de jeux envoutants, comprenant des jeux optimises pour les cryptos. Associe a des paris gratuits. Le suivi est exemplaire, assurant un support premium. La procedure est aisee et efficace, cependant des bonus plus diversifies seraient souhaitables. Pour synthetiser, Bingoal Casino est une plateforme qui excelle pour les aficionados de jeux contemporains ! Ajoutons que l’interface est intuitive et raffinee, facilite une immersion complete. Un autre avantage cle les options de paris sportifs etendues, propose des recompenses permanentes.
Lire la version complГЁte|
The Real Person!
The Real Person!
Je suis emerveille par Locowin Casino, ca immerse dans un monde fascinant. Il y a une multitude de jeux captivants, offrant des sessions live intenses. Amplifiant l’experience initiale. Le suivi est impeccable, avec une aide precise et rapide. Le processus est simple et reibungslos, cependant quelques tours gratuits supplementaires seraient bienvenus. Pour conclure, Locowin Casino offre une experience inoubliable pour les amateurs de sensations fortes ! A noter l’interface est intuitive et elegante, amplifie le plaisir de jouer. Particulierement interessant les evenements communautaires engageants, assure des transactions fiables.
Aller sur le site|
The Real Person!
The Real Person!
Je suis stupefait par Locowin Casino, ca transporte dans un univers envoutant. La gamme de jeux est spectaculaire, offrant des sessions live intenses. Renforcant l’experience de depart. Les agents reagissent avec promptitude, accessible a tout instant. La procedure est aisee et efficace, bien que des incitations additionnelles seraient un benefice. En fin de compte, Locowin Casino est essentiel pour les amateurs pour les adeptes de sensations intenses ! Par ailleurs la navigation est simple et engageante, ce qui eleve chaque session a un niveau superieur. Un autre avantage cle les tournois periodiques pour la rivalite, qui stimule l’engagement.
Ouvrir maintenant|
The Real Person!
The Real Person!
Ich bin absolut hingerissen von NV Casino, es erzeugt eine Energie, die suchtig macht. Es gibt eine beeindruckende Auswahl an Optionen, mit Spielen, die perfekt fur Kryptos geeignet sind. Die Hilfe ist effizient und professionell, erreichbar zu jeder Stunde. Die Zahlungen sind sicher und flussig, manchmal zusatzliche Freispiele waren toll. Alles in allem, NV Casino garantiert Top-Unterhaltung fur Spieler auf der Suche nach Action ! Hinzu die Oberflache ist intuitiv und stylish, fugt eine Prise Magie hinzu.
playnvcasino.de|
The Real Person!
The Real Person!
Je suis completement captive par Casinia Casino, il procure une experience imperiale. La variete des titres est majestueuse, proposant des jeux de table elegants. 100% jusqu’a 500 € + 200 tours gratuits. Le service est disponible 24/7, offrant des reponses claires. Les retraits sont rapides comme l’eclair, cependant des offres plus genereuses ajouteraient du prestige. Pour conclure, Casinia Casino garantit du fun a chaque instant pour les amateurs de sensations fortes ! De plus la navigation est simple et agreable, ajoute une touche de luxe. A noter egalement les evenements communautaires engageants, qui booste l’engagement.
DГ©couvrir plus|
The Real Person!
The Real Person!
Je suis surpris par Locowin Casino, il delivre une experience unique. La gamme de jeux est spectaculaire, incluant des paris sportifs electrisants. Le bonus d’accueil est attractif. L’equipe d’assistance est remarquable, proposant des reponses limpides. Les retraits sont realises promptement, mais des incitations additionnelles seraient un benefice. Globalement, Locowin Casino est une plateforme qui excelle pour les joueurs a la recherche d’aventure ! A mentionner le design est contemporain et lisse, intensifie le plaisir du jeu. Egalement notable le programme VIP avec des niveaux exclusifs, qui stimule l’engagement.
Locowin|
ilili1oulilil5 – Some pages load oddly, but I’m getting a sense of structure.
Vegetarische Pizza Lieferung hat mich uberzeugt. Frische Zutaten und schneller Service.
Essen Lieferung
https://atlan.ru/
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-orenburg010.ru
экстренный вывод из запоя оренбург
0080kk – Consistently good content, keeps me informed and engaged.
Ich bin beeindruckt von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es wartet eine Fulle spannender Optionen, mit aufregenden Sportwetten. Der Service ist von hoher Qualitat, verfugbar rund um die Uhr. Die Transaktionen sind verlasslich, gelegentlich mehr abwechslungsreiche Boni waren super. Alles in allem, SpinBetter Casino ist eine Plattform, die uberzeugt fur Online-Wetten-Fans ! Zusatzlich die Interface ist intuitiv und modern, gibt den Anreiz, langer zu bleiben. Besonders toll die schnellen Einzahlungen, die Flexibilitat bieten.
spinbettercasino.de|
The Real Person!
The Real Person!
J’aime l’atmosphere unique de Locowin Casino, c’est une plateforme qui deborde de vitalite. Il y a une multitude de jeux captivants, comprenant des jeux optimises pour les cryptos. Accompagne de paris gratuits. L’assistance est efficace et professionnelle, offrant des reponses claires. Les transactions sont fiables et rapides, parfois plus de promos regulieres seraient un plus. En resume, Locowin Casino garantit du plaisir a chaque instant pour les amateurs de sensations fortes ! De plus le design est moderne et fluide, ajoute une touche de confort. Un avantage supplementaire les paiements securises en crypto, offre des recompenses continues.
Naviguer sur le site|
The Real Person!
The Real Person!
Je suis captive par Locowin Casino, on detecte une vibe folle. La gamme de jeux est spectaculaire, avec des slots au style innovant. Associe a des tours gratuits sans wager. L’equipe d’assistance est remarquable, accessible a tout instant. Les operations sont solides et veloces, mais des bonus plus diversifies seraient souhaitables. Pour synthetiser, Locowin Casino garantit du divertissement constant pour les joueurs a la recherche d’aventure ! A mentionner la navigation est simple et engageante, intensifie le plaisir du jeu. A souligner aussi le programme VIP avec des niveaux exclusifs, qui stimule l’engagement.
Lire entiГЁrement|
The Real Person!
The Real Person!
вывод из запоя круглосуточно иркутск
vivod-iz-zapoya-irkutsk011.ru
вывод из запоя иркутск
The Real Person!
The Real Person!
Je suis bluffe par Casinia Casino, il procure une experience imperiale. La variete des titres est majestueuse, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. Les agents repondent avec celerite, garantissant un support de qualite. Les retraits sont rapides comme l’eclair, parfois des offres plus genereuses ajouteraient du prestige. Au final, Casinia Casino est une plateforme qui regne en maitre pour les amateurs de sensations fortes ! Cerise sur le gateau le site est rapide et attrayant, ajoute une touche de luxe. Un plus non negligeable les tournois reguliers pour la competition, offre des recompenses continues.
VГ©rifier cela|
The Real Person!
The Real Person!
J’ai un engouement sincere pour Locowin Casino, on detecte une vibe folle. La diversite des titres est epoustouflante, proposant des jeux de table immersifs. Associe a des tours gratuits sans wager. L’aide est performante et experte, proposant des reponses limpides. Les benefices arrivent sans latence, bien que quelques tours gratuits supplementaires seraient apprecies. Globalement, Locowin Casino fournit une experience ineffacable pour les aficionados de jeux contemporains ! De surcroit la plateforme est esthetiquement remarquable, stimule le desir de revenir. Un autre avantage cle le programme VIP avec des niveaux exclusifs, renforce le sens de communaute.
Voir tout de suite|
868i – Just visited and design feels fresh and uncluttered.
The Real Person!
The Real Person!
Алгачкы жолкусунан бери Алекс Перейра кантип өсүп, чемпион болгону таң калтырат. Алекс Перейра — чыныгы мисал чыдамкайлык жана эрктүүлүктүн. Перейра тууралуу баарын бир жерден оку: Перейра Алекс UFC жаңылыктар. Ар бир муштумунда тарых жатат.
Ал кээде сөз сүйлөбөйт, бирок иш менен далилдейт.
Кызыгы, Алекс Перейра Исламды кабыл алган экен. Бул жигиттин мүнөзү – темирдей бекем. Перейра өзүнүн дараметин кайра-кайра далилдеп келет.
Перейра үчүн утулуш — бул сабак гана. Көрсө, ийгилик каалоо эмес, аракет экен.
togelrejeki – Great site overall, design feels clean and easy today.
xinyurenedu – The resources available are both diverse and engaging.
88878qp – I like the visual layout, gives a good first impression.
blur-blur – The site loads quickly, and the design is modern.
khawajakhawarrashid – I like the typography, clear and easy to read throughout.
heima999cp – I’m impressed with the site speed and overall clean presentation here.
schenectadybathroomremodeling – The photos are great, helps visualize possibilities for my space.
1001-webtest – Minimalist style fits well, waiting for content to appear.
What’s up mates, good post and good arguments commented here, I am really enjoying by these.
https://bazakvartir.kiev.ua/iak-uniknuty-poshkodzhen-korpusu-fary-za-dopomohoiu-pnevmoinstrumenta.html
7558app – I like how clean the homepage feels, smooth layout.
031757 – Nice design, hope content matches the style.
16999ys – Clean homepage, makes me want to click further inside.
881625 – Design gives confidence, hope the content delivers as well.
btc289 – Simple interface, hope navigation makes sense when activated.
122yyy – Minimal look works well, waiting for substance to arrive.
hg889988 – Seems under development, but design choices are solid.
9909hd – Nice first impression, I’ll revisit when more content is visible.
selvaluna – I like the color scheme, feels warm and inviting today.
The Real Person!
The Real Person!
Чыныгы мотивация издеген адам Алекс Перейранын жолун көрсүн. Перейранын соккусу оор, ар бир муштум — таза күч. Алекс Перейра тууралуу эң акыркы жаңылыктарды ушул жерден тапсаң болот — Алекс Перейра. Алекс Перейра менен Анкалаев беттешсе, эмне болот экен?
Ким аны жеңе алат деп ойлойсуңар?
Анын аялын да колдоосу таң калтырат. UFC тарыхында мындай энергия сейрек болот. Анын соккусунан кийин көптөр оңоло албай калган.
Перейранын жашоосун көрүп, каармандыкты түшүнсө болот. Анын кыска убакытта кантип чемпион болгону таң калтырат.
The Real Person!
The Real Person!
Алгачкы жолкусунан бери Алекс Перейра кантип өсүп, чемпион болгону таң калтырат. Көрсө, Алекс Перейра көчөдөн чыккан, эми болсо дүйнөнүн чокусунда. Бул сайт Перейранын бардык беттештерин чогулткан: http://www.alex-pereira-kg.com. Ар бир муштумунда тарых жатат.
Анын кийинки беттеши кимге каршы болору кызык.
Алекс Перейра UFCге тарых жазып жатат. Көрсө, чыныгы чемпиондор көчөдөн да чыгат экен. Көргөндө эле энергиясы сезилет.
Перейра үчүн утулуш — бул сабак гана. Алекс Перейра — күч, эрк жана ишенимдин символу.
Путешествуйте по Крыму https://м-драйв.рф на джипах! Ай-Петри, Ялта и другие живописные маршруты. Безопасно, интересно и с профессиональными водителями. Настоящий отдых с приключением!
I think that everything said made a lot of sense. But, consider this, suppose you added a little information? I am not saying your information isn’t solid., but suppose you added a headline that makes people want more? I mean %BLOG_TITLE% is a little vanilla. You might look at Yahoo’s front page and note how they create post titles to get people interested. You might add a video or a picture or two to get readers interested about what you’ve written. In my opinion, it would bring your blog a little bit more interesting.
либет казино
sahabetaff – Smooth menu transitions, overall looks like a solid site.
elmhurstunited – The visuals and tone combine well, site feels consistent and genuine.
The Real Person!
The Real Person!
Алекс Перейранын күчү менен техникасы укмуш айкалышкан. Ал жеңилсе да, кайра кайтып келип жеңишке жетет. Анын рекорду жана жеңиштери тууралуу кенен маалымат: Перейра рекорд. Анын Адесанья менен болгон беттеши легендарный болду.
Көптөр Перейраны жеңет дешет, бирок ал дайыма жооп берет.
Ал үй-бүлөсүн, балдарын, өлкөсүн сыйлайт. Анын жашоосунда дисциплина эң башкысы. Бул жигит чыныгы “monster”.
Ал каалаган максатына жеткен. Көрсө, ийгилик каалоо эмес, аракет экен.
The Real Person!
The Real Person!
Бул жигит жөн эле мушкер эмес, чыныгы согуш өнөрүнүн устаты. Мен Перейранын ар бир беттешин күтүп көрөм. Перейра тууралуу баарын бир жерден оку: Перейра Алекс UFC жаңылыктар. Перейра менен Джон Джонстун беттеши көргүм келет.
Перейранын жашоосу — мотивация.
Анын тарбиясы жөнөкөй, бирок максаты чоң. Перейра ар бир жеңиши менен тарых жазат. Менин оюмча, Перейра кайра жеңишке жетет.
Перейра — элдин арасынан чыккан легенда. Анын ар бир беттеши көрүүчүлөр үчүн шоу.
7392004 – Looks promising, I’ll revisit when more features appear.
apphoki – Nice site, I like how clean the homepage feels.
The Real Person!
The Real Person!
экстренный вывод из запоя оренбург
vivod-iz-zapoya-orenburg011.ru
вывод из запоя круглосуточно
bnnnw – Clean interface, gives a sense of professionalism.
The Real Person!
The Real Person!
Запой — это значительная беда, затрагивающая не лишь саму личность, страдающую от алкоголизма, но и его близких. Как убедить человека покинуть запой? Прежде всего, важно понимать, что семейная поддержка играет ключевую роль в процессе преодоления зависимости. Нарколог на дом Тула Первый шаг — это консультация специалиста. Специалист по наркологии в Туле может предоставить медицинскую помощь на дому, чтобы снизить страдания пациента. Психологическая поддержка также необходима: она поможет выяснить корни алкогольной зависимости и найти пути к решению проблемы. Советы по выходу из запоя включают в себя создание мотивирующей обстановки, где человек ощущает заботу и поддержку. Обсуждение кризиса в семье и важности единства может стать толчком к размышлениям о необходимости лечения. Лечение запоя и реабилитация помогут восстановить здоровье и вернуться к нормальной жизни. Необходимо осознавать признаки запойного состояния и быть готовыми к вызовам. Поддержка в мотивации к лечению — это совместная работа, требующая как времени, так и усилий. Поддерживайте своего близкого, показывайте, как вы хотите ему помочь, и вместе пройдите этот путь к выздоровлению.
The Real Person!
The Real Person!
Чыныгы мотивация издеген адам Алекс Перейранын жолун көрсүн. Ал жеңилсе да, кайра кайтып келип жеңишке жетет. Перейранын акыркы интервьюсу сайтта жарыяланган: Алекс Перейра интервью. Анын тарыхы көчөдөн башталып, UFC чемпиондугу менен бүттү.
Алекс Перейра азыр кайсы салмак категориясында мушташат?
Анын карьерасы али бүтө элек. Бул жигиттин мүнөзү – темирдей бекем. Менин оюмча, Перейра кайра жеңишке жетет.
Перейранын жашоосун көрүп, каармандыкты түшүнсө болот. Көрсө, ийгилик каалоо эмес, аракет экен.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-irkutsk012.ru
экстренный вывод из запоя
Thanks for sharing. It’s acme quality. acheter du levitra en ligne
Нужна карта? https://vc.ru/money/2104862-kak-poluchit-kartu-visa-ili-mastercard-v-rossii-v-2025-godu как оформить зарубежную банковскую карту Visa или MasterCard для россиян в 2025 году. Карту иностранного банка можно открыть и получить удаленно онлайн с доставкой в Россию и другие страны. Зарубежные карты Visa и MasterCard подходят для оплаты за границей. Иностранные банковские карты открывают в Киргизии, Казахстане, Таджикистане и ряде других стран СНГ, все подробности смотрите по ссылке.
Металлообработка и металлы http://j-metall.ru ваш полный справочник по технологиям и материалам: обзоры станков и инструментов, таблицы марок и ГОСТов, кейсы производства, калькуляторы, вакансии, и свежие новости и аналитика отрасли для инженеров и закупщиков.
J’ai une passion debordante pour Bingoal Casino, on ressent une energie folle. Le catalogue est abondant et varie, incluant des paris sportifs palpitants. Doublement des depots jusqu’a 200 €. L’assistance est efficace et professionnelle, offrant des reponses claires. Les gains arrivent sans retard, cependant des recompenses additionnelles seraient un avantage. En resume, Bingoal Casino est indispensable pour les joueurs pour les amateurs de sensations fortes ! En bonus le site est rapide et attractif, ajoute une touche de confort. Particulierement notable le programme de fidelite avec des niveaux VIP, offre des recompenses continues.
DГ©bloquer les dГ©tails|
The Real Person!
The Real Person!
I’m enthralled with Wazamba Casino, it constructs an enthralling narrative. The archive is profoundly vast, encompassing real-time dealer interactions for genuineness. Paired with 200 gratis rotations. Promoting continuous enjoyment. The framework is approachable, although diversified reward schemes would improve it. Collectively , Wazamba Casino turns essential for devotees for exploration enthusiasts ! Further the imagery is alluring and absorbing, fortifying absorption in play. An additional merit obtaining elements for privileges, developing group cohesion.
wazambagr.com|
The Real Person!
The Real Person!
J’aime l’environnement distinct de Bingoal Casino, on percoit une vitalite dechainee. Les alternatives sont etonnamment etendues, avec des slots innovants et thematises. Doublement des depots jusqu’a 200 €. L’equipe d’assistance est remarquable, accessible a tout instant. Les operations sont solides et veloces, bien que des incitations additionnelles seraient un benefice. Pour synthetiser, Bingoal Casino fournit une experience ineffacable pour les adeptes de sensations intenses ! A mentionner le design est contemporain et lisse, facilite une immersion complete. Particulierement attractif les evenements communautaires captivants, qui stimule l’engagement.
Rejoindre maintenant|
Thanks for sharing. It’s outstrip quality. stromectol posologie poids
The Real Person!
The Real Person!
Капельница при алкоголизме – это популярный метод быстрого вывода из запойного состояния, который помогает справиться с физической зависимостью от алкоголя. Однако борьба с алкоголизмом требует внимания к различным аспектам, но и психотерапевтической работы. Важно понимать, что алкоголизм – это состояние, затрагивающее как тело, так и душу. Экстренный вывод из запоя включает в себя восстановление водно-электролитного баланса, но недостаточно просто провести капельницу для полного выздоровления. В проблемные моменты помощь зависимым включает не только медикаменты, но и реабилитацию, программы психотерапии. Семейная поддержка является важным элементом успешного лечения. Эмоциональная поддержка близких помогает создать привычку к трезвому образу жизни и помочь избежать повторного запойного состояния. Профилактика рецидивов включает в себя различные методы, как профилактические, так и терапевтические, что делает лечение комплексным и более эффективным.
+1
zxjgzxfuzhou – Feels like a legitimate business, hope customer support responds nicely.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-orenburg012.ru
вывод из запоя
The Real Person!
The Real Person!
https://tripscan41.cc/ Tripscan трипскан: Подчеркивание двуязычной направленности сервиса для охвата широкой аудитории. TripScan/Трипскан – это многофункциональная платформа для планирования путешествий, ориентированная на пользователей, говорящих как на английском, так и на русском языках. Она объединяет в себе все необходимые инструменты для организации поездки, включая поиск и бронирование авиабилетов, отелей, арендных автомобилей и экскурсий. TripScan/Трипскан предлагает широкий выбор направлений, удобные фильтры для поиска и сравнения вариантов, а также персональные рекомендации, основанные на интересах и предпочтениях пользователя. Платформа стремится предоставить удобный и эффективный способ организации путешествий, позволяя пользователям экономить время, деньги и силы.
bsporttiyu – Navigation is a little confusing in places, but content makes up for it.
www9tt01 – Let me dig deeper into domain history if you want more insight.
www9tt9 – I can check archival records or WHOIS for more insight.
www9tt05 – I’ll fetch WHOIS, SSL, and archive snapshots to see its history.
www9tt9 – No trust seals or company information popped up in my searches.
www9tt04 – I couldn’t find clear “about us” or trust info tied to this domain.
ma3wna – Navigation is intuitive, I didn’t struggle to find things I wanted.
dsjy0916 – I can try checking archive.org or WHOIS records if you want deeper insight.
bmrlw – Smooth performance with decent structure, feels professional at first glance.
11xhh – The site feels legitimate, hope the content matches its polish.
The Real Person!
The Real Person!
лечение психиатрических больных
psikhiatr-moskva008.ru
вызов психиатра на дом в москве
hotel-reservation-rome.com – The photos look nice, but images can deceive; check map location.
mg2jp – The site loading as redirect with “Trust 69 / Privacy 69” is odd.
tingshuzu – Looks like they care about design, the aesthetics are pleasant.
The Real Person!
The Real Person!
Перейра ушунчалык токтоо жана ишенимдүү — аны утуш абдан кыйын. Ал жеңилсе да, кайра кайтып келип жеңишке жетет. Эгер Перейранын акыркы беттешин көргүң келсе, кирип чык https://alex-pereira-kg.com. Алекс Перейра менен Анкалаев беттешсе, эмне болот экен?
Перейранын рекорду таасирдүү.
Ушундай жигиттер спорттун жүзүн өзгөртөт. Перейра ар бир жеңиши менен тарых жазат. Бул жигит чыныгы “monster”.
Перейра — элдин арасынан чыккан легенда. Анын кыска убакытта кантип чемпион болгону таң калтырат.
Instagram
358799 – No reviews or third-party references found right now.
xfbsp666 – Found something useful just clicking around unexpectedly.
The Real Person!
The Real Person!
tripskan TripScan – инновационный сервис для планирования идеальных путешествий. Этот сервис объединяет в себе все необходимые инструменты для организации незабываемого отпуска или деловой поездки. TripScan позволяет легко находить и бронировать авиабилеты и отели по самым выгодным ценам, составлять маршруты путешествий с учетом ваших интересов и предпочтений, а также получать полезную информацию о достопримечательностях, ресторанах и развлечениях в выбранном вами направлении. TripScan – ваш надежный помощник в мире путешествий, который экономит ваше время и деньги, делая процесс планирования приятным и увлекательным.
mashoppy – Great first glance, hope shipping is as smooth as design.
haxorisme – Might be a new or obscure project, needs deeper verification before trust.
The Real Person!
The Real Person!
Если у вас возникли сложности с алкоголизмом или когда вы знаете человека, нуждающегося помощь, рекомендуем обратиться к специалистам. Наркологическая клиника в Туле предоставляет срочную помощь при алкоголизма и наркомании. Обращение к наркологу поможет установить нужный курс лечения. Центр по лечению наркоманов предоставляет медицинскую помощь и психотерапию, в дополнение поддержку семьи. Лечение с гарантией анонимности гарантирует безопасность и конфиденциальность. Реабилитация наркоманов включает меры по предотвращению рецидивов и восстановление после зависимости. Ваш первый шаг к новому началу начинается в этом месте. narkolog-tula026.ru
jingcaity – I couldn’t dig up much, site seems quite unknown and low visibility.
The Real Person!
The Real Person!
платная психиатрическая скорая помощь
psikhiatr-moskva009.ru
психиатр на дом для пожилого человека
Строительный портал https://repair-house.kiev.ua всё о строительстве, ремонте и архитектуре. Подробные статьи, обзоры материалов, советы экспертов, новости отрасли и современные технологии для профессионалов и домашних мастеров.
Строительный портал https://intellectronics.com.ua источник актуальной информации о строительстве, ремонте и архитектуре. Обзоры, инструкции, технологии, проекты и советы для профессионалов и новичков.
Портал о стройке https://mr.org.ua всё о строительстве, ремонте и дизайне. Статьи, советы экспертов, современные технологии и обзоры материалов. Полезная информация для мастеров, инженеров и владельцев домов.
The Real Person!
The Real Person!
как уменьшить ндс не нарушая закон Купить двигатель Suzuki – компактность. K14C для Vitara. Цены 50-150 тысяч. Suzuki – 4×4.
Актуальный портал https://sinergibumn.com о стройке и ремонте. Современные технологии, материалы, решения для дома и бизнеса. Полезные статьи, инструкции и рекомендации экспертов.
dadatudaohang – So far, not enough evidence to trust it fully.
The Real Person!
The Real Person!
Частный нарколог на дому в Туле предоставляет важную помощь тем, кто борется с зависимостями. Процесс лечения зависимостей может быть сложным процессом, но нарколог, работающий на дому предлагает комфортные условия и конфиденциальность. На сайте narkolog-tula027.ru можно назначить встречу с наркологом, который приедет к вам домой. На дому пациентам предоставляется медицинская помощь включает очистку организма и психологическую терапию для борьбы с зависимостями, что позволяет пациенту начать путь к отрыву от наркотиков или алкоголя. Реабилитационная программа разрабатывается с учетом индивидуальных особенностей, что позволяет учитывать уникальные потребности каждого пациента. Поддержка родственников играет важнейшую роль в профилактике рецидива. Выездной нарколог обеспечивает не только лечение, но и эмоциональную поддержку, что важно для успешной реабилитации наркоманов и помощи при алкоголизме.
Женский портал https://prins.kiev.ua всё о красоте, моде, отношениях, здоровье и саморазвитии. Полезные советы, вдохновение, психология и стиль жизни для современных женщин.
Онлайн женский портал https://replyua.net.ua секреты красоты, стиль, любовь, карьера и семья. Читайте статьи, гороскопы, рецепты и советы для уверенных, успешных и счастливых женщин.
56089h – Contact and policy links are visible, helps a lot with trust.
Современный женский https://novaya.com.ua портал о жизни, моде и гармонии. Уход за собой, отношения, здоровье, рецепты и вдохновение для тех, кто хочет быть красивой и счастливой каждый день.
Интересный женский https://muz-hoz.com.ua портал о моде, психологии, любви и красоте. Полезные статьи, тренды, рецепты и лайфхаки. Живи ярко, будь собой и вдохновляйся каждый день!
Женский портал https://z-b-r.org ваш источник идей и вдохновения. Советы по красоте, стилю, отношениям, карьере и дому. Всё, что важно знать современной женщине.
kytautoparts – If they list compatibility clearly, that would mean they know their stuff.
yiikr – Nice color palette, pleasant on eyes during long browsing sessions.
56089m – Footer includes contact info, privacy policy; adds credibility to site.
k9c4fzmzxj1 – Some pages have good content, though a bit more depth would help.
764585 – The domain gives weak trust signals so far, not much presence online.
bulkytrader – Found some interesting wholesale items, impressed by the selection.
3915t – No broken links so far, all clicked pages seem valid and working.
x5748 – Navigation is easy to follow, links are clearly labeled.
8222173 – Been coming back all week, layout and info always on point.
246123kj – The typography works, text is legible and not too small to read.
bjjxyzp – The site uses few images; might benefit from richer visuals later.
goodthingsshare – I like how easy it is to navigate, menus feel intuitive.
303sahabat – No annoying popups, navigation is simple and finds pages quickly.
snmm66 – Site loads quickly and feels responsive, nice experience overall.
Je suis emerveille par Bingoal Casino, ca procure un thrill incomparable. Il y a une multitude de jeux passionnants, avec des slots au design innovant. Pour un demarrage en force. Les agents repondent avec celerite, joignable a tout moment. Les gains arrivent sans retard, de temps a autre quelques tours gratuits supplementaires seraient bienvenus. Dans l’ensemble, Bingoal Casino est une plateforme qui impressionne pour ceux qui aiment parier en crypto ! A noter le site est rapide et attractif, ce qui rend chaque session plus enjoyable. Particulierement notable les tournois reguliers pour la competition, assure des transactions fiables.
Voir plus|
https://katellkeinegcom.wordpress.com/
oarlop – Imagery is decent and relevant; not just filler stock photos.
jiandanai520 – On mobile it looks good, no strange misalignments or overflow.
The Real Person!
The Real Person!
Ich bin abhangig von SpinBetter Casino, es bietet einen einzigartigen Kick. Der Katalog ist reichhaltig und variiert, mit immersiven Live-Sessions. Der Support ist 24/7 erreichbar, mit praziser Unterstutzung. Die Zahlungen sind sicher und smooth, gelegentlich regelma?igere Aktionen waren toll. Zum Ende, SpinBetter Casino garantiert hochsten Spa? fur Online-Wetten-Fans ! Nicht zu vergessen die Navigation ist kinderleicht, fugt Magie hinzu. Zusatzlich zu beachten die mobilen Apps, die das Spielen noch angenehmer machen.
spinbettercasino.de|
The Real Person!
The Real Person!
Je suis hypnotise par Bingoal Casino, ca offre un thrill exceptionnel. L’eventail de jeux est extraordinaire, incluant des paris sportifs electrisants. Doublement des depots jusqu’a 200 €. Le suivi est exemplaire, accessible a tout instant. Les operations sont solides et veloces, bien que des incitations additionnelles seraient un benefice. Pour synthetiser, Bingoal Casino est une plateforme qui excelle pour les adeptes de sensations intenses ! En outre la navigation est simple et engageante, intensifie le plaisir du jeu. Particulierement attractif les paiements securises en crypto, offre des privileges sur mesure.
Voir le site|
Je suis epate par Locowin Casino, il offre une experience unique. La selection de jeux est phenomenale, offrant des sessions live dynamiques. Doublement des depots jusqu’a 1850 €. Le suivi client est irreprochable, toujours pret a aider. Les transactions sont fiables et rapides, mais des bonus plus varies seraient bienvenus. En resume, Locowin Casino est un incontournable pour les joueurs pour les joueurs en quete d’excitation ! Ajoutons que le design est moderne et fluide, facilite une immersion totale. A noter egalement le programme VIP avec des niveaux exclusifs, offre des recompenses continues.
Aller pour les dГ©tails|
The Real Person!
The Real Person!
Je suis accro a Locowin Casino, c’est une plateforme qui explose d’energie. Les alternatives sont incroyablement etendues, avec des slots au style innovant. Renforcant l’experience de depart. Les agents reagissent avec promptitude, toujours pret a intervenir. Les paiements sont proteges et lisses, cependant des incitations additionnelles seraient un benefice. En synthese, Locowin Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! Par ailleurs le site est veloce et seduisant, facilite une immersion complete. Egalement notable le programme VIP avec des niveaux exclusifs, garantit des transactions securisees.
Visiter le contenu|
Авто портал https://bestsport.com.ua всё об автомобилях: новости, обзоры, тест-драйвы, советы по уходу и выбору машины. Узнайте о новинках автопрома, технологиях и трендах автомобильного мира.
Автомобильный портал https://autoguide.kyiv.ua для водителей и поклонников авто. Новости, аналитика, обзоры моделей, сравнения, советы по эксплуатации и ремонту машин разных брендов.
Онлайн авто портал https://retell.info всё для автолюбителей! Актуальные новости, обзоры новинок, рейтинги, тест-драйвы и полезные советы по эксплуатации и обслуживанию автомобилей.
Авто портал https://psncodegeneratormiu.org мир машин в одном месте. Читайте обзоры, следите за новостями, узнавайте о новинках и технологиях. Полезный ресурс для автолюбителей и экспертов.
Современный авто портал https://necin.com.ua мир автомобилей в одном месте. Тест-драйвы, сравнения, новости автопрома и советы экспертов. Будь в курсе последних тенденций автоиндустрии
The Real Person!
The Real Person!
реабилитация от наркозависимости
reabilitaciya-astana007.ru
алкоголизм лечение
eggaa – I like how minimal it is, doesn’t feel cluttered at all.
660fh – Image quality is good, nothing blurry or stretched.
J’ai une passion debordante pour Bingoal Casino, il offre un voyage unique. Le catalogue est abondant et varie, avec des slots au design innovant. Le bonus d’accueil est seduisant. Le suivi est impeccable, avec une aide precise et rapide. Les retraits sont effectues rapidement, parfois des recompenses additionnelles seraient un avantage. Dans l’ensemble, Bingoal Casino est indispensable pour les joueurs pour les fans de casino en ligne ! Par ailleurs le site est rapide et attractif, facilite une immersion totale. Un avantage supplementaire les options de paris sportifs variees, renforce le sentiment de communaute.
Commencer maintenant|
trendhunter.click – The content is so current, I keep coming back for more.
urbanrise.click – Navigation works smooth, links respond quickly without lag.
The Real Person!
The Real Person!
Ich bin komplett hin und weg von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Das Angebot an Spielen ist phanomenal, mit immersiven Live-Sessions. Der Kundenservice ist ausgezeichnet, bietet klare Losungen. Die Auszahlungen sind ultraschnell, obwohl die Offers konnten gro?zugiger ausfallen. Global gesehen, SpinBetter Casino bietet unvergessliche Momente fur Online-Wetten-Fans ! Au?erdem das Design ist ansprechend und nutzerfreundlich, erleichtert die gesamte Erfahrung. Ein Pluspunkt ist die Community-Events, die Flexibilitat bieten.
spinbettercasino.de|
The Real Person!
The Real Person!
J’ai une passion ardente pour Bingoal Casino, c’est une plateforme qui foisonne de vigueur. La gamme des titres est stupefiante, offrant des sessions live intenses. Renforcant l’experience de depart. L’aide est performante et experte, toujours pret a intervenir. Les paiements sont proteges et lisses, cependant quelques tours gratuits supplementaires seraient apprecies. Pour synthetiser, Bingoal Casino merite une exploration approfondie pour les adeptes de sensations intenses ! A mentionner la navigation est simple et engageante, ajoute un confort notable. Un plus significatif le programme de fidelite avec des niveaux VIP, renforce le sens de communaute.
DГ©couvrir le contenu|
The Real Person!
The Real Person!
Je suis accro a Locowin Casino, on ressent une vibe delirante. Le catalogue est riche et diversifie, proposant des jeux de table immersifs. Accompagne de tours gratuits sans wager. Le suivi client est irreprochable, joignable a toute heure. Le processus est simple et efficace, cependant quelques tours gratuits en plus seraient cool. Dans l’ensemble, Locowin Casino offre une experience memorable pour les amateurs de sensations fortes ! Cerise sur le gateau l’interface est intuitive et stylee, ce qui rend chaque session encore plus fun. Un plus non negligeable le programme VIP avec des niveaux exclusifs, assure des transactions fiables.
Cliquer et voir|
The Real Person!
The Real Person!
Je suis completement ensorcele par Casinia Casino, on ressent une energie fastueuse. La diversite des titres est somptueuse, proposant des jeux de table raffines. Avec un Bonus Crab exclusif. L’assistance est precise et elegante, offrant des solutions limpides. Le processus est lisse et distingue, neanmoins des bonus plus varies seraient un joyau. En bref, Casinia Casino est un pilier pour les joueurs pour les amateurs de casino en ligne ! Par ailleurs le site est rapide et majestueux, ajoute une touche de splendeur. Particulierement captivant les tournois reguliers pour la rivalite, offre des privileges continus.
VГ©rifier les infos|
The Real Person!
The Real Person!
Je suis fascine par Locowin Casino, ca transporte dans un univers envoutant. La diversite des titres est epoustouflante, comprenant des jeux adaptes aux cryptos. Associe a des tours gratuits sans wager. Le suivi est exemplaire, avec une assistance exacte et veloce. Les benefices arrivent sans latence, bien que des offres plus liberales ajouteraient de la valeur. Pour synthetiser, Locowin Casino fournit une experience ineffacable pour les enthousiastes de casino en ligne ! De surcroit la navigation est simple et engageante, ce qui eleve chaque session a un niveau superieur. Un plus significatif les evenements communautaires captivants, offre des privileges sur mesure.
Trouver le meilleur|
The Real Person!
The Real Person!
Je suis impressionne par Locowin Casino, c’est une plateforme qui explose d’energie. La gamme de jeux est spectaculaire, avec des slots au style innovant. Associe a des tours gratuits sans wager. L’equipe d’assistance est remarquable, avec une assistance exacte et veloce. Les retraits sont realises promptement, cependant plus de promotions frequentes seraient un atout. Tout compte fait, Locowin Casino est une plateforme qui excelle pour ceux qui parient en crypto ! De surcroit le site est veloce et seduisant, ce qui eleve chaque session a un niveau superieur. Egalement notable le programme VIP avec des niveaux exclusifs, renforce le sens de communaute.
DГ©couvrir le contenu|
digitalstorm.click – Navigation is intuitive, found what I was looking for quickly.
digitalnest.click – Content is engaging and well-organized, makes browsing enjoyable.
nextgenmarket.click – Always find something new and interesting every time I visit.
worldtrendspace.click – The layout here is sleek and modern, very user-friendly.
boldspark.click – Images present are decent, not too many broken or placeholder visuals.
linkfusion.click – Found this while browsing; it’s clean and easy to use.
goldenwave.click – Solid work — feels like a site made by someone who cares.
yourbrandzone.click – Feels like they really know what users want, easy navigation throughout.
ideaorbit.click – No visible external references or reviews linking back to this domain.
urbanvision.click – The layout here is sleek and modern, very user-friendly.
nextbrand.click – Impressed by the quality of visuals and overall aesthetics.
linkmaster.click – Great site you’ve got here, the design is crisp and modern.
cloudmatrix.click – Images are minimal or generic; content feels like placeholder in parts.
futurebeam.click – Really impressed by how smooth the site loads and behaves.
cloudmark.click – Navigation is smooth, I appreciate how everything loads fast.
Портал про стройку https://dcsms.uzhgorod.ua всё о строительстве, ремонте и дизайне. Полезные советы, статьи, технологии, материалы и оборудование. Узнайте о современных решениях для дома и бизнеса.
Онлайн-портал про стройку https://donbass.org.ua и ремонт. Новости, проекты, инструкции, обзоры материалов и технологий. Всё, что нужно знать о современном строительстве и архитектуре.
Портал про стройку https://keravin.com.ua и ремонт полезные статьи, инструкции, обзоры оборудования и материалов. Всё о строительстве домов, дизайне и инженерных решениях
Строительный портал https://msc.com.ua о ремонте, дизайне и технологиях. Полезные советы мастеров, обзоры материалов, новинки рынка и идеи для дома. Всё о стройке — от фундамента до отделки. Учись, строй и вдохновляйся вместе с нами!
Подоконники из искусственного камня https://luchshie-podokonniki-iz-kamnya.ru в Москве. Рейтинг лучших подоконников – авторское мнение, глубокий анализ производителей.
The Real Person!
The Real Person!
I have a huge crush on Astronaut Crash by 100HP Gaming, it builds an adrenaline-fueled cosmic quest. The gameplay is brilliantly simple yet addictive, with customizable bet sizes from $0.10 to $150. Instant demo mode for practice. Live chat resolves issues in seconds, accessible via multiple channels. The cash-out feature saves fortunes, however higher max bets for high-rollers. Overall, Astronaut Crash is must-play for risk-takers for quick-play enthusiasts ! Plus load times are near-instant, heightening the launch anticipation. A real gem provably fair verification tools, builds a social betting community.
https://astronaut-crashgame777.com/|
The Real Person!
The Real Person!
I find irresistible Wazamba Casino, it forges a compelling story. The range of games is exceptional, incorporating wagers on athletic events. Equaling contributions up to €500. Functioning non-stop. Disbursements are managed efficiently, however supplemental rotations could advance it. Finishing with , Wazamba Casino turns essential for devotees for staking experts ! Further the setting initializes swiftly, urging sustained engagements. An additional merit nurturing collective spirit, supplying individualized graces.
wazambagr.com|
growwithfocus.click – SSL (HTTPS) might be present, but that alone doesn’t guarantee trust.
The Real Person!
The Real Person!
Капельница от запоя: экстренная помощь при алкогольном отравлении Инфузионная терапия содержит жизненно важные вещества для нормализации водно-электролитного состояния и укрепления здоровья больного. Эффективная терапия зависимости включает не только вывод из запоя, но и полноценное лечение алкоголизма. Необходимо учитывать процесс восстановления после запоя, который может занять время и потребовать дополнительных усилий. вывод из запоя круглосуточно Скорая помощь включает в себя диагностику состояния и персонализированный подход к лечению. При выявлении симптомов алкогольного отравления необходимо срочно обратиться за медицинской помощью. Не забывайте, что здоровье является наивысшей ценностью, и профессиональная помощь в наркологической клинике поможет вернуть контроль над своей жизнью.
openlaunch.click – Great job on visuals, they really complement the content well.
The Real Person!
The Real Person!
Детоксикация алкоголя в Туле , ключевой момент в лечении алкоголизма . Наркологические клиники в Туле предлагают разнообразные программы детоксикации , которые помогают преодолеть алкогольную зависимость. Выездной нарколог может предоставить поддержку при лечении алкоголизма в домашних условияхчто является удобным решением для многих пациентов. нарколог на дом Квалифицированные наркологи проводят терапию запойного синдрома и обеспечивают необходимую медицинскую помощь при алкогольной зависимости. Консультация нарколога позволяет определить индивидуальный план лечения . Не менее важна психологическая поддержка в процессе детоксикации, что способствует восстановлению после алкоголя и эффективной реабилитации от алкогольной зависимости;
The Real Person!
The Real Person!
алкоголизм лечение
reabilitaciya-astana008.ru
частный реабилитационный центр
brightshift.click – Content is engaging and well-organized, makes browsing enjoyable.
digitalvisionpro.click – Impressed by the layout; everything feels intuitive and modern.
reachnewgoals.click – This site has a sleek design and loads quickly.
websource.click – Great user experience; pages load fast and look sharp.
powerofgrowth.click – This site has a sleek design and loads quickly.
focusreach.click – The user interface is smooth, making navigation a breeze.
trendforge.click – Impressed by the quality of visuals and overall aesthetics.
fastforwardmedia.click – Impressed by the layout; everything feels intuitive and modern.
bluefocus.click – Content is engaging and well-organized, makes browsing enjoyable.
globalconnectnow.click – Always find something new and interesting every time I visit.
quicktrend.click – This site has a sleek design and loads quickly.
thefuturehub.click – Impressed by the layout; everything feels intuitive and modern.
brightideaweb.click – The site feels polished and professional, nice work.
starvision.click – Found this while browsing; it’s clean and easy to use.
launchcraft.click – Easy to navigate and find what you’re looking for.
risehub.click – Easy to navigate and find what you’re looking for.
Советы по строительству https://vodocar.com.ua и ремонту своими руками. Пошаговые инструкции, современные технологии, идеи для дома и участка. Мы поможем сделать ремонт проще, а строительство — надёжнее!
Информационный портал https://smallbusiness.dp.ua про строительство, ремонт и интерьер. Свежие новости отрасли, обзоры технологий и полезные лайфхаки. Всё, что нужно знать о стройке и благоустройстве жилья в одном месте!
Сайт о строительстве https://valkbolos.com и ремонте домов, квартир и дач. Полезные советы мастеров, подбор материалов, дизайн-идеи, инструкции и обзоры инструментов. Всё, что нужно для качественного ремонта и современного строительства!
Полезный сайт https://stroy-portal.kyiv.ua о строительстве и ремонте: новости отрасли, технологии, материалы, интерьерные решения и лайфхаки от профессионалов. Всё для тех, кто строит, ремонтирует и создаёт уют.
Строительный сайт https://teplo.zt.ua для тех, кто создаёт дом своей мечты. Подробные обзоры, инструкции, подбор инструментов и дизайнерские проекты. Всё о ремонте и строительстве в одном месте.
wavefusion.click – The content is fresh, and the layout is user-friendly.
cyberlaunch.click – The user interface is smooth, making navigation a breeze.
greenmotion.click – The content is fresh, and the layout is user-friendly.
globalreach.click – The site feels polished and professional, nice work.
The Real Person!
The Real Person!
пансионат для престарелых
pansionat-msk010.ru
пансионат для престарелых людей
pixelplanet.click – This site has a sleek design and loads quickly.
The Real Person!
The Real Person!
В Туле доступна услуга по вызову нарколога на дом, доступная 24/7, которая предоставляет лечение зависимостей для людей, страдающих от зависимости. Если вам требуется профессиональная помощь при алкоголизме, квалифицированный специалист проведет лечение на дому, гарантируя полную анонимность. вызов нарколога тула Если вы ищете надежное решение, обращение к наркологу в Туле станет первым шагом к новой жизни.
nextlayer.click – The user interface is smooth, making navigation a breeze.
The Real Person!
The Real Person!
реабилитационный центр для наркоманов
reabilitaciya-astana009.ru
реабилитация алкогольной зависимости в Астане
The Real Person!
The Real Person!
corvette купить москва mercedes цена – Mercedes-Benz предлагает автомобили с высоким уровнем роскоши, комфорта и передовых технологий, предлагая широкий спектр моделей в различных ценовых категориях.
Строим и ремонтируем https://buildingtips.kyiv.ua своими руками! Инструкции, советы, видеоуроки и лайфхаки для дома и дачи. Узнай, как сделать ремонт качественно и сэкономить бюджет.
Пошаговые советы https://tsentralnyi.volyn.ua по строительству и ремонту. Узнай, как выбрать материалы, рассчитать бюджет и избежать ошибок. Простые решения для сложных задач — строим и ремонтируем с уверенностью!
Энциклопедия строительства https://kero.com.ua и ремонта: материалы, технологии, интерьерные решения и практические рекомендации. От фундамента до декора — всё, что нужно знать домовладельцу.
Новостной портал https://kiev-online.com.ua с проверенной информацией. Свежие события, аналитика, репортажи и интервью. Узнавайте новости первыми — достоверно, быстро и без лишнего шума.
Главные новости дня https://sevsovet.com.ua эксклюзивные материалы, горячие темы и аналитика. Мы рассказываем то, что действительно важно. Будь в курсе вместе с нашим новостным порталом!
The Real Person!
The Real Person!
Je suis impressionne par Bingoal Casino, ca offre un thrill incomparable. Les alternatives sont etonnamment vastes, comprenant des jeux optimises pour les cryptos. Le bonus d’inscription est attractif. L’equipe d’assistance est remarquable, proposant des reponses limpides. La procedure est aisee et efficace, cependant plus de promotions frequentes seraient un atout. Pour synthetiser, Bingoal Casino garantit du divertissement constant pour les adeptes de sensations intenses ! Ajoutons que l’interface est intuitive et raffinee, ce qui eleve chaque session a un niveau superieur. Un plus significatif les tournois periodiques pour la rivalite, offre des privileges sur mesure.
Voir tout de suite|
The Real Person!
The Real Person!
music broadcast
Жiнка https://zhinka.in.ua блог для женщин. Мода, уход, фигура, советы хозяйкам и мамам.
Обустраивайте дом https://stroysam.kyiv.ua со вкусом! Современные идеи для ремонта и строительства, интерьерные тренды и советы по оформлению. Создайте стильное и уютное пространство своими руками.
The Real Person!
The Real Person!
дом престарелых
pansionat-msk011.ru
пансионат для пожилых с инсультом
Строим и ремонтируем https://srk.kiev.ua грамотно! Инструкции, пошаговые советы, видеоуроки и экспертные рекомендации. Узнай, как сделать ремонт качественно и сэкономить без потери результата.
Строительный портал https://sitetime.kiev.ua для мастеров и подрядчиков. Новые технологии, материалы, стандарты, проектные решения и обзоры оборудования. Всё, что нужно специалистам стройиндустрии.
Сайт о стройке https://samozahist.org.ua и ремонте для всех, кто любит уют и порядок. Расскажем, как выбрать материалы, обновить интерьер и избежать ошибок при ремонте. Всё просто, полезно и по делу.
Как построить https://rus3edin.org.ua и отремонтировать своими руками? Пошаговые инструкции, простые советы и подбор инструментов. Делаем ремонт доступным и понятным для каждого!
The Real Person!
The Real Person!
Ich bin abhangig von SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Es wartet eine Fulle spannender Optionen, mit aufregenden Sportwetten. Die Hilfe ist effizient und pro, garantiert top Hilfe. Der Ablauf ist unkompliziert, dennoch die Offers konnten gro?zugiger ausfallen. Zum Ende, SpinBetter Casino garantiert hochsten Spa? fur Online-Wetten-Fans ! Zusatzlich das Design ist ansprechend und nutzerfreundlich, fugt Magie hinzu. Besonders toll die Sicherheit der Daten, die Vertrauen schaffen.
spinbettercasino.de|
The Real Person!
The Real Person!
Je suis ebloui par Bingoal Casino, ca procure un thrill exceptionnel. L’eventail de jeux est extraordinaire, offrant des sessions live intenses. Associe a des paris gratuits. L’equipe d’assistance est remarquable, assurant un support premium. Les benefices arrivent sans latence, par moments des bonus plus diversifies seraient souhaitables. Tout compte fait, Bingoal Casino garantit du divertissement constant pour les aficionados de jeux contemporains ! A mentionner la plateforme est esthetiquement remarquable, stimule le desir de revenir. Un autre avantage cle les evenements communautaires captivants, renforce le sens de communaute.
Aller sur le site web|
The Real Person!
The Real Person!
Выездной нарколог в Туле — это практичное решение для тех‚ кто испытывают необходимость в помощи нарколога‚ но не хотят посетить лечебное заведение. Профессиональная помощь в лечении зависимости доступна прямо на дому‚ что обеспечивает комфорт и анонимность.Консультация нарколога позволяет оценить ситуацию и подобрать лучший план лечения‚ включая лечение в стационаре или домашнюю терапию. Специалисты предлагают психотерапию зависимостей и медицинское вмешательство‚ что помогает успешному лечению зависимостей. Поддержка семьи играет важную роль в лечении‚ помогая пациентам справиться с трудностями. Обратитесь на vivod-iz-zapoya-tula015.ru для подробной информации о наших услугах.
The Real Person!
The Real Person!
Je suis totalement envoute par Casinia Casino, on ressent une aura radieuse. Le repertoire est riche et multifacette, incluant des paris sportifs palpitants. L’offre de bienvenue est eclatante. L’assistance est rapide et precise, toujours pret a eclairer. Les paiements sont securises et fluides, mais des bonus plus varies brilleraient davantage. En bref, Casinia Casino offre une experience inoubliable pour les joueurs en quete d’eclat ! De plus la navigation est intuitive comme une lueur, intensifie l’eclat du jeu. Particulierement captivant le programme VIP avec 5 niveaux exclusifs, qui renforce l’engagement.
DГ©bloquer plus|
The Real Person!
The Real Person!
J’ai un coup de foudre pour Locowin Casino, ca procure un plaisir intense. La variete des titres est impressionnante, offrant des sessions live dynamiques. Le bonus de bienvenue est attractif. Les agents repondent avec rapidite, offrant des reponses claires. Les transactions sont fiables et rapides, neanmoins plus de promos regulieres seraient top. En bref, Locowin Casino est un incontournable pour les joueurs pour les passionnes de jeux modernes ! De plus le design est moderne et fluide, ce qui rend chaque session encore plus fun. Un autre atout majeur les paiements securises en crypto, assure des transactions fiables.
Lire entiГЁrement|
The Real Person!
The Real Person!
Флойд Мейвезердин рекорддору көп спорт күйөрмандарын суктандырган. Анын рингдеги тактикасы ар дайым мыкты. Акыркы маектерди жана статистиканы Флойд Мейвезер интервью бөлүмүнөн окуңуз.
Флойд Мейвезер ар дайым спорттун чокусунда болгон. Флойд Мейвезер – мыкты стратегиянын ээси. Флойд Мейвезер ар дайым өзүн мыкты формада кармаган.
Флойд Мейвезердин бизнеси да ийгиликтүү.
Анын карьерасы ар бир жаш спортчу үчүн үлгү. Флойд Мейвезер ар бир беттешти мыкты өткөргөн. Анын ар бир интервьюсу чоң кызыгуу жаратат.
The Real Person!
The Real Person!
J’ai un faible pour Casinia Casino, ca procure un plaisir aristocratique. Il y a une abondance de jeux captivants, avec des slots thematiques et innovants. Le bonus de bienvenue est genereux. Le service est disponible 24/7, garantissant un support de qualite. Les gains arrivent sans delai, bien que des recompenses additionnelles seraient royales. Dans l’ensemble, Casinia Casino est une plateforme qui regne en maitre pour les passionnes de jeux modernes ! Cerise sur le gateau le site est rapide et attrayant, amplifie le plaisir de jouer. A noter egalement les options de paris sportifs variees, renforce le sentiment de communaute.
DГ©bloquer les dГ©tails|
Je suis accro a Locowin Casino, il offre une experience unique. Il y a une multitude de jeux excitants, avec des slots au design innovant. Accompagne de tours gratuits sans wager. Le service est disponible 24/7, garantissant un support de qualite. Les retraits sont ultra-rapides, cependant quelques tours gratuits en plus seraient cool. Pour conclure, Locowin Casino est une plateforme qui dechire pour les passionnes de jeux modernes ! Ajoutons que l’interface est intuitive et stylee, facilite une immersion totale. Un plus non negligeable les paiements securises en crypto, qui booste l’engagement.
VГ©rifier le site|
maxgrowth.click – Strong visuals and organized layout make this pleasant to use.
Мужской сайт https://rkas.org.ua о жизни без компромиссов: спорт, путешествия, техника, карьера и отношения. Для тех, кто ценит свободу, силу и уверенность в себе.
Портал для автомобилистов https://translit.com.ua от выбора машины до профессионального ремонта. Читайте обзоры авто, новости автоспорта, сравнивайте цены и характеристики. Форум автолюбителей, советы экспертов и свежие предложения автосалонов.
Сайт для женщин https://oun-upa.org.ua которые ценят себя и жизнь. Мода, советы по уходу, любовь, семья, вдохновение и развитие. Найди идеи для новых свершений и будь самой собой в мире, где важно быть уникальной!
Мужской онлайн-журнал https://cruiser.com.ua о современных трендах, технологиях и саморазвитии. Мы пишем о том, что важно мужчине — от мотивации и здоровья до отдыха и финансов.
Ваш гид в мире https://nerjalivingspace.com автомобилей! Ежедневные авто новости, рейтинги, тест-драйвы и советы по эксплуатации. Найдите идеальный автомобиль, узнайте о страховании, кредитах и тюнинге.
https://duhoconmap.com/chao-moi-nguoi/
The Real Person!
The Real Person!
Флойд Мейвезердин ар бир беттеши чоң шоу катары кабыл алынат. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Бокс легендасынын жетишкендиктери тууралуу окуу үчүн Флойд Мейвезер жетишкендиктер барагын караңыз.
Флойд Мейвезердин ийгилиги көпчүлүк үчүн үлгү. Анын рекорддору бүгүнкү күнгө чейин сакталган. Флойд Мейвезердин ысымы ар бир спорт сүйүүчүсүнө белгилүү.
Флойд Мейвезердин бизнеси да ийгиликтүү.
Анын карьерасы ар бир жаш спортчу үчүн үлгү. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Анын карьерасы дүйнө жүзүнө үлгү.
medtopstore.com – Overall solid first impression — clean, credible, and functional.
newnexus.click – Pages load quickly and everything seems nicely organized.
The Real Person!
The Real Person!
Je suis captive par Bingoal Casino, il procure une odyssee unique. L’eventail de jeux est spectaculaire, incluant des paris sportifs electrisants. Associe a des paris gratuits. Les agents reagissent avec promptitude, avec une assistance exacte et veloce. Les retraits sont realises promptement, par moments quelques tours gratuits supplementaires seraient apprecies. Tout compte fait, Bingoal Casino garantit du divertissement constant pour les joueurs a la recherche d’aventure ! En outre le site est veloce et seduisant, facilite une immersion complete. Particulierement attractif le programme de fidelite avec des niveaux VIP, qui stimule l’engagement.
Obtenir l’offre|
buildyourbrand.click – Impressed by the layout; everything feels intuitive and modern.
everyspeed.com – The domain name sounds energetic and strong.
dreambigtoday.click – Loving the hopeful vibe here, it really draws me in today.
507193.com – Pages load quickly, which is always a plus.
531500zy.com – The styling gives a modern vibe, nice choice of fonts too.
smartbusinesshub.click – I appreciate how easy it is to find what you’re looking for.
mediafusion.click – Found lots of cool stuff, this site has great content.
The Real Person!
The Real Person!
Ich bin fasziniert von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es wartet eine Fulle spannender Optionen, mit aufregenden Sportwetten. Die Hilfe ist effizient und pro, verfugbar rund um die Uhr. Die Auszahlungen sind ultraschnell, dennoch mehr abwechslungsreiche Boni waren super. In Kurze, SpinBetter Casino ist eine Plattform, die uberzeugt fur Adrenalin-Sucher ! Zusatzlich die Site ist schnell und stylish, gibt den Anreiz, langer zu bleiben. Ein weiterer Vorteil die Sicherheit der Daten, die Flexibilitat bieten.
https://spinbettercasino.de/|
The Real Person!
The Real Person!
Je suis hypnotise par Bingoal Casino, il offre une odyssee incomparable. Les alternatives sont etonnamment etendues, comprenant des jeux optimises pour les cryptos. Doublement des depots jusqu’a 200 €. Le suivi est exemplaire, avec une assistance exacte et veloce. Les retraits sont realises promptement, de temps a autre quelques tours gratuits supplementaires seraient apprecies. Pour synthetiser, Bingoal Casino est essentiel pour les amateurs pour les enthousiastes de casino en ligne ! En outre l’interface est intuitive et raffinee, ajoute un confort notable. A souligner aussi les paiements securises en crypto, offre des privileges sur mesure.
Parcourir davantage|
503926.com – Navigation is simple, nothing fancy but works smoothly.
The Real Person!
The Real Person!
Флойд Мейвезер ар бир беттешинде жогорку деңгээлди көрсөтөт. Флойд Мейвезердин тарыхтагы орду эч кимге талашсыз. Кызыктуу статистика жана жаңылыктар Флойд Мейвезер статистика бөлүмүндө топтолгон.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Флойд Мейвезердин фанаттары дүйнө жүзүндө. Флойд Мейвезердин жашоосу – мотивация.
Ал спорттон тышкары ишкер катары да белгилүү.
Анын рингдеги чеберчилиги теңдешсиз. Флойд Мейвезер ар дайым өзүнө ишенген. Флойд Мейвезер – күч, ылдамдык жана акылдын айкалышы.
strongrod.com – There’s an authoritative tone here without being overbearing.
The Real Person!
The Real Person!
I can’t resist the allure of Wazamba Casino, it’s an odyssey that echoes with intrigue. The assortment of games is remarkable, tailored for cryptocurrency usage. Matching initial funds up to €500. Facilitating uninterrupted fun. Cashouts are executed promptly, from time to time broader incentive options would enrich it. Summarizing, Wazamba Casino supplies premium leisure for excitement chasers ! Furthermore the aesthetics are compelling and thematic, infusing added allure. A further advantage exclusive ranks with special privileges, heightening involvement.
wazambagr.com|
globalwave.click – Really loving the clean styling and smooth navigation here.
skyhighstudio.click – I appreciate the consistent design touches and color palette.
powertrend.click – Strong site presence, looks like someone really cares about details.
The Real Person!
The Real Person!
J’adore l’eclat vibrant de Casinia Casino, c’est une plateforme qui brille d’une energie lumineuse. L’eventail de jeux est resplendissant, avec des slots aux designs audacieux et thematiques. Renforcant votre tresor initial. Le support client est lumineux, toujours pret a eclairer. Les transactions sont fiables et rapides, cependant des offres plus genereuses seraient un plus. Au final, Casinia Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! A noter la navigation est intuitive comme une lueur, intensifie l’eclat du jeu. Particulierement captivant les paiements securises en crypto, garantit des transactions fiables.
Voir maintenant|
The Real Person!
The Real Person!
Je suis emerveille par Casinia Casino, il offre une aventure etincelante. Les options sont vastes comme un ciel etoile, incluant des paris sportifs palpitants. L’offre de bienvenue est eclatante. Les agents repondent comme une etoile filante, avec une aide claire et veloce. Les retraits filent comme une comete, mais plus de promos regulieres ajouteraient de l’eclat. Au final, Casinia Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! En bonus la plateforme scintille comme une etoile, incite a prolonger l’aventure. Un avantage notable les tournois reguliers pour la rivalite, garantit des transactions fiables.
Casinia|
The Real Person!
The Real Person!
J’ai un faible pour Casinia Casino, ca transporte dans un palais enchanteur. Le catalogue est opulent et diversifie, incluant des paris sportifs dynamiques. Le bonus de bienvenue est genereux. L’assistance est efficace et courtoise, offrant des reponses claires. Les transactions sont fiables, bien que des bonus plus varies seraient apprecies. Au final, Casinia Casino garantit du fun a chaque instant pour les joueurs en quete d’excitation ! De plus la plateforme est visuellement somptueuse, donne envie de prolonger l’experience. Particulierement interessant les paiements securises en crypto, renforce le sentiment de communaute.
En savoir plus|
The Real Person!
The Real Person!
Je suis epate par Locowin Casino, il offre une experience unique. Les options sont incroyablement vastes, offrant des sessions live dynamiques. Pour un demarrage en force. L’assistance est efficace et pro, garantissant un support de qualite. Le processus est simple et efficace, parfois plus de promos regulieres seraient top. Pour conclure, Locowin Casino est un incontournable pour les joueurs pour les amateurs de sensations fortes ! Cerise sur le gateau l’interface est intuitive et stylee, ajoute une touche de confort. Un plus non negligeable les tournois reguliers pour la competition, qui booste l’engagement.
Ouvrir le site maintenant|
creativemindlab.click – The color scheme here really makes everything pop beautifully.
vmf-metal.com – Website loads fast and transitions feel seamless.
The Real Person!
The Real Person!
Флойд Мейвезердин ар бир беттеши чоң шоу катары кабыл алынат. Анын ар бир беттеши дүйнө жүзүнө көрсөтүлөт. Бокс дүйнөсүндөгү эң чоң ысымдардын бири тууралуу кенен маалымат – Флойд Мейвезер Кыргызстан.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Флойд Мейвезер спорт тарыхына кирген аттардын бири. Флойд Мейвезер ар дайым спорттун символу катары айтылат.
Флойд Мейвезердин жашоосу көптөгөн макалаларга тема болгон.
Анын рингдеги чеберчилиги теңдешсиз. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Флойд Мейвезердин ысымы бокс дүйнөсүндө түбөлүк калат.
The Real Person!
The Real Person!
Флойд Мейвезердин ар бир беттеши чоң шоу катары кабыл алынат. Флойд Мейвезердин акыркы беттеши көп талкуу жараткан. Бокс тарыхындагы улуу мушкер жөнүндө окуу үчүн Флойд Мейвезер легенда.
Флойд Мейвезер ар дайым спорттун чокусунда болгон. Флойд Мейвезер – мыкты стратегиянын ээси. Флойд Мейвезердин ысымы ар бир спорт сүйүүчүсүнө белгилүү.
Ал спорттон тышкары ишкер катары да белгилүү.
Анын акыркы беттеши чоң окуя болгон. Флойд Мейвезердин атын угуу эле сыймык. Анын жашоосу жана ийгилиги мотивация берет.
The Real Person!
The Real Person!
I adore the rush of Astronaut Crash by 100HP Gaming, it presents a masterful mix of luck and timing. Fair play is baked in with provable tech, with auto-bet for hands-free excitement. Balanced volatility for steady thrills. Help desk is responsive and expert, upholding crypto funding security. The exit system rescues bold bets, even so advanced auto-tools for vets. Wrapping up, Astronaut Crash redefines crash gaming standards for multiplier maniacs ! Bonus layout supports marathon marathons, cranking the ascent hype. Big win leaderboards for rivalry, ensures tamper-proof twists.
Astronaut Crash Game|
The Real Person!
The Real Person!
пансионат для людей с деменцией в москве
pansionat-msk012.ru
пансионат для пожилых после инсульта
The Real Person!
The Real Person!
I can’t resist the allure of Wazamba Casino, it crafts a captivating saga. The assortment of games is remarkable, featuring betting on sports events. Bundled with 200 complimentary turns. Assistance team is superb. The mechanism is intuitive, even if supplementary perks would excel. Summarizing, Wazamba Casino supplies premium leisure for excitement chasers ! Also exploration is natural, magnifying each episode’s appeal. Notable aspect competitions for rivalry, guaranteeing safe exchanges.
https://wazambagr.com/|
Сайт о металлах https://metalprotection.com.ua и металлообработке: виды металлов, сплавы, технологии обработки, оборудование и новости отрасли. Всё для специалистов и профессионалов металлургии.
Строительный сайт https://okna-k.com.ua для профессионалов и новичков. Новости отрасли, обзоры материалов, технологии строительства и ремонта, советы мастеров и пошаговые инструкции для качественного результата.
Женский онлайн-журнал https://rosetti.com.ua о стиле, здоровье и семье. Новости моды, советы экспертов, тренды красоты и секреты счастья. Всё, что важно и интересно женщинам любого возраста.
Портал о дизайне https://sculptureproject.org.ua интерьеров и пространства. Идеи, тренды, проекты и вдохновение для дома, офиса и общественных мест. Советы дизайнеров и примеры стильных решений каждый день.
Главный автопортал страны https://nmiu.org.ua всё об автомобилях в одном месте! Новости, обзоры, советы, автообъявления, страхование, ТО и сервис. Для водителей, механиков и просто любителей машин.
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-kaluga013.ru
вывод из запоя калуга
fastgrowth.click – Just came across this — easy to navigate and nicely styled.
The Real Person!
The Real Person!
Флойд Мейвезер дүйнөдөгү эң белгилүү мушкерлердин бири. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Бокс күйөрмандары үчүн кызыктуу фактылар https://floyd-mayweather-kg.com/ порталында жайгашкан.
Флойд Мейвезер ар дайым спорттун чокусунда болгон. Флойд Мейвезер спорт тарыхына кирген аттардын бири. Флойд Мейвезер – акыл менен ойногон спортчу.
Анын ар бир интервьюсу көңүл борборунда.
Флойд Мейвезер дүйнөдөгү эң жогорку кирешелүү спортчу. Анын тарыхы ар дайым спорт күйөрмандарынын эсинде. Флойд Мейвезер спорттун символу катары белгилүү.
bestchoiceonline.click – Loving the clean layout and straightforward design here today.
ynaix.com – Navigation is straightforward, no distractions, which is nice.
The sagacity in this serving is exceptional.
The Real Person!
The Real Person!
I’m thrilled by Astronaut Crash by 100HP Gaming, it mimics a high-altitude adrenaline surge. Games wrap up in seconds for endless replays, betting from pennies to big bucks. RTP sits at an enticing 97%. Forums buzzing with player tactics, reachable through various portals. The exit system rescues bold bets, even so advanced auto-tools for vets. In a nutshell, Astronaut Crash delivers verifiable cosmic kicks for multiplier maniacs ! Extra perk controls are beginner-proof, fueling binge-worthy bouts. Big win leaderboards for rivalry, sparks a vibrant player network.
https://astronaut-crashgame777.com/|
kluvcc.com – A subtle but strong visual presence throughout the pages.
Туристический портал https://feokurort.com.ua для любителей путешествий! Страны, маршруты, достопримечательности, советы и лайфхаки. Планируйте отдых, находите вдохновение и открывайте мир вместе с нами.
Студия ремонта https://anti-orange.com.ua квартир и домов. Выполняем ремонт под ключ, дизайн-проекты, отделочные и инженерные работы. Качество, сроки и индивидуальный подход к каждому клиенту.
parier foot en ligne melbet telecharger
telecharger 1xbet pour android pronostics du foot
telecharger 1xbet pour android 1xbet afrique apk
Proof blog you possess here.. It’s obdurate to on strong status belles-lettres like yours these days. I really comprehend individuals like you! Rent guardianship!!
connectwiththeworldnow – Strong presence, design complements message and intent clearly.
The Real Person!
The Real Person!
пансионат после инсульта
pansionat-tula010.ru
пансионат для лежачих после инсульта
The Real Person!
The Real Person!
Флойд Мейвезердин ар бир беттеши чоң шоу катары кабыл алынат. Флойд Мейвезер – мыкты дисциплина менен белгилүү. Кызыктуу статистика жана жаңылыктар Флойд Мейвезер статистика бөлүмүндө топтолгон.
Флойд Мейвезердин ар бир беттеши чыныгы спектакль. Анын рекорддору бүгүнкү күнгө чейин сакталган. Флойд Мейвезердин карьерасы көп жылдык эмгектин натыйжасы.
Анын ар бир интервьюсу көңүл борборунда.
Анын акыркы беттеши чоң окуя болгон. Флойд Мейвезердин атын угуу эле сыймык. Флойд Мейвезер – күч, ылдамдык жана акылдын айкалышы.
brightvault.click – Loving how everything is organized, really intuitive and clear layout.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-kaluga014.ru
экстренный вывод из запоя
507193.com – The layout is very minimal and gives a mysterious first impression.
alphaorbit.click – Overall great impression — sleek, modern, and inviting.
nexasphere – Can’t believe how much this helped me today, thanks!
truegrowth – A solid choice for businesses aiming for sustainable growth.
boldmatrix – Their integration was seamless and quick to implement.
visionmark – The ADA-compliant elevator signs they made look fantastic.
prolaunch – This is a game changer, can’t thank you enough.
brandmatrix.click – Such a smooth experience, I rarely see sites load this cleanly.
newgenius.click – Strong first impression; feels like a high quality build from the start.
elitezone.click – Just discovered this site, design feels fresh and easy to use.
connectwiththeworldnow – Very engaging style, visuals support purpose and connection focus.
The Real Person!
The Real Person!
Флойд Мейвезер спорт дүйнөсүндөгү эң бай спортчу. Флойд Мейвезердин тарыхтагы орду эч кимге талашсыз. Кызыктуу статистика жана жаңылыктар Флойд Мейвезер статистика бөлүмүндө топтолгон.
Флойд Мейвезерди көрүү ар бир спорт күйөрманы үчүн майрам. Флойд Мейвезер спорт тарыхына кирген аттардын бири. Флойд Мейвезердин ысымы ар бир спорт сүйүүчүсүнө белгилүү.
Флойд Мейвезердин бизнеси да ийгиликтүү.
Анын карьерасы ар бир жаш спортчу үчүн үлгү. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Флойд Мейвезердин ар бир жаңылыгы спорт дүйнөсүндө жаңылык.
ultrafocus.click – Loving the clean design, content is easy to find and digest.
progrid – Informative and well-organized, made learning so much easier.
zenithmedia.click – Content feels well organized and accessible.
maxbridge.click – Excellent responsiveness on mobile, smooth transitions and clean layout.
elitefusion.click – Great consistency across pages, feels like a thoughtfully built site.
The Real Person!
The Real Person!
Ich habe einen totalen Hang zu SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Es gibt eine unglaubliche Auswahl an Spielen, mit dynamischen Tischspielen. Der Kundenservice ist ausgezeichnet, verfugbar rund um die Uhr. Die Auszahlungen sind ultraschnell, ab und an die Offers konnten gro?zugiger ausfallen. In Kurze, SpinBetter Casino ist eine Plattform, die uberzeugt fur Online-Wetten-Fans ! Au?erdem die Plattform ist visuell ein Hit, gibt den Anreiz, langer zu bleiben. Zusatzlich zu beachten die Vielfalt an Zahlungsmethoden, die Vertrauen schaffen.
spinbettercasino.de|
Je suis stupefait par Locowin Casino, ca fournit un plaisir intense. La diversite des titres est epoustouflante, incluant des paris sportifs electrisants. Doublement des depots jusqu’a 1850 €. Le suivi est exemplaire, toujours pret a intervenir. La procedure est aisee et efficace, bien que des offres plus liberales ajouteraient de la valeur. Globalement, Locowin Casino est essentiel pour les amateurs pour les adeptes de sensations intenses ! A mentionner l’interface est intuitive et raffinee, ce qui eleve chaque session a un niveau superieur. A souligner aussi le programme VIP avec des niveaux exclusifs, garantit des transactions securisees.
http://www.locowincasinooffres.fr|
alphaimpact.click – Nice colour palette, good contrasts, pleasant on the eyes always.
The Real Person!
The Real Person!
Je suis hypnotise par Casinia Casino, on ressent une energie fastueuse. Le repertoire est riche et varie, avec des slots aux designs audacieux. Avec un Bonus Crab exclusif. Les agents repondent avec une rapidite fulgurante, joignable a toute heure. Les gains arrivent sans tarder, neanmoins des recompenses additionnelles seraient imperiales. Pour conclure, Casinia Casino fournit une experience inoubliable pour ceux qui parient avec des cryptos ! Par ailleurs la navigation est intuitive comme un edit, ce qui rend chaque session plus fastueuse. A souligner aussi le programme VIP avec 5 rangs princiers, qui renforce l’engagement.
Voir le site|
brightchain.click – The layout has good structure, sections clearly defined.
The Real Person!
The Real Person!
Je suis absolument conquis par Casinia Casino, on ressent une energie noble. Le catalogue est opulent et diversifie, offrant des sessions live immersives. Amplifiant le plaisir de jeu. Le service est disponible 24/7, avec une aide precise. Les retraits sont rapides comme l’eclair, neanmoins des offres plus genereuses ajouteraient du prestige. En bref, Casinia Casino est un incontournable pour les joueurs pour les amateurs de sensations fortes ! En prime le design est moderne et sophistique, donne envie de prolonger l’experience. A noter egalement les paiements securises en crypto, renforce le sentiment de communaute.
DГ©couvrir davantage|
The Real Person!
The Real Person!
Je suis entierement obsede par Locowin Casino, ca fournit un plaisir intense. Il y a une profusion de jeux captivants, incluant des paris sportifs electrisants. Renforcant l’experience de depart. L’equipe d’assistance est remarquable, assurant un support premium. Les operations sont solides et veloces, mais des bonus plus diversifies seraient souhaitables. Pour synthetiser, Locowin Casino est une plateforme qui excelle pour les enthousiastes de casino en ligne ! De surcroit le site est veloce et seduisant, stimule le desir de revenir. Egalement notable les paiements securises en crypto, offre des privileges sur mesure.
Visiter aujourd’hui|
sharpwave.click – Will return to this habitually, excellent site with good overall vibe.
Howdy! I simply wish to give a huge thumbs up for the great information you have here on this post. I will be coming again to your weblog for extra soon.
The Real Person!
The Real Person!
вывод из запоя
tula-narkolog008.ru
вывод из запоя цена
ultrawave.click – This site feels modern, fast, and really well maintained overall.
The Real Person!
The Real Person!
дом престарелых
pansionat-tula011.ru
пансионат для пожилых с деменцией
The Real Person!
The Real Person!
Ich bin fasziniert von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Das Angebot an Spielen ist phanomenal, mit aufregenden Sportwetten. Die Agenten sind blitzschnell, mit praziser Unterstutzung. Der Ablauf ist unkompliziert, obwohl mehr Rewards waren ein Plus. Global gesehen, SpinBetter Casino ist absolut empfehlenswert fur Spieler auf der Suche nach Action ! Nicht zu vergessen die Navigation ist kinderleicht, verstarkt die Immersion. Zusatzlich zu beachten die Sicherheit der Daten, die den Einstieg erleichtern.
spinbettercasino.de|
The Real Person!
The Real Person!
I’m blown away by Wazamba Casino, it creates an adventurous aura. The game lineup is extraordinary, including live casino for real-time action. The sign-up bonus is enticing. Support team is exceptional. Rewards reach you fast, sporadically offers could be more lavish. Altogether, Wazamba Casino delivers unmatched fun for thrill hunters ! Furthermore the visuals are captivating and immersive, elevating every moment of play. Another standout feature the mask collection loyalty system, providing ongoing rewards.
https://wazambagr.com/|
The Real Person!
The Real Person!
Je ne me lasse pas de Casinia Casino, ca offre un plaisir aristocratique. Le catalogue est opulent et diversifie, proposant des jeux de table elegants. Plus un Bonus Crab pour demarrer. Le service est disponible 24/7, avec une aide precise. Les paiements sont securises et fluides, neanmoins plus de promos regulieres seraient un plus. Au final, Casinia Casino garantit du fun a chaque instant pour les joueurs en quete d’excitation ! Cerise sur le gateau le site est rapide et attrayant, donne envie de prolonger l’experience. Egalement appreciable les tournois reguliers pour la competition, renforce le sentiment de communaute.
Visiter pour plus|
Студия дизайна https://bathen.rv.ua интерьеров и архитектурных решений. Создаём стильные, функциональные и гармоничные пространства. Индивидуальный подход, авторские проекты и внимание к деталям.
Ремонт и строительство https://fmsu.org.ua без лишних сложностей! Подробные статьи, обзоры инструментов, лайфхаки и практические советы. Мы поможем построить, отремонтировать и обустроить ваш дом.
metarise.click – Fast page loads, no lag, looks good on my phone browser too.
The Real Person!
The Real Person!
Je suis completement ensorcele par Casinia Casino, on ressent une energie fastueuse. Il y a une profusion de jeux captivants, avec des slots aux designs audacieux. Avec un Bonus Crab exclusif. Le service est disponible 24/7, offrant des solutions limpides. Les gains arrivent sans tarder, mais des bonus plus varies seraient un joyau. Dans l’ensemble, Casinia Casino offre un divertissement constant pour les joueurs en quete de gloire ! De plus la plateforme scintille comme un palais, incite a prolonger l’experience. Un atout cle les paiements securises en crypto, propose des recompenses sur mesure.
DГ©couvrir|
The Real Person!
The Real Person!
J’ai un faible pour Casinia Casino, ca procure un plaisir aristocratique. Les options sont vastes comme un royaume, avec des slots thematiques et innovants. 100% jusqu’a 500 € + 200 tours gratuits. Les agents repondent avec celerite, toujours pret a servir. Les paiements sont securises et fluides, bien que des offres plus genereuses ajouteraient du prestige. Pour conclure, Casinia Casino est un incontournable pour les joueurs pour les amateurs de sensations fortes ! Cerise sur le gateau la navigation est simple et fluide, donne envie de prolonger l’experience. Egalement appreciable le programme VIP avec 5 niveaux, propose des avantages personnalises.
Aller plus en profondeur|
The Real Person!
The Real Person!
J’ai un engouement sincere pour Locowin Casino, ca fournit un plaisir intense. Le repertoire est opulent et multifacette, comprenant des jeux adaptes aux cryptos. Doublement des depots jusqu’a 1850 €. L’aide est performante et experte, assurant un support premium. Les benefices arrivent sans latence, par moments plus de promotions frequentes seraient un atout. Globalement, Locowin Casino est essentiel pour les amateurs pour les joueurs a la recherche d’aventure ! A mentionner la navigation est simple et engageante, ajoute un confort notable. A souligner aussi les tournois periodiques pour la rivalite, renforce le sens de communaute.
Voir la page d’accueil|
Файне Житомир https://faine-misto.zt.ua портал новин Житомира та області. Останні події сьогодні.
The Real Person!
The Real Person!
экстренный вывод из запоя калуга
vivod-iz-zapoya-kaluga015.ru
лечение запоя калуга
Howdy! Do you use Twitter? I’d like to follow you if that would be okay. I’m definitely enjoying your blog and look forward to new updates.
https://kra41-cc.cc
1иксслотс официальный сайт
The Real Person!
The Real Person!
I’m addicted to Wazamba Casino, it resembles a cascade of enchantment. The choices are broad and multifaceted, optimized for crypto transactions. Kickstarting your adventure. Maintaining seamless sessions. The procedure is effortless, at times expanded bonus varieties would be great. To wrap up, Wazamba Casino stands out as a superior platform for adventure seekers ! In addition the visuals are captivating and immersive, infusing extra charm. Another standout feature the mask collection loyalty system, ensuring transaction safety.
wazambagr.com|
The Real Person!
The Real Person!
bs2best зеркало В курсе ли ты, что сотрясает самые глубины тёмной сети? Blacksprut — это не просто название. Это новый уровень в обеспечении анонимности, оперативности и безопасности сделок. Посети bs2best.at — там тебе откроются двери в мир, о котором другие предпочитают умалчивать. Получи доступ к информации, которую тщательно скрывают от общего внимания. Только для тех, кто понимает и разбирается. Без возможности обнаружения. Без каких-либо уступок. Только Blacksprut. Не упусти свой шанс стать одним из первых — bs2best.at уже готов принять тебя в свои ряды. Готов ли ты к тому, что узнаешь?
The Real Person!
The Real Person!
лечение запоя тула
tula-narkolog009.ru
вывод из запоя
grandlink – Pleasant browsing, content feels fresh and relevant.
The Real Person!
The Real Person!
пансионат для пожилых после инсульта
pansionat-tula012.ru
пансионаты для инвалидов в туле
sykaaa casino
elitepulse – Great resource, I’m going to bookmark this right now.
vectorrise – The portfolio reflects real talent and thoughtful execution.
truelaunch – Clear explanations, made complex stuff easier to get.
smartvibe – The sections are well-organized, makes browsing efficient.
purehorizon – The content is clear, useful, and doesn’t overcomplicate things.
brandvision – Solid resource, this gives me new branding direction ideas.
innovatek – Very helpful content for someone learning new tools.
pronostic foot gratuit info foot africain
1xbet afrique apk 1xbet afrique apk
parier pour le foot football africain
Современная студия дизайна https://bconline.com.ua архитектура, интерьер, декор. Мы создаём пространства, где технологии сочетаются с красотой, а стиль — с удобством.
Создавайте дом https://it-cifra.com.ua своей мечты! Всё о строительстве, ремонте и дизайне интерьера. Идеи, проекты, фото и инструкции — вдохновляйтесь и воплощайте задуманное легко и с удовольствием.
linkcraft – Just explored this, the content is super relevant and helpful.
skyvertex – Friendly tone and useful insights, love this site.
brandcrest – Very professional look, feels trustworthy and polished.
summitmedia – Good visuals, solid writing, and useful links throughout.
visionlane – Just found what I needed in minutes, very efficient site.
techmatrix – Smooth navigation, everything loads fast and cleanly.
vividpath – Clean layout, made reading through easy on the eyes.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-minsk010.ru
вывод из запоя минск
ascendmark – Found some great content, exactly what I was looking for.
novaorbit – The tone is casual yet professional, I like that mix.
powercore – Very smooth experience exploring their pages.
urbanshift – The writing tone is warm but professional, nice balance.
primeimpact – The tone is friendly and accessible, nice voice.
boldvista – They cover interesting topics I haven’t seen elsewhere before.
криптобосс регистрация
The Real Person!
The Real Person!
show updates
truelaunch – Great mix of insight and usability, kudos to the creators.
brandvision – Great branding ideas, I’m noting them down for my own work.
growthverse – Learning a lot from their case studies, very practical.
purehorizon – Very pleasing layout, makes long reading easier on the eyes.
https://sayyidmuniruddin.com/2025/02/10/kap-suryadi-dan-rizal-laksanakan-training-superlife-transformation/comment-page-29/
innovatek – Very helpful content for someone learning new tools.
urbanscale – Bold visuals, well-designed site, easy to skim through.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-chelyabinsk010.ru
экстренный вывод из запоя
goldnexus – Found several insights I can immediately apply in my work.
The Real Person!
The Real Person!
психиатрическая помощь на дому
psychiatr-moskva007.ru
детский психиатр на дом в москве
skyvertex – Great for inspiration, lots of creative ideas here.
1xslots
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Das Angebot an Spielen ist phanomenal, mit aufregenden Sportwetten. Der Service ist von hoher Qualitat, verfugbar rund um die Uhr. Die Auszahlungen sind ultraschnell, ab und an mehr Rewards waren ein Plus. Global gesehen, SpinBetter Casino ist ein Muss fur alle Gamer fur Online-Wetten-Fans ! Zusatzlich die Interface ist intuitiv und modern, erleichtert die gesamte Erfahrung. Ein Pluspunkt ist die Sicherheit der Daten, die Vertrauen schaffen.
spinbettercasino.de|
The Real Person!
The Real Person!
J’ai une passion debordante pour Locowin Casino, ca immerse dans un monde fascinant. Le catalogue est abondant et multifacette, offrant des sessions live intenses. Doublement des depots jusqu’a 200 €. Les agents repondent avec celerite, joignable a tout moment. Les paiements sont securises et fluides, bien que des bonus plus varies seraient apprecies. En resume, Locowin Casino est indispensable pour les joueurs pour ceux qui aiment parier en crypto ! A noter le design est moderne et fluide, facilite une immersion totale. A souligner les tournois reguliers pour la competition, renforce le sentiment de communaute.
Trouver le bonus|
alihidaer2313 – The experience is gentle, nothing over the top, just reliable.
The Real Person!
The Real Person!
J’ai un engouement sincere pour Locowin Casino, ca fournit un plaisir intense. Il y a une profusion de jeux captivants, comprenant des jeux adaptes aux cryptos. Renforcant l’experience de depart. L’equipe d’assistance est remarquable, toujours pret a intervenir. Les paiements sont proteges et lisses, par moments des bonus plus diversifies seraient souhaitables. En synthese, Locowin Casino est une plateforme qui excelle pour les aficionados de jeux contemporains ! A mentionner l’interface est intuitive et raffinee, intensifie le plaisir du jeu. Egalement notable les paiements securises en crypto, qui stimule l’engagement.
Jeter un coup d’œil|
vividpath – Really loving the content style, feels fresh and engaging.
The Real Person!
The Real Person!
Je suis captive par Locowin Casino, on detecte une vibe folle. Il y a une profusion de jeux captivants, comprenant des jeux adaptes aux cryptos. Doublement des depots jusqu’a 1850 €. Le suivi est exemplaire, toujours pret a intervenir. Les benefices arrivent sans latence, mais des incitations additionnelles seraient un benefice. En fin de compte, Locowin Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! De surcroit la plateforme est esthetiquement remarquable, stimule le desir de revenir. Particulierement attractif les tournois periodiques pour la rivalite, propose des recompenses permanentes.
Apprendre ici|
ultraconnect – Found some tools I’ll definitely use in my next project.
ascendmark – Really impressed by the design, feels sleek and modern.
quantumreach – Found several useful ideas I’m going to try out.
urbanshift – Navigation is so smooth, I never felt lost exploring.
focuslab – Bookmarking this — looks like a resource I’ll use often.
valuevision – Seems like a solid brand idea; content just needs fleshing out.
powercore – Clean design and intuitive navigation, nice work.
solidvision – Looking forward to seeing their design and offerings.
alphaunity – Content looks crisp and the color scheme is pleasant on eyes.
primeimpact – Really appreciate the depth in their articles.
bluetrail – Love the layout and how everything is well organized.
Мир архитектуры https://vineyardartdecor.com и дизайна в одном месте! Лучшие идеи, проекты и вдохновение для дома, офиса и города. Узнай, как создаются красивые и функциональные пространства.
urbanmatrix – Overall a positive experience, feels trustworthy and solid.
novaorbit – Pleasant browsing experience, content feels useful and genuine.
boldnetwork – Could be great for networking or community-focused content, maybe.
sharpbridge – Maybe more visuals would help, currently mostly text.
urbannexus – The writing is clear and easy to follow, nice job.
trendforge – Good potential, hope the content lives up to the name.
goldbridge – The domain looks solid, hoping the content matches up well.
boldimpact – The domain looks strong, hoping content is up soon.
smartgrid – If content matches the name, this could become useful resource.
skyportal – Articles are informative, I keep coming back every week.
The Real Person!
The Real Person!
вывод из запоя
vivod-iz-zapoya-minsk011.ru
вывод из запоя круглосуточно минск
nextrealm – Clean URL suggests professional intentions, looking forward.
zenithlabs – The content is engaging, and the site is responsive.
maxvision – Great user experience, everything loads so smoothly and fast.
nextlayer – A pleasure to browse, everything is well-organized and clear.
thinkbeyondtech – The site is well-structured, making information easy to find.
greenmotion – Offers valuable insights, and the interface is top-notch.
openlaunch – Just browsed the site, looks promising with neat design.
growwithfocus – Clean and catchy name, perfect for growth or learning resources.
elitegrowth – Impressed by the layout, feels modern and user-friendly.
The Real Person!
The Real Person!
Ich bin abhangig von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es wartet eine Fulle spannender Optionen, mit innovativen Slots und fesselnden Designs. Der Service ist von hoher Qualitat, immer parat zu assistieren. Die Gewinne kommen prompt, obwohl die Offers konnten gro?zugiger ausfallen. In Kurze, SpinBetter Casino ist ein Muss fur alle Gamer fur Spieler auf der Suche nach Action ! Daruber hinaus das Design ist ansprechend und nutzerfreundlich, was jede Session noch besser macht. Ein Pluspunkt ist die schnellen Einzahlungen, die Flexibilitat bieten.
https://spinbettercasino.de/|
cloudmatrix – Great user experience, everything loads so smoothly and fast.
fastgrowth – The content is engaging, and the site is responsive.
The Real Person!
The Real Person!
J’aime l’atmosphere unique de Locowin Casino, c’est une plateforme qui deborde de vitalite. Il y a une multitude de jeux captivants, comprenant des jeux optimises pour les cryptos. Doublement des depots jusqu’a 200 €. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les retraits sont effectues rapidement, mais plus de promos regulieres seraient un plus. En bref, Locowin Casino est indispensable pour les joueurs pour les amateurs de sensations fortes ! Ajoutons que la plateforme est visuellement impressionnante, ajoute une touche de confort. Particulierement interessant le programme de fidelite avec des niveaux VIP, offre des recompenses continues.
Plongez dГЁs maintenant|
The Real Person!
The Real Person!
I’m amazed by Wazamba Casino, it’s an adventure that throbs with excitement. The options are extensive and diverse, including slots with tribal themes. Enhancing your initial experience. Customer support is outstanding. Payments are secure and reliable, nevertheless more frequent promotions could enhance it. Overall, Wazamba Casino provides guaranteed enjoyment for online betting fans ! In addition the site is fast and responsive, adds a layer of excitement. Notably the loyalty program with masks, offering more ways to win.
wazambagr.com|
анлим казино
The Real Person!
The Real Person!
Je suis surpris par Locowin Casino, c’est une plateforme qui explose d’energie. Les alternatives sont incroyablement etendues, incluant des paris sportifs electrisants. Le bonus d’accueil est attractif. Le suivi est exemplaire, toujours pret a intervenir. Les retraits sont realises promptement, cependant des bonus plus diversifies seraient souhaitables. En synthese, Locowin Casino fournit une experience ineffacable pour les enthousiastes de casino en ligne ! De surcroit le design est contemporain et lisse, stimule le desir de revenir. A souligner aussi les paiements securises en crypto, renforce le sens de communaute.
Poursuivre la lecture|
The Real Person!
The Real Person!
Je suis stupefait par Locowin Casino, il delivre une experience unique. Les alternatives sont incroyablement etendues, offrant des sessions live intenses. Pour un lancement puissant. L’equipe d’assistance est remarquable, assurant un support premium. Les operations sont solides et veloces, de temps a autre plus de promotions frequentes seraient un atout. En fin de compte, Locowin Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! Ajoutons que la navigation est simple et engageante, stimule le desir de revenir. Particulierement attractif les evenements communautaires captivants, offre des privileges sur mesure.
Explorer tout|
blueorbit – Just browsed their resources, found several helpful ones today.
futurelink – Just explored the site, found several useful tools and ideas.
Amo a vibracao de PlayPIX Casino, transporta para um mundo de folia cativante. Ha uma explosao de jogos emocionantes, incluindo apostas esportivas vibrantes. Eleva a diversao do jogo. O acompanhamento e impecavel, acessivel a qualquer hora. Os saques sao rapidos como um raio, as vezes recompensas adicionais seriam festivas. Para concluir, PlayPIX Casino garante diversao a cada momento para jogadores em busca de adrenalina ! Vale notar a plataforma e visualmente espetacular, adiciona um toque de conforto. Igualmente impressionante os pagamentos seguros em cripto, fortalece o senso de comunidade.
Verificar isso|
The Real Person!
The Real Person!
частный психиатр на дом в москве
psychiatr-moskva008.ru
психиатрическая клиника стационар
The Real Person!
The Real Person!
I’m hooked on Wazamba Casino, it seems like a whirlwind of delight. The catalog is absolutely massive, featuring over 5,000 titles from top providers. The welcome bonus is generous. Ensuring smooth gameplay. Transactions are hassle-free, sometimes lower wagering requirements would be ideal. To sum up, Wazamba Casino is a platform that excels for casino enthusiasts ! In addition the interface is intuitive and themed, adds a layer of excitement. Notably crypto-friendly banking options, elevating the engagement.
https://wazambagr.com/|
The Real Person!
The Real Person!
экстренный вывод из запоя челябинск
vivod-iz-zapoya-chelyabinsk011.ru
лечение запоя
Τα online καζίνο αποτελούν σήμερα μια από τις πιο δημοφιλείς μορφές ψυχαγωγίας στην Ελλάδα. Η αγάπη των Ελλήνων παικτών για αυτή τη μορφή διασκέδασης μεγαλώνει συνεχώς, και μαζί με αυτή την αύξηση, πολλαπλασιάζονται και οι επιλογές σε διαδικτυακά καζίνο. Αν θέλεις να βρεις τα καλύτερα και πιο ασφαλή καζίνο για να παίξεις, είναι σημαντικό να ξέρεις πώς να ξεχωρίζεις τις αξιόπιστες πλατφόρμες από τις υπόλοιπες. Στο παρακάτω άρθρο θα βρεις χρήσιμες πληροφορίες και συμβουλές για να κάνεις την καλύτερη επιλογή και να απολαύσεις το παιχνίδι με ασφάλεια και διασκέδαση.
valuevision – Found some good sections, wish there was more detailed content.
1xslots
Amo a vibracao de PlayPIX Casino, e uma plataforma que pulsa com energia festiva. A variedade de titulos e deslumbrante, com sessoes ao vivo dinamicas. Eleva a diversao do jogo. O acompanhamento e impecavel, oferecendo respostas claras. Os ganhos chegam sem demora, no entanto bonus mais variados seriam bem-vindos. No geral, PlayPIX Casino vale uma visita epica para jogadores em busca de adrenalina ! Vale notar a interface e fluida e elegante, aumenta o prazer de jogar. Igualmente impressionante os eventos comunitarios envolventes, que impulsiona o engajamento.
Ler os detalhes|
skyportal – User experience is smooth, links and menus work perfectly.
futurelink – Clean layout and easy navigation made me stick around.
The Real Person!
The Real Person!
https://bsm-e.at
quantumreach – The writing is clear and not overly complicated, love that.
The Real Person!
The Real Person!
лечение запоя минск
vivod-iz-zapoya-minsk012.ru
вывод из запоя
1xslots рабочее зеркало
The Real Person!
The Real Person!
консультация врача психиатра
psychiatr-moskva009.ru
принудительное лечение в психиатрической больнице
alphaunity – The visuals complement the text nicely, very balanced design.
Автоматические гаражные ворота давно перестали быть роскошью и стали необходимым элементом комфортной жизни. Наши автоматические ворота сочетают надёжность проверенных европейских механизмов с элегантным дизайном, который гармонично впишется в архитектуру любого здания. Мы предлагаем полный цикл услуг: от профессиональной консультации и точного замера до установки под ключ и гарантийного обслуживания. Доверьте безопасность своего дома профессионалам — получите бесплатный расчёт стоимости уже сегодня: Тут
sharpbridge – Navigation feels intuitive, didn’t struggle finding anything so far.
The Real Person!
The Real Person!
https://b-s2best.at
The Real Person!
The Real Person!
лечение запоя челябинск
vivod-iz-zapoya-chelyabinsk012.ru
вывод из запоя цена
bluetrail – Overall positive vibes, I’m glad I checked this out.
solidvision – Clean branding, simple domain—can work well.
elitegrowth – The content is engaging, and the site is responsive.
The Real Person!
The Real Person!
Je suis epate par Locowin Casino, il offre une experience unique. Le catalogue est riche et diversifie, avec des slots au design innovant. Le bonus de bienvenue est attractif. L’assistance est efficace et pro, avec une aide precise et rapide. Les transactions sont fiables et rapides, cependant des bonus plus varies seraient bienvenus. En resume, Locowin Casino est une plateforme qui dechire pour les joueurs en quete d’excitation ! Notons aussi l’interface est intuitive et stylee, amplifie le plaisir de jouer. Un plus non negligeable les paiements securises en crypto, offre des recompenses continues.
Lancer le site|
cloudmatrix – Navigation is seamless, and the visuals are appealing.
сукааа казино
fastgrowth – Offers valuable insights, and the interface is top-notch.
innovatewithus – Content is super useful, I learned something new today.
The Real Person!
The Real Person!
Je suis surpris par Locowin Casino, ca transporte dans un univers envoutant. Il y a une profusion de jeux captivants, offrant des sessions live intenses. Le bonus d’accueil est attractif. Les agents reagissent avec promptitude, assurant un support premium. La procedure est aisee et efficace, par moments des bonus plus diversifies seraient souhaitables. En fin de compte, Locowin Casino est une plateforme qui excelle pour les joueurs a la recherche d’aventure ! Par ailleurs l’interface est intuitive et raffinee, intensifie le plaisir du jeu. Particulierement attractif le programme VIP avec des niveaux exclusifs, garantit des transactions securisees.
Plonger dedans|
sharpwave – Content feels high quality, very informative and credible overall.
metarise – Very user friendly, easy to find what I need each visit.
kluvcc – Really nice site, design is clean and feels professional.
ynaix – The aesthetic is clean and minimal, which works very well.
bestchoiceonline – Wish list option is helpful, good for planning future purchases.
The Real Person!
The Real Person!
Je suis impressionne par Locowin Casino, ca transporte dans un univers envoutant. Il y a une profusion de jeux captivants, avec des slots au style innovant. Renforcant l’experience de depart. L’aide est performante et experte, avec une assistance exacte et veloce. Les operations sont solides et veloces, bien que des incitations additionnelles seraient un benefice. Globalement, Locowin Casino est essentiel pour les amateurs pour les aficionados de jeux contemporains ! Par ailleurs le design est contemporain et lisse, stimule le desir de revenir. Un autre avantage cle les tournois periodiques pour la rivalite, qui stimule l’engagement.
Ouvrir la page|
brightchain – Found helpful sections, resources seem well thought out.
maxvision – A pleasure to browse, everything is well-organized and clear.
nextbrand – Overall feels polished, would return for more content here.
futurebeam – Loving the energy here, feels futuristic yet approachable.
cloudmark – The homepage is elegant, feels welcoming and well-designed.
coreimpact – Gives a good first impression, polished and organized.
ultrawave – Contents are clear and helpful, no filler, just solid insight.
wavefusion – Visual hierarchy is good, important stuff stands out immediately.
cyberlaunch – Feels trustworthy and tech-savvy, would revisit often.
alphaimpact – Handy resources are placed well; easy access to what matters.
strongrod – The tone feels confident and trustworthy, not overbearing.
brightideaweb – Overall nice polish, site feels professional and thoughtfully designed.
yourbrandzone – Content is valuable, clearly written and well organized.
digitalstorm – Pages load quickly, even complex graphics appear smooth.
boldspark – Typography is bold and readable, headings stand out clearly.
zenithmedia – Typography is crisp, spacing makes reading comfortable.
pixelplanet – Love the creativity here, visuals are really on point from first glance.
linkmaster – Navigation is straightforward, I got around without confusion.
discoveramazingthingsonline – Color choices are bright and engaging, mood booster when browsing.
everythingyouneedtoknow – Navigation is well-thought, found deep info without confusion.
explorecreativeideasdaily – Love the inspiration, so many new ideas every visit.
The Real Person!
The Real Person!
J’ai un coup de foudre pour Locowin Casino, ca procure un plaisir intense. Le catalogue est riche et diversifie, comprenant des jeux compatibles avec les cryptos. Pour un demarrage en force. Le service est disponible 24/7, avec une aide precise et rapide. Les gains arrivent sans delai, cependant des bonus plus varies seraient bienvenus. Pour conclure, Locowin Casino offre une experience memorable pour les amateurs de sensations fortes ! De plus le site est rapide et attrayant, ajoute une touche de confort. Egalement appreciable le programme VIP avec des niveaux exclusifs, offre des recompenses continues.
Voir les nouveautГ©s|
The Real Person!
The Real Person!
Je suis surpris par Locowin Casino, on detecte une vibe folle. Le repertoire est opulent et multifacette, avec des slots au style innovant. Le bonus d’accueil est attractif. Les agents reagissent avec promptitude, accessible a tout instant. Les benefices arrivent sans latence, mais des bonus plus diversifies seraient souhaitables. Globalement, Locowin Casino garantit du divertissement constant pour les adeptes de sensations intenses ! Ajoutons que la plateforme est esthetiquement remarquable, facilite une immersion complete. A souligner aussi les tournois periodiques pour la rivalite, renforce le sens de communaute.
Tout accГ©der|
The Real Person!
The Real Person!
J’ai un engouement pour Locowin Casino, on detecte une vibe folle. Il y a une profusion de jeux captivants, incluant des paris sportifs electrisants. Pour un lancement puissant. Le service est operationnel 24/7, assurant un support premium. Les paiements sont proteges et lisses, par moments des bonus plus diversifies seraient souhaitables. En fin de compte, Locowin Casino est une plateforme qui excelle pour les joueurs a la recherche d’aventure ! Par ailleurs la plateforme est esthetiquement remarquable, intensifie le plaisir du jeu. Un autre avantage cle les paiements securises en crypto, qui stimule l’engagement.
Visiter pour plus|
The Real Person!
The Real Person!
Me encantei pelo ritmo de BR4Bet Casino, tem um ritmo de jogo que acende como uma tocha. Os jogos formam um clarao de entretenimento. com caca-niqueis modernos que encantam como lanternas. Os agentes sao rapidos como um raio de farol. com solucoes precisas e instantaneas. As transacoes sao faceis como um brilho. de vez em quando mais giros gratis seriam brilhantes. Ao final, BR4Bet Casino vale explorar esse cassino ja para os apaixonados por slots modernos! E mais a interface e fluida e brilha como um farol. criando uma experiencia de cassino reluzente.
tempo de saque br4bet|
The Real Person!
The Real Person!
лечение запоя
vivod-iz-zapoya-omsk010.ru
вывод из запоя омск
The Real Person!
The Real Person!
платный психиатр
psikhiatr-moskva007.ru
психиатрическая помощь
The Real Person!
The Real Person!
Fiquei fascinado com BETesporte Casino, leva a um universo de apostas dinamico. As opcoes sao amplas como um campo de futebol, incluindo apostas esportivas que aceleram o coracao. 100% ate R$600 + apostas gratis. Os agentes respondem com rapidez, sempre pronto para entrar em campo. As transacoes sao confiaveis, as vezes bonus mais variados seriam um golaco. Em sintese, BETesporte Casino garante diversao constante para fas de cassino online ! Vale destacar o design e moderno e vibrante, instiga a prolongar o jogo. Outro destaque os pagamentos seguros em cripto, oferece recompensas continuas.
Explorar o site|
The Real Person!
The Real Person!
Sou viciado na pista de F12.Bet Casino, tem um ritmo de jogo que derrapa como um drift. Tem um pit stop de jogos de cassino irados. com caca-niqueis modernos que roncam como motores. O suporte e um engenheiro de pit stop. disponivel por chat ou e-mail. Os saques aceleram como um turbo. entretanto mais bonus seriam um diferencial turbo. Em sintese, F12.Bet Casino oferece uma experiencia que e puro ronco para os apaixonados por slots modernos! Como extra a plataforma acelera com um visual veloz. fazendo o cassino roncar como um motor.
baixar app f12 bet|
The Real Person!
The Real Person!
Galera, quero registrar aqui no 4PlayBet Casino porque nao e so mais um cassino online. A variedade de jogos e simplesmente incrivel: jogos ao vivo imersivos, todos rodando lisos. O suporte foi amigavel, responderam em minutos pelo chat, algo que vale elogio. Fiz saque em Bitcoin e o dinheiro entrou muito rapido, ponto fortissimo. Se tivesse que criticar, diria que mais brindes fariam falta, mas isso nao estraga a experiencia. Enfim, o 4PlayBet Casino tem diferencial real. Recomendo sem medo.
celular motog 4play|
The Real Person!
The Real Person!
Sou viciado em PlayPIX Casino, oferece um prazer eletrizante. As opcoes sao amplas como um festival, com sessoes ao vivo cheias de emocao. Com uma oferta inicial para impulsionar. O suporte ao cliente e excepcional, com suporte rapido e preciso. As transacoes sao confiaveis, no entanto bonus mais variados seriam incriveis. Resumindo, PlayPIX Casino oferece uma experiencia memoravel para fas de cassino online ! Acrescentando que a navegacao e intuitiva e envolvente, tornando cada sessao mais vibrante. Outro destaque o programa VIP com niveis exclusivos, fortalece o senso de comunidade.
Saber mais|
Частный заем денег мфо домашние деньги альтернатива банковскому кредиту. Быстро, безопасно и без бюрократии. Получите нужную сумму наличными или на карту за считанные минуты.
ynaix – Overall very polished, definitely feels like a premium service offering.
Все спортивные новости http://sportsat.ru в реальном времени. Итоги матчей, трансферы, рейтинги и обзоры. Следите за событиями мирового спорта и оставайтесь в курсе побед и рекордов!
The Real Person!
The Real Person!
Sou louco pela vibracao de BR4Bet Casino, e uma onda de diversao que cintila como um farol. O leque do cassino e um brilho de delicias. com jogos adaptados para criptomoedas. O atendimento e solido como um farol. disponivel por chat ou e-mail. Os pagamentos sao lisos como uma chama. as vezes mais giros gratis seriam uma loucura iluminada. Ao final, BR4Bet Casino promete uma diversao que e uma chama para os fas de adrenalina brilhante! E mais a interface e fluida e brilha como um farol. dando vontade de voltar como uma chama eterna.
cassino br4bet|
The Real Person!
The Real Person!
Tenho uma paixao vibrante por BETesporte Casino, sinto um rugido de emocao. Ha uma explosao de jogos emocionantes, suportando jogos compativeis com criptomoedas. O bonus de boas-vindas e empolgante. Os agentes respondem com rapidez, garantindo atendimento de alto nivel. O processo e simples e direto, no entanto bonus mais variados seriam um golaco. Resumindo, BETesporte Casino vale uma aposta certa para fas de cassino online ! Tambem o site e veloz e envolvente, facilita uma imersao total. Um diferencial importante os eventos comunitarios envolventes, oferece recompensas continuas.
Abrir agora|
The Real Person!
The Real Person!
Estou alucinado com MarjoSports Casino, explode com uma vibe de cassino esportiva. As escolhas sao vibrantes como um apito. com slots tematicos de esportes. Os agentes voam como jogadores. com ajuda que marca gol como um penalti. Os ganhos chegam rapido como um gol. as vezes mais bonus seriam um diferencial de campo. Em sintese, MarjoSports Casino garante um jogo que vibra como uma torcida para os fas de adrenalina de campo! Vale dizer o site e uma obra-prima de estilo esportivo. dando vontade de voltar como um craque.
contato marjosports|
The Real Person!
The Real Person!
Galera, nao podia deixar de comentar no 4PlayBet Casino porque nao e so mais um cassino online. A variedade de jogos e bem acima da media: poquer estrategico, todos com graficos de primeira. O suporte foi rapido, responderam em minutos pelo chat, algo que raramente vi. Fiz saque em PIX e o dinheiro entrou mais ligeiro do que imaginei, ponto fortissimo. Se tivesse que criticar, diria que podia ter mais promocoes semanais, mas isso nao estraga a experiencia. Resumindo, o 4PlayBet Casino vale demais a pena. Recomendo sem medo.
4play strip|
The Real Person!
The Real Person!
вывод из запоя цена
vivod-iz-zapoya-cherepovec013.ru
экстренный вывод из запоя
The Real Person!
The Real Person!
Estou totalmente fascinado por PlayPIX Casino, oferece um prazer eletrizante. A variedade de titulos e estonteante, com slots de design inovador. Com uma oferta inicial para impulsionar. Os agentes respondem com agilidade, acessivel a qualquer momento. Os saques sao rapidos como um raio, de vez em quando bonus mais variados seriam incriveis. No fim, PlayPIX Casino garante diversao constante para jogadores em busca de adrenalina ! Tambem a navegacao e intuitiva e envolvente, adiciona um toque de conforto. Outro destaque os eventos comunitarios envolventes, que aumenta o engajamento.
Ver os detalhes|
sportwetten paysafecard ohne oasis online
paypal
wavefusion – Found useful content, everything seems well organized.
cyberlaunch – Visuals are sharp, layout is uncluttered and pleasing.
metarise – Found great resources, very useful and clearly presented here.
sharpwave – Thoughtful layout, sections well separated and easy to scan.
Suchen Sie Immobilien? https://www.montenegro-immobilien-kaufen.com wohnungen, Villen und Grundstucke mit Meerblick. Aktuelle Preise, Fotos, Auswahlhilfe und umfassende Transaktionsunterstutzung.
brightchain – Content strikes a good balance, informative yet easy to read.
The Real Person!
The Real Person!
вывод из запоя круглосуточно омск
vivod-iz-zapoya-omsk011.ru
лечение запоя
innovatewithus – Videos and articles both are engaging, love that mix.
findwhatyouarelookingfor – Visuals support the message well, nothing feels out of place.
learnsomethingneweveryday – Overall experience is uplifting and motivating, I’ll return daily.
thebestplacetostarttoday – Layout is intuitive, I always know where to click next.
cloudmark – Navigation is slick, found what I needed intuitively.
everythingaboutsuccess – Found useful advice, not fluffy, straight to things I can use.
thefutureofinnovation – Great layout, content is well organized and easy to follow.
discoverendlessinspiration – Visuals are beautiful and inspiring, brightens my mood instantly.
yourtrustedguideforlife – Overall very comforting presence, makes me trust the content here.
joinourcreativecommunity – The tone invites participation, feels more like a conversation than lecture.
getreadytoexplore – Navigation is smooth, sections are easy to find without effort.
growyourbusinessfast – Tone is motivational yet practical, not overhyped.
everythingyouneedtoknow – Typography is very readable, spacing gives breathing room.
discoveramazingthingsonline – Pages load nicely, no wait time, even on my phone.
stayupdatedwithtrends – Featured posts stand out clearly, what’s trending is easy to catch.
makingeverymomentcount – Overall feel is uplifting, makes me want to revisit often.
makeimpactwithideas – The layout is engaging, visuals and text balance nicely.
explorecreativeideasdaily – Articles are stimulating and short enough to digest easily.
digitalstorm – Content is compelling and detailed, not just superficial.
makelifebettereveryday – Hope the content is practical, not just motivational fluff, that matters.
The Real Person!
The Real Person!
J’adore l’ambiance de Locowin Casino, c’est une plateforme qui bouillonne d’energie. Le catalogue est riche et diversifie, proposant des jeux de table immersifs. Doublement des depots jusqu’a 1850 €. Le service est disponible 24/7, garantissant un support de qualite. Le processus est simple et efficace, parfois des offres plus genereuses ajouteraient du piquant. En resume, Locowin Casino garantit du fun a chaque instant pour les passionnes de jeux modernes ! Notons aussi la plateforme est visuellement top, ajoute une touche de confort. Egalement appreciable les options de paris sportifs variees, propose des avantages personnalises.
AccГ©der au site|
learnshareandsucceed – The tone feels friendly but knowledgeable, confidence without arrogance.
LearnWithConfidence – Very approachable lessons, perfect for beginners wanting to improve efficiently.
TogetherWeCreateChange – Great initiative, the guides and tips feel practical and usable.
GrowWithConfidenceHere – Navigation is smooth, finding resources for projects is super fast.
BuildABetterTomorrow – It’s inspiring to see how they empower local communities.
expandyourhorizons – Navigation looks intuitive, discovering new ideas seems smooth.
The Real Person!
The Real Person!
J’adore l’eclat vibrant de Casinia Casino, ca distille un plaisir eclatant. La diversite des titres est eblouissante, offrant des sessions live captivantes. L’offre de bienvenue est eclatante. Le support client est lumineux, toujours pret a eclairer. Les transactions sont fiables et rapides, de temps a autre des bonus plus varies brilleraient davantage. En somme, Casinia Casino merite une exploration eblouissante pour les passionnes de frissons lumineux ! De plus la navigation est intuitive comme une lueur, facilite une immersion totale. A souligner aussi les evenements communautaires captivants, offre des privileges continus.
Regarder de prГЁs|
discoveramazingstories – Curious about categories; mystery, adventure, personal tales could really shine.
CreateInspireAndGrow – I find the tutorials engaging and easy to follow along.
YourTrustedSourceOnline – I love how they integrate technology into their initiatives.
https://blog.369usa.com/1158-2/
BuildSomethingMeaningful – Their approach to education is both innovative and impactful.
SimpleWaysToBeHappy – This platform offers valuable insights for boosting happiness.
The Real Person!
The Real Person!
J’adore l’ambiance de Locowin Casino, ca transporte dans un monde captivant. La selection de jeux est phenomenale, proposant des jeux de table immersifs. Le bonus de bienvenue est attractif. Le support client est exceptionnel, joignable a toute heure. Les paiements sont securises et fluides, cependant des bonus plus varies seraient bienvenus. Au final, Locowin Casino est un incontournable pour les joueurs pour les passionnes de jeux modernes ! De plus la plateforme est visuellement top, ce qui rend chaque session encore plus fun. Particulierement interessant le programme VIP avec des niveaux exclusifs, propose des avantages personnalises.
Trouver les dГ©tails|
The Real Person!
The Real Person!
вывод из запоя череповец
vivod-iz-zapoya-cherepovec014.ru
лечение запоя череповец
The Real Person!
The Real Person!
Je suis totalement fou de Locowin Casino, il offre une experience unique. Il y a une multitude de jeux excitants, offrant des sessions live dynamiques. Amplifiant l’experience initiale. Le suivi client est irreprochable, garantissant un support de qualite. Les retraits sont ultra-rapides, cependant quelques tours gratuits en plus seraient cool. Au final, Locowin Casino est un incontournable pour les joueurs pour les amateurs de sensations fortes ! Cerise sur le gateau le design est moderne et fluide, facilite une immersion totale. Un autre atout majeur les evenements communautaires engageants, renforce le sentiment de communaute.
Emmenez-moi lГ -bas|
The Real Person!
The Real Person!
Me encantei pelo nado de Brazino Casino, parece um abismo de adrenalina subaquatica. O leque do cassino e um recife de delicias. oferecendo sessoes ao vivo que brilham como aguas cristalinas. Os agentes nadam como golfinhos. garantindo suporte direto e sem correntezas. Os saques sao velozes como um mergulho. ocasionalmente mais giros gratis seriam uma loucura marinha. No fim das contas, Brazino Casino e um recife de emocoes para os exploradores de jogos online! Vale dizer o layout e vibrante como uma perola. tornando cada sessao ainda mais aquatica.
brazino777 apostas|
The Real Person!
The Real Person!
J’adore l’eclat vibrant de Casinia Casino, ca transporte dans un palais scintillant. La diversite des titres est eblouissante, proposant des jeux de table raffines. 100% jusqu’a 500 € + 200 tours gratuits. Le suivi est irreprochable, toujours pret a eclairer. Les retraits filent comme une comete, de temps a autre des offres plus genereuses seraient un plus. Pour conclure, Casinia Casino merite une exploration eblouissante pour les aficionados de jeux modernes ! De plus la navigation est intuitive comme une lueur, ce qui rend chaque session plus eclatante. A souligner aussi le programme VIP avec 5 niveaux exclusifs, propose des recompenses sur mesure.
DГ©couvrir les offres|
The Real Person!
The Real Person!
Acho simplesmente ardente Verabet Casino, tem um ritmo de jogo que danca como labaredas. O catalogo de jogos e um altar de prazeres. incluindo jogos de mesa com um toque de ritual. O atendimento esta sempre ativo 24/7. com solucoes precisas e instantaneas. Os saques sao velozes como um ritual de fogo. as vezes mais giros gratis seriam incendiarios. No fim das contas, Verabet Casino e um altar de emocoes para os exploradores de jogos online! De bonus o visual e uma explosao de chamas. fazendo o cassino queimar como uma fogueira.
vera bet app|
The Real Person!
The Real Person!
Adoro a labareda de Fogo777 Casino, pulsa com uma forca de cassino digna de um xama. Tem uma enxurrada de jogos de cassino irados. com slots tematicos de cerimonias antigas. O suporte e um fulgor reluzente. garantindo suporte direto e sem cinzas. Os saques sao velozes como um ritual de fogo. entretanto mais giros gratis seriam uma loucura de fogo. Na real, Fogo777 Casino e o point perfeito pros fas de cassino para os apaixonados por slots modernos! De lambuja o site e uma obra-prima de estilo ardente. fazendo o cassino queimar como uma fogueira.
www fogo777|
The Real Person!
The Real Person!
Je suis accro a Locowin Casino, ca procure un plaisir intense. Il y a une multitude de jeux excitants, offrant des sessions live dynamiques. Accompagne de tours gratuits sans wager. Le suivi client est irreprochable, offrant des reponses claires. Les paiements sont securises et fluides, parfois quelques tours gratuits en plus seraient cool. Pour conclure, Locowin Casino est un incontournable pour les joueurs pour les fans de casino en ligne ! Notons aussi la plateforme est visuellement top, ajoute une touche de confort. Particulierement interessant les paiements securises en crypto, offre des recompenses continues.
Commencer Г lire|
The Real Person!
The Real Person!
Je trouve absolument enivrant BankOnBet Casino, it’s a vault of fun that locks in wins. L’assortiment de jeux du casino est un tresor de delices. proposant des slots de casino a theme financier. Le support du casino est disponible 24/7. repondant en un cliquetis de serrure. Les paiements du casino sont securises et fluides. parfois more free spins would be a smart bet. En somme, BankOnBet Casino c’est un casino a miser sans hesiter pour ceux qui cherchent l’adrenaline securisee du casino! En plus l’interface du casino est fluide et securisee comme un coffre. amplifie l’immersion totale dans le casino.
bankonbet pl|
The Real Person!
The Real Person!
Sou totalmente viciado em BETesporte Casino, transporta para um universo de apostas eletrizante. A selecao de jogos e fenomenal, suportando jogos compativeis com criptomoedas. Fortalece seu saldo inicial. A assistencia e eficiente e amigavel, garantindo atendimento de alto nivel. Os ganhos chegam sem atraso, as vezes promocoes mais frequentes dariam um toque extra. Para finalizar, BETesporte Casino oferece uma experiencia inesquecivel para fas de cassino online ! Adicionalmente a interface e fluida e energetica, tornando cada sessao mais competitiva. Um diferencial importante os torneios regulares para rivalidade, fortalece o senso de comunidade.
Ir para a web|
The Real Person!
The Real Person!
Fiquei fascinado com PlayPIX Casino, leva para um universo de pura excitacao. Ha uma explosao de jogos emocionantes, com slots de design inovador. O bonus de boas-vindas e cativante. O acompanhamento e impecavel, acessivel a qualquer momento. Os saques sao rapidos como um raio, embora promocoes mais frequentes dariam um toque extra. No fim, PlayPIX Casino garante diversao constante para jogadores em busca de adrenalina ! Tambem a plataforma e visualmente espetacular, facilita uma imersao total. Um diferencial significativo os pagamentos seguros em cripto, oferece recompensas continuas.
Saber mais|
joinourcreativecommunity – Designs are vibrant and personable, I enjoy scrolling through.
The Real Person!
The Real Person!
Me encantei pelo nado de Brazino Casino, explode com uma vibe aquatica eletrizante. Os jogos formam um coral de diversao. com slots tematicos de aventuras marinhas. O suporte e uma concha de eficiencia. assegurando apoio sem turbulencia aquatica. As transacoes sao simples como uma bolha. porem as ofertas podiam ser mais generosas. No geral, Brazino Casino oferece uma experiencia que e puro mergulho para os apaixonados por slots modernos! Adicionalmente a navegacao e facil como uma corrente marinha. fazendo o cassino reluzir como um recife.
brazino777 nude|
The Real Person!
The Real Person!
Acho simplesmente digital PlayPix Casino, oferece uma aventura que glitcha como um render 8-bit. A selecao de titulos e um buffer de prazeres. com caca-niqueis que reluzem como bytes. O atendimento esta sempre ativo 24/7. respondendo rapido como um buffer. O processo e claro e sem crashes. porem mais giros gratis seriam vibrantes. Em resumo, PlayPix Casino e um cassino online que e uma matriz de diversao para os apaixonados por slots modernos! Por sinal a plataforma reluz com um visual glitch. dando vontade de voltar como um byte.
painel playpix|
The Real Person!
The Real Person!
Me encantei pelo fulgor de Fogo777 Casino, oferece uma aventura que reluz como brasas vivas. A colecao e um ritual de entretenimento. com caca-niqueis que reluzem como brasas. O suporte e um fulgor reluzente. respondendo veloz como uma faisca. Os saques queimam como brasas. porem mais giros gratis seriam incendiarios. No geral, Fogo777 Casino garante um jogo que reluz como chamas para os cacadores de vitorias incendiarias! Alem disso o site e uma obra-prima de estilo ardente. dando vontade de voltar como uma labareda.
jogar fogo777|
The Real Person!
The Real Person!
Estou completamente encantado com BETesporte Casino, e uma plataforma que vibra como um estadio em dia de final. Ha uma explosao de jogos emocionantes, com sessoes ao vivo cheias de energia. Fortalece seu saldo inicial. A assistencia e eficiente e amigavel, garantindo atendimento de alto nivel. O processo e simples e direto, as vezes mais apostas gratis seriam incriveis. No fim, BETesporte Casino vale uma aposta certa para entusiastas de jogos modernos ! Acrescentando que o site e veloz e envolvente, adiciona um toque de estrategia. Outro destaque os torneios regulares para rivalidade, fortalece o senso de comunidade.
Encontrar os detalhes|
The Real Person!
The Real Person!
Amo a energia selvagem de PlayPIX Casino, proporciona uma aventura eletrizante. Ha uma explosao de jogos emocionantes, com sessoes ao vivo cheias de energia. O bonus de boas-vindas e cativante. O acompanhamento e impecavel, acessivel a qualquer momento. O processo e simples e elegante, no entanto promocoes mais frequentes dariam um toque extra. Resumindo, PlayPIX Casino e essencial para jogadores para entusiastas de jogos modernos ! Adicionalmente a navegacao e intuitiva e envolvente, tornando cada sessao mais vibrante. Igualmente impressionante os eventos comunitarios envolventes, fortalece o senso de comunidade.
Acessar o site|
The Real Person!
The Real Person!
вывод из запоя круглосуточно череповец
vivod-iz-zapoya-cherepovec015.ru
экстренный вывод из запоя череповец
The Real Person!
The Real Person!
https://bsmen.at
The Real Person!
The Real Person!
https://1-bs2best.lat
The Real Person!
The Real Person!
Pomimo swojej ograniczonej przeszłości, aby pamiętać. Sugar rush Symbole slotów według ich wartości tony G ma wieloletnie doświadczenie osobiste i zawodowe przy stołach pokerowych i kasynach, że możesz używać konta lub karty kredytowej tylko wtedy. Czeka tu na Ciebie sporo do odkrycia, więc nie traćmy czasu i zanurzmy się w recenzji slotu Sugar Rush, aby poznać jego zmienność, RTP, specjalne funkcje oraz dowiedzieć się, jak je aktywować i gdzie zagrać na prawdziwe pieniądze lub w wersji demo! Formularz wykorzystuj wyłącznie w celu pozostawienia opinii o produkcie lub zadania pytania na jego temat. 10 min czytania Diabły są przedstawione w stylu kreskówki z Lat 90, ponieważ oferują one bardziej wyrafinowane mobilne doświadczenie hazardowe. Nie tylko jest to ekscytujące grać, choć mobilne kasyna są nadal zalecane. Madame jest znana z dużych bonusów na automatach między innymi od BoomingGames i Playson, gdzie Corman jasno przedstawił swój cel wyeliminowania wszystkich gier zręcznościowych. Przetestuj inny tytuł Gamomat, że jest to bukmacher.
https://fora.babinet.cz/profile.php?id=94671
Jeśli intrygują Cię Pragmatic Play slots i chciałbyś przetestować kilka najlepszych, mamy dla Ciebie listę najciekawszych pozycji tego studia. Oto tabela 10 najlepszych gier kasynowych na żywo od Pragmatic Play z ich istotnymi statystykami. Legalny Hazard Online Klasyczne gry na automatach z nowoczesną technologią, nacisk na prostotę i łatwość obsługi, a także wysokie RTP, zaangażowanie w innowacje, w tym tytuły z portfolio Pragmatic Play, jak zdrapka i scatter. Discover the universe of Starburst at BDMBet, a place where fun meets luxury in the vastness of online gaming. The casino offers a plush welcome package up to €450 and 250 free spins, making it a stellar starting point for any space adventurer. Plus, daily cashbacks and a loyalty program that’s simply out of this world! Jump into real money play and don’t just reach for the stars—spin them! And remember, even a supernova starts with a single spin—make yours at BDMBet.
The Real Person!
The Real Person!
https://2-bs2best.lat
The Real Person!
The Real Person!
blsp at Заинтересован, что будоражит тёмную сеть? Blacksprut — это не просто название, это эталон анонимности, оперативности и уверенности. Добро пожаловать на bs2best.at — место, где открывают то, о чём принято умалчивать. Здесь ты получишь доступ к запретному плоду. Только для избранных. Без улик. Без уступок. Только Blacksprut. Не упусти возможность стать первопроходцем — bs2best.at уже готов раскрыть свои секреты. Хватит ли тебе смелости взглянуть правде в лицо?
The Real Person!
The Real Person!
Алкогольная зависимость — это важная проблема, которая требует квалифицированной помощи. В Красноярске предлагаются услуги вывода из запоя, которые гарантируют безопасное очищение организма и восстановление. Клиники наркологии предлагают круглосуточную поддержку и анонимные методы лечения, что обеспечивает высокий уровень конфиденциальности. вывод из запоя Красноярск
The Real Person!
The Real Person!
Funkcja Zakup bonusowy w Sugar Rush 1000 oferuje natychmiastowy dostęp do rundy darmowych spinów za 100-krotność zakładu lub Super darmowych spinów za 500-krotność, w których mnożniki są już na siatce, aczkolwiek z niższym RTP wynoszącym 96,52%. Aby potwierdzić, Wind Creek oferuje wiele opcji kasyna. Kasyno 21 Bet nie ma żadnych witryn siostrzanych, wygrywając 5-4 i 4-1 i stawiając się z powrotem w wyścigu o mistrzostwo. Street Fighter II to jedna z najlepiej sprzedających się gier zręcznościowych w historii, ale jego wpływ wzrósł. Są to zazwyczaj niewielkie kwoty, zwycięskie kombinacje w automacie sugar rush co najlepsze. Ma trochę wymarzony motyw, dzięki imponującemu portfolio wysokiej jakości gier.
http://extension.unimagdalena.edu.co/extension/Lists/Contactenos/DispForm.aspx?ID=1721231&Source=http%3A%2F%2Fextension%2Eunimagdalena%2Eedu%2Eco%2Fextension%2FLists%2FContactenos%2FAllItems%2Easpx
Polscy gracze korzystający z BetOnRed Casino napotykają na szeroki wybór alternatyw zakładów dostosowanych do regionalnych preferencji. Betonred na polskim rynku kładzie nacisk na obszerne relacje sportowe z lokalnych lig i międzynarodowych rozgrywek. Dzięki mobilnej przeglądarce Bet On Red wszystkie funkcje zakładów pozostają dostępne niezależnie od lokalizacji. Jak zostać Sugar rush profesjonalistą w kasynach online? Gniazda muszą być ponownie skonfigurowane, które nadal są warte zachodu. Branża gier online nadal ewoluuje, a oferty premium, takie jak Fontan Casino, stają się obecne na rynku. Platforma ta obsługuje ponad 3000 tytułów od uznanych dostawców, w tym NetEnt i Microgaming. Nowi gracze otrzymują znaczne zachęty – bonus 15 € bez depozytu i opcje dopasowania do 500 €. Interfejs kasyna integruje klasyczne automaty, gry stołowe i doświadczenia z krupierem na żywo w usprawnionym środowisku. Połączenie różnorodności gier i struktury bonusów uzasadnia dalsze badanie.
The Real Person!
The Real Person!
круглосуточный вывод из запоя
Unlock your images with WebPtoJPGHero.com, the simple, free solution for solving WebP compatibility issues. While the WebP format helps websites load faster, it can leave you with files you can’t easily edit or share. Our online tool provides an instant fix, transforming any WebP image into a high-quality JPG that works seamlessly across all your devices and applications. You can fine-tune the output quality to balance clarity and file size, and even save time by converting an entire batch of photos at once. It’s all done quickly and privately right in your browser — no software required.
newseasoncollection.shop – Found the perfect outfit for the event, thanks for the great selection.
inspireeverydaylife.shop – Great deals and fast shipping, very satisfied with my purchases.
Matç öncəsi statistik blokda komanda forması, H2H və zədəlilər siyahısı toplanıb.
VIP proqramda səviyyə artdıqca keşbek faizi və çıxarış prioriteti yüksəlir. Lokal ödənişlər və AZN balansı üçün pinco az bölməsinə keçin, limitsiz canlı mərc rahatdır. Komissiya və limit cədvəlləri müştəri razılaşmasında açıq yazılıb. Canlı kazino bölməsində blackjack, rulet və bakara masaları 24/7 açıqdır.
Slotlarda volatilite seçimi qısa və ya uzun sessiyalara görə dəyişdirilə bilər. Mobil interfeys jestlərlə işləyir, kupon sürüşdürmə ilə redaktə olunur.
Messencer kanallarına keçidlər rəsmi səhifədə yerləşir. Region qaydalarına uyğun lokal variantlar və hüquqi məlumat verilir. Daimi istifadəçilər üçün loyallıq mağazasında fribet və spinlərə dəyişdirilə bilən xal sistemi var.
shopthelatesttrend.shop – Shipping was prompt and packaging was on point, very pleased.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-smolensk022.ru
вывод из запоя круглосуточно смоленск
hardman4schools – I like the positive tone; it radiates passion and responsibility.
everytrendinone.shop – Great pricing and stylish items, totally worth the time spent browsing.
connectdiscovergrow.shop – Informative posts and helpful insights, perfect for anyone who loves learning.
dreamcreateinspire.shop – Will definitely return for more, this store became a new favourite of mine.
trendyfindshub.shop – Highly recommend for anyone looking for quality products and service.
createandgrow.click – The layout is clean and easy to navigate, made exploring so simple.
bestdealsforlife.click – Found some really solid deals here, made my day discovering them.
theperfectgiftshop.click – The items look even better in person than they did online—very pleased.
The Real Person!
The Real Person!
https://adseon.online/code-promo-inscription-1xbet-2026-100-e130/
smartchoiceoutlet.click – User-friendly website layout, found what I needed without any hassle.
The Real Person!
The Real Person!
Kuponda multi-bet, sistem və tez düzəliş funksiyaları var, səhv əmsal da avtomatik düzəlir.
Promokod bölməsinə daxil olmaq asandır, aktiv kodlar avtomatik tanınır. Tez giriş üçün bu varianta da klik edə bilərsiniz: http://pinco-casino-azerbaijan.biz.ua; pinco az giris burada təhlükəsizdir. Risk idarəsi üçün gündəlik və aylıq xərcləmə limitləri qurmaq mümkündür. Top oyunlar və yeni relizlər ayrı vitrinlərdə təqdim edilir.
Canlı mərclərdə gecikməni nəzərə alıb əmsal kilidlərinə diqqət etmək lazımdır. Matç mərkəzi canlı statistika və animasiya ilə təqvimi birləşdirir.
E-poçt dəstəyi müraciət nömrəsi ilə gəlir və izləmək rahatdır. Məsuliyyətli oyun üçün yaş təsdiqi və limitlər məcburidir. Sürətli ödəniş axını və şəffaf limitlər rahat təcrübə yaradır.
uniquetrendstore.click – Highly recommend this site for anyone looking for trendy and reliable shopping.
dreamcreateinspire.click – The experience feels trustworthy and reliable, very satisfied with my visit.
exploreopportunitiesnow.click – Website layout is smooth and user-friendly, browsing felt effortless today.
brightfuturedeals.shop – Found some really good deals here, price and quality both solid.
joinourcreativeworld.click – Found a few unique ideas I’m excited to try out this week.
discovernewworld.click – Customer service responded quickly when I had a small question earlier today.
thinkcreategrow – Shared this with a friend who’s looking to innovate and they liked it too.
changetheworld – Layout mobile-friendly, images load well, writing is clear and helpful.
discoverendlessideas – Loved the downloadable resources section; useful for planning and idea generation.
The Real Person!
The Real Person!
Pinco casino-da futbol və e-idman mərcləri üçün koeffisentlər tez-tez yenilənir, ona görə canlı rejimdə qərar vermək rahatdır.
VIP proqramda səviyyə artdıqca keşbek faizi və çıxarış prioriteti yüksəlir. Tez giriş üçün bu varianta da klik edə bilərsiniz: http://pinco-casino-azerbaijan.biz.ua; pinco az giris burada təhlükəsizdir. Hesab təhlükəsizliyi üçün iki faktorlu giriş və cihazların siyahısı var. Sürətli oyunlar və crash-mekanikalar qısa sessiyalar üçün ideal həll təqdim edir.
Riskli kombinlərdə cash-out planı əvvəlcədən müəyyənləşdirilməlidir. APK yenilənməsi tətbiqdaxili xəbərdarlıqla gəlir və bir dəqiqəyə tamamlanır.
Şikayət forması və cavab müddətləri xidmət qaydalarında göstərilib. Şəxsi məlumatların işlənməsi siyasətində məqsəd və saxlanma müddəti göstərilir. Seçim etməkdə çətinlik varsa, tematik toplular — futbol mərcləri, slot hitləri və canlı oyunlar — kömək edir.
hardman4schools – Very clear communication, every section feels honest and approachable.
The Real Person!
The Real Person!
Pinco casino-da futbol və e-idman mərcləri üçün koeffisentlər tez-tez yenilənir, ona görə canlı rejimdə qərar vermək rahatdır.
Yeni istifadəçilər üçün pulsuz fırlatmalar və sığortalı kupon kampaniyaları tez-tez verilir. Aktiv promosları qaçırmamaq üçün pinco bonus bölməsinə baxın, canlı izləmə zamanı əlavə fribetlər çıxır. Məlumatlar TLS şifrələnməsi ilə qorunur, sessiya avtomatik vaxtı bitdikdə bağlanır. Turnirlər slotlarda aktivliyi artırır və real hədiyyələr verir.
Hesab idarəsində xərcləmə hesabatlarını aylıq yükləyib analiz etmək rahatdır. APK yenilənməsi tətbiqdaxili xəbərdarlıqla gəlir və bir dəqiqəyə tamamlanır.
Şikayət forması və cavab müddətləri xidmət qaydalarında göstərilib. Özünü istisna etdikdən sonra dəstək geri aktivləşdirməni minimum 24 saatdan əvvəl etmir. Sürətli ödəniş axını və şəffaf limitlər rahat təcrübə yaradır.
The Real Person!
The Real Person!
Məsuliyyətli oyun alətləri — limitlər, pauza və self-exclusion — profil ayarlarında əlçatandır.
Qeydiyyat e-mail və ya telefonla 1 dəqiqədən az çəkir, təsdiqdən sonra başlanğıc bonusu aktivləşir. Aktiv promosları qaçırmamaq üçün pinco bonus bölməsinə baxın, canlı izləmə zamanı əlavə fribetlər çıxır. Depozit bonusunun qaydaları dildə qısa və misallarla izah edilib. Sürətli oyunlar və crash-mekanikalar qısa sessiyalar üçün ideal həll təqdim edir.
Mobil tətbiqdə bildirişləri yalnız maraqlandığınız liqalara açmaq məsləhətdir. iOS istifadəçiləri üçün PWA veb-tətbiqi ikonaya əlavə etməklə bir toxunuşda açılır.
Hesab bərpası SMS və ya e-poçt vasitəsilə aparılır. Şəxsi məlumatların işlənməsi siyasətində məqsəd və saxlanma müddəti göstərilir. Yüksək əmsal axtaranlar üçün gündəlik yüksəldilmiş xətt ayrıca işarələnib.
The Real Person!
The Real Person!
лечение запоя смоленск
vivod-iz-zapoya-smolensk023.ru
вывод из запоя круглосуточно смоленск
yourtrustedonlinestore – Content is varied and well‑written—it’s clear effort was put into this.
everytrendinone – Totally worth bookmarking: found five ideas today I will try out.
The Real Person!
The Real Person!
Məsuliyyətli oyun alətləri — limitlər, pauza və self-exclusion — profil ayarlarında əlçatandır.
Çoxlu lokal ödəniş üsulu dəstəklənir, komissiyalar real vaxt göstərilir. Canlı futbol statistikalarını izləyərkən pinco-casino-azerbaijan.biz.ua pəncərəsini açıq saxlayın — pinco bonus elanları tez-tez yenilənir. Sürətli ödəniş üçün sevimli metodları saxlayıb bir kliklə istifadə etmək olur. Minimum və maksimum mərc diapazonları həm yeni başlayanlar, həm də high-rollerlər üçün uyğundur.
Oyun qaydaları hər masada ayrıca açılır və suallara cavab verir. Matç mərkəzi canlı statistika və animasiya ilə təqvimi birləşdirir.
Azərbaycan dilində konsultasiya mövcuddur və texniki suallar tez həll olunur. Valideyn nəzarəti üçün marşrutlar və məsləhət linkləri toplanıb. Seçim etməkdə çətinlik varsa, tematik toplular — futbol mərcləri, slot hitləri və canlı oyunlar — kömək edir.
findwhatyoulove – I shared one article with family, they found it super helpful too.
newseasoncollection – The seasonal collection really fits the current fashion trends perfectly right now.
yourjourneybegins – Easy to read, very encouraging, makes me feel ready to act.
makelifebetter – I enjoy reading the posts, very uplifting and positive vibes today.
buildyourdreamtoday – The tips here are practical and realistic, not just vague inspiration.
Could not disagree with the main ideas. Wonder how things will develop over the coming years.
trendyfindshub – Very user-friendly interface, things load fast, content is engaging and fresh.
learnshareconnect – Navigation is smooth, the layout is clean, content loads without distraction.
globalmarketplacehub – Overall this is a promising hub for connecting buyers and sellers across borders.
successpartnersgroup – Excellent content and well-presented, I’ll be returning regularly for more insights.
successpartnersgroup – Very informative and useful, makes business planning much easier today.
nextgeninnovation – Excellent presentation, very professional, I’ll be returning regularly for updates.
https://factava.com.ua/prykmety/do-choho-vyie-sobaka-prykmety-iaki-varto-znaty-kozhnomu-vlasnyku/
shopandsmilealways – Sharing with colleagues because it’s inspiring and worth a browse during breaks.
urbanwearzone – Easy checkout process, I will definitely shop here again soon.
shopandshineeveryday – This platform shows so many fresh ideas, I’m impressed every time.
inspireeverydaylife – Found exactly what I needed today: a fresh perspective and new inspiration.
globalideasnetwork – Interesting concepts shared here, totally worth reading for everyone interested.
discovernewideas – Always discover unique concepts that spark my creativity.
inspiredgrowthteam – The content provided is both informative and actionable for teams.
shopthelatesttrend – Comments seem authentic, community is interactive, feels like a real hub.
buildyourdreamtoday – Feels uplifting every time I visit—makes me want to keep going.
discoveryourmoment – This site offers so many surprising ideas that grabbed my attention fast.
hardman4schools – I like how transparent and sincere the campaign presentation feels.
thebestplacetoshop – The tone is friendly and approachable, doesn’t feel overly formal or salesy.
The Real Person!
The Real Person!
Pinco casino-da futbol və e-idman mərcləri üçün koeffisentlər tez-tez yenilənir, ona görə canlı rejimdə qərar vermək rahatdır.
Turnirlərdə liderlər cədvəli şəffafdır, mükafatlar və qaydalar əvvəlcədən açıqlanır. Azərbaycan bazarı üçün lokal təkliflər pinco-casino-azerbaijan.biz.ua səhifəsində toplanıb — limitlər AZN üzrə göstərilir. Məlumatlar TLS şifrələnməsi ilə qorunur, sessiya avtomatik vaxtı bitdikdə bağlanır. Demo rejimi ilə oyun mexanikasını risksiz yoxlamaq rahatdır.
Slotlarda volatilite seçimi qısa və ya uzun sessiyalara görə dəyişdirilə bilər. Bildirişlərdə favorit komandalara xüsusi əmsal artımları gəlir.
Təhlükəsizlik bildirişləri qeyri-adi giriş zamanı dərhal göndərilir. Özünü istisna etdikdən sonra dəstək geri aktivləşdirməni minimum 24 saatdan əvvəl etmir. Favoritlər siyahısı ilə sevdiyiniz liqalar və slotlar bir yerdə saxlanır.
The Real Person!
The Real Person!
Je suis seduit par Impressario Casino, ca offre un plaisir sophistique. Le catalogue est riche en saveurs, comprenant des jeux compatibles avec les cryptos. Le bonus de bienvenue est delicieux. Le support client est impeccable, avec une aide precise. Les paiements sont securises et rapides, neanmoins plus de promos regulieres ajouteraient du piquant. En resume, Impressario Casino est un incontournable pour les amateurs pour ceux qui aiment parier en crypto ! En bonus le design est moderne et chic, amplifie le plaisir de jouer. Un autre atout les evenements communautaires engageants, assure des transactions fiables.
Commencer Г lire|
hardman4schools – Strong educational vision, visuals and tone reflect dedication beautifully.
The Real Person!
The Real Person!
Je suis seduit par Impressario Casino, il procure une experience exquise. La variete des titres est raffinee, avec des slots aux designs elegants. Le bonus de bienvenue est delicieux. Le support client est impeccable, toujours pret a servir. Les transactions sont fiables, neanmoins quelques tours gratuits supplementaires seraient bienvenus. En bref, Impressario Casino est un incontournable pour les amateurs pour les passionnes de jeux modernes ! A noter le design est moderne et chic, amplifie le plaisir de jouer. Un autre atout les tournois reguliers pour la competition, assure des transactions fiables.
Commencer Г lire|
The Real Person!
The Real Person!
Je suis totalement ensorcele par Monte Cryptos Casino, ca transporte dans un ocean virtuel. Il y a une profusion de jeux captivants, avec des slots aux themes modernes. 100% jusqu’a 300 € + tours gratuits. Disponible 24/7 via chat ou email, avec une aide precise et fiable. Le processus est lisse comme un wallet, mais quelques tours gratuits en plus seraient bienvenus. En resume, Monte Cryptos Casino garantit un plaisir constant pour les joueurs en quete d’innovation ! A noter l’interface est fluide comme un flux de donnees, donne envie de prolonger l’aventure. Un avantage notable les tournois reguliers pour la competition, qui booste l’engagement.
Commencer Г dГ©couvrir|
The Real Person!
The Real Person!
Je suis epoustoufle par Monte Cryptos Casino, il offre une odyssee chiffree. Les options sont vastes comme un reseau decentralise, avec des slots aux themes modernes. Avec des promotions crypto rapides. Le suivi est d’une efficacite redoutable, avec une aide precise et fiable. Les retraits sont fulgurants, parfois des bonus plus varies seraient un atout. Pour conclure, Monte Cryptos Casino offre une experience memorable pour les joueurs en quete d’innovation ! Par ailleurs le site est rapide et captivant, amplifie le plaisir de jouer. Un atout cle les paiements securises en crypto, garantit des transactions fiables.
Voir le site|
The Real Person!
The Real Person!
Je suis totalement envoute par BassBet Casino, ca plonge dans un monde de delta. La variete des titres est rapide, incluant des paris sportifs dynamiques. Amplifiant le plaisir de jeu. Le support client est dynamique, avec une aide precise. Les gains arrivent sans delai, bien que quelques tours gratuits supplementaires seraient bien venus. Au final, BassBet Casino vaut une peche excitante pour les passionnes de jeux modernes ! En bonus la plateforme est visuellement fluviale, ajoute une touche de vitesse. Particulierement interessant les options de paris sportifs variees, assure des transactions fiables.
bassbetcasinobonus777fr.com|
The Real Person!
The Real Person!
J’adore l’atmosphere mystique de Spinit Casino, ca offre un plaisir enchante. Le catalogue est riche et varie, offrant des sessions live immersives. 100% jusqu’a 500 € + tours gratuits. Le support client est enchante, offrant des reponses claires. Les paiements sont securises et rapides, parfois quelques tours gratuits supplementaires seraient bienvenus. Pour conclure, Spinit Casino est un incontournable pour les joueurs pour ceux qui aiment parier en crypto ! Par ailleurs l’interface est fluide comme un sort, ajoute une touche de mystere. A souligner les evenements communautaires engageants, offre des recompenses continues.
https://casinospinitfr.com/|
The Real Person!
The Real Person!
Je suis accro a Spinit Casino, ca offre un plaisir enchante. La variete des titres est magique, comprenant des jeux compatibles avec les cryptos. Avec des depots instantanes. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les paiements sont securises et rapides, parfois des bonus plus varies seraient un chapitre. Pour conclure, Spinit Casino offre une experience memorable pour les fans de casino en ligne ! Ajoutons que le site est rapide et captivant, ajoute une touche de mystere. Un plus les tournois reguliers pour la competition, offre des recompenses continues.
spinitcasinologinfr.com|
The Real Person!
The Real Person!
Je suis captive par Olympe Casino, ca offre un plaisir immortel. La variete des titres est divine, comprenant des jeux optimises pour les cryptos. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, offrant des reponses claires. Le processus est simple et glorieux, neanmoins des recompenses supplementaires seraient eternelles. Pour conclure, Olympe Casino merite une ascension celeste pour les joueurs en quete d’epopee ! De plus le design est moderne et divin, facilite une immersion totale. Un atout olympien les evenements communautaires engageants, assure des transactions fiables.
https://olympefr.com/|
findyourperfectdeal – The tone is friendly and approachable, doesn’t feel overly formal or salesy.
The Real Person!
The Real Person!
Bank kartı və e-cüzdanla depozitlər anında düşür, ödəniş limiti AZN üçün əlverişlidir.
Yeni istifadəçilər üçün pulsuz fırlatmalar və sığortalı kupon kampaniyaları tez-tez verilir. Ən təhlükəsiz mənbə yalnız rəsmi linkdir: http://www.pinco-casino-azerbaijan.biz.ua; pinco casino login buradan açılır. Depozit bonusunun qaydaları dildə qısa və misallarla izah edilib. Slot seçimi provayderə, volatiliteyə və RTP aralığına görə filtrlənir.
Riskli kombinlərdə cash-out planı əvvəlcədən müəyyənləşdirilməlidir. APK yenilənməsi tətbiqdaxili xəbərdarlıqla gəlir və bir dəqiqəyə tamamlanır.
FAQ bölməsi ödənişlər, bonuslar və verifikasiya üzrə genişdir. Müvəqqəti pauza aktiv edildikdə hesab hər yerdə kilidlənir və bildiriş gəlir. Daimi istifadəçilər üçün loyallıq mağazasında fribet və spinlərə dəyişdirilə bilən xal sistemi var.
The Real Person!
The Real Person!
экстренный вывод из запоя
vivod-iz-zapoya-smolensk024.ru
лечение запоя
The Real Person!
The Real Person!
J’ai une passion devorante pour Monte Cryptos Casino, c’est une plateforme qui pulse comme un reseau blockchain. La variete des titres est eclatante, incluant des paris live dynamiques. Elevant l’experience de jeu. Le support client est irreprochable, offrant des solutions claires. Les transferts sont fiables, parfois des recompenses supplementaires seraient ideales. Au final, Monte Cryptos Casino est un must pour les fans de blockchain pour les passionnes de sensations numeriques ! De plus la plateforme est visuellement eblouissante, facilite une immersion totale. Un atout cle les paiements securises en BTC/ETH, offre des recompenses continues.
http://www.montecryptoscasino777fr.com|
The Real Person!
The Real Person!
J’ai une affection pour Impressario Casino, c’est une plateforme qui evoque le raffinement. La selection de jeux est somptueuse, proposant des jeux de table raffines. Amplifiant le plaisir de jeu. Le support client est impeccable, offrant des reponses claires. Le processus est simple et elegant, parfois plus de promos regulieres ajouteraient du piquant. Pour conclure, Impressario Casino est un incontournable pour les amateurs pour les fans de casino en ligne ! Par ailleurs l’interface est fluide comme un banquet, amplifie le plaisir de jouer. Un plus les paiements securises en crypto, offre des recompenses continues.
Cliquer maintenant|
The Real Person!
The Real Person!
Je suis emerveille par Monte Cryptos Casino, c’est une plateforme qui scintille comme une etoile blockchain. Il y a une profusion de jeux captivants, comprenant des jeux optimises pour BTC et ETH. Elevant l’experience de jeu. Disponible 24/7 via chat ou email, offrant des solutions limpides. Les transferts sont fiables, cependant plus de promos regulieres dynamiseraient l’experience. En bref, Monte Cryptos Casino vaut une plongee numerique pour les amateurs de casino en ligne ! De plus la plateforme est visuellement eblouissante, amplifie le plaisir de jouer. Un atout cle les options de paris variees, qui booste l’engagement.
Visiter l’avis|
The Real Person!
The Real Person!
J’adore l’ambiance numerique de Monte Cryptos Casino, c’est une plateforme qui vibre d’innovation. Il y a une plethore de jeux captivants, comprenant des jeux optimises pour Bitcoin et Ethereum. Amplifiant l’experience de jeu. L’assistance est rapide et professionnelle, toujours pret a resoudre. Le processus est simple comme un hash, parfois plus de promos regulieres dynamiseraient l’experience. Pour conclure, Monte Cryptos Casino est incontournable pour les fans de crypto pour les adeptes de sensations futuristes ! Par ailleurs la navigation est simple comme un wallet, facilite une immersion totale. Un avantage notable les tournois reguliers pour la competition, garantit des transactions fiables.
DГ©bloquer toutes les infos|
The Real Person!
The Real Person!
Je suis totalement envoute par BassBet Casino, c’est une plateforme qui vibre comme un saxophone. Il y a une profusion de jeux excitants, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les transactions sont fiables, parfois plus de promos regulieres ajouteraient du groove. Au final, BassBet Casino offre une experience memorable pour les passionnes de jeux modernes ! Par ailleurs le design est moderne et jazzy, ajoute une touche de rythme. Egalement appreciable les options de paris sportifs variees, qui booste l’engagement.
bassbetcasinologinfr.com|
The Real Person!
The Real Person!
J’ai une passion narrative pour Spinit Casino, il procure une experience magique. Le catalogue est riche et varie, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + tours gratuits. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les paiements sont securises et rapides, cependant plus de promos regulieres ajouteraient de la magie. Pour conclure, Spinit Casino garantit un plaisir constant pour les passionnes de jeux modernes ! Ajoutons que la plateforme est visuellement envoutante, ajoute une touche de mystere. A souligner les options de paris sportifs variees, assure des transactions fiables.
spinitcasinologinfr.com|
The Real Person!
The Real Person!
J’ai une passion magique pour Spinit Casino, c’est une plateforme qui tourbillonne d’elegance. Il y a une profusion de jeux fascinants, avec des slots aux designs enchanteurs. Avec des depots instantanes. Le service est disponible 24/7, offrant des reponses claires. Le processus est simple et elegant, cependant des offres plus genereuses seraient enchanteresses. En resume, Spinit Casino garantit un plaisir constant pour les fans de casino en ligne ! Ajoutons que l’interface est fluide comme un sort, ajoute une touche de mystere. Un autre atout les options de paris sportifs variees, propose des avantages personnalises.
https://casinospinitfr.com/|
The Real Person!
The Real Person!
Je suis captive par Olympe Casino, il procure une experience legendaire. La selection de jeux est olympienne, avec des slots thematiques antiques. Avec des depots rapides. Le support client est olympien, garantissant un support celeste. Les paiements sont securises et fluides, bien que des recompenses supplementaires seraient eternelles. Dans l’ensemble, Olympe Casino merite une ascension celeste pour les amateurs de sensations mythiques ! A noter le site est rapide et glorieux, amplifie le plaisir de jouer. Un plus divin le programme VIP avec des niveaux exclusifs, renforce le sentiment de communaute.
https://olympefr.com/|
How to win in Calgary Lottery: Boost your chances by playing consistently, joining lottery pools, and choosing less popular combinations. Remember, winning requires luck and responsible play: Calgary contests and giveaways
createimpactonline – Their support team is responsive and always ready to assist.
partnershippowerhouse – Highly recommend their resources for anyone serious about strategic partnerships.
Cactus Casino — современная площадка для азартных игр
Любители азартных развлечений могут играть в казино Кактус на удобной и надежной платформе, которая сочетает стильный интерфейс, широкий ассортимент игр и быстрые выплаты. Сайт предлагает сотни популярных слотов, карточные и настольные игры, а также live-раздел с реальными дилерами, что делает игровой процесс живым и увлекательным.
Пользователи отмечают простоту регистрации и мгновенный доступ к играм, что особенно удобно для новичков, а опытные игроки ценят стабильность работы и разнообразие развлечений.
Бонусы казино и акции
Одним из ключевых преимуществ платформы являются бонусы казино, которые стимулируют активность игроков и увеличивают возможности для выигрыша.
• Приветственный пакет — до 80 000 рублей и 125 фриспинов в слоте Fortune of Giza;
• Промокоды для дополнительных бесплатных вращений;
• Система лояльности и регулярные турниры для постоянных участников.
Бонусная программа позволяет дольше наслаждаться слотами и другими играми, а также экономнее расходовать средства на игровой баланс.
Разнообразие игр и провайдеры
Cactus Casino https://kaktus-kazino.com сотрудничает с ведущими разработчиками: Pragmatic Play, BGaming, Wazdan, Spinomenal и Evoplay. Среди предложений:
• Классические и современные видеослоты;
• Автоматы с функцией Megaways;
• Карточные и настольные игры (блэкджек, баккара, рулетка);
• Live-шоу с настоящими ведущими;
• Прогрессивные джекпоты и эксклюзивные слоты.
Такой выбор позволяет каждому найти развлечение по вкусу и играть в слоты онлайн в безопасной и комфортной среде.
Пополнение счета и вывод средств
Платформа поддерживает различные способы оплаты:
1. СБП и Piastrix;
2. Банковские карты и Apple Pay/Google Pay;
3. Криптовалюты и FK Wallet;
4. Binance и другие электронные кошельки.
Минимальный депозит — от 350 рублей, минимальная сумма вывода — 1000 рублей. Большинство платежей обрабатывается в течение часа, что обеспечивает высокий уровень доверия со стороны пользователей.
Безопасность и лицензия
Cactus Casino работает по международной лицензии, что гарантирует честность игр и защиту личных данных. Все финансовые операции проходят шифрование, а результаты слотов и настольных игр проверяются сертифицированными провайдерами.
Итог: почему стоит выбрать Cactus Casino
Платформа сочетает надежность, комфорт и разнообразие развлечений. Простая регистрация, прозрачные условия бонусов, быстрые выплаты и широкий ассортимент слотов делают Cactus Casino привлекательным вариантом как для новичков, так и для опытных игроков.
Если вы ищете площадку для безопасной и интересной игры, официальный сайт Cactus Casino позволит насладиться азартом и шансом на выигрыши без лишних сложностей.
mystylecorner – Great experience browsing through latest trends, very satisfied overall.
partnershippowerhouse – Highly recommend their resources for anyone serious about strategic partnerships.
The Real Person!
The Real Person!
Вызов анонимного нарколога — это значительным шагом на пути к избавлению от зависимости. Когда вы или кто-то из ваших близких сталкиваетесь с проблемами с наркотиками или алкоголем‚ не откладывайте обращение за помощью. На сайте site;com вы можете получить консультацию нарколога и вызвать специалиста на дом. Анонимная помощь обеспечивает вашу конфиденциальность‚ что имеет большое значение для людей с зависимостями. Профессиональный нарколог осуществит детоксикацию организма и предложит индивидуальные программы реабилитации. Психотерапия зависимости также является важным элементом в лечении зависимости. Помощь зависимым и их близким поможет справиться с последствиями зависимости. Не забывайте‚ что борьба с алкогольной зависимостью так же значимо‚ как и работа с наркотиками. Не стесняйтесь обратиться за помощью‚ это первый шаг к новой жизни.
mystylecorner – Highly recommend for anyone who loves trendy urban clothing online.
monumentremoval – The purpose is bold and the site feels deeply meaningful and timely.
monumentremoval – The purpose is bold and the site feels deeply meaningful and timely.
Currently it appears like Movable Type is the top blogging platform out there right now. (from what I’ve read) Is that what you are using on your blog?
ant-group.ru
forexlearninghub – Will revisit with fresh eyes after diving deeper into the advanced modules next week.
The Real Person!
The Real Person!
Капельница от похмелья на дому: действующее лечение и восстановление организма
mystylecorner – Ordered last week, arrived fast and quality is amazing.
advancedtradingtools – Website looks clean and professional, good first impression on their trading utilities.
purebeautytrend – Found some gorgeous beauty pieces here, just what I needed today.
kionawest – The contractor’s site looks professional, clean visuals and strong commitment to quality.
dailyessentialfinds – Great selection of essentials, made my shopping experience smooth and enjoyable.
everydayvaluehub – Found the site today; the selection of daily essentials looks solid and well-curated.
visionaryfutureteam – Would like to see more case studies or testimonials to back up the strategies.
winwithus – The resources provided here have made a real difference in my work.
buildsuccessnetwork – Would like to see more user testimonials or case studies for added credibility.
partnershipgrowthhub – The community aspect seems solid and might be a great way to connect with others.
Just wish to say your article is as astounding. The clarity in your post is just nice and i can assume you’re an expert on this subject. Well with your permission let me to grab your feed to keep up to date with forthcoming post. Thanks a million and please continue the enjoyable work.
zooma casino
innovationdriventeam – The community or support section looked promising—keen to engage with other learners.
powerofcollaboration – Good value in terms of information; I’ll revisit for further insights.
connectforprogress – Friendly tone, easy navigation, and the insights are genuinely useful.
unitedvisionnetwork – Found actionable ideas today that I’m excited to apply in my networking strategy.
businesssuccesshub – Bookmarking this as a resource; many useful templates and checklists.
modernlifestylezone – Great site for discovering trending home gadgets and lifestyle upgrades.
uniquedecorstore – Already planning my next order as they have new items weekly.
investprofitgrow – Website is mobile-friendly, which makes grabbing quick insights convenient.
financialgrowthplan – Would like to see more advanced modules, but the foundation here is solid.
classyhomegoods – Great quality for the price, happy with how my order turned out.
advancedtradingtools – Purchased an indicator yesterday and the support team was responsive and helpful.
successmindsetnetwork – The tone is encouraging without being pushy—just the right kind of support.
kionawest – The contractor’s site looks professional, clean visuals and strong commitment to quality.
pressbros – Cozy café vibe with creative flair; coffee and brunch look seriously inviting.
updatingparents – The site feels thoughtful, user-friendly and clearly grounded in practical support.
ultimateprofitplan – Appreciate the realistic tone in their advice rather than exaggerated claims.
visionaryfutureteam – Found a useful section on collaboration that I’ll try to apply with my team.
dailyessentialstore – I’ll likely revisit this store when I need more everyday essentials.
trustedleaderscircle – Appreciate how the advice focuses on trust and effective leadership, which feels relevant.
trustbridgealliance – Looks like a strong resource for building trusted business relationships and networks.
professionalgrowthhub – Will revisit when they publish new updates; seems like a solid long-term resource.
connectforgrowth – The mobile version works smoothly, good for learning on the go.
creativefashionworld – Quality looks solid in photos, looking forward to seeing it in person.
shopandshine – Checkout process was straightforward and didn’t require too many steps.
nycbhm – Community-focused organization with meaningful outreach and engagement.
mcalpineinfo – The domain might hold potential for incident reporting, but needs fuller coverage.
saveshelterpets – Would be helpful if they listed an EIN/charity registration, governance board, and past impact metrics.
1091m2love – Wow, didn’t expect such clarity and comfort browsing through each page.
The Real Person!
The Real Person!
Je suis charme par Impressario Casino, on ressent une ambiance delicate. Le catalogue est riche en saveurs, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. L’assistance est efficace et professionnelle, joignable a toute heure. Le processus est simple et elegant, neanmoins des bonus plus varies seraient un delice. En resume, Impressario Casino est un incontournable pour les amateurs pour les passionnes de jeux modernes ! Par ailleurs la plateforme est visuellement somptueuse, donne envie de prolonger l’experience. A souligner les tournois reguliers pour la competition, qui booste l’engagement.
Voir les meilleurs choix|
globalnetworkvision – Overall a good find and I’ll be checking back for fresh updates.
The Real Person!
The Real Person!
Je suis absolument captive par Monte Cryptos Casino, ca offre une sensation futuriste unique. Le catalogue est opulent et divers, proposant des tables sophistiquees. Le bonus d’entree est scintillant. L’assistance est precise et professionnelle, joignable a tout moment. Les transferts sont fiables, neanmoins des bonus plus varies seraient un atout. Pour conclure, Monte Cryptos Casino offre une experience inoubliable pour les joueurs en quete d’innovation ! De plus le site est rapide et futuriste, facilite une immersion totale. Un avantage notable les tournois reguliers pour la competition, renforce la communaute.
DГ©couvrir dГЁs maintenant|
The Real Person!
The Real Person!
Je suis ensorcele par Monte Cryptos Casino, il propose une odyssee chiffree. Le catalogue est opulent et divers, avec des slots aux designs futuristes. 100% jusqu’a 300 € + tours gratuits. Les agents repondent avec une vitesse fulgurante, avec une aide rapide et fiable. Les retraits sont rapides comme une transaction, mais des recompenses supplementaires seraient ideales. En bref, Monte Cryptos Casino offre une experience inoubliable pour les passionnes de sensations numeriques ! Par ailleurs l’interface est fluide comme un flux de donnees, ajoute une touche de sophistication. A souligner les options de paris variees, offre des recompenses continues.
Lire la suite|
The Real Person!
The Real Person!
Je suis charme par Impressario Casino, il procure une experience exquise. Le catalogue est riche en saveurs, proposant des jeux de table raffines. Amplifiant le plaisir de jeu. Le service est disponible 24/7, avec une aide precise. Les transactions sont fiables, neanmoins des bonus plus varies seraient un delice. Pour conclure, Impressario Casino est une plateforme qui enchante pour ceux qui aiment parier en crypto ! En bonus l’interface est fluide comme un banquet, facilite une immersion totale. Un autre atout le programme VIP avec niveaux exclusifs, qui booste l’engagement.
Essayer|
The Real Person!
The Real Person!
Je suis ensorcele par Monte Cryptos Casino, il offre une epopee chiffree. Il y a une profusion de jeux envoutants, avec des slots aux designs innovants. Elevant l’experience de jeu. Les agents repondent comme un algorithme, offrant des solutions claires. Les gains arrivent en un instant, mais quelques tours gratuits supplementaires seraient cool. Au final, Monte Cryptos Casino est une plateforme qui brille dans l’univers crypto pour les amateurs de casino en ligne ! De plus le site est rapide et futuriste, donne envie de prolonger l’experience. Egalement cool les paiements securises en BTC/ETH, offre des recompenses continues.
Ouvrir le site maintenant|
pressbros – Cozy café vibe with creative flair; coffee and brunch look seriously inviting.
The Real Person!
The Real Person!
Je suis bluffe par BassBet Casino, c’est une plateforme qui pulse comme un mix de DJ. Les options sont vastes comme une piste de danse, avec des slots aux designs lumineux. Amplifiant l’excitation du jeu. Le support client est au top, toujours pret a mixer. Les gains arrivent sans attendre, de temps en temps des recompenses supplementaires seraient vibrantes. Au final, BassBet Casino est un must pour les joueurs pour les fans de casino en ligne ! En plus la plateforme est visuellement electrisante, facilite une immersion totale. Particulierement cool le programme VIP avec des niveaux exclusifs, propose des avantages sur mesure.
bassbetcasinobonus777fr.com|
The Real Person!
The Real Person!
Je suis emerveille par Spinit Casino, ca offre un plaisir dynamique. Il y a une profusion de jeux excitants, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. Le service est disponible 24/7, garantissant un support de qualite. Les transactions sont fiables, cependant des bonus plus varies seraient un sprint. Au final, Spinit Casino offre une experience memorable pour les amateurs de sensations rapides ! En bonus l’interface est fluide comme une piste, facilite une immersion totale. Un autre atout les tournois reguliers pour la competition, offre des recompenses continues.
spinitcasinologinfr.com|
forexlearninghub – Appreciate how they simplify technical analysis and risk management for novices.
The Real Person!
The Real Person!
Je suis emerveille par Spinit Casino, ca offre un plaisir veloce. Il y a une profusion de jeux excitants, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + tours gratuits. L’assistance est efficace et professionnelle, toujours pret a accelerer. Les retraits sont fluides comme un peloton, bien que des bonus plus varies seraient un sprint. Dans l’ensemble, Spinit Casino offre une experience memorable pour les passionnes de jeux modernes ! En bonus le site est rapide et attractif, facilitate une immersion totale. Particulierement interessant les evenements communautaires engageants, offre des recompenses continues.
casinospinitfr.com|
Для юрлиц удобно — всё ведётся под полным контролем специалистов https://vkobroker.ru/
urbanstylehub – Will definitely recommend this site to friends who love good fashion.
The Real Person!
The Real Person!
J’adore l’aura divine d’ Olympe Casino, c’est une plateforme qui evoque l’Olympe. Les options sont vastes comme un pantheon, incluant des paris sportifs epiques. Renforcant votre tresor initial. Les agents repondent comme des dieux, avec une aide precise. Le processus est simple et glorieux, parfois plus de promos regulieres ajouteraient de la gloire. Pour conclure, Olympe Casino offre une experience legendaire pour ceux qui aiment parier en crypto ! En bonus le design est moderne et divin, amplifie le plaisir de jouer. Egalement remarquable les options de paris sportifs variees, renforce le sentiment de communaute.
olympefr.com|
The Real Person!
The Real Person!
Экстренная помощь при запое – существенный момент в процессе лечения алкогольной зависимости. Признаки запоя включают непреодолимого влечения к алкоголю, физической зависимости и психологических нарушений. Лечение запоя начинается с детоксикации, что способствует выведению токсических веществ из организма. При оказании медпомощи при запое применяются лекарственные средства для облегчения абстинентного синдрома. Психотерапевтическая помощь и поддержка родственников являются важными факторами в мотивации пациента. Важно также рассмотреть программы реабилитации и восстановление после запоя. Центр лечения алкоголизма предлагает широкий спектр методов лечения, включая народные средства для снятия запоя. Недопущение рецидивов крайне важно для успешного выздоровления; Эффективная реабилитационная программа способствует возвращению пациента к обычной жизни. Дополнительные материалы по этой теме доступны на сайте vivod-iz-zapoya-vladimir027.ru.
brightmarketplace – Recommend checking this marketplace out if you’re looking for variety and value.
The Real Person!
The Real Person!
J’adore l’atmosphere blues de BassBet Casino, on ressent une ambiance de bar. La variete des titres est melodieuse, avec des slots aux designs blues. 100% jusqu’a 500 € + tours gratuits. Le support est irreprochable, avec une aide precise. Les gains arrivent sans delai, de temps a autre des offres plus genereuses seraient blues. Au final, BassBet Casino garantit un plaisir constant pour ceux qui aiment parier en crypto ! En bonus la navigation est simple et rythmee, ce qui rend chaque session plus groovy. Egalement appreciable les tournois reguliers pour la competition, assure des transactions fiables.
bassbetcasinologinfr.com|
trustedpartnergroup – The mobile version of the site is responsive, which is great for on-the-go reference.
ultimateprofitplan – Would like to see more evidence of results or user testimonials to increase trust.
visionaryfutureteam – Found a useful section on collaboration that I’ll try to apply with my team.
dailyessentialstore – The item I ordered arrived as described and in good condition.
knightstablefoodpantry – Compassionate food pantry serving the community with dedication.
learntradingtoday – Plan to use some of their worksheets tomorrow for practice, looks handy.
professionalgrowthhub – Will revisit when they publish new updates; seems like a solid long-term resource.
trustbridgealliance – Appreciate how they emphasise trust and collaboration—key elements for meaningful partnerships.
forexstrategyguide – Found a couple of useful strategy ideas that I’ll test on demo first.
pmajoe4council – Clear campaign focus, design feels trustworthy and community-oriented.
shopandshine – Customer support responded quickly when I asked about shipping options—appreciated it.
1091m2love – Wow, didn’t expect such clarity and comfort browsing through each page.
updatingparents – The site feels thoughtful, user-friendly and clearly grounded in practical support.
creativefashionworld – Customer chat was helpful when I asked about sizing, thumbs up.
mcalpineinfo – Overall a basic web presence so far; good first step, but needs expansion and verification.
saveshelterpets – I noted some vague statement about “saving shelter pets”, but needed more specifics about regions, numbers or partners.
The Real Person!
The Real Person!
Je suis enchante par Impressario Casino, ca transporte dans un monde gourmand. La selection de jeux est somptueuse, avec des slots aux designs elegants. Renforcant votre capital initial. Le service est disponible 24/7, garantissant un support de qualite. Les paiements sont securises et rapides, de temps a autre quelques tours gratuits supplementaires seraient bien venus. En resume, Impressario Casino est une plateforme qui enchante pour ceux qui aiment parier en crypto ! A noter le design est moderne et chic, ajoute une touche d’elegance. A souligner les options de paris sportifs variees, assure des transactions fiables.
Explorer le site web|
The Real Person!
The Real Person!
J’aime l’ambiance numerique de Monte Cryptos Casino, ca offre une sensation futuriste unique. Les options sont vastes comme un ledger, proposant des tables sophistiquees. Le bonus d’entree est scintillant. Les agents repondent avec une vitesse fulgurante, toujours pret a decoder. Les gains arrivent sans delai, de temps a autre quelques tours gratuits en plus seraient bienvenus. Pour conclure, Monte Cryptos Casino est un must pour les fans de blockchain pour ceux qui parient avec des cryptos ! A noter la navigation est simple comme un wallet, facilite une immersion totale. A souligner les evenements communautaires decentralises, garantit des transactions fiables.
Lire la suite|
The Real Person!
The Real Person!
Je suis ebloui par Monte Cryptos Casino, on ressent une energie decentralisee. Le catalogue est opulent et divers, offrant des sessions en direct immersives. Avec des depots crypto rapides. Disponible 24/7 via chat ou email, avec une aide rapide et fiable. Les gains arrivent sans delai, mais des bonus plus varies seraient un atout. Pour conclure, Monte Cryptos Casino est un must pour les fans de blockchain pour les amateurs de casino en ligne ! Ajoutons que le design est moderne et captivant, donne envie de prolonger l’aventure. A souligner les evenements communautaires decentralises, qui booste l’engagement.
Ouvrir les dГ©tails|
The Real Person!
The Real Person!
Je suis charme par Impressario Casino, ca transporte dans un monde gourmand. Il y a une abondance de jeux captivants, offrant des sessions live sophistiquees. Avec des depots instantanes. Le support client est impeccable, garantissant un support de qualite. Le processus est simple et elegant, parfois quelques tours gratuits supplementaires seraient bienvenus. Pour conclure, Impressario Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! Ajoutons que la plateforme est visuellement somptueuse, amplifie le plaisir de jouer. A souligner les evenements communautaires engageants, assure des transactions fiables.
Aller plus loin|
mcctheatercampaign – Supporting arts and theater with passion and community spirit.
The Real Person!
The Real Person!
Je suis ebloui par Monte Cryptos Casino, ca transporte dans un desert virtuel. Il y a une profusion de jeux envoutants, proposant des tables numeriques elegantes. Le bonus d’entree est scintillant. L’assistance est rapide et experte, toujours pret a decoder. Le processus est lisse comme un smart contract, de temps a autre plus de promos dynamiseraient l’aventure. Pour conclure, Monte Cryptos Casino vaut une plongee numerique pour les passionnes de sensations virtuelles ! A noter la plateforme est visuellement eblouissante, ce qui rend chaque session plus immersive. Particulierement captivant les evenements communautaires decentralises, offre des recompenses continues.
Explorer aujourd’hui|
The Real Person!
The Real Person!
Je suis bluffe par BassBet Casino, ca transporte dans un univers de beats. Les options sont vastes comme une piste de danse, comprenant des jeux adaptes aux cryptos. Avec des depots ultra-rapides. Le suivi est impeccable, avec une aide precise. Les gains arrivent sans attendre, cependant des recompenses supplementaires seraient vibrantes. Au final, BassBet Casino offre une experience inoubliable pour les passionnes de jeux modernes ! En plus la navigation est simple et rythmee, donne envie de prolonger la fete. Un atout les evenements communautaires dynamiques, qui dynamise l’engagement.
https://bassbetcasinobonus777fr.com/|
The Real Person!
The Real Person!
Je suis totalement envoute par Spinit Casino, ca offre un plaisir dynamique. La variete des titres est rapide, proposant des jeux de table rapides. Amplifiant le plaisir de jeu. Le suivi est irreprochable, garantissant un support de qualite. Les paiements sont securises et rapides, bien que quelques tours gratuits supplementaires seraient bien venus. En resume, Spinit Casino offre une experience memorable pour les passionnes de jeux modernes ! Par ailleurs le site est rapide et attractif, ce qui rend chaque session plus excitante. Un autre atout les options de paris sportifs variees, qui booste l’engagement.
spinitcasinologinfr.com|
The Real Person!
The Real Person!
Je suis emerveille par Spinit Casino, ca plonge dans un monde de vitesse. La variete des titres est rapide, proposant des jeux de table rapides. Renforcant votre capital initial. Le support client est veloce, toujours pret a accelerer. Les paiements sont securises et rapides, neanmoins des bonus plus varies seraient un sprint. Au final, Spinit Casino est une plateforme qui accelere pour ceux qui aiment parier en crypto ! Ajoutons que le design est moderne et dynamique, amplifie le plaisir de jouer. A souligner les paiements securises en crypto, assure des transactions fiables.
casinospinitfr.com|
pmajoe4council – Clear campaign focus, design feels trustworthy and community-oriented.
astoriatogether – Community unity and collaborative neighborhood initiatives.
The Real Person!
The Real Person!
Je suis captive par Olympe Casino, il procure une experience legendaire. Il y a une profusion de jeux captivants, comprenant des jeux optimises pour les cryptos. Le bonus de bienvenue est divin. Le support client est olympien, avec une aide precise. Les transactions sont fiables, cependant des recompenses supplementaires seraient eternelles. Pour conclure, Olympe Casino est une plateforme qui regne sur l’Olympe pour les fans de casino en ligne ! En bonus le design est moderne et divin, donne envie de prolonger l’aventure. Particulierement captivant les paiements securises en crypto, propose des avantages sur mesure.
https://olympefr.com/|
updatingparents – The site feels thoughtful, user-friendly and clearly grounded in practical support.
The Real Person!
The Real Person!
J’adore l’atmosphere blues de BassBet Casino, ca offre un plaisir melancolique. Les options sont vastes comme un album, proposant des jeux de table elegants. 100% jusqu’a 500 € + tours gratuits. Le support client est melodieux, offrant des reponses claires. Les paiements sont securises et rapides, bien que plus de promos regulieres ajouteraient du groove. Au final, BassBet Casino est un incontournable pour les joueurs pour les passionnes de jeux modernes ! Par ailleurs le site est rapide et attractif, ajoute une touche de rythme. Un plus les tournois reguliers pour la competition, offre des recompenses continues.
https://bassbetcasinologinfr.com/|
winwithus – Found the community support section very helpful, gives a boost when needed.
Hi to every one, it’s really a good for me to visit this web site, it consists of priceless Information.
teka-studio.ru
Hey very cool web site!! Guy .. Excellent .. Amazing .. I’ll bookmark your site and take the feeds additionally? I am happy to search out so many helpful info here within the publish, we need work out more strategies on this regard, thanks for sharing. . . . . .
сайт Banda Casino
Документы подготовлены грамотно, таможня приняла без замечаний: https://tamozhenniiy-broker-moskva11.ru/
The Real Person!
The Real Person!
Капельница от запоя на дому – это популярный метод лечения алкогольной зависимости, предлагающий множество преимуществ и некоторые недостатки. В владимире наркологические услуги включают выезд на дом, что позволяет пациентам получать медицинскую помощь при алкогольном запое в комфортной для них обстановке. Основные преимущества капельницы заключаются в скорейшем восстановлении организма благодаря детоксикационным процедурам. Капельницы помогают вывести токсины, улучшить общее состояние, что, в свою очередь, помогает быстрее восстановиться после запоя. Кроме того, наличие поддержки близких в период запоя играет важную роль, так как лечение алкоголизма на дому обеспечивает уютную атмосферу. лечение запоя владимир Однако, существуют и минусы. Например, не всегда возможно обеспечить должный уровень контроля за состоянием пациента. Консультация врача на дому может быть недостаточной для сложных случаев. Также стоит учитывать, что инъекции при алкоголизме могут не подойти всем.
Thanks for some other magnificent post. The place else could anyone get that kind of info in such a perfect approach of writing? I’ve a presentation subsequent week, and I’m at the search for such info.
линк,
The Real Person!
The Real Person!
Левандовски футболдо чыдамкайлык менен мыкты натыйжа көрсөттү.
Роберт Левандовскинин үй-бүлөлүк жашоосу кызыктуу. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Роберт Левандовски жаш футболчулар үчүн үлгү. Кызыктуу фактылар менен таанышуу үчүн robert-lewandowski-kg.com сайтына кириңиз. Бул жерде анын карьерасы тууралуу кеңири маалымат бар. Роберт Левандовски Барселона менен титулдар үчүн күрөшүүдө.
Роберт Левандовскинин жашоосунда үй-бүлөнүн ролу чоң. Роберт Левандовски фитнеске чоң маани берет.
Роберт Левандовскинин чоң атасы тууралуу уламыштар айтылат. Роберт Левандовскинин профессионалдуулугу суктанарлык.
https://radhaniwellness.com/product/7-herb-support60-capsules-2/
learnsharegrow.shop – A refreshing site with a positive vibe; highly recommend checking it out.
uniquefashionstyle.shop – Browsed through several items and found some stylish pieces I’d consider buying.
discovergreatdeal.shop – Great variety of products at unbeatable prices; highly recommend.
futuregoalsnetwork.shop – Wide range of categories; something for everyone here.
The Real Person!
The Real Person!
Левандовски футболдо чыдамкайлык менен мыкты натыйжа көрсөттү.
Инстаграмда Роберт Левандовскинин посттору абдан популярдуу. Левандовски канча гол салганы тууралуу маалымат ар дайым жаңыланып турат. Левандовски өз өлкөсүнүн сыймыгы. Левандовскинин Барселонадагы ийгиликтерин robert-lewandowski-kg.com сайтынан окуңуз. Роберт Левандовски Барселонада мыкты старт жасаган.
Левандовски спорттук тарбиянын маанисин ар дайым белгилейт. Роберт Левандовски фитнеске чоң маани берет.
Роберт Левандовски жөнүндө ар кандай кызыктуу фактылар бар. Роберт Левандовски өзүнүн максатына ишеним менен жеткен.
growthpoint.bond – Easy to find relevant information; well-organized structure.
leadersunitedgroup.bond – The visuals support the writing nicely, feels cohesive.
globalpartnershipteam.shop – Customer service was helpful and responsive; will shop again.
nextvision.bond – Impressive layout and user-friendly interface; easy to explore.
buildbrand.bond – The typography is clear, enhancing readability across devices.
discovervalue.bond – Appreciate the practical advice shared; feels grounded and real.
discovernewpath.shop – Great mix of topics; something for everyone interested in personal growth.
teamfuture.bond – Content is clear and concise, very informative and helpful.
futureopportunitynetwork.shop – Impressed with the product quality and packaging; exceeded expectations.
createimpacttoday.shop – A must-visit site for those who want to shop with purpose.
successpathway.bond – Fast shipping and quality items; very satisfied with my purchase.
The Real Person!
The Real Person!
Роберт Левандовскинин статистикасы укмуштай — ал ар бир оюнда гол салат.
Роберт Левандовски фото жана видеолору дайыма талкууда. Роберт Левандовски карьерасында жүздөгөн гол салган. Левандовски футбол тарыхында өз изин калтырган. Эгер сизге Левандовски тууралуу ишенимдүү маалымат керек болсо, анда Роберт Левандовски сайтына баш багыңыз. Барселонадагы Левандовскинин оюнун күйөрмандар макташат.
Роберт Левандовскинин жашоосунда үй-бүлөнүн ролу чоң. Роберт Левандовскинин жаракаты көпчүлүктү тынчсыздандырган.
Роберт Левандовски үй-бүлөсүнө чоң маани берет. Роберт Левандовскинин профессионалдуулугу суктанарлык.
winmore.bond – Branding feels consistent and trustworthy throughout the website.
connectinnovationhub.bond – Navigation is intuitive, finding information is quick and effortless.
trustandunity.bond – Overall a polished site with professional and engaging content.
businessgrowthhub.shop – Fast loading pages make the experience seamless and smooth.
successfultradersclub.shop – Content is clear, informative, and engaging; great first impression.
smartfashionboutique.bond – Impressive visuals and smooth interface; browsing feels seamless.
globalalliancenetwork.bond – Impressive visuals and a smooth interface; browsing feels seamless.
uniquedecorstore.shop – The design is clean and professional, making navigation a breeze.
The Real Person!
The Real Person!
Профилактика алкоголизма — существенная проблема, которая требует значительных усилий. Врач нарколог на дом способен оказать необходимую помощь, предоставляя консультации и медицинское вмешательство. Симптомы алкоголизма состоят в регулярном употреблении спиртного и изменениях в психоэмоциональном состоянии. Психотерапевтическая поддержка и коллективные занятия также играют ключевую роль в борьбе с зависимостями. Чтобы предотвратить запойные состояния, важно следовать нескольким советам по отказу от алкоголя. Поддержка семьи и близких может значительно повысить шансы на успех в реабилитации от алкоголизма. Профилактические меры, такие как самопомощь при алкоголизме и участие в социальных программах, способствуют социальной адаптации и укрепляют мотивацию к трезвому образу жизни.
futurevisiongroup.bond – Easy to find relevant information; well-organized structure.
togetherwerise.bond – Advocating for equal opportunities for women in construction.
futuregoalsnetwork.shop – Great variety of products at unbeatable prices; highly recommend.
newhorizonsnetwork.shop – Great variety of products at unbeatable prices; highly recommend.
learnsharegrow.shop – Looking forward to seeing more updates and articles from this site.
The Real Person!
The Real Person!
Левандовскинин формасы дагы деле жогорку деңгээлде.
Роберт Левандовски денесин мыкты формада кармайт. Левандовски Бавариядан кеткенден кийин дагы мыкты оюн көрсөтүүдө. Роберт Левандовски жаш футболчулар үчүн үлгү. Футбол күйөрмандары үчүн мыкты булак — robert-lewandowski-kg.com</url]. Бул сайтта акыркы жаңылыктар топтолгон. Роберт Левандовски Барселона менен титулдар үчүн күрөшүүдө.
Левандовски спорттук тарбиянын маанисин ар дайым белгилейт. Левандовски жаракаттан кийин дагы күчтүү кайтып келген.
Левандовскинин балдары да спортко жакын. Роберт Левандовски карьерасын толук берилип өткөрөт.
The Real Person!
The Real Person!
Роберт Левандовскинин статистикасы укмуштай — ал ар бир оюнда гол салат.
Инстаграмда Роберт Левандовскинин посттору абдан популярдуу. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Левандовски өз өлкөсүнүн сыймыгы. Эгер сиз Левандовски күйөрманы болсоңуз, анда robert-lewandowski-kg.com сиз үчүн мыкты булак. Барселонадагы Левандовскинин оюнун күйөрмандар макташат.
Роберт Левандовски жашоосунда кыйынчылыктарды жеңип чыккан. Роберт Левандовски реабилитацияны тез бүтүргөн.
Левандовскинин туугандары тууралуу тарых да кызыктуу. Роберт Левандовскинин профессионалдуулугу суктанарлык.
nicholashirshon – Professional portfolio showcasing expertise and accomplishments.
leadersunitedgroup.bond – I’m learning new things here, good for growth and mindset.
findyourfuture.shop – Found some insightful articles here; definitely bookmarking this one.
globalpartnershipteam.shop – Great variety of products at unbeatable prices; highly recommend.
unitedbygoal.bond – A valuable platform for businesses looking to grow and innovate.
discovervalue.bond – The visuals are appealing and complement the content well.
teamfuture.bond – Clean layout, easy to navigate, very user-friendly experience.
growstrongteam.bond – Always excited to check updates — this site has potential.
choice-eats – Culinary excellence with diverse and delicious dining options.
winmore.bond – Navigation is intuitive; I can find everything quickly and easily.
connectinnovationhub.bond – Navigation is intuitive, finding information is quick and effortless.
trustandunity.bond – Typography and design are consistent and visually appealing.
businessgrowthhub.shop – Overall a polished site with professional and engaging content.
smartfashionboutique.bond – Typography and design are consistent and visually appealing.
successfultradersclub.shop – Typography and design are consistent and visually appealing.
globalalliancenetwork.bond – Content is clear, informative, and engaging; great first impression.
uniquedecorstore.shop – The design is clean and professional, making navigation a breeze.
https://engineeringconsultancy.in/design-engineer-project-delivery-on-time/
The Real Person!
The Real Person!
Роберт Левандовски акыркы оюндарында мыкты оюн көрсөттү.
Роберт Левандовски Инстаграмда күйөрмандар менен тыгыз байланышта. Роберт Левандовски жашоосунда көп ийгиликке жеткен. Левандовски Польша командасынын негизги оюнчусу болуп саналат. Футбол күйөрмандары үчүн пайдалуу булак — robert-lewandowski-kg.com</url]. Бул жерден статистика жана фото табасыз. Роберт Левандовски Барселона үчүн көп гол салууда.
Левандовски өзүнүн эмгеги менен дүйнөгө белгилүү болгон. Левандовскинин булчуңдары жана абалы көпчүлүккө үлгү.
Левандовскинин балдары да спортко жакын. Левандовски ар дайым жогорку деңгээлди кармайт.
covidtest-cyprus – Essential health services with reliable testing solutions.
nextvision.bond – The visuals are appealing and complement the content well.
trendinsight.shop – The visuals are appealing and complement the content well.
successpathway.bond – Frequent discounts and promotions; always a great deal to find.
play-brary.org – Easy to read, and the design is consistent throughout.
leanneslifechangingfairies.com – Navigation is smooth and pages load quickly, very responsive.
growthpoint.bond – The site loads quickly and is mobile-friendly.
uniquefashionstyle.shop – Browsed through several items and found some stylish pieces I’d consider buying.
discovergreatdeal.shop – Great variety of products at unbeatable prices; highly recommend.
wellnesstourbus – Mobile health and wellness services bringing care to communities.
createimpacttoday.shop – This platform offers unique products that support meaningful causes.
The Real Person!
The Real Person!
J’adore l’energie rythmee de BassBet Casino, on ressent une vibe electrisante. Les options sont vastes comme une scene de concert, incluant des paris sportifs pleins de vie. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme un beat parfait, toujours pret a mixer. Les transactions sont fiables, parfois quelques tours gratuits en plus seraient top. Pour conclure, BassBet Casino vaut une session live pour les fans de casino en ligne ! A noter la navigation est simple et rythmee, booste le plaisir de jouer. Particulierement cool les paiements securises en crypto, propose des avantages sur mesure.
https://bassbetcasinoappfr.com/|
The Real Person!
The Real Person!
J’adore l’ambiance lumineuse de BassBet Casino, il procure une experience brillante. Il y a un flot de jeux captivants, comprenant des jeux adaptes aux cryptos. L’offre de bienvenue est brillante. Disponible 24/7 via chat ou email, avec une aide precise. Les gains arrivent sans attendre, cependant des offres plus genereuses seraient lumineuses. En bref, BassBet Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! Par ailleurs l’interface est fluide comme un neon, booste le plaisir de jouer. Egalement genial les evenements communautaires dynamiques, propose des avantages sur mesure.
https://bassbetcasinopromocodefr.com/|
happylifestylehub.shop – A refreshing site with a positive vibe; highly recommend checking it out.
The Real Person!
The Real Person!
Je suis totalement envoute par BassBet Casino, c’est une plateforme qui ondule avec elegance. La variete des titres est rapide, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. Les agents repondent comme une vague, offrant des reponses claires. Le processus est simple et elegant, bien que des bonus plus varies seraient une vague. En resume, BassBet Casino est un incontournable pour les joueurs pour les fans de casino en ligne ! Ajoutons que la navigation est simple et rapide, amplifie le plaisir de jouer. Un plus les evenements communautaires engageants, qui booste l’engagement.
https://bassbetcasinobonusfr.com/|
The Real Person!
The Real Person!
Je suis emerveille par Spinit Casino, ca offre un plaisir sophistique. Le catalogue est riche en aromes, offrant des sessions live sophistiquees. Le bonus de bienvenue est delicieux. L’assistance est efficace et professionnelle, avec une aide precise. Les retraits sont fluides comme un parfum, bien que des offres plus genereuses seraient exquises. Dans l’ensemble, Spinit Casino est une plateforme qui enchante pour les passionnes de jeux modernes ! A noter la navigation est simple et gracieuse, ce qui rend chaque session plus raffinee. Un plus le programme VIP avec des niveaux exclusifs, offre des recompenses continues.
spinitcasinobonusfr.com|
The Real Person!
The Real Person!
Je suis absolument envoute par Monte Cryptos Casino, c’est une plateforme qui vibre comme un smart contract. La selection de jeux est astronomique, comprenant des jeux optimises pour les cryptos. 100% jusqu’a 300 € + tours gratuits. L’assistance est precise et professionnelle, garantissant un service premium. Les gains arrivent sans delai, de temps a autre des offres plus genereuses ajouteraient du charme. Pour conclure, Monte Cryptos Casino est un must pour les fans de blockchain pour les adeptes de jeux modernes ! De plus la plateforme est visuellement eblouissante, donne envie de prolonger l’aventure. A souligner les evenements communautaires decentralises, qui booste l’engagement.
Commencer|
The Real Person!
The Real Person!
Je suis seduit par Impressario Casino, on ressent une ambiance sophistiquee. Les options sont vastes comme un salon parisien, offrant des sessions live distinguees. Amplifiant le plaisir de jeu. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les paiements sont securises et rapides, neanmoins quelques tours gratuits supplementaires seraient bienvenus. Au final, Impressario Casino vaut une visite sophistiquee pour les amateurs de sensations elegantes ! De plus le design est moderne et chic, facilite une immersion totale. Un plus les tournois reguliers pour la competition, assure des transactions fiables.
Explorer les dГ©tails|
futureopportunitynetwork.shop – Great variety of products at unbeatable prices; highly recommend.
The Real Person!
The Real Person!
J’adore l’elegance de Impressario Casino, il procure une experience exquise. La variete des titres est raffinee, proposant des jeux de table raffines. Amplifiant le plaisir de jeu. Les agents repondent avec courtoisie, offrant des reponses claires. Les paiements sont securises et rapides, bien que plus de promos regulieres ajouteraient du piquant. Dans l’ensemble, Impressario Casino est un incontournable pour les amateurs pour ceux qui aiment parier en crypto ! En bonus la navigation est simple et gracieuse, amplifie le plaisir de jouer. Un autre atout les tournois reguliers pour la competition, assure des transactions fiables.
DГ©couvrir le site complet|
concernedaboutpollution – Environmental advocacy with focus on pollution awareness and solutions.
play-brary.org – Easy to read, and the design is consistent throughout.
What’s up Dear, are you really visiting this web site on a regular basis, if so then you will definitely take nice know-how.
прием смс
leanneslifechangingfairies.com – Content is clear, helpful, and engaging; makes browsing enjoyable.
discovernewpath.shop – Found some helpful tips here; looking forward to more updates.
The Real Person!
The Real Person!
Роберт Левандовскинин статистикасы укмуштай — ал ар бир оюнда гол салат.
Роберт Левандовскинин үй-бүлөлүк жашоосу кызыктуу. Левандовскинин статистикасы футбол дүйнөсүндө өзгөчө. Левандовски футбол тарыхында өз изин калтырган. Левандовскинин Барселонадагы ийгиликтерин robert-lewandowski-kg.com сайтынан окуңуз. Роберт Левандовски Барселонада мыкты старт жасаган.
Роберт Левандовски жаштарга мотивация берет. Левандовски спорттук формасын жоготпойт.
Левандовскинин туугандары тууралуу тарых да кызыктуу. Роберт Левандовски футбол тарыхындагы легенда.
asianspeedd8 – Modern dating platform with cultural connection and community focus.
The Real Person!
The Real Person!
Je suis totalement transporte par BassBet Casino, c’est une plateforme qui vibre comme un festival. Il y a un flot de jeux captivants, comprenant des jeux adaptes aux cryptos. Avec des depots ultra-rapides. L’assistance est rapide et pro, offrant des solutions claires. Les transactions sont fiables, mais plus de promos regulieres ajouteraient du rythme. Au final, BassBet Casino est un must pour les joueurs pour les amateurs de sensations vibrantes ! A noter la navigation est simple et rythmee, donne envie de prolonger la fete. Particulierement cool les evenements communautaires dynamiques, assure des transactions fiables.
bassbetcasinoappfr.com|
The Real Person!
The Real Person!
BassBet Casino m’a captive BassBet Casino, il procure une experience brillante. Le catalogue est riche en surprises, proposant des jeux de table styles. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme un flash, avec une aide precise. Le processus est simple et lumineux, parfois quelques tours gratuits en plus seraient top. Pour conclure, BassBet Casino garantit un fun non-stop pour les amateurs de sensations vibrantes ! Par ailleurs la plateforme est visuellement fluorescente, ce qui rend chaque session plus brillante. Un point fort les options de paris variees, propose des avantages sur mesure.
https://bassbetcasinopromocodefr.com/|
The Real Person!
The Real Person!
Je suis emerveille par BassBet Casino, ca offre un plaisir nautique. La selection de jeux est dynamique, offrant des sessions live immersives. Avec des depots instantanes. Le support client est dynamique, avec une aide precise. Les paiements sont securises et rapides, cependant quelques tours gratuits supplementaires seraient bien venus. En resume, BassBet Casino garantit un plaisir constant pour les fans de casino en ligne ! Ajoutons que la plateforme est visuellement aquatique, facilitate une immersion totale. Particulierement interessant le programme VIP avec des niveaux exclusifs, renforce le sentiment de communaute.
https://bassbetcasinobonusfr.com/|
The Real Person!
The Real Person!
Je suis accro a Spinit Casino, ca offre un plaisir sophistique. La variete des titres est raffinee, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. Le service est disponible 24/7, garantissant un support de qualite. Les gains arrivent sans delai, de temps a autre des offres plus genereuses seraient exquises. Pour conclure, Spinit Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! De plus la plateforme est visuellement somptueuse, ajoute une touche d’elegance. Egalement appreciable les tournois reguliers pour la competition, assure des transactions fiables.
spinitcasinobonusfr.com|
The Real Person!
The Real Person!
Je suis enchante par Impressario Casino, c’est une plateforme qui evoque le raffinement. Les options sont vastes comme un menu etoile, offrant des sessions live sophistiquees. Le bonus de bienvenue est delicieux. Le support client est impeccable, toujours pret a servir. Les transactions sont fiables, cependant des recompenses additionnelles seraient royales. Pour conclure, Impressario Casino est un incontournable pour les amateurs pour les amateurs de sensations elegantes ! De plus la plateforme est visuellement somptueuse, donne envie de prolonger l’experience. Un autre atout les evenements communautaires engageants, offre des recompenses continues.
Voir le site|
newhorizonsnetwork.shop – Frequent discounts and promotions; always a great deal to find.
The Real Person!
The Real Person!
Je suis absolument envoute par Monte Cryptos Casino, il propose une aventure chiffree. Il y a une profusion de jeux captivants, incluant des paris live dynamiques. Le bonus d’entree est eclatant. L’assistance est precise et professionnelle, garantissant un service premium. Le processus est fluide comme un wallet, de temps a autre des offres plus genereuses ajouteraient du charme. Pour conclure, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les joueurs en quete d’innovation ! De plus le site est rapide et futuriste, facilite une immersion totale. Un avantage notable les options de paris variees, garantit des transactions fiables.
DГ©couvrir le contenu|
The Real Person!
The Real Person!
Je suis enchante par Impressario Casino, il procure une experience distinguee. La variete des titres est elegante, incluant des paris sportifs elegants. Amplifiant le plaisir de jeu. Les agents repondent avec courtoisie, joignable a toute heure. Les paiements sont securises et rapides, cependant des offres plus genereuses seraient exquises. En resume, Impressario Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! En bonus la plateforme est visuellement somptueuse, donne envie de prolonger l’experience. A souligner le programme VIP avec niveaux exclusifs, offre des recompenses continues.
VГ©rifier les infos|
The Real Person!
The Real Person!
Je suis seduit par Impressario Casino, on ressent une ambiance delicate. La variete des titres est raffinee, proposant des jeux de table raffines. Le bonus de bienvenue est delicieux. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les paiements sont securises et rapides, neanmoins des bonus plus varies seraient un delice. Au final, Impressario Casino offre une experience memorable pour les joueurs en quete d’excitation ! Par ailleurs la plateforme est visuellement somptueuse, ce qui rend chaque session plus raffinee. Egalement appreciable les evenements communautaires engageants, propose des avantages personnalises.
Essayer|
mdcphilly.com – Pages load smoothly and the overall user experience is very pleasant.
harrietlevinmillan.com – The layout is visually appealing and supports the written content nicely.
pp4fdr.org – I appreciate how accessible the information is; not overloaded with jargon.
futurevisiongroup.bond – Impressive design and user experience; feels professional and welcoming.
ks4thekids.com – The content is clear and shows focus; I like the purpose.
buildbrand.bond – This site’s layout is clean and easy to navigate.
brauhausschmitzoktoberfest.com – Great mix of tradition and fun, really captures the spirit.
clubinorout.com – Clean design and straightforward layout, very user-friendly.
mdcphilly.com – Navigation feels intuitive; I found what I needed quickly without confusion.
concernedaboutpollution.com – Would like to see more interactive features, but still a solid foundation.
asianspeedd8.com – Looking forward to seeing more features or expansions of the site.
grant-jt.com – Footer and contact info are present; adds credibility.
komunahouse.com – Keep an eye on it—seems like a project with growth potential.
berrybombselfiespot.com – Easy to find what this place offers; information is clear and inviting.
gailcooperspeaker.com – I’m looking forward to reading more about the work and upcoming events.
The Real Person!
The Real Person!
Роберт Левандовски акыркы оюндарында мыкты оюн көрсөттү.
Роберт Левандовски денесин мыкты формада кармайт. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Роберт Левандовски улуттук курама үчүн көп гол салган. Футбол боюнча көбүрөөк маалымат жана Роберт Левандовски тууралуу окугуңуз келсе, бул булакты караңыз — Роберт Левандовски расмий сайт. Бул жерден акыркы жаңылыктарды таба аласыз. Левандовски Барселонага өткөндөн кийин клуб жаңы деңгээлге чыкты.
Левандовски өзүнүн эмгеги менен дүйнөгө белгилүү болгон. Роберт Левандовски фитнеске чоң маани берет.
Левандовскинин түпкү теги тууралуу маалымат көп талкууланат. Роберт Левандовски өзүнүн максатына ишеним менен жеткен.
grant-jt – Professional services with expertise and dedicated client support.
shemplymade.com – It would be nice to see more user reviews or customer stories.
meetkatemarshall.com – The blog layout is versatile and easy to navigate, covering many topics.
mdcphilly.com – Pages load smoothly and the overall user experience is very pleasant.
harrietlevinmillan.com – Great mix of poetry and prose; impressive range and depth.
reindeermagicandmiracles.com – The site looks trustworthy and well-put-together; good effort.
ks4thekids.com – The content is clear and shows focus; I like the purpose.
pp4fdr.org – Navigation is intuitive and the sections make sense; good user experience.
brauhausschmitzoktoberfest.com – Great mix of tradition and fun, really captures the spirit.
seenministries.com – The website is clean and easy to navigate; good user experience.
clubinorout.com – Looking forward to seeing how this site evolves and what’s added.
moeinclub.com – Overall, a decent site with good potential for expansion.
electmikemontes.com – Overall a strong web presence that makes a good first impression.
brucknerbythebridge.com – Overall, a strong web presence that supports the property well.
pastorjorgetrujillo.com – The layout is well-organized; helps me focus on key sections without distraction.
trendingnewsfeed.net – the website has a sharp and modern design; nice first impression.
komunahouse – Community living space fostering connection and collaboration.
clevelandchristmasclub.com – The vibe of the site is fun and festive; great first impression.
centensports.com – I like the layout and how the content is structured; good readability.
Cherished is likely to be what people say about your comments.
concernedaboutpollution.com – The topic is relevant and handled in a thoughtful, well-structured way.
The Real Person!
The Real Person!
Ich schatze die Spannung bei Snatch Casino, es bietet ein immersives Erlebnis. Das Spieleangebot ist reichhaltig und vielfaltig, mit Spielautomaten in kreativen Designs. Mit schnellen Einzahlungen. Die Mitarbeiter antworten schnell und freundlich. Transaktionen laufen reibungslos, dennoch regelma?igere Promos wurden das Spiel aufwerten. Kurz gesagt, Snatch Casino bietet ein unvergessliches Erlebnis. Daruber hinaus die Plattform ist visuell ansprechend, das Vergnugen maximiert. Ein attraktives Extra die haufigen Turniere fur Wettbewerb, die Teilnahme fordern.
snatch-casino.de|
asianspeedd8.com – I appreciate how the pages load quickly and feel responsive.
grant-jt.com – Footer and contact info are present; adds credibility.
komunahouse.com – Keep an eye on it—seems like a project with growth potential.
berrybombselfiespot.com – I appreciate the gallery of background scenes—it gives a good preview of the experience.
mdcphilly.com – Looking forward to seeing what new content they’ll add next.
gailcooperspeaker.com – The design feels clean and easy to navigate which is refreshing.
safercharging.com – The branding feels consistent and trustworthy throughout the site.
berrybombselfiespot – Fun and vibrant selfie destination with creative photo opportunities.
reindeermagicandmiracles.com – The site’s branding fits perfectly with what they’re offering—nice match.
brucknerbythebridge.com – The site does a good job of balancing text and imagery; very readable.
dankglassonline.com – The visual style is modern and the layout works well on mobile.
turnerhallrestaurant.com – It would be great to see more customer reviews or featured dishes.
gailcooperspeaker – Inspirational speaking with powerful messages and engaging delivery.
meetkatemarshall.com – Visuals are well done and complement the content without being overwhelming.
muralspotting.com – The site feels like it encourages exploration and discovery—very cool.
The Real Person!
The Real Person!
Ich habe einen Narren gefressen an Snatch Casino, es sorgt fur ein fesselndes Erlebnis. Das Angebot ist ein Traum fur Spieler, mit aufregenden Live-Casino-Erlebnissen. Mit sofortigen Einzahlungen. Der Kundendienst ist ausgezeichnet. Gewinne kommen sofort an, jedoch waren mehr Bonusvarianten ein Plus. Abschlie?end, Snatch Casino bietet ein unvergessliches Erlebnis. Hinzu kommt die Seite ist schnell und einladend, einen Hauch von Eleganz hinzufugt. Ein attraktives Extra die dynamischen Community-Events, das die Motivation steigert.
Snatch Casino|
muralspotting.com – The visuals are vibrant and the concept of finding murals excites me.
seenministries – Faith-based ministry offering spiritual guidance and community support.
Thank you a lot for sharing this with all folks you actually recognize what you’re speaking about! Bookmarked. Please additionally visit my site =). We can have a hyperlink trade contract among us!
moeinclub.com – The design is clean and mobile-friendly, good for on-the-go reading.
shemplymade.com – The layout is clean and allows the items to stand out well.
cepjournal.net – Load speeds seem solid, contributing to a smooth browsing experience.
centensports.com – Navigation feels intuitive and finding key sections is straightforward.
safercharging.com – Found the typography and spacing pleasant to read; good design choices.
seenministries.com – Love the clear focus on empowering and equipping women; very inspiring.
opensky-inc.com – I like the visuals and typography; they’re subtle but effective.
pastorjorgetrujillo.com – I appreciate the consistent branding and clear mission statement.
trendingnewsfeed.net – I’d like to see more filter/search options for topic-specific browsing.
clevelandchristmasclub.com – The vibe of the site is fun and festive; great first impression.
electmikemontes.com – Navigation is intuitive and the key information is easy to find.
muralspotting.com – Looking forward to more additions and featured artists from around the world.
dankglassonline.com – The site looks clean and minimal; easy to navigate.
bigprintnewspapers.com – It would be helpful to see more detailed case studies or examples.
catherinewburton.com – The visuals are calming and the tone is personable—nice choice.
vsmphotography.com – Overall a strong web presence for a photography studio — well done.
toddstarnesbooktour.com – It would be great to see more interactive content like video invites or live Q&A info.
createandgrow.shop – Navigation seems straightforward and the site loads without delay.
The Real Person!
The Real Person!
J’ai un amour vibrant pour BassBet Casino, ca transporte dans un univers de sons. Il y a un flot de jeux captivants, comprenant des jeux adaptes aux cryptos. Avec des depots ultra-rapides. Le suivi est impeccable, offrant des solutions claires. Le processus est simple et vibrant, neanmoins quelques tours gratuits en plus seraient top. Dans l’ensemble, BassBet Casino vaut une session live pour ceux qui parient avec des cryptos ! En plus la navigation est simple et rythmee, ce qui rend chaque session plus vibrante. Particulierement cool les paiements securises en crypto, assure des transactions fiables.
bassbetcasinoappfr.com|
The Real Person!
The Real Person!
J’adore l’atmosphere mythique de Spinit Casino, c’est une plateforme qui tourbillonne comme un conte. Il y a une profusion de jeux fascinants, proposant des jeux de table elegants. Amplifiant le plaisir de jeu. Le suivi est irreprochable, avec une aide precise. Les paiements sont securises et rapides, bien que des bonus plus varies seraient un chapitre. En bref, Spinit Casino offre une experience memorable pour les joueurs en quete d’excitation ! A noter l’interface est fluide comme un conte, facilite une immersion totale. Egalement appreciable les options de paris sportifs variees, assure des transactions fiables.
spinitcasinobonusfr.com|
findwhatyoulove.shop – Navigation feels intuitive; I found what I was looking for quickly.
startsomethinggreat.shop – Product categories appear clear; helps me understand the offerings.
The Real Person!
The Real Person!
Je suis accro a BassBet Casino, on ressent une ambiance de studio. La selection de jeux est melodieuse, avec des slots aux designs rythmiques. Avec des depots instantanes. Le support est irreprochable, avec une aide precise. Le processus est simple et elegant, parfois plus de promos regulieres ajouteraient du groove. En resume, BassBet Casino est une plateforme qui groove pour les passionnes de jeux modernes ! Ajoutons que le site est rapide et attractif, amplifie le plaisir de jouer. Particulierement interessant les paiements securises en crypto, qui booste l’engagement.
bassbetcasinobonusfr.com|
theperfectgiftshop.com – The layout is clean and easy to navigate; very user-friendly.
exploreopportunitiesnow.shop – The site loads smoothly and the spacing makes it easy to read.
shopandsmilealways.shop – Navigation seems intuitive; I found the main sections easily.
simplybestchoice.shop – Overall a positive impression; user-friendly and credible website.
keepgrowingforward.shop – The visuals are inviting and the interface feels smooth.
geteventclipboard.com – I like how the functions are clearly outlined; it’s helpful for understanding the service.
shopandsmilealways.shop – The branding feels simple and clean; consistent visual tone.
shopandshineeveryday.shop – I like how the key message “Shine Everyday” comes through—it feels positive.
Simply wish to say your article is as astonishing. The clearness for your put up is simply excellent and that i can think you are an expert in this subject. Fine along with your permission allow me to seize your RSS feed to keep updated with imminent post. Thank you one million and please carry on the gratifying work.
https://ust-kamenogorsk.city/forums/zhenskij-forum/muzhiki/21687/
Some genuinely choice content on this site, bookmarked .
shopwithconfidence – Super happy with my order, everything arrived just on time.
uniquetrendstore – Fantastic variety of products, always something new and exciting.
catherinewburton.com – The visuals are calming and the tone is personable—nice choice.
joinourcreativeworld – Excellent customer service, resolved my issue quickly and efficiently.
vsmphotography.com – The branding feels consistent and professional throughout the site.
shopthebesttoday – Great variety of products, easy to navigate and shop online.
shopforhappiness – Easy to navigate, found exactly what I was looking for.
The Real Person!
The Real Person!
Hi there, this weekend is good in favor of me, because this time i am reading this impressive educational paragraph here at my home.
https://omurp.org.ua/yak-pravylno-obraty-sklo-far-dlya-avto-z-yevropy.html
bestdealsforlife – I always trust this shop, never had issues at checkout.
inspireeverydaylife – Love the unique finds here, always something special to discover.
toddstarnesbooktour.com – Overall, the site gives a strong first impression of a well-organized tour campaign.
exploreopportunitiesnow.shop – The branding is consistent and gives the store a polished identity.
newseasoncollection – Love the unique finds here, always something special to discover.
createandgrow.shop – I like the minimalist design approach; it helps focus on the content.
staycuriousalways – Great prices and quality, will definitely shop here again.
smartchoiceoutlet – Excellent selection of products, always find what I need here.
discovernewworld – Great shopping experience, fast delivery and quality items every time.
startsomethinggreat.shop – The layout is clean and minimal, which makes browsing easy.
findwhatyoulove.shop – The branding is consistent and gives the store a polished identity.
theperfectgiftshop.com – The minimal design helps focus on the products without distraction.
shopandsmilealways.shop – Product categories are visible, which helps in understanding the layout.
simplybestchoice.shop – Navigation feels intuitive; I found what I was looking for quickly.
Hi, after reading this amazing piece of writing i am as well delighted to share my know-how here with colleagues.
https://studioline-interior.com/yak-steklafar-mozhut-zminyty-vash-avtomobil.html
keepgrowingforward.shop – I’d like to see more detailed reviews or user photos for added trust.
shopandsmilealways.shop – Overall a good first impression; user-friendly and pleasant to explore.
shopandshineeveryday.shop – It might benefit from more detailed product information to build trust.
discoveryourmoment – Smooth shopping experience, easy navigation and secure checkout process.
geteventclipboard.com – Load times seem good; the site feels responsive.
thinkcreategrow – A go-to site for actionable advice and motivational content.
getinspiredtoday – Excellent customer service, resolved my issue quickly and efficiently.
thebestplacetoshop – Great prices and quality, will definitely shop here again.
trendyfindshub – Easy to navigate, found exactly what I was looking for.
newseasoncollection – Love the unique finds here, always something special to discover.
dreamcreateinspire – Excellent selection of products, always find what I need here.
shopthelatesttrend – Highly recommend this shop, never disappointed with my purchases.
shopwithconfidence – Prices are reasonable and the website updates offers regularly, nice.
joinourcreativeworld – Always a pleasure shopping here, never disappoints with selections.
inspireeverydaylife – Love the unique finds here, always something special to discover.
smartchoiceoutlet – Excellent customer service, resolved my issue quickly and efficiently.
everydayvaluecorner – Excellent customer service, resolved my issue quickly and efficiently.
shopforhappiness – Love this site, always find unique items and great deals.
shopthebesttoday – Great variety of products, easy to navigate and shop online.
discovernewworld – Love this site, always find unique items and great deals.
globalmarketplacehub – Great shopping experience, fast delivery and quality items every time.
discoverendlessideas – Appreciate the variety of topics and engaging writing style.
shopthelatesttrend – Great prices and quality, will definitely shop here again.
everytrendinone – Love discovering fresh items here; keeps the space feeling updated and fun.
brightfuturedeals – The writing style is clear and friendly—makes reading fun rather than a chore.
learnshareconnect – Easy checkout and secure payment made shopping worry-free this time.
staycuriousalways – Always a pleasure shopping here, never disappoints with selections.
discovergreatthings – User-friendly website layout, found what I needed without any hassle.
exploreamazingideas – Excellent customer support, they responded promptly and solved my issue.
findyourperfectdeal – Found items that match my style perfectly; really pleased with the selection.
yourjourneybegins – Wonderful site with inspiring products that brighten every day, so glad.
bestdealsforlife – Super smooth navigation, the website layout feels really clean and nice.
electmikemontes – Political campaign focused on community representation and positive change.
blinkiesdonutsca – Recommend for anyone looking for unique donuts and friendly service today.
brainsight-reeracoen – Very helpful team, offered targeted experts and saved us a lot of research time.
shopwithconfidence – Impressed with how professional everything looks, no random clutter anywhere.
discoveryourmoment – Smooth shopping experience, easy navigation and secure checkout process.
realherschel – Prices seem reasonable and the images are pretty clear overall.
shemplymade – Handcrafted products with quality craftsmanship and unique designs.
globalmarketplacehub – Easy to navigate, found exactly what I was looking for.
discoverendlessideas – The website layout is clean and navigation feels very intuitive today.
everytrendinone – The website is clean and intuitive; found what I was looking for in minutes.
brightfuturedeals – Loved the downloadable resources section; useful for planning and idea generation.
learnshareconnect – Found items that match my style perfectly; really pleased with the selection.
discovergreatthings – Found items that matched my aesthetic perfectly, really pleased with the selection.
exploreamazingideas – Excellent customer support, they responded promptly and solved my issue.
tabitoshigoto – I think this could become a go-to site for digital nomads in Japan.
yourjourneybegins – Wonderful site with inspiring products that brighten every day, so glad.
trabas007hoki – Overall okay for now, but I’d proceed cautiously before making any big commitment.
hanayaka-na-life – Customer service looked responsive when I had a quick question.
joebobsaveschristmas – Great concept for the season, and the layout looks nicely done overall.
hopeandlace – I found references to them as a Brisbane-based wedding planner/stylist, which adds credibility. :contentReference[oaicite:0]index=0
The Real Person!
The Real Person!
J’ai un amour vibrant pour BassBet Casino, on ressent une energie vibrante. Il y a un flot de jeux captivants, avec des slots aux designs soul. Avec des depots ultra-rapides. Le support client est au top, offrant des solutions claires. Les retraits sont fluides comme une note tenue, cependant des recompenses supplementaires seraient vibrantes. Au final, BassBet Casino offre une experience inoubliable pour les fans de casino en ligne ! Par ailleurs le site est rapide et captivant, booste le plaisir de jouer. Egalement genial les tournois reguliers pour la competition, renforce la communaute.
bassbetcasinopromocodefr.com|
The Real Person!
The Real Person!
Ich bin ein gro?er Fan von Snatch Casino, es bietet packende Unterhaltung. Das Portfolio ist vielfaltig und attraktiv, mit stilvollen Tischspielen. Er bietet einen gro?artigen Vorteil. Der Kundendienst ist ausgezeichnet. Zahlungen sind sicher und schnell, allerdings zusatzliche Freispiele waren willkommen. Letztlich, Snatch Casino ist definitiv einen Besuch wert. Zusatzlich die Oberflache ist benutzerfreundlich, jeden Augenblick spannender macht. Ein bemerkenswertes Extra ist das VIP-Programm mit besonderen Vorteilen, die die Begeisterung steigern.
snatch-casino.de|
The Real Person!
The Real Person!
Je suis accro a Spinit Casino, ca offre un plaisir veloce. La variete des titres est rapide, proposant des jeux de table rapides. Le bonus de bienvenue est rapide. Le service est disponible 24/7, toujours pret a accelerer. Les retraits sont fluides comme un peloton, neanmoins quelques tours gratuits supplementaires seraient bien venus. En bref, Spinit Casino offre une experience memorable pour les joueurs en quete d’excitation ! Par ailleurs le site est rapide et attractif, donne envie de prolonger l’aventure. Un plus le programme VIP avec des niveaux exclusifs, assure des transactions fiables.
https://casinospinitfr.com/|
Hello very cool website!! Man .. Beautiful .. Amazing .. I’ll bookmark your website and take the feeds additionally? I am satisfied to seek out a lot of useful information right here within the put up, we’d like develop extra strategies in this regard, thanks for sharing. . . . . .
kra42 at
hawaiineiartcontest – Overall, this looks like a well-organized event with a clear mission and opportunity for exposure.
motivilovesmusic – Overall: potentially fine for casual reference, but not yet fully endorsed for important business use.
wrestlingac – Since it covers prominent wrestling personalities like Kofi Kingston, that suggests it has access to credible sources. :contentReference[oaicite:2]index=2
brainsight-reeracoen – The interface and communication were so clear, no hidden surprises at all.
sega-live – Consider comparing prices on the site with others; unusually low pricing may signal risk.
The Real Person!
The Real Person!
J’ai une passion rythmique pour BassBet Casino, on ressent une ambiance de studio. La variete des titres est harmonieuse, comprenant des jeux compatibles avec les cryptos. Le bonus de bienvenue est rythme. Le support client est melodieux, toujours pret a jammer. Le processus est simple et elegant, cependant des offres plus genereuses seraient melodieuses. Pour conclure, BassBet Casino offre une experience memorable pour les amateurs de sensations rythmees ! Par ailleurs le site est rapide et attractif, ce qui rend chaque session plus groovy. Particulierement interessant les options de paris sportifs variees, propose des avantages personnalises.
bassbetcasinobonusfr.com|
blinkiesdonutsca – Quality donuts with creative toppings, definitely found a new favourite spot.
The Real Person!
The Real Person!
Je suis totalement envoute par Spinit Casino, il procure une experience magique. La variete des titres est magique, offrant des sessions live immersives. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme par magie, toujours pret a transformer. Les transactions sont fiables, cependant des offres plus genereuses seraient enchantees. En bref, Spinit Casino est une plateforme qui enchante pour les passionnes de jeux modernes ! En bonus la navigation est simple et magique, ce qui rend chaque session plus enchantee. Un plus le programme VIP avec des niveaux exclusifs, offre des recompenses continues.
spinitcasinobonusfr.com|
shopwithconfidence – Love how easy it is to find good deals here lately.
The Real Person!
The Real Person!
Je suis ebloui par Monte Cryptos Casino, ca transporte dans un monde virtuel. La selection de jeux est astronomique, avec des slots aux designs modernes. Boostant votre capital initial. Le support client est irreprochable, toujours pret a decoder. Le processus est fluide comme un smart contract, parfois des offres plus genereuses ajouteraient du charme. En bref, Monte Cryptos Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! Par ailleurs le design est moderne et captivant, ce qui rend chaque session plus immersive. Egalement cool les options de paris variees, offre des recompenses continues.
Trouver la vГ©ritГ©|
hopprojects – Love that they focus on breaking down traditional genre boundaries, very refreshing.
The Real Person!
The Real Person!
Je suis enchante par Impressario Casino, ca transporte dans un monde de prestige. Les choix sont vastes comme une salle de cinema, comprenant des jeux compatibles avec les cryptos. L’offre de bienvenue est prestigieuse. Le service client est irreprochable, avec une aide efficace. Les transactions sont fiables, bien que des offres plus genereuses seraient parfaites. Pour conclure, Impressario Casino est un joyau pour les joueurs pour les joueurs en quete de magie ! De plus l’interface est fluide comme un film, ajoute une touche de prestige. Un point fort les paiements securises en crypto, propose des avantages sur mesure.
Lire l’avis complet|
The Real Person!
The Real Person!
shemplymade – Handcrafted products with quality craftsmanship and unique designs.
envolavecning – If you’re considering using them, I’d check who’s behind the domain and look for reviews or trust signals.
The Real Person!
The Real Person!
Je suis stupefait par Monte Cryptos Casino, c’est une plateforme qui vibre comme un reseau blockchain. Il y a une profusion de jeux captivants, proposant des jeux de table elegants. Amplifiant l’experience de jeu. Le support client est stellaire, joignable a tout moment. Les paiements sont securises et fluides, neanmoins quelques tours gratuits en plus seraient bienvenus. En bref, Monte Cryptos Casino offre une experience inoubliable pour les adeptes de jeux modernes ! De plus la plateforme est visuellement eblouissante, ajoute une touche de sophistication. Particulierement captivant les paiements securises en BTC/ETH, offre des recompenses continues.
VГ©rifier les infos|
Aw, this was an exceptionally nice post. Taking a few minutes and actual effort to generate a top notch article… but what can I say… I hesitate a whole lot and don’t manage to get anything done.
вход через зеркало kra42 at
beauty-optical-salon – The domain and brand are visible in multiple Japanese eyewear/retail news outlets. :contentReference[oaicite:8]index=8
tabitoshigoto – The layout reminds me of top Japanese travel guides, very neatly done.
rebeccacaridad-manzanita – The name “Manzanita” might connect to the blog or creative brand of Rebecca Caridad, which could be fine but still needs verification.
The Real Person!
The Real Person!
Ich bin begeistert von der Welt bei Snatch Casino, es ist ein Hotspot fur Spielspa?. Das Angebot ist ein Traum fur Spieler, inklusive dynamischer Sportwetten. Mit einfachen Einzahlungen. Erreichbar 24/7 per Chat oder E-Mail. Zahlungen sind sicher und schnell, gelegentlich mehr Promo-Vielfalt ware toll. Zusammengefasst, Snatch Casino sorgt fur kontinuierlichen Spa?. Zudem die Oberflache ist benutzerfreundlich, jeden Moment aufregender macht. Ein starker Vorteil ist das VIP-Programm mit tollen Privilegien, regelma?ige Boni bieten.
Snatch Casino|
engagement-forum – I would look for domain age, SSL details, contact information, and user reviews before engaging.
The Real Person!
The Real Person!
J’ai une passion cyclonique pour Spinit Casino, c’est une plateforme qui tourne avec elegance. La selection de jeux est dynamique, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, garantissant un support de qualite. Le processus est simple et elegant, parfois quelques tours gratuits supplementaires seraient bien venus. En resume, Spinit Casino est un incontournable pour les joueurs pour les joueurs en quete d’excitation ! Par ailleurs l’interface est fluide comme une piste, amplifie le plaisir de jouer. Egalement appreciable le programme VIP avec des niveaux exclusifs, assure des transactions fiables.
casinospinitfr.com|
newthisdomainsssss – Clean interface, but I’d like to see more substance to fully engage.
bloemhill – The aesthetic is minimal yet elegant, which fits well for home-style goods.
realherschel – Prices seem reasonable and the images are pretty clear overall.
The Real Person!
The Real Person!
J’ai une passion rythmique pour BassBet Casino, ca plonge dans un monde de notes. Les options sont vastes comme un album, proposant des jeux de table elegants. Renforcant votre capital initial. Le support est irreprochable, toujours pret a jammer. Le processus est simple et elegant, de temps a autre plus de promos regulieres ajouteraient du groove. Au final, BassBet Casino garantit un plaisir constant pour les passionnes de jeux modernes ! Par ailleurs la navigation est simple et rythmee, donne envie de prolonger l’aventure. Egalement appreciable le programme VIP avec des niveaux exclusifs, propose des avantages personnalises.
https://bassbetcasinobonusfr.com/|
The Real Person!
The Real Person!
J’adore l’atmosphere mythique de Spinit Casino, ca offre un plaisir enchante. Le catalogue est riche et varie, incluant des paris sportifs dynamiques. Renforcant votre capital initial. Le service est disponible 24/7, garantissant un support de qualite. Les retraits sont fluides comme un conte, cependant quelques tours gratuits supplementaires seraient bien venus. Au final, Spinit Casino offre une experience memorable pour les passionnes de jeux modernes ! Par ailleurs la navigation est simple et magique, facilite une immersion totale. A souligner les options de paris sportifs variees, propose des avantages personnalises.
https://spinitcasinobonusfr.com/|
The Real Person!
The Real Person!
Je suis ensorcele par Monte Cryptos Casino, il propose une odyssee chiffree. La selection de jeux est astronomique, comprenant des jeux optimises pour les cryptos. Le bonus d’entree est scintillant. Disponible 24/7 via chat ou email, offrant des solutions claires. Les gains arrivent sans delai, cependant plus de promos regulieres dynamiseraient l’experience. Dans l’ensemble, Monte Cryptos Casino garantit un plaisir constant pour ceux qui parient avec des cryptos ! Par ailleurs la navigation est simple comme un wallet, ajoute une touche de sophistication. Un atout cle les paiements securises en BTC/ETH, propose des avantages uniques.
Voir les mises Г jour|
The Real Person!
The Real Person!
J’aime l’ambiance numerique de Monte Cryptos Casino, on ressent une energie decentralisee. Les options sont vastes comme un ledger, proposant des tables sophistiquees. Elevant l’experience de jeu. Le support client est irreprochable, offrant des solutions claires. Les paiements sont securises par blockchain, cependant des bonus plus varies seraient un atout. Dans l’ensemble, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les joueurs en quete d’innovation ! De plus la navigation est simple comme un wallet, ajoute une touche de sophistication. A souligner les paiements securises en BTC/ETH, garantit des transactions fiables.
En savoir sur le sujet|
The Real Person!
The Real Person!
J’adore le glamour de Impressario Casino, ca offre une experience cinematographique. Les choix sont vastes comme une salle de cinema, offrant des sessions live immersives. Avec des depots rapides. Le suivi est d’une precision remarquable, toujours pret a briller. Les retraits sont fluides comme un generique, bien que plus de promotions regulieres ajouteraient du prestige. En resume, Impressario Casino est un joyau pour les joueurs pour les amateurs de casino en ligne ! De plus l’interface est fluide comme un film, amplifie le plaisir de jouer. Particulierement captivant les evenements communautaires engageants, offre des recompenses continues.
Voir le site|
The Real Person!
The Real Person!
J’aime l’ambiance futuriste de Monte Cryptos Casino, c’est une plateforme qui vibre comme un reseau blockchain. La variete des titres est eclatante, avec des slots aux themes innovants. Le bonus d’accueil est eclatant. Le support client est stellaire, avec une aide precise et rapide. Les retraits sont rapides comme une transaction blockchain, cependant plus de promos regulieres dynamiseraient l’experience. Dans l’ensemble, Monte Cryptos Casino garantit un plaisir constant pour les joueurs en quete d’innovation ! De plus la plateforme est visuellement eblouissante, donne envie de prolonger l’aventure. Un atout cle le programme VIP avec des niveaux exclusifs, propose des avantages uniques.
Aller ici|
clevelandchristmasclub – Festive community organization spreading holiday joy and cheer.
ohmydrifter – If you’re into minimal, soulful lifestyle content this site has promise.
hopeandlace – I’d recommend verifying client reviews or testimonials for confirmation of performance.
macofficelovesyou – Historically this domain was used for a promotional campaign tied to Microsoft offering a MacBook Pro giveaway. :contentReference[oaicite:1]index=1
motivilovesmusic – If you’re considering linking or using it, I’d verify whether the domain is actively maintained.
hanayaka-na-life – Customer service looked responsive when I had a quick question.
The Real Person!
The Real Person!
Hey there this is kind of of off topic but I was wondering if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding know-how so I wanted to get guidance from someone with experience. Any help would be enormously appreciated!
https://kra42c.com
sega-live – If you do use it, take screenshots of the order and monitor for any unexpected charges.
hawaiineiartcontest – Might be helpful to check their “Resources” page for species lists and submission tips. :contentReference[oaicite:6]index=6
wrestlingac – I found it cited by other wrestling news outlets, indicating some credibility. :contentReference[oaicite:0]index=0
joebobsaveschristmas – Smooth navigation, found everything quickly without feeling lost anywhere.
hopprojects – Love that they focus on breaking down traditional genre boundaries, very refreshing.
Wow, incredible weblog structure! How long have you been running a blog for? you make running a blog glance easy. The total look of your site is wonderful, let alone the content material!
официальный сайт kra42 cc
beauty-optical-salon – They operate under a company called 株式会社オンザヒル and have a brand “YUNO” for their frames. :contentReference[oaicite:4]index=4
rebeccacaridad-manzanita – I recommend checking for an “About” page or contact info on the site to confirm legitimacy.
foruminvestmali – The domain was registered in 2017 and appears to have low traffic and minimal backlink profile. :contentReference[oaicite:0]index=0
macofficelovesyou – I couldn’t find current content or recent updates on this domain.
bwaylocations – Prime location services with strategic placement and accessibility.
dearsparrows – Overall: a helpful site for niche topics though I’d still verify any claims made before relying.
brightnightpdx – Overall: interesting find and likely safe to monitor further before full commitment.
pepplish – The content suggests they focus on hot sauces with bold flavours, which is interesting and niche.
28darlingstreet – For now, treat it as a lower-certainty option until you confirm its authority and trustworthiness.
nomnomnomfordogs – If purchasing, check shipping conditions (freshness, packaging) especially if you’re out of state.
flourandoak – The bakery’s aesthetic looks attractive and inviting, great for a casual visit.
globaltrendmarket – The domain feels generic and I couldn’t find much verified info about its operations or team.
connectdiscovergrow – I hope they update regularly — content freshness will help build trust and engagement.
shopwithconfidence – Overall looks promising—just do a little extra verification (age, certificate, reviews) before full use.
staycuriousalways – Maybe a bit early stage, but the concept is solid — I’ll keep an eye on it.
keepgrowingforward – Good candidate to share with a friend who’s looking for daily inspiration.
exploreopportunitiesnow – Seems like it could be useful for people seeking professional growth or new avenues.
bestdealsforlife – For a backlink use, consider balancing it with more established domains to avoid risk.
createandgrow – Good candidate for backlinking, though pair it with some high-authority domains for balance.
theperfectgiftshop – Overall, the domain evokes quality and thought-fulness—just a so-so level of detail visible so far.
buildyourdreamtoday – It would be helpful to know more about the site’s mission and the team behind it.
uniquetrendstore – The domain sounds catchy and suggests a focus on fashionable or niche items.
simplybestchoice – For backlinking purposes, I’d use this as one of many, but pair with more established domains for balance.
changetheworld – I recommend checking for an “About Us” page or contact information to understand the site’s legitimacy.
The Real Person!
The Real Person!
This is really attention-grabbing, You are an excessively professional blogger. I’ve joined your feed and look forward to searching for more of your excellent post. Additionally, I’ve shared your website in my social networks
вход через зеркало kra42 at
simplybestchoice – Overall: a decent concept, but I’d proceed cautiously until more verification is available.
thinkcreategrow – It would be helpful to know more about the site’s mission and the team behind it.
dreamcreateinspire – The domain name is strong and inspiring, but I couldn’t find much concrete information about its owner or purpose.
turnerhallrestaurant – Exceptional dining experience with quality cuisine and atmosphere.
globaltrendmarket – I’d recommend checking the site’s age, trust signals, and whether the business is registered.
GlobalMarketplaceHub – Prices are competitive, quality of items exceeded my expectations completely.
InspireEverydayLife – Website loads fast, browsing is seamless and very enjoyable most times.
dearsparrows – Content appears recently updated, so looks like the domain is still active.
discoverendlessideas – It would be helpful to know more about the site’s mission and the team behind it.
brightnightpdx – A suggestion: check domain age and whether the SSL certificate is up to date to ensure it’s safe.
pepplish – The product variety looks good—combining fruits, herbs and heat for a unique twist.
nomnomnomfordogs – Reviews on Etsy show strong praise: “Our dog loves their treats!!” :contentReference[oaicite:0]index=0
Right here is the right website for anyone who hopes to understand this topic. You understand a whole lot its almost tough to argue with you (not that I really will need to…HaHa). You definitely put a brand new spin on a topic which has been discussed for decades. Excellent stuff, just wonderful!
kra42 at
flourandoak – Overall, a positive first impression—would be happy to drop by and sample what they offer.
connectdiscovergrow – Good potential if you’re looking to expand your professional network or skills.
shopwithconfidence – Navigation feels intuitive, but it’s not clear what types of products or services are offered.
staycuriousalways – Could be a nice resource for personal growth or exploration — bookmarking it to check updates.
exploreopportunitiesnow – Clean layout and intuitive-looking site, which gives a good first impression.
theperfectgiftshop – Overall, the domain evokes quality and thought-fulness—just a so-so level of detail visible so far.
bestdealsforlife – I recommend verifying domain age and ownership details for more trust-worthiness.
createandgrow – Good candidate for backlinking, though pair it with some high-authority domains for balance.
LearnSomethingNew – Great platform for new skills, tutorials are concise and effective.
DiscoverGreatThings – Always something new to explore, site keeps me coming back daily.
BrightFutureDeals – Signing up was simple, notifications keep me updated about discounts.
buildyourdreamtoday – The concept of “building your dream today” is appealing, but the execution needs more transparency.
spiritoftheaerodrome – Aviation heritage and historical preservation with passion.
discovernewworld – I recommend checking for an “About Us” page or contact information to understand the site’s legitimacy.
The Real Person!
The Real Person!
Hi there every one, here every person is sharing these experience, so it’s nice to read this web site, and I used to pay a quick visit this web site every day.
https://kra41c.com
ShopTheBestToday – Customer support helped promptly, answered all questions politely and solved efficiently.
uniquetrendstore – Overall, the concept is promising—worth keeping an eye on, but proceed with caution until more validation is found.
ExploreAmazingIdeas – Website is reliable, deals and tips are always up to date.
The Real Person!
The Real Person!
Hmm is anyone else encountering problems with the images on this blog loading? I’m trying to determine if its a problem on my end or if it’s the blog. Any feed-back would be greatly appreciated.
kra41 cc
FindYourPerfectDeal – Always something new to discover, makes online shopping fun and exciting.
TheBestPlaceToShop – Great selection of items, site makes finding deals really easy.
TrendyFindsHub – Always something new to discover, makes online shopping fun and exciting.
ShopAndSmileAlways – Love this site, always find great deals and unique items.
changeyourworld – Great selection of items, site makes finding deals really easy.
startsomethinggreat – Always something new to discover, makes online shopping fun and exciting.
InspireEverydayLife – Daily posts are inspiring, website always leaves me feeling motivated afterward.
learnshareconnect – Supportive community members who actively contribute to discussions and learning.
findwhatyoulove – Fast shipping, products arrived on time and in perfect condition today.
makelifebetter – Deals are updated frequently, site is reliable and trustworthy overall.
ShopTheLatestTrend – Deals are updated frequently, site is reliable and trustworthy overall.
34crooke – Unique address with distinctive character and presence.
everytrendinone – I keep returning here, always find interesting products quickly and easily.
newseasoncollection – Customer support was helpful, answered all my questions promptly and politely.
yourjourneybegins – Great selection of items, site makes finding deals really easy.
everydayvaluecorner – Easy navigation, site layout makes shopping smooth and enjoyable overall.
DiscoverYourMoment – Signing up was smooth, notifications keep me updated with new content daily.
simplybestchoice – Before relying on it for business or advertising, check for any user reviews or complaints found online.
The Real Person!
The Real Person!
Je suis captive par Olympe Casino, on ressent une energie divine. Les options sont vastes comme un orchestre, proposant des jeux de table glorieux. Avec des depots rapides. Le support client est olympien, offrant des reponses claires. Les gains arrivent sans delai, parfois des recompenses supplementaires seraient eternelles. Au final, Olympe Casino garantit un plaisir divin pour les amateurs de sensations mythiques ! Ajoutons que le site est rapide et glorieux, facilite une immersion totale. Egalement remarquable le programme VIP avec des niveaux exclusifs, renforce le sentiment de communaute.
olympefr.com|
ShopTheBestToday – Fast delivery, products arrived safely and exactly as described online today.
GlobalMarketplaceHub – Signing up was effortless, notifications keep me informed about latest deals.
thinkcreategrow – The concept of “thinking, creating, and growing” is appealing, but the execution needs more transparency.
loftsonlex – Modern urban living with stylish loft accommodations.
makelifebetter – Highly recommend this platform, shopping experience is smooth and enjoyable.
discoverendlessideas – I recommend checking for an “About Us” page or contact information to understand the site’s legitimacy.
changeyourworld – Deals are updated frequently, site is reliable and trustworthy overall.
TheBestPlaceToShop – Prices are reasonable, found exactly what I wanted without any hassle.
startsomethinggreat – Highly recommend this platform, shopping experience is smooth and enjoyable.
ShopAndSmileAlways – Customer support was helpful, answered all my questions promptly and politely.
TrendyFindsHub – Customer support was helpful, answered all my questions promptly and politely.
The Real Person!
The Real Person!
Ich bin beeindruckt von Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Es gibt eine Fulle an aufregenden Titeln, inklusive dynamischer Sportwetten. Der Willkommensbonus ist ein Highlight. Der Support ist schnell und freundlich. Der Prozess ist unkompliziert, jedoch ein paar Freispiele mehr waren super. Insgesamt, Cat Spins Casino sorgt fur kontinuierlichen Spa?. Au?erdem die Oberflache ist glatt und benutzerfreundlich, das Spielvergnugen steigert. Ein starkes Plus sind die schnellen Krypto-Transaktionen, regelma?ige Boni bieten.
Mit dem Surfen beginnen|
dreamcreateinspire – The layout looks clean and modern, but it lacks depth of content, which raises caution.
findwhatyoulove – Always something new to discover, makes online shopping fun and exciting.
The Real Person!
The Real Person!
Ich schatze die Energie bei Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Das Spieleangebot ist reichhaltig und vielfaltig, mit modernen Slots in ansprechenden Designs. Er sorgt fur einen starken Einstieg. Der Kundendienst ist hervorragend. Gewinne werden schnell uberwiesen, von Zeit zu Zeit waren mehr Bonusvarianten ein Plus. Alles in allem, Cat Spins Casino ist ideal fur Spielbegeisterte. Au?erdem die Navigation ist einfach und klar, einen Hauch von Eleganz hinzufugt. Ein tolles Feature die dynamischen Community-Veranstaltungen, die die Gemeinschaft starken.
Zum Ansehen klicken|
everytrendinone – Customer support was helpful, answered all my questions promptly and politely.
everydayvaluecorner – Easy navigation, site layout makes shopping smooth and enjoyable overall.
newseasoncollection – Prices are reasonable, found exactly what I wanted without any hassle.
YourTrustedOnlineStore – Prices are competitive, always find what I need without any trouble.
The Real Person!
The Real Person!
Je suis captive par Ruby Slots Casino, ca offre une experience immersive. Les jeux proposes sont d’une diversite folle, offrant des sessions live palpitantes. Avec des depots fluides. Le suivi est impeccable. Les paiements sont securises et rapides, a l’occasion des recompenses supplementaires seraient parfaites. En fin de compte, Ruby Slots Casino offre une experience inoubliable. Ajoutons aussi la navigation est fluide et facile, apporte une touche d’excitation. Un element fort les evenements communautaires pleins d’energie, renforce le lien communautaire.
Lire plus|
The Real Person!
The Real Person!
Je suis emerveille par Olympe Casino, c’est une plateforme qui resonne comme une lyre. Les options sont vastes comme un orchestre, incluant des paris sportifs epiques. 100% jusqu’a 500 € + tours gratuits. L’assistance est efficace et sage, garantissant un support celeste. Les transactions sont fiables, parfois des bonus plus varies seraient un nectar. Pour conclure, Olympe Casino est une plateforme qui regne sur l’Olympe pour les amateurs de sensations mythiques ! Par ailleurs la plateforme est visuellement olympienne, amplifie le plaisir de jouer. A souligner les options de paris sportifs variees, propose des avantages sur mesure.
https://olympefr.com/|
yourjourneybegins – Prices are reasonable, found exactly what I wanted without any hassle.
The Real Person!
The Real Person!
J’adore la vibe de Sugar Casino, on y trouve une energie contagieuse. Il y a une abondance de jeux excitants, offrant des experiences de casino en direct. 100% jusqu’a 500 € avec des spins gratuits. Le support est fiable et reactif. Les retraits sont simples et rapides, rarement des offres plus consequentes seraient parfaites. En resume, Sugar Casino est un immanquable pour les amateurs. A noter la plateforme est visuellement vibrante, booste l’excitation du jeu. Un plus les evenements communautaires pleins d’energie, assure des transactions fluides.
Aller sur le site|
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Ruby Slots Casino, ca transporte dans un monde d’excitation. Le catalogue de titres est vaste, offrant des experiences de casino en direct. Il booste votre aventure des le depart. Les agents repondent avec rapidite. Le processus est transparent et rapide, toutefois des bonus varies rendraient le tout plus fun. Au final, Ruby Slots Casino merite un detour palpitant. Par ailleurs le site est rapide et engageant, booste l’excitation du jeu. Egalement super le programme VIP avec des recompenses exclusives, qui motive les joueurs.
Obtenir plus|
The Real Person!
The Real Person!
Ich liebe das Flair von Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Die Auswahl ist einfach unschlagbar, mit stilvollen Tischspielen. Er bietet einen gro?artigen Vorteil. Der Service ist von hochster Qualitat. Der Prozess ist klar und effizient, in manchen Fallen gro?ere Angebote waren super. Im Gro?en und Ganzen, Cat Spins Casino garantiert langanhaltenden Spa?. Nebenbei die Seite ist schnell und einladend, das Vergnugen maximiert. Ein gro?es Plus ist das VIP-Programm mit exklusiven Stufen, reibungslose Transaktionen sichern.
Jetzt starten|
The Real Person!
The Real Person!
Ich bin absolut begeistert von Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Es gibt eine riesige Vielfalt an Spielen, mit Spielen, die Krypto unterstutzen. Mit schnellen Einzahlungen. Der Kundendienst ist ausgezeichnet. Auszahlungen sind blitzschnell, dennoch zusatzliche Freispiele waren willkommen. Im Gro?en und Ganzen, Cat Spins Casino bietet ein unvergessliches Erlebnis. Zudem die Benutzeroberflache ist klar und flussig, eine Prise Spannung hinzufugt. Ein attraktives Extra ist das VIP-Programm mit exklusiven Stufen, ma?geschneiderte Vorteile liefern.
http://www.catspins777.de|
LearnSomethingNew – Support team was quick to respond and very helpful today.
The Real Person!
The Real Person!
Je suis fascine par Ruby Slots Casino, il procure une sensation de frisson. Le choix de jeux est tout simplement enorme, comprenant des jeux crypto-friendly. Le bonus de depart est top. Le service d’assistance est au point. Les transactions sont fiables et efficaces, cependant des offres plus consequentes seraient parfaites. En conclusion, Ruby Slots Casino est une plateforme qui pulse. En plus le site est rapide et engageant, incite a prolonger le plaisir. Egalement top les transactions en crypto fiables, propose des privileges sur mesure.
Visiter la page web|
The Real Person!
The Real Person!
Je suis captive par Sugar Casino, ca donne une vibe electrisante. Les options sont aussi vastes qu’un horizon, avec des slots aux designs captivants. 100% jusqu’a 500 € avec des free spins. Les agents sont toujours la pour aider. Les paiements sont surs et fluides, bien que des recompenses supplementaires seraient parfaites. En somme, Sugar Casino est une plateforme qui fait vibrer. Pour completer la navigation est claire et rapide, permet une plongee totale dans le jeu. Un point cle les transactions crypto ultra-securisees, propose des privileges sur mesure.
Lire les dГ©tails|
The Real Person!
The Real Person!
J’adore l’ambiance electrisante de Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. La selection de jeux est impressionnante, incluant des paris sur des evenements sportifs. Il offre un coup de pouce allechant. Les agents sont toujours la pour aider. Les paiements sont securises et instantanes, cependant des bonus plus varies seraient un plus. Dans l’ensemble, Ruby Slots Casino est un lieu de fun absolu. Par ailleurs la navigation est fluide et facile, donne envie de continuer l’aventure. Un point fort le programme VIP avec des niveaux exclusifs, propose des privileges sur mesure.
Commencer Г lire|
BuildStrongRelationship – Site design is clean, navigation feels smooth and intuitive overall.
BusinessConnectWorld – Signing up was quick, interface is intuitive and user-friendly.
Hi there, yup this post is truly nice and I have learned lot of things from it regarding blogging. thanks.
https://bursa138slot.net/melbet-zaregistrirovatsya-2025/
ProfessionalGrowthHub – Customer support answered my questions quickly and made everything clear.
dailyprofitupdate – The daily summaries here really help me keep my strategy consistent.
smartbuytoday – Everything looks modern and organized, really easy to browse without confusion.
classytrendstore – Found some really classy outfits here, quality and fit were both amazing.
smarttradingmentor – I like that the tips are realistic and grounded in actual experience.
brightmarketplace – I like how everything is organized, makes finding items really simple.
bestdealcorner – Everything feels organized and simple, perfect for quick smart shopping.
The Real Person!
The Real Person!
https://zumo-spin-games.com/
bestdealcorner – Prices here are unbeatable, seriously one of the best places to shop.
trendandstyle – Customer support was super helpful when I had a sizing question.
freshstylemarket – The product variety is amazing, always something new to check out.
olympicsbrooklyn – Sports and athletic excellence with community engagement.
MarketTrendAlerts – I keep coming back because the insights actually make a difference.
purebeautyoutlet – Love how clean and soft the design feels, really inviting store.
visionpartnersclub – Great platform for connecting with like-minded people and sharing success tips.
discovergreatvalue – The items look exactly like the photos, no surprises which I love.
protraderacademy – Excellent resource overall, makes trading education actually interesting and approachable.
For most recent news you have to pay a quick visit the web and on world-wide-web I found this site as a finest web page for most up-to-date updates.
https://www.ohlor.com/skachat-melbet-na-telefon-2025/
freshfashionfinds – Love how trendy everything looks, the styles feel current and bold.
uniquefashionboutique – My order came beautifully packed, quality exceeded what I was expecting.
classyhomegoods – Perfect place to find stylish, well-made items that truly elevate any space.
futuregrowthteam – The articles are simple yet powerful, encouraging growth with every read.
yourtradingmentor – I check this site every morning, it keeps me focused and calm.
nextleveltrading – I appreciate how transparent this site is with its trading tips.
modernvaluecorner – My order arrived on time and matched exactly what I saw online.
connectforprogress – I like how the posts encourage growth and self-improvement consistently.
tradingmasterclass – Appreciate how everything is explained simply, even complex chart patterns make sense.
smarttradingmentor – Love the straightforward approach here, trading advice feels clear and actionable.
trendinggiftcorner – Love how this shop has so many unique gift ideas every week.
trendandstyle – Definitely my new go-to shop for stylish, on-point fashion every season.
stylemebetter – I keep coming back here for inspiration, love their seasonal picks.
smartbuytoday – Great customer support experience, quick response and super friendly assistance.
shoplocaltrend – Great browsing experience, feels personal and friendly all the way through.
freshstylemarket – I like how fresh and clean the layout feels, easy to navigate.
bestdealcorner – My package arrived fast, and everything was exactly as described, super happy.
bestdealcorner – Love the product range here, there’s truly something for every category.
visionpartnersclub – This site really pushes positivity and shared vision, love the concept.
brightmarketplace – Found some amazing deals here, everything looks trendy and well-priced.
discovergreatvalue – Great prices and fast delivery, everything came just as described.
freshfashionfinds – Great experience overall, items looked even better in real life honestly.
classyhomegoods – I love browsing this shop, it gives me serious home decor inspiration.
uniquefashionboutique – The pieces I found here are so different from regular stores, amazing.
futuregrowthteam – Great resource for anyone looking to build stronger business partnerships.
dailyprofitupdate – Feels like a trustworthy source for market signals and insights daily.
trendhunterplace – Feels like a treasure hunt every visit, love discovering new stuff.
brandonlangexperts – Expert consulting services with proven results and expertise.
BuildStrongRelationship – Signing up was easy, content is clear and very helpful.
BusinessConnectWorld – Tools are practical and effective, helped me reach new markets.
yourtradingmentor – I check this site every morning, it keeps me focused and calm.
connectforprogress – Great place to find new ideas and connect with positive thinkers.
purebeautyoutlet – I trust this site now after ordering twice, quality stayed consistent.
nextleveltrading – The tutorials are super clear, I learned a lot just reading around.
tradingmasterclass – The course layout feels organized and user-friendly, great for self-paced learning.
trendinggiftcorner – Great quality items, feels like they actually care about presentation and detail.
modernvaluecorner – Feels like they really care about quality, everything arrives perfectly packed.
investsmarttoday – Great place for quick financial tips that actually make sense in practice.
Been checking out keyword lately and it’s actually not bad. Their game selection is pretty decent – spent some time on slots like ‘Book of Wild’ and ‘Coin Win: Hold The Spin.’ The site runs smoothly, which is more than I can say for some เย็ด pgslot other places I’ve tried.
everydaytrendshop – My order arrived quickly and everything looked exactly like the pictures.
teamworkinnovation – The content feels inspiring, packed with ideas for improving teamwork.
globalchoicehub – Definitely one of my go-to shops now for quality and convenience combined.
brahmanshome – Enjoyed reading these posts, ideas are simple yet very useful.
forexstrategyguide – The insights here are practical and actually useful for real trading setups.
yourtradingmentor – Great resource overall, full of useful insights for daily market analysis.
simplelivinghub – Great experience overall, browsing the site actually feels relaxing and organized.
The Real Person!
The Real Person!
Hi, I think your website might be having browser compatibility issues. When I look at your website in Ie, it looks fine but when opening in Internet Explorer, it has some overlapping. I just wanted to give you a quick heads up! Other then that, fantastic blog!
https://devopswithmike.tech/melbet-na-android-2025-obzor-igra/
teamworksuccesspath – Love how they focus on shared growth and mutual understanding in business.
successnetworkgroup – Every section feels well thought out, focusing on community-driven growth.
learnandtrade – Every article feels written with real experience, not just copied theory.
globalmarketinsight – I check this site daily now, helps me understand global shifts better.
uniquedecorstore – Great selection of items that really elevate the look of any space.
successfultradersclub – Love how practical the trading advice is, easy to follow for beginners.
markettrendalerts – I like how the content combines charts, data, and expert analysis efficiently.
88fc là nền tảng cá cược thể thao và casino trực tuyến hàng đầu tại châu Á, mang đến trải nghiệm giải trí an toàn và minh bạch cho người chơi.
The Real Person!
The Real Person!
J’adore l’harmonie de Olympe Casino, c’est une plateforme qui resonne comme une lyre. Les options sont vastes comme un orchestre, avec des slots thematiques antiques. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, offrant des reponses claires. Les gains arrivent sans delai, bien que des bonus plus varies seraient un nectar. Pour conclure, Olympe Casino merite une ascension celeste pour ceux qui aiment parier en crypto ! En bonus l’interface est fluide comme un nectar, ce qui rend chaque session plus celeste. Egalement remarquable les paiements securises en crypto, offre des recompenses eternelles.
https://olympefr.com/|
Heya this is kind of of off topic but I was wondering if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding knowledge so I wanted to get guidance from someone with experience. Any help would be enormously appreciated!
https://artiq.com.co/melbet-prilozhenie-skachat-2025/
jonathanfinngamino – Content is fresh, practical, and engaging for anyone working at home.
motocitee – Enjoyed reading these posts, ideas are simple yet very useful.
adawebcreative – The site’s responsiveness across devices showcases thoughtful design considerations.
paws21airbrushstudio – The attention to detail in their artwork is remarkable.
raspinakala – Site layout is clean, making it very easy to follow instructions.
forexstrategyguide – Clear writing, real examples, and practical strategies make this site outstanding.
utti-dolci – Enjoyed reading these posts, ideas are simple yet very useful.
brahmanshome – Excellent resource, practical guides make home projects feel stress-free always.
pgmbconsultancy – Found exactly what I needed, instructions were clear and concise.
kivurc – Great website, navigation feels smooth and everything is easy today.
abbysauce – Found exactly what I needed, instructions were clear and concise.
everydaytrendshop – This shop feels current and creative, love how they follow latest styles.
The Real Person!
The Real Person!
Ich bin vollig uberzeugt von Cat Spins Casino, es ist ein Ort, der begeistert. Die Spiele sind abwechslungsreich und spannend, inklusive dynamischer Sportwetten. 100 % bis zu 500 € plus Freispiele. Der Kundendienst ist hervorragend. Auszahlungen sind blitzschnell, manchmal mehr Aktionen waren ein Gewinn. Kurz gesagt, Cat Spins Casino ist ein Top-Ziel fur Spieler. Au?erdem die Seite ist schnell und einladend, eine tiefe Immersion ermoglicht. Ein super Vorteil ist das VIP-Programm mit tollen Privilegien, die Gemeinschaft starken.
Cat Spins|
The Real Person!
The Real Person!
Ich liebe das Flair von Cat Spins Casino, es schafft eine mitrei?ende Stimmung. Die Spielauswahl ist ein echtes Highlight, mit Krypto-kompatiblen Spielen. Er macht den Einstieg unvergesslich. Die Mitarbeiter antworten prazise. Gewinne kommen sofort an, gelegentlich mehr Aktionen wurden das Erlebnis steigern. Abschlie?end, Cat Spins Casino ist perfekt fur Casino-Liebhaber. Ubrigens ist das Design stilvoll und modern, das Vergnugen maximiert. Ein Hauptvorteil die regelma?igen Wettbewerbe fur Spannung, die die Motivation erhohen.
Einen Blick werfen|
teamworkinnovation – Every article pushes motivation, teamwork, and forward-thinking solutions.
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es bietet packende Unterhaltung. Es gibt eine enorme Vielfalt an Spielen, mit aufregenden Live-Casino-Erlebnissen. Mit schnellen Einzahlungen. Der Service ist absolut zuverlassig. Auszahlungen sind blitzschnell, manchmal mehr Promo-Vielfalt ware toll. Zum Schluss, Cat Spins Casino sorgt fur ununterbrochenen Spa?. Daruber hinaus ist das Design stilvoll und modern, jeden Moment aufregender macht. Ein starkes Plus die dynamischen Community-Events, zuverlassige Transaktionen sichern.
Inhalt entdecken|
globalchoicehub – The site feels professional and easy to use, really smooth shopping process.
shopandshine – Feels like a premium boutique online, love the classy presentation of items.
simplelivinghub – The site design itself reflects the brand—clean, balanced, and easy to use.
abbysauce – Found exactly what I needed, instructions were clear and concise.
teamworksuccesspath – Love how they focus on shared growth and mutual understanding in business.
successnetworkgroup – Great design overall, makes reading and connecting with others super easy.
dividedheartsofamericafilm – Engaging documentary fostering dialogue on a deeply divisive issue.
uniquedecorstore – I enjoy browsing the collections, keeps giving me fresh decorating inspiration.
The Real Person!
The Real Person!
J’adore l’harmonie de Olympe Casino, ca offre un plaisir melodieux. La variete des titres est divine, avec des slots thematiques antiques. Avec des depots rapides. Le suivi est irreprochable, offrant des reponses claires. Le processus est simple et glorieux, neanmoins des bonus plus varies seraient un nectar. Dans l’ensemble, Olympe Casino offre une experience legendaire pour les fans de casino en ligne ! Ajoutons que la navigation est simple comme un oracle, amplifie le plaisir de jouer. Egalement remarquable le programme VIP avec des niveaux exclusifs, propose des avantages sur mesure.
olympefr.com|
globalmarketinsight – The analysis here feels solid, data-backed, and genuinely useful for traders.
successfultradersclub – Great platform for traders looking to improve consistently with structured guidance.
markettrendalerts – Feels like a professional tool for anyone serious about trading effectively.
The Real Person!
The Real Person!
J’adore la vibe de Ruby Slots Casino, ca offre un plaisir vibrant. Les titres proposes sont d’une richesse folle, incluant des paris sportifs en direct. Il rend le debut de l’aventure palpitant. Le support est pro et accueillant. Les gains arrivent sans delai, quelquefois quelques tours gratuits en plus seraient geniaux. En resume, Ruby Slots Casino est un must pour les passionnes. Notons egalement la navigation est claire et rapide, ce qui rend chaque partie plus fun. Egalement super les paiements en crypto rapides et surs, offre des bonus constants.
Commencer ici|
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Sugar Casino, ca invite a plonger dans le fun. Il y a un eventail de titres captivants, comprenant des titres adaptes aux cryptomonnaies. 100% jusqu’a 500 € + tours gratuits. Le support client est irreprochable. Les paiements sont surs et fluides, mais des offres plus genereuses seraient top. En somme, Sugar Casino assure un fun constant. En extra la plateforme est visuellement vibrante, incite a prolonger le plaisir. Egalement excellent le programme VIP avec des privileges speciaux, offre des recompenses regulieres.
AccГ©der Г la page|
conorjmurphy – Really helpful tips here, saved me hours of unnecessary work.
The Real Person!
The Real Person!
Je suis sous le charme de Ruby Slots Casino, ca invite a l’aventure. Les titres proposes sont d’une richesse folle, offrant des experiences de casino en direct. 100% jusqu’a 500 € plus des tours gratuits. Le service d’assistance est au point. Les retraits sont fluides et rapides, mais encore des bonus plus frequents seraient un hit. Globalement, Ruby Slots Casino garantit un amusement continu. En complement la plateforme est visuellement captivante, facilite une experience immersive. Un plus les transactions en crypto fiables, renforce la communaute.
Aller Г la page|
The Real Person!
The Real Person!
J’ai un faible pour Ruby Slots Casino, ca transporte dans un monde d’excitation. Les options de jeu sont infinies, comprenant des titres adaptes aux cryptomonnaies. 100% jusqu’a 500 € + tours gratuits. Le suivi est d’une precision remarquable. Les paiements sont securises et instantanes, mais encore des recompenses supplementaires seraient parfaites. En resume, Ruby Slots Casino garantit un amusement continu. Par ailleurs l’interface est simple et engageante, facilite une experience immersive. Egalement genial le programme VIP avec des niveaux exclusifs, offre des recompenses regulieres.
Visiter pour plus|
bigjanuarycleanup – Great website, navigation feels smooth and everything is easy today.
learnandtrade – I’ve learned a lot about risk management just from a few sessions here.
The Real Person!
The Real Person!
Ich bin suchtig nach Cat Spins Casino, es entfuhrt in eine Welt voller Nervenkitzel. Das Spieleportfolio ist unglaublich breit, mit klassischen Tischspielen. Er bietet einen tollen Startvorteil. Verfugbar 24/7 fur alle Fragen. Gewinne kommen ohne Verzogerung, manchmal regelma?igere Promos wurden das Spiel aufwerten. Im Gro?en und Ganzen, Cat Spins Casino ist ein Ort, der begeistert. Au?erdem ist das Design stilvoll und modern, zum Verweilen einladt. Ein tolles Feature die regelma?igen Turniere fur Wettbewerbsspa?, die Gemeinschaft starken.
Weiterlesen|
The Real Person!
The Real Person!
Ich bin beeindruckt von der Qualitat bei Cat Spins Casino, es bietet ein mitrei?endes Spielerlebnis. Es gibt eine beeindruckende Anzahl an Titeln, mit eleganten Tischspielen. Er macht den Einstieg unvergesslich. Der Support ist schnell und freundlich. Transaktionen sind immer sicher, gelegentlich mehr Aktionen wurden das Erlebnis steigern. Am Ende, Cat Spins Casino ist perfekt fur Casino-Liebhaber. Zudem die Navigation ist einfach und klar, eine Prise Stil hinzufugt. Ein gro?artiges Plus die vielfaltigen Sportwetten-Optionen, kontinuierliche Belohnungen bieten.
Jetzt besuchen|
The Real Person!
The Real Person!
Ich bin suchtig nach Cat Spins Casino, es schafft eine aufregende Atmosphare. Das Angebot ist ein Paradies fur Spieler, mit dynamischen Wettmoglichkeiten. Er macht den Start aufregend. Der Service ist einwandfrei. Gewinne werden schnell uberwiesen, dennoch mehr regelma?ige Aktionen waren toll. Im Gro?en und Ganzen, Cat Spins Casino ist ein Top-Ziel fur Casino-Fans. Zusatzlich die Plattform ist optisch ansprechend, zum Verweilen einladt. Ein bemerkenswertes Feature ist das VIP-Programm mit einzigartigen Belohnungen, die die Begeisterung steigern.
Tauchen Sie ein|
biotecmedics – Great website, navigation feels smooth and everything is easy today.
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Ruby Slots Casino, on y trouve une energie contagieuse. Le choix est aussi large qu’un festival, offrant des tables live interactives. Il offre un coup de pouce allechant. Les agents sont toujours la pour aider. Les retraits sont simples et rapides, occasionnellement des offres plus importantes seraient super. Au final, Ruby Slots Casino offre une aventure memorable. D’ailleurs la navigation est claire et rapide, donne envie de prolonger l’aventure. Particulierement cool les nombreuses options de paris sportifs, renforce la communaute.
Aller en ligne|
jonathanfinngamino – Love browsing this site, content is always practical and clear.
dinahshorewexler – Really helpful tips here, saved me hours of unnecessary work.
The Real Person!
The Real Person!
J’ai un faible pour Sugar Casino, on y trouve une energie contagieuse. Les jeux proposes sont d’une diversite folle, proposant des jeux de casino traditionnels. Il amplifie le plaisir des l’entree. Les agents repondent avec rapidite. Le processus est transparent et rapide, parfois plus de promos regulieres dynamiseraient le jeu. En fin de compte, Sugar Casino vaut une visite excitante. En complement le site est fluide et attractif, apporte une touche d’excitation. Un point cle les options variees pour les paris sportifs, renforce la communaute.
http://www.sugarcasinoappfr.com|
The Real Person!
The Real Person!
Je suis captive par Ruby Slots Casino, on y trouve une energie contagieuse. Les titres proposes sont d’une richesse folle, proposant des jeux de table sophistiques. Avec des depots rapides et faciles. Le service d’assistance est au point. Les retraits sont ultra-rapides, mais encore des bonus varies rendraient le tout plus fun. Au final, Ruby Slots Casino offre une experience hors du commun. Ajoutons aussi le site est rapide et immersif, ce qui rend chaque moment plus vibrant. Un point cle le programme VIP avec des niveaux exclusifs, qui motive les joueurs.
Voir le site|
paws21airbrushstudio – A go-to studio for unique, high-quality airbrush designs.
ucbstriketowin – A must-visit site for understanding the dynamics of academic labor.
motocitee – Enjoyed reading these posts, ideas are simple yet very useful.
kivurc – Helpful ideas posted here, I’ll definitely share with all friends.
raspinakala – Content is fresh, practical, and engaging for anyone working at home.
alexanderbuonointl – Enjoyed reading these posts, ideas are simple yet very useful.
You are one talented writer thank you for the post.
adawebcreative – A well-crafted site that balances aesthetics and usability effectively.
inkorswimtattoocruise – Excellent resource, practical guides make home projects feel stress-free always.
oldschoolopen – Love browsing this site, content is always practical and clear.
sjydtech – Very good user experience, functionality and look are well balanced.
zz-meta – Excellent explanations, made complex topics feel much easier instantly.
ibdesignstreet – Site layout is clean, making it very easy to follow instructions.
letter4reform – Engaging articles that challenge conventional views on societal issues.
The Real Person!
The Real Person!
kraken сайт Kraken ссылка – это как нить Ариадны, ведущая сквозь темный лес цифрового мира к самому сердцу платформы. Однако, прежде чем довериться этой нити, убедитесь в её прочности и надёжности, ведь в сети подстерегают коварные обманщики, готовые воспользоваться вашей неосторожностью. Проверяйте каждый символ, каждое слово, чтобы не стать жертвой фишинга и не потерять свои сбережения. Помните, что безопасность – это прежде всего ваша личная ответственность.
lotsofonlinepeople – The layout is clean, making browsing through content enjoyable.
wooziewear – Found exactly what I needed, instructions were clear and concise.
chopchopgrubshop – The ambiance complements the flavorful dishes perfectly, creating a memorable experience.
pgmbconsultancy – Found exactly what I needed, instructions were clear and concise.
expandyourhorizon – I appreciate the depth and quality of the articles shared.
Good day! This is kind of off topic but I need some advice from an established blog. Is it very difficult to set up your own blog? I’m not very techincal but I can figure things out pretty quick. I’m thinking about creating my own but I’m not sure where to begin. Do you have any points or suggestions? Cheers
купить номер виртуальный
abbysauce – Great website, navigation feels smooth and everything is easy today.
chopchopgrubshop – Always a pleasure to visit, never disappointed with the meals.
ieeb – Always a pleasure to read, informative and well-written articles.
ieeb – Their posts are timely and relevant, a valuable resource.
lastminute-corporate – User-friendly design enhances the corporate travel booking experience significantly.
kazhanmaster – The content is diverse and engaging, always something new to explore.
broodbase – The layout is clean and user-friendly, making navigation effortless.
conorjmurphy – Helpful ideas posted here, I’ll definitely share with all friends.
wooziewear – Enjoyed reading these posts, ideas are simple yet very useful.
expandyourhorizon – I find their perspectives unique and refreshing, highly informative.
inkorswimtattoocruise – Site layout is clean, making it very easy to follow instructions.
oldschoolopen – Found exactly what I needed, instructions were clear and concise.
lotsofonlinepeople – I enjoy the variety of topics covered here regularly.
letter4reform – Engaging articles that challenge conventional views on societal issues.
chopchopgrubshop – Consistently great food and a welcoming environment every visit.
zz-meta – The team really cares, content quality is consistently impressive.
chopchopgrubshop – Consistently great food and a welcoming environment every visit.
ieeb – I appreciate the depth of analysis in each post.
ieeb – I find their perspectives unique and refreshing, highly informative.
lastminute-corporate – Reliable platform ensuring timely bookings for business trips worldwide.
broodbase – A fantastic platform for gaining knowledge on various topics.
kazhanmaster – I find their perspectives unique and refreshing, highly informative.
Well, I don’t know if that’s going to work for me, but definitely worked for you! 🙂 Excellent post!
sjydtech – Just landed here today, and it already feels welcoming.
buildabetterworld – Navigation is smooth, page loads fast, user experience is solid.
The Real Person!
The Real Person!
code promo 1xbet pour bonus The specific demands reveal nuanced strategies. Gamblers aren’t just looking for any code; they seek the best – “meilleur code promo 1xBet.” The appeal of freebies is undeniable (“code promo 1xBet gratuit,” “code promo 1xBet sans depot”), reflecting a desire to minimize risk and maximize potential returns. Validation is crucial (“code promo 1xBet valide”), assurance that the offered benefit is genuine and not merely a marketing ploy. The “code promo 1xBet 130€” exemplifies the tangible rewards users hope to secure.
makelifesimpler – Great resource for anyone looking to simplify and organize.
styleforliving – Clean layout, great variety and easy to navigate shopping experience.
discovergreatdeals – Product images are clear and the site loads fast.
everydaystylecorner – Found a few items worth adding to my wish-list.
dreambuycollection – The website layout is clean and products look nicely curated.
yourcreativejourney – The checkout process was smooth and payment options looked safe.
theworldawaits – Appreciate the clear shipping info and friendly tone throughout.
shopfornewthings – The product photos are clear and descriptions are helpful.
learnandgrowtoday – I’m looking forward to exploring more of the topics available.
learnshareandgrow – I appreciate how practical and relatable many of the tips are.
findbetteroptions – Pricing looks competitive and the checkout process seems user-friendly so far.
happyshoppingplace – Fun and lively design, the name fits perfectly with the vibe.
The Real Person!
The Real Person!
Ich liebe die Atmosphare bei Cat Spins Casino, es bietet ein mitrei?endes Spielerlebnis. Es gibt eine beeindruckende Anzahl an Titeln, mit spannenden Sportwetten-Angeboten. Er gibt Ihnen einen Kickstart. Die Mitarbeiter sind schnell und kompetent. Zahlungen sind sicher und schnell, manchmal ein paar zusatzliche Freispiele waren klasse. Abschlie?end, Cat Spins Casino bietet ein unvergessliches Erlebnis. Ubrigens ist das Design zeitgema? und attraktiv, das Vergnugen maximiert. Ein super Vorteil die regelma?igen Turniere fur Wettbewerbsspa?, reibungslose Transaktionen sichern.
Details prГјfen|
Автоматические выключатели
buildabetterworld – Will revisit when I’m looking for more unique items.
The Real Person!
The Real Person!
Ich bin absolut begeistert von Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Es gibt zahlreiche spannende Spiele, mit Krypto-kompatiblen Spielen. Mit sofortigen Einzahlungen. Der Service ist absolut zuverlassig. Der Prozess ist unkompliziert, ab und zu mehr Bonusvielfalt ware ein Vorteil. Alles in allem, Cat Spins Casino bietet ein gro?artiges Erlebnis. Daruber hinaus die Plattform ist visuell ansprechend, und ladt zum Verweilen ein. Ein bemerkenswertes Feature ist das VIP-Programm mit exklusiven Stufen, personliche Vorteile bereitstellen.
Plattform besuchen|
styleforliving – I found several items that caught my eye and felt unique.
discovernewthings – Reasonable pricing and fast-loading pages make the experience pleasant.
The Real Person!
The Real Person!
Ich bin total angetan von Cat Spins Casino, es ist ein Ort voller Energie. Das Angebot an Titeln ist riesig, mit dynamischen Wettmoglichkeiten. Er macht den Start aufregend. Der Kundendienst ist hervorragend. Transaktionen sind zuverlassig und effizient, trotzdem gro?ere Boni waren ideal. Zum Schluss, Cat Spins Casino ist ein Highlight fur Casino-Fans. Au?erdem die Seite ist schnell und einladend, jede Session unvergesslich macht. Ein klasse Bonus die dynamischen Community-Events, die Gemeinschaft starken.
Inhalt entdecken|
The Real Person!
The Real Person!
Ich habe eine Leidenschaft fur Cat Spins Casino, es bietet eine Welt voller Action. Das Spieleportfolio ist unglaublich breit, inklusive dynamischer Sportwetten. Er steigert das Spielvergnugen sofort. Erreichbar 24/7 per Chat oder E-Mail. Transaktionen sind zuverlassig und klar, ab und zu regelma?igere Promos wurden das Spiel aufwerten. Im Gro?en und Ganzen, Cat Spins Casino ist perfekt fur Casino-Liebhaber. Hinzu kommt ist das Design modern und einladend, und ladt zum Verweilen ein. Ein starkes Feature die breiten Sportwetten-Angebote, sichere Zahlungen garantieren.
Plattform besuchen|
The Real Person!
The Real Person!
J’adore la vibe de Sugar Casino, il cree un monde de sensations fortes. La variete des jeux est epoustouflante, offrant des sessions live palpitantes. Le bonus initial est super. Le suivi est toujours au top. Les retraits sont lisses comme jamais, bien que quelques free spins en plus seraient bienvenus. Dans l’ensemble, Sugar Casino garantit un plaisir constant. En bonus la navigation est claire et rapide, amplifie l’adrenaline du jeu. A noter le programme VIP avec des niveaux exclusifs, qui booste la participation.
DГ©couvrir davantage|
The Real Person!
The Real Person!
J’adore l’energie de Ruby Slots Casino, il offre une experience dynamique. La selection de jeux est impressionnante, avec des slots aux graphismes modernes. 100% jusqu’a 500 € + tours gratuits. Disponible 24/7 pour toute question. Le processus est fluide et intuitif, en revanche plus de promotions frequentes boosteraient l’experience. En fin de compte, Ruby Slots Casino offre une experience inoubliable. En bonus le design est moderne et energique, incite a prolonger le plaisir. Un avantage le programme VIP avec des niveaux exclusifs, propose des privileges personnalises.
http://www.rubyslotscasinopromocodefr.com|
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Es gibt eine riesige Vielfalt an Spielen, inklusive dynamischer Sportwetten. Er gibt Ihnen einen tollen Boost. Der Service ist immer zuverlassig. Auszahlungen sind schnell und reibungslos, trotzdem mehr Bonusangebote waren spitze. Alles in allem, Cat Spins Casino ist ein absolutes Highlight. Zusatzlich die Benutzeroberflache ist klar und flussig, das Spielerlebnis bereichert. Besonders erwahnenswert die breiten Sportwetten-Angebote, individuelle Vorteile liefern.
Website besuchen|
makelifesimpler – The tips are refreshing and genuinely easy to implement.
The Real Person!
The Real Person!
J’ai une affection particuliere pour Sugar Casino, ca pulse comme une soiree animee. On trouve une profusion de jeux palpitants, offrant des tables live interactives. Avec des depots rapides et faciles. Le suivi est impeccable. Les retraits sont simples et rapides, de temps en temps plus de promotions frequentes boosteraient l’experience. Dans l’ensemble, Sugar Casino est un must pour les passionnes. En complement la navigation est simple et intuitive, booste l’excitation du jeu. Un point cle les paiements securises en crypto, cree une communaute soudee.
Commencer Г naviguer|
yourcreativejourney – A great new shop that seems full of inventive and practical products.
The Real Person!
The Real Person!
Je suis totalement conquis par Ruby Slots Casino, il cree une experience captivante. Il y a une abondance de jeux excitants, avec des machines a sous visuellement superbes. Il offre un coup de pouce allechant. Le suivi est d’une precision remarquable. Les retraits sont ultra-rapides, en revanche plus de promotions variees ajouteraient du fun. Pour conclure, Ruby Slots Casino offre une experience inoubliable. Ajoutons aussi l’interface est intuitive et fluide, ce qui rend chaque partie plus fun. A signaler les tournois reguliers pour la competition, qui booste la participation.
Entrer maintenant|
theworldawaits – The branding feels strong and the overall vibe is trustworthy.
The Real Person!
The Real Person!
Je suis bluffe par Sugar Casino, c’est un lieu ou l’adrenaline coule a flots. Les jeux proposes sont d’une diversite folle, comprenant des jeux crypto-friendly. Il donne un elan excitant. Le service client est de qualite. Les gains sont transferes rapidement, par contre plus de promos regulieres dynamiseraient le jeu. En somme, Sugar Casino vaut une visite excitante. En complement la navigation est simple et intuitive, facilite une immersion totale. Un avantage les options variees pour les paris sportifs, propose des avantages sur mesure.
DГ©couvrir les faits|
discovergreatdeals – The product range is solid and the interface feels polished.
learnandgrowtoday – Great mix of topics and clearly organized for practical use.
The Real Person!
The Real Person!
Ich bin ganz hin und weg von Cat Spins Casino, es verspricht ein einzigartiges Abenteuer. Die Spielauswahl ist beeindruckend, mit Spielautomaten in kreativen Designs. 100 % bis zu 500 € plus Freispiele. Der Kundensupport ist top. Transaktionen sind zuverlassig und klar, ab und zu gro?zugigere Angebote waren klasse. Insgesamt, Cat Spins Casino garantiert dauerhaften Spielspa?. Daruber hinaus ist das Design modern und einladend, eine vollstandige Immersion ermoglicht. Ein starkes Feature die vielfaltigen Wettmoglichkeiten, fortlaufende Belohnungen bieten.
Zur Seite gehen|
The Real Person!
The Real Person!
Ich liebe die Atmosphare bei Cat Spins Casino, es schafft eine aufregende Atmosphare. Die Auswahl ist atemberaubend vielfaltig, mit interaktiven Live-Spielen. Mit schnellen Einzahlungen. Der Kundendienst ist ausgezeichnet. Gewinne werden ohne Wartezeit uberwiesen, allerdings mehr Aktionen waren ein Gewinn. Am Ende, Cat Spins Casino ist ein Ort, der begeistert. Zusatzlich die Benutzeroberflache ist flussig und intuitiv, zum Verweilen einladt. Ein starker Vorteil ist das VIP-Programm mit besonderen Vorteilen, die die Community enger zusammenschwei?en.
Startseite ansehen|
staycuriousdiscover – Inspiring tone, layout encourages creativity and exploration beautifully.
The Real Person!
The Real Person!
Ich bin vollig uberzeugt von Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Es gibt eine beeindruckende Anzahl an Titeln, mit traditionellen Tischspielen. Er bietet einen gro?artigen Vorteil. Die Mitarbeiter antworten schnell und freundlich. Der Prozess ist unkompliziert, dennoch mehr Aktionen wurden das Erlebnis steigern. In Summe, Cat Spins Casino ist ein Top-Ziel fur Spieler. Nebenbei die Navigation ist unkompliziert, eine Note von Eleganz hinzufugt. Ein gro?artiges Bonus die vielfaltigen Sportwetten-Optionen, sichere Zahlungen garantieren.
Details prГјfen|
The Real Person!
The Real Person!
Ich freue mich sehr uber Cat Spins Casino, es ladt zu spannenden Spielen ein. Die Auswahl ist einfach unschlagbar, mit Spielautomaten in kreativen Designs. Er steigert das Spielvergnugen sofort. Der Kundendienst ist hervorragend. Zahlungen sind sicher und schnell, aber haufigere Promos wurden begeistern. Zum Schluss, Cat Spins Casino ist ein Muss fur Spieler. Zusatzlich die Seite ist schnell und ansprechend, und ladt zum Verweilen ein. Ein super Vorteil die regelma?igen Wettbewerbe fur Spannung, die Community enger verbinden.
Jetzt entdecken|
The Real Person!
The Real Person!
J’ai une affection particuliere pour Sugar Casino, c’est une plateforme qui pulse avec energie. Le choix est aussi large qu’un festival, avec des slots aux designs captivants. 100% jusqu’a 500 € plus des tours gratuits. Le suivi est d’une precision remarquable. Les gains sont verses sans attendre, parfois des bonus plus varies seraient un plus. Au final, Sugar Casino est un lieu de fun absolu. En complement le site est fluide et attractif, ajoute une touche de dynamisme. Un atout les evenements communautaires dynamiques, garantit des paiements rapides.
DГ©couvrir dГЁs maintenant|
The Real Person!
The Real Person!
Je suis epate par Ruby Slots Casino, il cree un monde de sensations fortes. La variete des jeux est epoustouflante, offrant des sessions live immersives. Il rend le debut de l’aventure palpitant. Le support est fiable et reactif. Le processus est clair et efficace, de temps a autre des recompenses en plus seraient un bonus. Au final, Ruby Slots Casino est un lieu de fun absolu. D’ailleurs le design est moderne et energique, amplifie l’adrenaline du jeu. Un plus le programme VIP avec des privileges speciaux, propose des avantages uniques.
Aller voir|
The Real Person!
The Real Person!
Je suis completement seduit par Sugar Casino, on y trouve une energie contagieuse. Les jeux proposes sont d’une diversite folle, offrant des experiences de casino en direct. Il donne un elan excitant. Le suivi est d’une precision remarquable. Le processus est simple et transparent, par moments plus de promos regulieres ajouteraient du peps. Dans l’ensemble, Sugar Casino vaut une visite excitante. Par ailleurs le design est moderne et energique, ce qui rend chaque moment plus vibrant. Un atout les options variees pour les paris sportifs, cree une communaute vibrante.
Essayer maintenant|
exploreendlessideas – Modern look, design sparks imagination and a sense of wonder.
The Real Person!
The Real Person!
J’ai un veritable coup de c?ur pour Ruby Slots Casino, il cree une experience captivante. La variete des jeux est epoustouflante, offrant des sessions live immersives. 100% jusqu’a 500 € avec des free spins. Le suivi est d’une fiabilite exemplaire. Les retraits sont fluides et rapides, mais des bonus plus varies seraient un plus. Globalement, Ruby Slots Casino garantit un amusement continu. A souligner le design est tendance et accrocheur, donne envie de continuer l’aventure. Particulierement fun les tournois reguliers pour s’amuser, qui booste la participation.
https://rubyslotscasinologinfr.com/|
shopthetrendtoday – Overall positive first impression—looking forward to seeing how product quality and service match up.
The Real Person!
The Real Person!
J’adore le dynamisme de Sugar Casino, ca invite a l’aventure. La variete des jeux est epoustouflante, avec des slots aux graphismes modernes. Le bonus d’inscription est attrayant. Les agents repondent avec efficacite. Les transactions sont toujours fiables, par ailleurs des bonus plus frequents seraient un hit. En resume, Sugar Casino est une plateforme qui fait vibrer. Notons egalement le design est moderne et attrayant, incite a prolonger le plaisir. Un atout les evenements communautaires vibrants, renforce le lien communautaire.
Obtenir les dГ©tails|
shopfornewthings – Found some neat items I hadn’t seen elsewhere before.
discoverandcreate – Balanced visuals, message promotes creativity and curiosity together.
getinspireddaily – I found the visuals clean and navigation intuitive in initial browsing.
globalstylemarket – Checkout process seems straightforward and payment options appear visible.
connectlearncreate – I’ll bookmark this site for when I’m ready to explore more deeply.
exploreandcreate – The product variety looks good—something for different tastes.
uniquelifestylehub – Checkout appeared straightforward and payment options were clearly displayed.
growwithconfidence – Customer information appears accessible which adds confidence for using this site.
changeyourdirection – Customer support or contact info is accessible — that adds important trust.
discoveryourpotential – Good first impression—hope the content or products deliver value as promised.
fashionloverszone – Customer support/contact information looks accessible, which builds trust.
modernlivingstore – Found some interesting items that seem thoughtfully selected and stylish.
shopwithstylehub – The checkout process seems straightforward and payment options are clearly displayed.
connectwithideas – Checkout seems straightforward and payment options appear transparent.
findjoytoday – I found a few items that might make good gifts—nice variety.
makeimpacttoday – Navigation through the site is smooth and browsing is enjoyable.
dreambuyoutlet – Customer support/contact details visible – good sign for trust and reliability.
findyourdreamplace – Product images are sharp, and descriptions seem helpful and readable.
buildabetterworld – Uplifting concept, site feels full of purpose and positivity.
modernhomefinds – The site presents a clean, stylish layout and feels well-curated.
discoverandcreate – I found the layout is clean and browsing items is pleasant.
imaginelearnexplore – Customer support or contact info appears accessible, which adds trust.
discoveramazingoffers.shop – Great variety of products, really enjoy finding deals every time.
urbanwearstudio – The product range looks appealing—some standout pieces in the roster.
yourmomenttoshine – Pricing and selection look reasonable; hoping product quality and service match.
changeyourdirection – I’ll bookmark this site for the future and revisit when ready to dive in.
purefashionmarket – Overall a positive first impression; looking forward to seeing how service and quality perform.
growwithconfidence – I’ll bookmark this page for future visits when ready to grow.
getinspireddaily – I’ll bookmark this site and check back for quality & shipping practice.
globalstylemarket – Customer support contact appears available, which builds confidence in ordering.
uniquelifestylehub – Pricing seems fair; delivery and quality will determine the full value.
connectlearncreate – Product visuals are sharp and descriptions appear thorough from my quick review.
exploreandcreate – Product visuals are sharp, making browsing feel easy and pleasant.
changeyourperspective – The site feels fresh and inspiring; great message behind the brand.
fashionloverszone – The site has a stylish feel and looks easy to navigate.
modernlivingstore – Checkout appears straightforward and payment options are visible and well-laid-out.
shopwithstylehub – Product visuals are sharp and descriptions appear helpful and clear.
connectwithideas – I’ll bookmark this site for later when I’m ready to explore more.
findjoytoday – Customer support/contact info appears accessible, which adds confidence in using the store.
makeimpacttoday – Overall positive first impression; looking forward to seeing how quality and service perform.
dreambuyoutlet – The layout is clean and inviting; promising selection of items.
inspirechangealways – The site looks encouraging and the layout feels modern and positive.
discoveryourpotential – Good first impression—hope the content or products deliver value as promised.
urbanwearstudio – Trendy and sharp, captures streetwear energy in a polished way.
The Real Person!
The Real Person!
7k casino регистрация игровые автоматы 7к известны высокими выплатами и интересными сюжетами, что привлекает множество игроков.
findsomethingnew – Pricing appears reasonable and the overall site gives a professional, trustworthy impression.
createinspirelead – Clean website layout and inviting theme — promising start.
The Real Person!
The Real Person!
kraken сайт Kraken ссылка – это ключ к заветной двери, ведущей в глубины цифрового пространства, где правят свои законы. Убедитесь в подлинности источника, прежде чем доверить ему свои данные, ведь мошенники не дремлют. Остерегайтесь фишинговых сайтов и поддельных зеркал, чтобы ваше путешествие не обернулось разочарованием. Будьте бдительны и осмотрительны, и тогда сможете избежать неприятностей и насладиться всеми преимуществами платформы.
discoveramazingthings – Exciting name, visuals and content give a sense of adventure.
The Real Person!
The Real Person!
https://body-system.ru/ 7к официальный сайт постоянно пополняется новыми играми и обновлениями для удобства пользователей.
modernhomefinds – Checkout appears easy and payment options are clearly displayed.
happylivingstore.shop – Simple layout makes it easy to find what I want.
findyourdreamplace – The site looks inviting with a fresh design and clear navigation.
discoveramazingoffers.shop – Really enjoy browsing here, so many cool things every week.
urbanwearstudio – Pricing looks competitive, the brand feels aligned with current fashion trends.
The Real Person!
The Real Person!
kraken сайт Kraken market – это пестрый калейдоскоп предложений, где каждый может найти то, что ищет. Но, как и на любом рынке, здесь встречаются не только честные торговцы, но и мошенники, готовые обмануть доверчивых покупателей. Поэтому, прежде чем совершить покупку, тщательно изучите репутацию продавца, сравните цены и не стесняйтесь задавать вопросы. Помните, что ваша бдительность – лучшая защита от обмана и разочарования. Kraken market – это мир возможностей, но и мир ответственности.
findsomethinggreat.shop – Found an amazing gadget today, shipping was fast and reliable.
explorewithpassion – Creative tone, design reflects motivation and energetic discovery.
imaginelearnexplore – The vibe is modern and aligns with growth, learning and creativity.
discoverandcreate – I found the layout is clean and browsing items is pleasant.
purefashionmarket – Product photos are crisp and descriptions appear clear and helpful.
yourmomenttoshine – The overall vibe is clean and modern; a solid first impression.
changan uni v купить https://changan-v-spb.ru
smartshoppinghub – Clean and practical, ideal for modern online shoppers and deals.
discovernewthings – Bright visuals and curious tone make this site engaging.
findjoyeveryday.shop – Website runs smooth, everything feels easy and full of happiness.
classyfindsonline.shop – Beautiful store overall, feels sophisticated yet affordable at the same time.
buildsomethingnew.shop – Perfect place for creative minds, love exploring new building ideas.
findyourmoments.click – Inspiring site overall, encourages living with presence and calm awareness.
smartfashionboutique.shop – Found my new favorite dress here, quality exceeded my expectations.
trendinghomeideas.shop – Perfect spot for interior lovers, every visit brings new inspiration.
startanewjourney.shop – Love the vibe here, truly feels like a fresh new chapter.
yourdailyshoppinghub.shop – Really convenient website, makes everyday shopping fast and stress-free always.
simplevalueplace.shop – Great site overall, simple layout and truly affordable everyday items.
modernlivingtrend.shop – Love the modern home ideas here, everything feels so elegant.
trendingstyleworld.shop – Amazing range of clothes, love how modern and chic everything looks.
staycuriousdiscover.shop – Love the theme here, really encourages learning and open exploration.
yourmomentstarts.click – Love the message here, feels uplifting and full of fresh motivation.
freshfashioncorner.shop – Clothes fit perfectly, love how updated their fashion sense is.
modernlivingstore.click – The collection is impressive, modern yet cozy and inviting overall.
yourmomentstarts.shop – Inspiring and clean design, makes browsing feel uplifting and motivating.
dreambelieveachieve.shop – Truly inspiring space, love the positive energy this site spreads.
classychoiceoutlet.shop – Beautifully curated selection, makes online shopping simple and satisfying always.
thebestpick – Clear branding, every detail feels user-friendly and neatly arranged.
findjoyeveryday.shop – Great selection of uplifting items, makes every purchase feel special.
findyourmoments.click – Great reminder to pause sometimes and enjoy simple happy moments.
changeyourperspective.click – Love how this site challenges my mindset in refreshing ways today.
yourdailyshoppinghub.shop – Prices are awesome, totally my go-to place for online deals.
createyourfuture.click – Inspiring site overall, helps me stay motivated toward long-term goals.
classyfindsonline.shop – Site feels smooth and refined, just like the items they sell.
buildsomethingnew.shop – Every visit gives me fresh inspiration for small weekend projects lately.
trendinghomeideas.shop – This site gives fresh design inspiration, everything looks so modern.
simplevalueplace.shop – Great site overall, simple layout and truly affordable everyday items.
smartfashionboutique.shop – Stylish site design, makes browsing through fashion collections super fun.
startanewjourney.shop – Always a good reminder to stay hopeful and start again confidently.
trendingstyleworld.shop – Love this boutique vibe, everything screams fashion-forward and effortlessly trendy.
modernlivingtrend.shop – The decor trends here always inspire me to redecorate my space.
modernhomefinds.click – Love the stylish home ideas here, everything feels fresh and cozy.
findyourpathnow.shop – Helpful content overall, great place to reset and find inspiration.
staycuriousdiscover.shop – Inspiring content overall, keeps me motivated to explore new ideas.
yourmomentstarts.click – Great vibe overall, reminds me to stay focused and positive daily.
modernlivingstore.click – Great prices for the quality, delivery came faster than expected too.
everydayshoppingzone.shop – Fantastic experience overall, definitely bookmarking this for future orders.
discoveryourpath.shop – I like how it focuses on growth, really feels authentic today.
freshfashioncorner.shop – This site nails the balance between affordability and great modern style.
yourmomentstarts.shop – Really nice concept, everything encourages action and positive daily habits.
exploreendlessideas.shop – Keeps me motivated to try new things, love this creative corner.
dreambelieveachieve.shop – Great reminder to stay focused, everything feels encouraging and uplifting.
getmoreforshop.shop – Love this outlet, always manage to get something useful and cheap.
createinspirelead – Customer support/contact information seems available, which adds confidence for shoppers.
classychoiceoutlet.shop – Found a few amazing deals, delivery was quick and smooth too.
How to win in Calgary Lottery: Boost your chances by playing consistently, joining lottery pools, and choosing less popular combinations. Remember, winning requires luck and responsible play: official win Calgary website
discoveryourpotential.click – Great platform for personal growth, love the positive and uplifting tone.
The Real Person!
The Real Person!
Galera, preciso compartilhar minha experiencia no 4PlayBet Casino porque me pegou de surpresa. A variedade de jogos e de cair o queixo: blackjack envolvente, todos funcionando perfeito. O suporte foi atencioso, responderam em minutos pelo chat, algo que vale elogio. Fiz saque em Bitcoin e o dinheiro entrou na mesma hora, ponto fortissimo. Se tivesse que criticar, diria que mais brindes fariam falta, mas isso nao estraga a experiencia. No geral, o 4PlayBet Casino tem diferencial real. Vale experimentar.
4play massager|
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es bietet einen einzigartigen Kick. Der Katalog ist reichhaltig und variiert, mit immersiven Live-Sessions. Der Service ist von hoher Qualitat, immer parat zu assistieren. Die Transaktionen sind verlasslich, dennoch die Offers konnten gro?zugiger ausfallen. Zum Ende, SpinBetter Casino garantiert hochsten Spa? fur Krypto-Enthusiasten ! Au?erdem die Plattform ist visuell ein Hit, erleichtert die gesamte Erfahrung. Besonders toll die schnellen Einzahlungen, die das Spielen noch angenehmer machen.
spinbettercasino.de|
The Real Person!
The Real Person!
Ich habe einen Narren gefressen an Cat Spins Casino, es verspricht pure Spannung. Es gibt unzahlige packende Spiele, mit klassischen Tischspielen. Er steigert das Spielvergnugen sofort. Der Service ist absolut zuverlassig. Gewinne kommen ohne Verzogerung, aber mehr Bonusangebote waren ideal. Letztlich, Cat Spins Casino ist perfekt fur Casino-Liebhaber. Daruber hinaus die Navigation ist klar und flussig, eine tiefe Immersion ermoglicht. Ein bemerkenswertes Extra ist das VIP-Programm mit einzigartigen Belohnungen, ma?geschneiderte Vorteile liefern.
Website starten|
The Real Person!
The Real Person!
I love the electric vibe at Pinco, it runs on pure adrenaline. The game choice is simply massive, offering interactive live tables. It supercharges your first session. Customer support is world-class. Payments are secure and instant, but a few extra spins would be dope. To close, Pinco is a platform that rocks. Also the site is fast and stylish, keeps you in the zone. One major perk are the broad sports betting markets, that sparks excitement.
Check the site|
The Real Person!
The Real Person!
Je suis bluffe par Sugar Casino, c’est une plateforme qui pulse avec energie. La bibliotheque est pleine de surprises, avec des slots aux designs captivants. Avec des transactions rapides. Le suivi est d’une precision remarquable. Le processus est clair et efficace, toutefois des offres plus consequentes seraient parfaites. En bref, Sugar Casino garantit un amusement continu. Pour couronner le tout la plateforme est visuellement vibrante, incite a rester plus longtemps. Egalement top les options de paris sportifs variees, garantit des paiements rapides.
Aller en ligne|
The Real Person!
The Real Person!
Je suis accro a Sugar Casino, ca transporte dans un monde d’excitation. Les options de jeu sont incroyablement variees, incluant des options de paris sportifs dynamiques. Avec des transactions rapides. Le support est pro et accueillant. Les transactions sont toujours fiables, par contre plus de promos regulieres dynamiseraient le jeu. En fin de compte, Sugar Casino offre une experience inoubliable. Pour couronner le tout la navigation est intuitive et lisse, ajoute une vibe electrisante. A noter les tournois reguliers pour la competition, propose des avantages uniques.
DГ©couvrir les offres|
findsomethinggreat.click – Great place to browse, always end up discovering something unexpectedly cool.
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Sugar Casino, il procure une sensation de frisson. Les options de jeu sont incroyablement variees, offrant des experiences de casino en direct. Il donne un avantage immediat. Les agents repondent avec efficacite. Les paiements sont securises et instantanes, neanmoins des recompenses additionnelles seraient ideales. En resume, Sugar Casino garantit un amusement continu. En extra l’interface est fluide comme une soiree, donne envie de continuer l’aventure. Egalement genial le programme VIP avec des recompenses exclusives, renforce la communaute.
Continuer ici|
classychoiceoutlet.click – Stylish collection overall, love the elegant and affordable fashion pieces.
shopandshinehub.click – Great variety here, products look amazing and prices are reasonable.
The Real Person!
The Real Person!
https://idol.st/user/93203/gratuitscodes/
findbetteroptions.click – Love this site, helps compare and choose smarter shopping decisions easily.
discoveryourpath.click – Beautifully designed site, helps me focus on personal growth daily.
everydayshoppingzone.shop – Checkout process was smooth, received items quickly without any trouble.
dreambuycollection.click – Beautiful selections here, quality items that truly match their descriptions.
The Real Person!
The Real Person!
Ich bin abhangig von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es gibt eine unglaubliche Auswahl an Spielen, mit dynamischen Tischspielen. Der Support ist 24/7 erreichbar, mit praziser Unterstutzung. Die Transaktionen sind verlasslich, gelegentlich mehr Rewards waren ein Plus. In Kurze, SpinBetter Casino ist ein Muss fur alle Gamer fur Casino-Liebhaber ! Zusatzlich die Site ist schnell und stylish, was jede Session noch besser macht. Ein weiterer Vorteil die mobilen Apps, die den Einstieg erleichtern.
https://spinbettercasino.de/|
The Real Person!
The Real Person!
Ich schatze die Spannung bei Cat Spins Casino, es bietet packende Unterhaltung. Das Portfolio ist vielfaltig und attraktiv, mit stilvollen Tischspielen. Er gibt Ihnen einen tollen Boost. Der Kundendienst ist hervorragend. Gewinne kommen sofort an, von Zeit zu Zeit zusatzliche Freispiele waren willkommen. Zusammenfassend, Cat Spins Casino ist perfekt fur Casino-Liebhaber. Hinzu kommt die Plattform ist optisch ein Highlight, das Spielvergnugen steigert. Besonders erwahnenswert ist das VIP-Programm mit tollen Privilegien, personliche Vorteile bereitstellen.
Hier tippen|
The Real Person!
The Real Person!
I’m absolutely hooked on Pinco, it’s a platform that never sleeps. The diversity is mind-blowing, including live sports betting action. 100% up to $500 with bonus spins. Agents respond fast and friendly. Winnings are paid out instantly, rarely more rewards would power up the action. In short, Pinco offers an unforgettable ride. To add the site is fast and immersive, maximizes the fun factor. Particularly notable are the regular tournaments for rivalry, that builds a strong community.
Start exploring|
The Real Person!
The Real Person!
Je suis enthousiasme par Ruby Slots Casino, il cree un monde de sensations fortes. Le choix est aussi large qu’un festival, avec des slots aux graphismes modernes. Avec des depots fluides. Le support est efficace et amical. Les paiements sont surs et efficaces, malgre tout des offres plus genereuses rendraient l’experience meilleure. En resume, Ruby Slots Casino vaut une exploration vibrante. En complement la navigation est fluide et facile, ce qui rend chaque session plus excitante. Particulierement attrayant les paiements en crypto rapides et surs, qui dynamise l’engagement.
Savoir plus|
The Real Person!
The Real Person!
Je suis captive par Ruby Slots Casino, on y trouve une vibe envoutante. Le catalogue est un tresor de divertissements, avec des slots aux designs captivants. Avec des depots rapides et faciles. Disponible 24/7 pour toute question. Les retraits sont lisses comme jamais, mais encore quelques spins gratuits en plus seraient top. En bref, Ruby Slots Casino merite une visite dynamique. Ajoutons aussi l’interface est intuitive et fluide, ce qui rend chaque session plus palpitante. Un element fort les evenements communautaires engageants, qui motive les joueurs.
Aller à l’intérieur|
The Real Person!
The Real Person!
J’adore l’energie de Sugar Casino, on y trouve une vibe envoutante. Les options de jeu sont infinies, incluant des paris sportifs en direct. 100% jusqu’a 500 € + tours gratuits. Disponible 24/7 par chat ou email. Les transactions sont toujours securisees, mais encore quelques tours gratuits supplementaires seraient cool. Dans l’ensemble, Sugar Casino vaut une visite excitante. En extra l’interface est simple et engageante, donne envie de prolonger l’aventure. Particulierement fun les paiements en crypto rapides et surs, qui dynamise l’engagement.
Voir le site|
startsomethingtoday – Feels like a gentle reminder that every goal starts with today.
changeyourdirection – This page feels like a quiet push to start moving differently today.
startsomethingtoday – I like how this site pushes small steps toward real daily progress.
findyourpathnow – The advice here feels grounded and actually doable in real life.
findjoytoday – This feels like a gentle friend reminding me that joy is everywhere.
freshfashioncorner – The collections featured here actually look wearable, not just runway stuff.
happylivingstore – The layout is simple and cheerful, easy to navigate without clutter.
shopwithstylehub – Great fashion finds here, honestly better than what I expected overall.
simplevalueplace – Such a calm layout, easy to read and browse through anytime.
explorethefuture – I like the tone here, it’s curious but still grounded in reality.
learnandgrowtoday – Great resource for anyone trying to stay curious and improve a little daily.
inspirechangealways – The writing style feels open, warm, and totally connects with readers easily.
startanewjourney – I keep revisiting this site because it helps me reset and refocus often.
trendingstyleworld – I enjoy the balance of everyday fashion and high-end inspiration here.
purevalueoutlet – Love how simple the layout is, makes shopping smooth and frustration-free.
modernlivingstore.click – Great store overall, stylish selections perfect for modern home lovers.
getmoreforshop – Found some amazing products here without spending too much, very happy!
everydaygiftcorner.click – Perfect site for gifts, found some lovely options for friends.
connectlearncreate.click – Amazing community vibe, love the focus on creativity and learning together.
everydaystylecorner – The posts feel practical and easy to replicate, very approachable fashion advice.
createinspirelead.click – Love the empowering tone here, truly encourages creativity and leadership.
happylivingstore – Browsing here feels like a mood boost, everything looks fresh and peaceful.
inspirechangealways – Love the name, feels positive and makes me think differently today.
startanewjourney – Great read for anyone going through a transition or new beginning now.
changeyourdirection – I love how honest and encouraging the tone feels throughout the posts.
findjoytoday – Such a positive space, even short reads here lift my mood instantly.
shopwithstylehub – Everything looks sleek and modern, definitely my kind of shopping vibe.
startsomethingtoday – Love how motivating everything feels here, perfect for morning inspiration vibes.
simplevalueplace – Found some great budget-friendly ideas here that still look amazing too.
explorethefuture – Love how the articles mix innovation with real-world examples and ideas.
learnandgrowtoday – Great resource for anyone trying to stay curious and improve a little daily.
startsomethingtoday – I like how this site pushes small steps toward real daily progress.
trendingstyleworld – Great resource for finding what’s trending without endless scrolling or ads.
findyourpathnow – Every post here feels like a gentle nudge toward personal clarity.
freshfashioncorner – Every post gives me styling confidence and that fresh fashion spark again.
purevalueoutlet – Love how simple the layout is, makes shopping smooth and frustration-free.
modernlivingstore.click – Great prices for the quality, delivery came faster than expected too.
yourcreativejourney – Perfect place for anyone looking to unlock new creative energy consistently.
smartfashionboutique.click – Stylish and modern pieces, definitely my go-to for classy fashion.
globalstylemarket – I like how everything blends culture and fashion in such a modern way.
yourdailytrend.click – Great source for everyday fashion inspiration, always something new featured.
everydaystylecorner.click – Love browsing here, always find stylish items that suit my taste.
индексирование страниц
https://algoritmyseo.ru/
getmoreforshop – Found some amazing products here without spending too much, very happy!
trendinghomeideas – The layouts and designs shared feel modern, practical, and totally doable.
everydaystylecorner – Browsing here always gives me fresh inspiration for my everyday wardrobe choices.
buildsomethingnew.click – Inspiring projects here, really motivates me to start new creative ideas.
The Real Person!
The Real Person!
1000 рублей за регистрацию вывод сразу без вложений в казино с выводом без депозита
expandyourhorizon.click – Great site for learning and exploring fresh perspectives every day.
theworldawaits.click – Inspiring content, makes me want to explore and travel everywhere.
inspirechangealways.click – Nice layout and easy to navigate, posts are very thoughtful.
9e2seattle.com – Love the local coverage here, very informative and well-presented overall.
The Real Person!
The Real Person!
1000 рублей за регистрацию в казино без депозита вывод сразу
orourkeforphilly.com – I appreciate that they include a detailed biography and track record; builds trust.
localphlmarket.com – Easy to navigate and checkout worked smoothly, thanks for the good user experience.
nataliakerbabian.com – Overall a thoughtful site that highlights preservation in a modern, artistic way.
garymasino.com – The layout works well on mobile and desktop, good user experience overall.
electcateriarmccabe.com – The biography section highlights relevant legal and public-service experience, nice to see.
chrishallforjudge.com – I appreciate the candidate’s experience and the emphasis on fairness in courts.
smyrnafestival.com – Great resource for planning a visit, thanks for the clear information and visuals.
cumberlandcountynjvaccination.org – Shared this link with friends in the county, they appreciated it too.
connectwithideas.click – Amazing platform, really encourages collaboration and sharing of new innovative ideas.
uncommittednj.org – I liked the volunteer signup section, simple and effective call‑to‑action.
oneillforda.com – Thanks for the clear donation instructions, easy to navigate and secure.
happylifestylehub.shop – I’d recommend monitoring this site over a few weeks for updates before using it fully.
trustedleaderscircle.bond – Easy navigation and clear call to actions made the experience straightforward.
newhorizonsnetwork.shop – Overall impression: solid e-commerce site, I’ll revisit when they update inventory.
successpathway.bond – Overall, a commendable site—looks well done, I’ll revisit for updates.
dibrunobottleshop.com – This site’s offerings are easy to browse, really liked the user experience.
joinourcreativeworld.shop – I didn’t see clear branding or product categories, so navigation is unclear.
shopthelatesttrend.shop – Until full functionality is confirmed, anchoring here might not provide strong value or credibility.
thinkcreategrow.click – The domain is active, but current page shows a directory listing rather than a full site.
exploreamazingideas.click – It’s wise to revisit later to confirm finished content, branding, and trust signals.
inspirechangealways.click – This blog always motivates me, content is inspiring every day.
nsfeg.org – Great resource for learning, very informative and well-structured content overall.
chrishallforjudge.com – It’s good that biographical and policy details are present and easy to find.
remiphl.com – Love the little touches in their plating and the flavors really pop.
The Real Person!
The Real Person!
J’apprecie enormement 7BitCasino, ca procure une sensation de casino unique. Le catalogue est incroyablement vaste, proposant des jeux de table elegants et classiques. Le service d’assistance est de premier ordre, avec un suivi de qualite. Les transactions en cryptomonnaies sont instantanees, bien que les bonus pourraient etre plus reguliers, ou des tournois avec des prix plus eleves. Pour conclure, 7BitCasino est un incontournable pour les amateurs de casino en ligne ! Par ailleurs le design est visuellement attrayant avec une touche vintage, renforce l’immersion totale.
7bitcasino free spins|
kionnawest.com – Inspiring platform, clearly shares vision and values in a compelling way.
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Es gibt eine beeindruckende Anzahl an Titeln, inklusive dynamischer Sportwetten. Er gibt Ihnen einen Kickstart. Der Service ist absolut zuverlassig. Zahlungen sind sicher und schnell, dennoch regelma?igere Promos wurden das Spiel aufwerten. Insgesamt, Cat Spins Casino ist definitiv einen Besuch wert. Zusatzlich die Benutzeroberflache ist klar und flussig, das Vergnugen maximiert. Ein tolles Extra die breiten Sportwetten-Angebote, zuverlassige Transaktionen sichern.
Bringen Sie mich dorthin|
The Real Person!
The Real Person!
Ich bin abhangig von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es wartet eine Fulle spannender Optionen, mit Spielen, die fur Kryptos optimiert sind. Der Support ist 24/7 erreichbar, mit praziser Unterstutzung. Die Auszahlungen sind ultraschnell, ab und an die Offers konnten gro?zugiger ausfallen. Zusammengefasst, SpinBetter Casino bietet unvergessliche Momente fur Casino-Liebhaber ! Daruber hinaus das Design ist ansprechend und nutzerfreundlich, gibt den Anreiz, langer zu bleiben. Besonders toll die Sicherheit der Daten, die Vertrauen schaffen.
spinbettercasino.de|
orourkeforphilly.com – Donation process seems simple and secure, which is important for transparency.
yourdailyshoppinghub.click – Convenient and well-organized store, makes daily shopping fast and enjoyable.
smyrnafestival.com – The photo gallery gave me a good sense of the festival’s atmosphere and energy.
nataliakerbabian.com – Clean design, strong visuals, and a clear voice; makes a standout impression.
happylifestylehub.shop – Navigation appears minimal; users might feel uncertain about site credibility at this stage.
The Real Person!
The Real Person!
Je suis completement seduit par Ruby Slots Casino, ca offre un plaisir vibrant. Le choix de jeux est tout simplement enorme, proposant des jeux de table classiques. Avec des depots instantanes. Disponible 24/7 par chat ou email. Les gains arrivent en un eclair, rarement des recompenses supplementaires seraient parfaites. Au final, Ruby Slots Casino assure un divertissement non-stop. Notons egalement la navigation est claire et rapide, ce qui rend chaque session plus palpitante. Particulierement fun le programme VIP avec des privileges speciaux, offre des bonus constants.
Lire les dГ©tails|
The Real Person!
The Real Person!
Je suis completement seduit par Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. Le choix de jeux est tout simplement enorme, offrant des experiences de casino en direct. Il donne un elan excitant. Le support est rapide et professionnel. Les transactions sont toujours securisees, parfois plus de promos regulieres ajouteraient du peps. En conclusion, Ruby Slots Casino est une plateforme qui pulse. A noter la plateforme est visuellement electrisante, ajoute une vibe electrisante. Un point fort les evenements communautaires vibrants, offre des recompenses continues.
Jeter un coup d’œil|
The Real Person!
The Real Person!
Je suis completement seduit par Sugar Casino, on ressent une ambiance festive. Les titres proposes sont d’une richesse folle, avec des machines a sous visuellement superbes. Il donne un avantage immediat. Le suivi est d’une precision remarquable. Le processus est clair et efficace, en revanche des bonus plus varies seraient un plus. Pour faire court, Sugar Casino offre une experience inoubliable. Pour couronner le tout la navigation est claire et rapide, ajoute une vibe electrisante. Un avantage notable le programme VIP avec des avantages uniques, offre des bonus exclusifs.
Ouvrir le site|
localphlmarket.com – Supporting local Philly artists and makers is such a meaningful initiative.
garymasino.com – I appreciate the clear presentation of his specialties and industry experience.
electcateriarmccabe.com – Donation and contact pages are easy to find, very user-friendly.
trustedleaderscircle.bond – I found the site and the mission feels meaningful with trusted leadership ideals.
newhorizonsnetwork.shop – Appreciate the clear presentation of product images and descriptions — helpful.
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Ruby Slots Casino, on y trouve une vibe envoutante. Les options de jeu sont infinies, comprenant des titres adaptes aux cryptomonnaies. Il donne un elan excitant. Le support est pro et accueillant. Les gains arrivent en un eclair, par contre des bonus varies rendraient le tout plus fun. Pour faire court, Ruby Slots Casino est un choix parfait pour les joueurs. A mentionner la navigation est intuitive et lisse, facilite une experience immersive. A signaler le programme VIP avec des recompenses exclusives, qui motive les joueurs.
Consulter les dГ©tails|
successpathway.bond – Overall, a commendable site—looks well done, I’ll revisit for updates.
joinourcreativeworld.shop – If you’re planning to link here, certifying the store and its offerings might be wise.
shopthelatesttrend.shop – The server-response is a generic file list, indicating the site may be under development.
thinkcreategrow.click – Better to anchor to domains with visible audience, authority and complete site architecture.
exploreamazingideas.click – If you choose to link now, consider it as “pending” until the site completes functionality.
The Real Person!
The Real Person!
Ich bin total angetan von Cat Spins Casino, es sorgt fur pure Unterhaltung. Das Spieleangebot ist reichhaltig und vielfaltig, mit Spielautomaten in beeindruckenden Designs. Der Willkommensbonus ist gro?zugig. Die Mitarbeiter sind immer hilfsbereit. Auszahlungen sind blitzschnell, allerdings mehr Aktionen waren ein Gewinn. Insgesamt, Cat Spins Casino sorgt fur kontinuierlichen Spa?. Ubrigens die Seite ist schnell und attraktiv, eine vollstandige Immersion ermoglicht. Ein weiteres Highlight sind die schnellen Krypto-Transaktionen, reibungslose Transaktionen sichern.
http://www.catspins24.com|
yourdailytrend.shop – Love the trendy picks here, perfect for keeping up with fashion.
cycleforsci.org – Great to see women and diverse scientists featured in your outreach efforts.
Нужно было быстро сгенерировать изображение для презентации, и статья помогла найти идеальное решение. Попробовал несколько вариантов из списка, остановился на самом удобном. Остальные инструменты тоже взял в закладки: https://vc.ru/top_rating/2301994-luchshie-besplatnye-nejroseti-dlya-generatsii-izobrazheniy
haginsforphilly.org – I shared this link with friends because the vision feels grounded and honest.
monumentremoval.org – Important initiative, clearly explains the process and historical context respectfully.
1911phl.com – Great information on local events, keeps me updated with ease.
oneillforda.com – The issues page is well written and easy to follow, good job.
uncommittednj.org – User‑friendly layout, helped me understand the goals without any jargon.
bestvaluecollection.click – Found some excellent deals, products are great quality for affordable prices.
dibrunobottleshop.com – Shipping was prompt and packaging solid, quite impressed with service.
remiphl.com – Great spot for authentic pasta and relaxed vibes, loved it.
n3rdmarket.com – A really good find for the community, love that it supports small makers.
mcalpineinfo.com – Love the detailed info here, very helpful and easy to follow.
natashaforjudge.com – Excellent campaign site, communicates priorities and experience clearly and effectively.
The Real Person!
The Real Person!
Ich freue mich riesig uber Cat Spins Casino, es ist ein Ort, der begeistert. Es gibt zahlreiche spannende Spiele, mit Krypto-freundlichen Titeln. 100 % bis zu 500 € plus Freispiele. Der Kundensupport ist erstklassig. Gewinne kommen sofort an, gelegentlich mehr Bonusoptionen waren top. Insgesamt, Cat Spins Casino ist ein Ort, der begeistert. Nebenbei die Oberflache ist glatt und benutzerfreundlich, eine Prise Stil hinzufugt. Ein gro?er Pluspunkt die regelma?igen Wettbewerbe fur Spannung, die die Community enger zusammenschwei?en.
Hier fortfahren|
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es begeistert mit Dynamik. Es gibt eine enorme Vielfalt an Spielen, mit stilvollen Tischspielen. Der Willkommensbonus ist ein Highlight. Der Kundensupport ist top. Auszahlungen sind schnell und reibungslos, in seltenen Fallen zusatzliche Freispiele waren ein Bonus. Letztlich, Cat Spins Casino ist ideal fur Spielbegeisterte. Zusatzlich die Navigation ist einfach und klar, eine Prise Spannung hinzufugt. Ein wichtiger Vorteil die regelma?igen Turniere fur Wettbewerbsspa?, fortlaufende Belohnungen bieten.
http://www.catspinsbonus.com|
The Real Person!
The Real Person!
Ich habe einen totalen Hang zu SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Der Katalog ist reichhaltig und variiert, mit dynamischen Tischspielen. Der Kundenservice ist ausgezeichnet, bietet klare Losungen. Die Gewinne kommen prompt, trotzdem mehr Rewards waren ein Plus. Alles in allem, SpinBetter Casino ist ein Muss fur alle Gamer fur Casino-Liebhaber ! Zusatzlich die Plattform ist visuell ein Hit, erleichtert die gesamte Erfahrung. Ein weiterer Vorteil die Vielfalt an Zahlungsmethoden, die Vertrauen schaffen.
spinbettercasino.de|
imaginelearnexplore.click – Inspiring content overall, really helps spark creativity and curiosity daily.
The Real Person!
The Real Person!
J’adore le dynamisme de Ruby Slots Casino, on ressent une ambiance festive. Les jeux proposes sont d’une diversite folle, incluant des paris sportifs en direct. Il donne un elan excitant. Disponible a toute heure via chat ou email. Les retraits sont ultra-rapides, neanmoins des recompenses supplementaires dynamiseraient le tout. Dans l’ensemble, Ruby Slots Casino est une plateforme qui pulse. Notons aussi l’interface est lisse et agreable, facilite une experience immersive. Un point fort les evenements communautaires vibrants, garantit des paiements securises.
Savoir plus|
The Real Person!
The Real Person!
J’adore le dynamisme de Sugar Casino, il offre une experience dynamique. Le catalogue de titres est vaste, comprenant des jeux compatibles avec les cryptos. Il offre un coup de pouce allechant. Le service est disponible 24/7. Les transactions sont fiables et efficaces, rarement plus de promos regulieres dynamiseraient le jeu. En conclusion, Sugar Casino assure un fun constant. Notons egalement le site est rapide et style, booste le fun du jeu. Un avantage les paiements en crypto rapides et surs, qui stimule l’engagement.
Trouver les dГ©tails|
The Real Person!
The Real Person!
Je ne me lasse pas de Ruby Slots Casino, il cree un monde de sensations fortes. Les jeux proposes sont d’une diversite folle, avec des machines a sous aux themes varies. Il amplifie le plaisir des l’entree. Le support est fiable et reactif. Les transactions sont toujours securisees, cependant des bonus plus varies seraient un plus. En bref, Ruby Slots Casino garantit un amusement continu. Pour couronner le tout le site est rapide et style, booste le fun du jeu. Egalement super le programme VIP avec des niveaux exclusifs, garantit des paiements securises.
Poursuivre la lecture|
createandgrow.shop – Happy to help find alternatives that are fully live and ready for backlink use right now.
findwhatyoulove.shop – For stronger backlinking, it’s usually better to anchor to domains with visible product listings, trust signals, and a solid design.
hardman4schools.com – Great campaign site, clearly communicates vision and priorities for education.
pmajoe4council.com – Informative site, provides clear insight into policies and council initiatives.
The Real Person!
The Real Person!
Ich bin suchtig nach Cat Spins Casino, es schafft eine mitrei?ende Stimmung. Die Spiele sind abwechslungsreich und spannend, mit eleganten Tischspielen. Er sorgt fur einen starken Einstieg. Die Mitarbeiter sind immer hilfsbereit. Gewinne kommen sofort an, manchmal regelma?igere Promos wurden das Spiel aufwerten. Alles in allem, Cat Spins Casino ist ein Ort, der begeistert. Daruber hinaus die Seite ist schnell und ansprechend, und ladt zum Verweilen ein. Ein gro?er Pluspunkt sind die sicheren Krypto-Zahlungen, die die Community enger zusammenschwei?en.
https://catspins24.com/|
makeimpacttoday.click – Motivational platform, encourages me to take action and make a difference.
modernlivingstyle.shop – If this becomes a full storefront it could be a go-to for décor.
Took me time to read all the comments, but I really enjoyed the article. It proved to be Very helpful to me and I am sure to all the commenters here It’s always nice when you can not only be informed, but also entertained I’m sure you had fun writing this article.
simplybestchoice.click – For now the website feels like it’s just getting started, interesting.
createandgrow.shop – If you do link now, consider it “pending” until the site completes structure and content.
findwhatyoulove.shop – Alternatively, I can find other domains that are **already fully operational** for linking right now.
discovergreatdeals.click – Love this site, always find amazing deals on quality products.
sparxcle.com – Interesting products here, really innovative ideas and practical solutions overall.
modernlivingstyle.shop – Will bookmark this to check back for new collections and stylish finds.
simplybestchoice.click – If the site delivers strong content, it could be worth bookmarking.
The Real Person!
The Real Person!
Rush Street Interactive Colombia SAS, con domicilio principal en la ciudad de Bogotá, Calle 81 No. 11 – 55 Torre Norte Piso 9 – autorizado para operar según contrato de concesión C1972 con Coljuegos. Fecha inicio: 08 06 2023 – Fecha terminación: 07 06 2028 Juega bien. Ser responsable es parte del juego. Jugar sin control causa adicción. Estos programas de software sofisticado garantizan la aleatoriedad de cada resultado, por lo que nos complace tener la opción de ampliar adicionalmente la oferta de crupieres en vivo de Hollywoodbets para abordar los problemas de las reuniones explícitas de clientes. El calendario deportivo se ha desnudado en todos los rincones del mundo, los pronósticos son optimistas. El símbolo comodín del cartel buscado puede reemplazar a todos los demás símbolos, un juego de tragamonedas de video no progresivo de 5 carretes y 9 líneas de pago. Sin embargo, dados.
https://tbablogs.com/sugar-rush-resena-de-un-juego-dulce-y-vibrante-de-pragmatic-play-para-argentina/
de la tercera capa para activar los giros gratis, y comenzarás con 10 giros . Cada símbolo scatter adicional por encima del mínimo de 4 te otorga 1 giro gratis adicional además del mínimo de 10.Reseña de Orb of Osiris La mecánica de capas parece tan obvia cuando la ves en acción, que es difícil creer que ningún desarrollador haya pensado en esto antes. Tal vez sí, por lo que sabemos, pero Live 5 es el primer estudio en convertir la innovación en una realidad tangible. Por alguna razón, no parecen haber registrado la función como marca registrada, y simplemente se llama “Capas” en el juego. Ganas si consigues 3 a 5 símbolos iguales de la tercera capa para activar los giros gratis, y comenzarás con 10 giros . Cada símbolo scatter adicional por encima del mínimo de 4 te otorga 1 giro gratis adicional además del mínimo de 10.Reseña de Orb of Osiris La mecánica de capas parece tan obvia cuando la ves en acción, que es difícil creer que ningún desarrollador haya pensado en esto antes. Tal vez sí, por lo que sabemos, pero Live 5 es el primer estudio en convertir la innovación en una realidad tangible. Por alguna razón, no parecen haber registrado la función como marca registrada, y simplemente se llama “Capas” en el juego. Ganas si consigues 3 a 5 símbolos iguales
leanneslifechangingfairies.com – Beautiful and imaginative site, full of magical ideas for everyone.
simonscider.co.uk – Delicious cider options, love the quality and unique flavor choices here.
globalmarketplacehub.click – On mobile the site loads slowly and lacks clear navigation—user experience is weak.
dankglassonline.com – If you’re into glass gear, this domain might be worth bookmarking for updates.
findyourperfectdeal.click – On mobile also it shows minimal information; user experience could improve.
dailyessentialfinds.bond – For now the trust signals (about page, reviews) are thin or absent.
The Real Person!
The Real Person!
shopthebesttoday.click – Great online store, easy navigation and fantastic product deals daily.
motivilovesmusic.com – Shared this with a friend who loves music brands—they liked the name.
purebeautytrend.shop – On mobile the page loaded but content was minimal and sparse.
everydayvaluecorner.shop – Could be a strong resource once more items are added and trust signals appear.
trustedleaderscircle.bond – The mission of building trusted leadership networks really resonates with me.
successpathway.bond – The branding is clean, yet more content and trust elements should be added.
The Real Person!
The Real Person!
бездепозитный бонус 1000 рублей в казино
learntradingtoday.bond – Excellent resource for beginners, explains trading concepts clearly and simply.
mdcphilly.com – Great local content, keeps community members informed about events and updates.
maltonfestivalofracing.co.uk – Amazing festival coverage, really captures the excitement of racing events beautifully.
everydayvaluecorner.shop – On mobile it loaded well but I wish there were more user reviews and details.
globalmarketplacehub.click – On mobile the site loads slowly and lacks clear navigation—user experience is weak.
findyourperfectdeal.click – The domain is active, but I didn’t find a full storefront or clear branding yet.
dailyessentialfinds.bond – The site caught my eye, though it seems early-stage and content is sparse.
The Real Person!
The Real Person!
1000 бездепозитный бонус
purebeautytrend.shop – If the site builds out product offerings and detailed content, it could be a strong anchor.
motivilovesmusic.com – If they add more content this could be a great resource for fans.
trustedleaderscircle.bond – It would be helpful to see more visible content and member stories soon.
successpathway.bond – If this grows into a complete site, it could be a strong anchor domain.
simplybestchoice.shop – Great product variety, everything feels affordable and high quality overall.
iswrphilly.com – Very professional organization, provides helpful resources and information for all users.
nomnomnomfordogs.com – I shared this with a friend who has a pup—she was very interested.
trabas007hoki – Love the layout here, everything loads super fast and smooth.
playfuel.co.uk – Great gaming products here, everything feels fun, creative, and high-quality.
inspireeverydaylife.shop – The clean layout and design hint at something special; good first impression.
pepplish – Just discovered this brand and the website feels super polished.
learntradingtoday – The lessons feel practical and not overly complicated, just how I like it.
dreambuyworld – The site loads fairly quickly, which gives a good first impression on performance.
flourandoak – I’m looking forward to placing an order, your products seem fantastic.
unitedbygoal – The navigation is intuitive, I found what I needed without fuss.
wrestlingac – I like the subtle aesthetic touches—they feel modern without being flashy.
buildyourdreamtoday – The site loads fast and navigation is smooth, big plus.
cepjournal – While the site has lots of posts, many entries appear to focus on casino-/gambling-type content. :contentReference[oaicite:3]index=3
strategicgrowthalliance – One suggestion: maybe include more testimonials or user feedback for credibility.
forexlearninghub – The branding looks slick and professional, that’s a plus for sure.
thebestplacetoshop.shop – I found something I liked, added to wishlist to see if discount appears.
foruminvestmali – I’d recommend contacting prior participants or organizers directly to gauge the real network value you’ll get.
bestdealsforlife.shop – When it launches fully with products and trust elements, it could become a decent anchor.
togetherwerise – The site looks clean and promising, but I couldn’t find detailed team bios or track record.
The Real Person!
The Real Person!
Estou vidrado no BacanaPlay Casino, e um cassino online que explode como um desfile de carnaval. Tem uma enxurrada de jogos de cassino irados, com slots de cassino tematicos de carnaval. Os agentes do cassino sao rapidos como um passista na avenida, com uma ajuda que brilha como serpentinas. Os saques no cassino sao velozes como um carro alegorico, de vez em quando mais giros gratis no cassino seria uma loucura. Resumindo, BacanaPlay Casino vale demais sambar nesse cassino para os folioes do cassino! Alem disso a navegacao do cassino e facil como um passo de frevo, eleva a imersao no cassino ao ritmo de um tamborim.
casino bacanaplay bГіnus|
nomnomnomfordogs.com – Overall great concept for pet treats, definitely worth checking out.
The Real Person!
The Real Person!
Ich freue mich sehr uber Cat Spins Casino, es verspricht ein einzigartiges Abenteuer. Die Spielesammlung ist uberwaltigend, mit aufregenden Live-Casino-Erlebnissen. Der Bonus fur Neukunden ist attraktiv. Der Service ist absolut zuverlassig. Transaktionen sind zuverlassig und klar, trotzdem regelma?igere Promos wurden das Spiel aufwerten. Kurz gesagt, Cat Spins Casino ist ein Ort, der begeistert. Au?erdem die Seite ist schnell und einladend, einen Hauch von Eleganz hinzufugt. Ein weiteres Highlight die vielfaltigen Wettmoglichkeiten, die Teilnahme fordern.
Fakten entdecken|
dreambuyworld – If you’re ordering internationally, check shipping times and return policy carefully.
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Die Titelvielfalt ist uberwaltigend, mit Spielen, die fur Kryptos optimiert sind. Der Service ist von hoher Qualitat, bietet klare Losungen. Der Ablauf ist unkompliziert, ab und an mehr Rewards waren ein Plus. In Kurze, SpinBetter Casino ist ein Muss fur alle Gamer fur Casino-Liebhaber ! Nicht zu vergessen die Plattform ist visuell ein Hit, erleichtert die gesamte Erfahrung. Hervorzuheben ist die Sicherheit der Daten, die das Spielen noch angenehmer machen.
https://spinbettercasino.de/|
learntradingtoday – Appreciate the clean layout and helpful visuals, makes it more engaging.
harrietlevinmillan.com – Informative and well-written content, really highlights expertise and knowledge clearly.
wrestlingac – I like the subtle aesthetic touches—they feel modern without being flashy.
buildyourdreamtoday – Love how minimal and straightforward everything is, nice user experience.
unitedbygoal – Browsing here was smooth and I appreciated the meaningful tone.
asterixfilm.co.uk – Fantastic movie content, perfect for fans of animation and classic films.
The Real Person!
The Real Person!
Je suis captive par Sugar Casino, on y trouve une energie contagieuse. On trouve une gamme de jeux eblouissante, proposant des jeux de table classiques. Avec des depots instantanes. Disponible 24/7 pour toute question. Le processus est simple et transparent, toutefois des bonus diversifies seraient un atout. Pour conclure, Sugar Casino est un incontournable pour les joueurs. A mentionner l’interface est simple et engageante, donne envie de prolonger l’aventure. Un element fort les paiements securises en crypto, propose des privileges personnalises.
Entrer maintenant|
значення імені міріам
The Real Person!
The Real Person!
J’ai une passion debordante pour Sugar Casino, ca offre un plaisir vibrant. La selection est riche et diversifiee, offrant des experiences de casino en direct. Avec des transactions rapides. Le support client est irreprochable. Les transactions sont fiables et efficaces, parfois des offres plus importantes seraient super. Pour finir, Sugar Casino est un lieu de fun absolu. A noter le site est rapide et style, incite a rester plus longtemps. Un avantage notable les evenements communautaires dynamiques, propose des avantages uniques.
Lancer le site|
strategicgrowthalliance – The visuals and fonts feel modern, good aesthetics overall.
pepplish – This site gives me serious foodie vibes, definitely bookmarking it.
The Real Person!
The Real Person!
Je suis completement seduit par Ruby Slots Casino, ca transporte dans un monde d’excitation. Il y a une abondance de jeux excitants, proposant des jeux de table sophistiques. Le bonus de bienvenue est genereux. Le suivi est impeccable. Les retraits sont ultra-rapides, par moments plus de promotions variees ajouteraient du fun. Globalement, Ruby Slots Casino est un must pour les passionnes. En extra le design est style et moderne, ajoute une touche de dynamisme. Un bonus les tournois reguliers pour s’amuser, qui stimule l’engagement.
Tout apprendre|
forexlearninghub – The branding looks slick and professional, that’s a plus for sure.
The Real Person!
The Real Person!
Je suis completement seduit par Ruby Slots Casino, il propose une aventure palpitante. On trouve une gamme de jeux eblouissante, incluant des paris sur des evenements sportifs. Avec des depots rapides et faciles. Le support est pro et accueillant. Les paiements sont surs et efficaces, neanmoins quelques free spins en plus seraient bienvenus. En bref, Ruby Slots Casino assure un divertissement non-stop. Par ailleurs le design est tendance et accrocheur, donne envie de continuer l’aventure. Particulierement fun le programme VIP avec des recompenses exclusives, garantit des paiements rapides.
DГ©couvrir dГЁs maintenant|
nextrealm.click – Interesting concept, love the futuristic vibe and creative presentation overall.
foruminvestmali – On the user-experience side the site loads okay and has the basic infos, yet I noticed the domain metrics show very low organic traffic. :contentReference[oaicite:2]index=2
inspireeverydaylife.shop – Shared this with my friends who love lifestyle content — they liked the name too.
trabas007hoki – Noticed great improvement lately, site runs smoother than last month.
flourandoak – The website design is clean and inviting, feels very professional.
The Real Person!
The Real Person!
Je suis enthousiasme par Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. La selection de jeux est impressionnante, incluant des paris sportifs pleins de vie. 100% jusqu’a 500 € avec des free spins. Le service client est excellent. Les retraits sont lisses comme jamais, de temps en temps des offres plus consequentes seraient parfaites. En resume, Ruby Slots Casino vaut une exploration vibrante. En extra la navigation est claire et rapide, ce qui rend chaque moment plus vibrant. Un avantage notable le programme VIP avec des privileges speciaux, assure des transactions fiables.
Continuer ici|
The Real Person!
The Real Person!
Ich habe einen Narren gefressen an Cat Spins Casino, es schafft eine mitrei?ende Stimmung. Das Angebot ist ein Paradies fur Spieler, inklusive dynamischer Sportwetten. Mit einfachen Einzahlungen. Der Service ist von hochster Qualitat. Der Prozess ist unkompliziert, gelegentlich mehr Bonusoptionen waren top. Alles in allem, Cat Spins Casino sorgt fur kontinuierlichen Spa?. Daruber hinaus ist das Design stilvoll und einladend, jeden Augenblick spannender macht. Besonders erwahnenswert die breiten Sportwetten-Angebote, kontinuierliche Belohnungen bieten.
Online besuchen|
thebestplacetoshop.shop – Bookmarking to watch for new arrivals, this one looks promising.
dearsparrows.com – Shared this link with a friend who follows betting-industry commentary—they liked it.
bestdealsforlife.shop – When it launches fully with products and trust elements, it could become a decent anchor.
dreamcreateinspire.shop – Beautiful design, love the inspiring message and creative energy here.
pp4fdr.org – Important initiative, shares valuable guidance and updates in a clear manner.
urbanmatrix.click – Modern and stylish concept, offers great insights into urban innovation.
The Real Person!
The Real Person!
Estou completamente apaixonado por BacanaPlay Casino, parece uma festa carioca cheia de axe. A gama do cassino e simplesmente um sambodromo de prazeres, oferecendo sessoes de cassino ao vivo que sambam com energia. O suporte do cassino ta sempre na ativa 24/7, com uma ajuda que brilha como serpentinas. Os pagamentos do cassino sao lisos e blindados, mas mais recompensas no cassino seriam um diferencial festivo. Resumindo, BacanaPlay Casino oferece uma experiencia de cassino que e puro axe para os viciados em emocoes de cassino! De bonus o site do cassino e uma obra-prima de estilo carioca, torna a experiencia de cassino uma festa inesquecivel.
bacanaplay deposit|
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es bietet ein mitrei?endes Spielerlebnis. Die Spielesammlung ist uberwaltigend, mit stilvollen Tischspielen. Er gibt Ihnen einen tollen Boost. Erreichbar 24/7 per Chat oder E-Mail. Der Prozess ist klar und effizient, dennoch mehr Bonusangebote waren spitze. Insgesamt, Cat Spins Casino garantiert dauerhaften Spielspa?. Ubrigens die Navigation ist klar und flussig, zum Verweilen einladt. Ein starkes Feature die haufigen Turniere fur mehr Spa?, ma?geschneiderte Vorteile liefern.
Jetzt entdecken|
trendandstyle.bond – If you plan to link this domain now, consider it “pending readiness” for full value.
DRINKIO приятно удивил профессионализмом и качеством обслуживания. Заказ доставили точно в срок, всё аккуратно упаковано. Курьеры вежливые, видно, что работают с ответственностью. Ассортимент большой, цены разумные. Особенно радует круглосуточная работа — всегда можно оформить заказ. Отличная доставка алкоголя на дом в Москве 24/7 – https://drinkio105.ru/catalog/category/pivo/
ks4thekids.com – Wonderful platform for children, lots of creative learning opportunities provided.
The Real Person!
The Real Person!
Ich schatze die Energie bei Cat Spins Casino, es sorgt fur ein fesselndes Erlebnis. Die Spielauswahl ist ein echtes Highlight, mit traditionellen Tischspielen. Der Willkommensbonus ist ein Highlight. Die Mitarbeiter antworten schnell und freundlich. Gewinne werden schnell uberwiesen, trotzdem mehr Bonusvarianten waren ein Hit. In Summe, Cat Spins Casino ist definitiv einen Besuch wert. Nebenbei die Seite ist schnell und attraktiv, eine vollstandige Eintauchen ermoglicht. Ein besonders cooles Feature sind die schnellen Krypto-Transaktionen, fortlaufende Belohnungen bieten.
Website erkunden|
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Der Katalog ist reichhaltig und variiert, mit innovativen Slots und fesselnden Designs. Die Hilfe ist effizient und pro, immer parat zu assistieren. Die Zahlungen sind sicher und smooth, trotzdem zusatzliche Freispiele waren ein Highlight. Zusammengefasst, SpinBetter Casino ist eine Plattform, die uberzeugt fur Krypto-Enthusiasten ! Nicht zu vergessen die Navigation ist kinderleicht, fugt Magie hinzu. Ein weiterer Vorteil die Community-Events, die den Spa? verlangern.
https://spinbettercasino.de/|
The Real Person!
The Real Person!
Je suis emerveille par Sugar Casino, ca transporte dans un monde d’excitation. Les jeux proposes sont d’une diversite folle, offrant des sessions live immersives. Avec des depots rapides et faciles. Le suivi est impeccable. Le processus est transparent et rapide, a l’occasion plus de promotions frequentes boosteraient l’experience. Pour conclure, Sugar Casino offre une experience hors du commun. D’ailleurs la plateforme est visuellement captivante, booste l’excitation du jeu. Egalement super les nombreuses options de paris sportifs, offre des recompenses continues.
Aller au site|
The Real Person!
The Real Person!
J’ai un veritable coup de c?ur pour Ruby Slots Casino, on ressent une ambiance festive. Le choix est aussi large qu’un festival, avec des slots aux designs captivants. 100% jusqu’a 500 € + tours gratuits. Les agents sont rapides et pros. Le processus est simple et transparent, par contre des offres plus genereuses seraient top. En resume, Ruby Slots Casino est un incontournable pour les joueurs. En bonus le site est rapide et style, ajoute une touche de dynamisme. Un avantage notable les evenements communautaires pleins d’energie, renforce la communaute.
DГ©couvrir les faits|
The Real Person!
The Real Person!
J’adore l’ambiance electrisante de Ruby Slots Casino, on ressent une ambiance de fete. La bibliotheque de jeux est captivante, incluant des paris sur des evenements sportifs. Il booste votre aventure des le depart. Le suivi est impeccable. Les paiements sont surs et fluides, bien que quelques free spins en plus seraient bienvenus. En resume, Ruby Slots Casino est un incontournable pour les joueurs. Notons aussi le site est rapide et immersif, booste le fun du jeu. Un point fort le programme VIP avec des recompenses exclusives, assure des transactions fiables.
Visiter la plateforme|
The Real Person!
The Real Person!
Ich freue mich sehr uber Cat Spins Casino, es ist ein Ort voller Energie. Das Angebot an Titeln ist riesig, mit eleganten Tischspielen. Er bietet einen gro?artigen Vorteil. Der Kundendienst ist hervorragend. Auszahlungen sind schnell und reibungslos, trotzdem mehr Promo-Vielfalt ware toll. Am Ende, Cat Spins Casino sorgt fur ununterbrochenen Spa?. Daruber hinaus die Benutzeroberflache ist klar und flussig, eine immersive Erfahrung ermoglicht. Ein klasse Bonus sind die schnellen Krypto-Transaktionen, die die Motivation erhohen.
Jetzt ansehen|
envolavecning – One suggestion: more background story or designer bio would help me connect deeper.
rebeccacaridad-manzanita – The mix of color, light, and texture in the visuals is very well executed.
theperfectgiftshop – Great for finding gift ideas, just make sure payment and delivery options suit your region.
lifestyleinspirationhub – Browsing was smooth, categories are clear and site loads quickly.
engagement-forum – It may well be a worthwhile opportunity, just treat it with measured optimism until more info is available.
yourdailyupdate – The homepage looks polished, but I couldn’t immediately verify much detail about the business behind it.
moesboardwalk – The site feels casual and fun—great for a night out or group event.
bloemhill – Love the aesthetic: clean, fresh and very boutique-style.
trendinsight – The homepage has a good first impression, clean layout and modern visuals.
trendyfindshub – Overall, a promising e-shop with stylish appeal—just do a bit of due diligence before purchasing.
connectdiscovergrow – The visuals are clean and modern, which builds confidence at first glance.
getinspiredtoday – One suggestion: add more company background or transparency about the business to build trust.
rosetemplates – If you’re doing design work or branding this could be a useful resource.
joebobsaveschristmas – Checked out the site and the holiday horror theme is really fun and unique.
sega-live – On the positive side, the website is professionally designed and loads well, which is a good start.
28darlingstreet – If you’re travelling internationally, check the menu specials or seasonal changes so you pick the right time to go.
madmadedesigns – The website looks crisp and the product photos are very clean and appealing.
The Real Person!
The Real Person!
интим бутик премиум класса Секс-шоп Erross интим-магазин товаров для взрослых с анонимной доставкой по Москве и РФ.
rosetemplates – Might be worth checking whether the licensing is clear for commercial use and downloads.
28darlingstreet – I appreciate the sample menus—they make me confident I know what to expect. :contentReference[oaicite:3]index=3
lifestyleinspirationhub – Would love to see more detailed product info and user reviews for confidence.
moesboardwalk – Suggestion: post some attendee reviews or event clips to build more trust and hype.
bloemhill – Great visuals—but it would help if they included customer reviews or sample projects.
theperfectgiftshop – Great for finding gift ideas, just make sure payment and delivery options suit your region.
yourdailyupdate – If you’re planning to purchase, I’d recommend checking payment security and return policy carefully.
The Real Person!
The Real Person!
kraken сайт Kraken ссылка – это навигатор по бескрайним просторам даркнета, указывающий путь к заветной цели. Однако, доверять первой попавшейся ссылке равносильно прыжку в пропасть с завязанными глазами. Прежде чем сделать этот шаг, убедитесь в её подлинности, проверьте репутацию источника и подготовьтесь к возможным неожиданностям. Безопасность превыше всего, и только внимательность и осторожность помогут вам избежать неприятных сюрпризов.
trendinsight – I like the range of content, but I couldn’t immediately find strong “about us” or company background details.
trendyfindshub – If they offer international shipping, that would be a major plus for global customers.
connectdiscovergrow – The visuals are clean and modern, which builds confidence at first glance.
getinspiredtoday – I’d proceed, but start with a smaller order to test how smoothly everything works (delivery, service, quality).
XN88 là một cổng game đổi thưởng và nhà cái trực tuyến mới thành lập (2025), cung cấp hơn 2.500 tựa game đa nền tảng, nổi bật với bảo mật mạnh, giao diện thân …
envolavecning – The brand’s name “Envol Avec Ning” means taking off with Ning, which is a nice touch of identity. :contentReference[oaicite:1]index=1
rebeccacaridad-manzanita – Just visited the site and the artistic photography and painting sections caught my eye immediately.
brauhausschmitzoktoberfest.com – Fantastic festival coverage, really captures the fun and lively Oktoberfest spirit.
engagement-forum – For users outside the core region it serves: check logistic factors, time zones, costs and refund policies.
Lc88 – Nền tảng giải trí trực tuyến ăn khách nhất châu Á, nơi người chơi tận hưởng trải nghiệm cá độ thể thao, casino và slot game trong môi trường an toàn.
madmadedesigns – Overall a promising site with good visual appeal—just proceed with standard caution for new-to-you online shops.
The Real Person!
The Real Person!
kraken сайт Kraken market – это пестрый калейдоскоп предложений, где каждый может найти то, что ищет. Но, как и на любом рынке, здесь встречаются не только честные торговцы, но и мошенники, готовые обмануть доверчивых покупателей. Поэтому, прежде чем совершить покупку, тщательно изучите репутацию продавца, сравните цены и не стесняйтесь задавать вопросы. Помните, что ваша бдительность – лучшая защита от обмана и разочарования. Kraken market – это мир возможностей, но и мир ответственности.
I really like your writing style, excellent info , thanks for putting up : D.
joebobsaveschristmas – The branding is memorable and the URL stands out which is nice.
hopprojects – I appreciate efforts to make “white walls” less exclusive and art more relatable — great idea.
sega-live – Without strong evidence of fulfilment history, I’d treat it as a higher-risk choice rather than a trusted brand.
macofficelovesyou – The site name made me smile, and the layout feels very nostalgic yet modern.
clubinorout.com – Great nightlife guide, keeps me updated on clubs and events easily.
The Real Person!
The Real Person!
smartshoppingplace – The site feels like a solid all-in-one store, easy to browse and inviting.
beauty-optical-salon – The product photography is stunning, makes me want to visit the store.
amazingdealscorner – Solid deal-site vibe; browsing was smooth and categories clearly laid out.
everydayinnovation – The brand name sounds inspiring and modern; curious to browse their collection and see how it lives up to the promise.
The Real Person!
The Real Person!
Estou alucinado com BetorSpin Casino, parece uma explosao cosmica de adrenalina. O catalogo de jogos do cassino e uma nebulosa de emocoes, com slots de cassino tematicos de espaco sideral. O servico do cassino e confiavel e brilha como uma galaxia, dando solucoes na hora e com precisao. Os pagamentos do cassino sao lisos e blindados, porem as ofertas do cassino podiam ser mais generosas. Resumindo, BetorSpin Casino garante uma diversao de cassino que e uma supernova para os apaixonados por slots modernos de cassino! Alem disso a interface do cassino e fluida e reluz como uma constelacao, torna a experiencia de cassino uma viagem espacial.
betorspin bГіnus|
jojoanime10th – Looks like a fun anime-themed project; the visuals really pop with color.
The Real Person!
The Real Person!
Ich habe einen Narren gefressen an Cat Spins Casino, es schafft eine elektrisierende Atmosphare. Es gibt eine beeindruckende Anzahl an Titeln, mit interaktiven Live-Spielen. 100 % bis zu 500 € und Freispiele. Der Support ist effizient und professionell. Gewinne kommen sofort an, von Zeit zu Zeit mehr Aktionen waren ein Gewinn. In Summe, Cat Spins Casino sorgt fur ununterbrochenen Spa?. Au?erdem die Seite ist schnell und attraktiv, jede Session unvergesslich macht. Ein super Vorteil die dynamischen Community-Events, kontinuierliche Belohnungen bieten.
http://www.catspinsbonus.com|
The Real Person!
The Real Person!
Ich habe einen totalen Hang zu SpinBetter Casino, es bietet einen einzigartigen Kick. Das Angebot an Spielen ist phanomenal, mit dynamischen Tischspielen. Die Hilfe ist effizient und pro, bietet klare Losungen. Die Zahlungen sind sicher und smooth, gelegentlich regelma?igere Aktionen waren toll. Alles in allem, SpinBetter Casino bietet unvergessliche Momente fur Adrenalin-Sucher ! Daruber hinaus die Interface ist intuitiv und modern, gibt den Anreiz, langer zu bleiben. Zusatzlich zu beachten die Community-Events, die den Spa? verlangern.
spinbettercasino.de|
The Real Person!
The Real Person!
Je suis accro a Sugar Casino, il propose une aventure palpitante. Il y a un eventail de titres captivants, proposant des jeux de cartes elegants. Il offre un coup de pouce allechant. Le service client est excellent. Le processus est fluide et intuitif, de temps a autre quelques free spins en plus seraient bienvenus. Au final, Sugar Casino est un immanquable pour les amateurs. De surcroit l’interface est fluide comme une soiree, ajoute une vibe electrisante. Particulierement fun les evenements communautaires pleins d’energie, qui stimule l’engagement.
Consulter les dГ©tails|
The Real Person!
The Real Person!
J’adore l’energie de Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. Le choix est aussi large qu’un festival, incluant des paris sportifs en direct. 100% jusqu’a 500 € avec des spins gratuits. Les agents repondent avec efficacite. Les transactions sont fiables et efficaces, occasionnellement des recompenses supplementaires dynamiseraient le tout. En conclusion, Ruby Slots Casino merite un detour palpitant. Pour completer la plateforme est visuellement electrisante, ce qui rend chaque session plus excitante. Egalement excellent les nombreuses options de paris sportifs, propose des avantages uniques.
Continuer ici|
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Sugar Casino, ca invite a l’aventure. Les options sont aussi vastes qu’un horizon, incluant des paris sportifs pleins de vie. Le bonus initial est super. Le suivi est d’une precision remarquable. Les gains sont verses sans attendre, neanmoins quelques tours gratuits en plus seraient geniaux. En somme, Sugar Casino est un incontournable pour les joueurs. D’ailleurs l’interface est lisse et agreable, booste le fun du jeu. Un element fort les tournois frequents pour l’adrenaline, propose des avantages uniques.
Aller sur le site|
The Real Person!
The Real Person!
Ich liebe die Atmosphare bei Cat Spins Casino, es sorgt fur ein fesselndes Erlebnis. Es gibt unzahlige packende Spiele, mit Spielautomaten in kreativen Designs. Er macht den Start aufregend. Der Service ist einwandfrei. Der Prozess ist einfach und transparent, manchmal gro?ere Boni waren ein Highlight. Alles in allem, Cat Spins Casino ist ein Highlight fur Casino-Fans. Daruber hinaus ist das Design stilvoll und einladend, jeden Moment aufregender macht. Ein gro?es Plus die vielfaltigen Sportwetten-Optionen, die Community enger verbinden.
Genauer ansehen|
shapeyourdreams – The phrase gets me motivated! Would like to see more about the product story or what makes it unique.
iswrphilly – The community-focused vibe here feels genuine, great to see local engagement online.
That’s some inspirational stuff. Never knew that opinions might be this varied. Thanks for all the enthusiasm to supply such helpful information here.
dreamdealsstore – Found some amazing bargains today, really happy with this shop.
findsomethingamazing – Smooth shopping experience, great finds and easy checkout process.
thebestvalue – Really enjoy browsing here, found some excellent deals and reliable offers.
The Real Person!
The Real Person!
Ich bin vollig uberzeugt von Cat Spins Casino, es bietet ein immersives Erlebnis. Das Portfolio ist vielfaltig und attraktiv, mit Spielen fur Kryptowahrungen. Er bietet einen tollen Startvorteil. Der Kundendienst ist hervorragend. Gewinne werden schnell uberwiesen, manchmal mehr Bonusvarianten waren ein Hit. Letztlich, Cat Spins Casino ist ein absolutes Highlight. Ubrigens die Seite ist schnell und einladend, das Spielerlebnis bereichert. Ein tolles Extra die spannenden Community-Aktionen, personliche Vorteile bereitstellen.
Informationen erhalten|
brightstylecorner – Love how colorful and inspiring the collection looks this season.
findyourowngrowth – Such a refreshing site, always encouraging people to be their best self.
globalfashionfinds – This site gives major fashion inspo, love browsing through the looks.
findbetteropportunities – Love the clear layout and powerful ideas shared across this site.
findyourfocus – Great site for mental clarity tips, everything’s short, simple, and useful.
everydaychoicehub – Found some helpful tips and featured choices, really enjoyed browsing through.
discovernewhorizons – Everyday reading that feels fresh and exciting, love the vibe of this site.
shapeyourdreams – Always end up feeling motivated after visiting this space — thank you!
trendylifestylehub – The visuals here are so clean and the writing very friendly and relatable.
globalfashionfinds – Always end up finding cool fashion ideas whenever I visit this site.
findyourinspiration – The writing style here feels genuine and not just generic-inspirational, which I appreciate.
The Real Person!
The Real Person!
Ich bin beeindruckt von der Qualitat bei Cat Spins Casino, es entfuhrt in eine Welt voller Spa?. Es gibt eine enorme Vielfalt an Spielen, mit Live-Sportwetten. Er gibt Ihnen einen tollen Boost. Der Support ist effizient und professionell. Gewinne werden ohne Wartezeit uberwiesen, allerdings regelma?igere Promos wurden das Spiel aufwerten. Zum Schluss, Cat Spins Casino bietet ein gro?artiges Erlebnis. Au?erdem die Seite ist schnell und ansprechend, eine vollstandige Eintauchen ermoglicht. Ein starker Vorteil sind die sicheren Krypto-Transaktionen, reibungslose Transaktionen sichern.
https://catspinsbonus.com/|
trendsettersparadise – The layout is clean, modern, and the fashion picks are spot on.
The Real Person!
The Real Person!
Ich bin komplett hin und weg von SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Der Katalog ist reichhaltig und variiert, mit innovativen Slots und fesselnden Designs. Der Kundenservice ist ausgezeichnet, bietet klare Losungen. Die Auszahlungen sind ultraschnell, obwohl regelma?igere Aktionen waren toll. Alles in allem, SpinBetter Casino ist ein Muss fur alle Gamer fur Krypto-Enthusiasten ! Hinzu kommt das Design ist ansprechend und nutzerfreundlich, fugt Magie hinzu. Hervorzuheben ist die Sicherheit der Daten, die das Spielen noch angenehmer machen.
https://spinbettercasino.de/|
The Real Person!
The Real Person!
лучший секс шоп России Секс-шоп Erross интим-магазин товаров для взрослых с анонимной доставкой по Москве и РФ.
The Real Person!
The Real Person!
J’ai un faible pour Sugar Casino, on ressent une ambiance de fete. Le choix de jeux est tout simplement enorme, proposant des jeux de casino traditionnels. Le bonus d’inscription est attrayant. Le suivi est impeccable. Les retraits sont lisses comme jamais, cependant plus de promos regulieres ajouteraient du peps. En conclusion, Sugar Casino offre une experience hors du commun. Par ailleurs la plateforme est visuellement captivante, ce qui rend chaque partie plus fun. A souligner les options de paris sportifs diversifiees, renforce la communaute.
Apprendre les dГ©tails|
The Real Person!
The Real Person!
J’ai un veritable coup de c?ur pour Ruby Slots Casino, ca offre une experience immersive. Il y a un eventail de titres captivants, incluant des paris sportifs en direct. Il donne un avantage immediat. Le suivi est d’une fiabilite exemplaire. Les retraits sont simples et rapides, cependant des bonus varies rendraient le tout plus fun. Pour finir, Ruby Slots Casino offre une aventure memorable. Pour completer le design est style et moderne, permet une immersion complete. Particulierement fun les competitions regulieres pour plus de fun, qui booste la participation.
Aller en ligne|
hanayaka-na-life – Elegant and serene design, the Japanese aesthetic truly shines through beautifully.
everydaychoicehub – “Choice hub” feels like a central spot for variety—nice for finding items you might not expect.
everymomentmatters – The name hits emotionally, good for meaningful purchases; just check the fine details.
The Real Person!
The Real Person!
Je suis bluffe par Sugar Casino, ca transporte dans un monde d’excitation. Le catalogue est un paradis pour les joueurs, proposant des jeux de casino traditionnels. Avec des transactions rapides. Le support client est irreprochable. Le processus est transparent et rapide, neanmoins des bonus plus varies seraient un plus. Pour finir, Sugar Casino est un incontournable pour les joueurs. Pour couronner le tout la plateforme est visuellement captivante, apporte une touche d’excitation. Egalement super les tournois reguliers pour la competition, cree une communaute vibrante.
Lire la suite|
The Real Person!
The Real Person!
Ich bin begeistert von der Welt bei Cat Spins Casino, es schafft eine elektrisierende Atmosphare. Die Spielauswahl ist beeindruckend, mit eleganten Tischspielen. Mit einfachen Einzahlungen. Die Mitarbeiter sind immer hilfsbereit. Gewinne kommen ohne Verzogerung, jedoch ein paar zusatzliche Freispiele waren klasse. Im Gro?en und Ganzen, Cat Spins Casino garantiert langanhaltenden Spa?. Zudem die Plattform ist optisch ein Highlight, das Spielerlebnis steigert. Ein tolles Feature ist das VIP-Programm mit besonderen Vorteilen, zuverlassige Transaktionen sichern.
Hier klicken|
dreamdealsstore – Fast-loading site, neat design and amazing daily offers to grab.
findsomethingamazing – Love browsing here, every visit brings something unique and creative.
thebestvalue – The site looks professional and the value is obvious from the first glance.
brightstylecorner – Great layout and clean design, browsing through products feels smooth.
everydaychoicehub – Everyday choices feel simplified and smart through this platform, nice find.
modernstylemarket – Great name and the design looks sharp—perfect for trend-savvy shoppers.
shopwithhappiness – Love the cheerful tone; browsing here actually feels positive and easygoing.
discovernewhorizons – Clear design, meaningful messages—this site stands out for thoughtful content.
findbetteropportunities – The writing style feels honest and motivating, really like the message.
shapeyourdreams – Simple yet powerful content, exactly what I needed this morning.
trendylifestylehub – Easy to navigate site, found some new favorites to follow and bookmark.
globalfashionfinds – The quality and selection are really impressive for an online store.
findyourinspiration – Found practical advice here that actually felt relatable and useful today.
findyourowngrowth – Great reads here, every article feels inspiring and really well written.
discovergreatoffers – Straight to the point: you’re promised “great offers”—good for bargain-hunters if service and shipping align.
globalfashionfinds – Always end up finding cool fashion ideas whenever I visit this site.
findyourfocus – Always find great insights here about productivity and mindful living.
trendsettersparadise – Always find something unexpected and stylish when I visit this site.
urbanwearoutlet – Stylish urban fashion outlet feel, lots of potential if the sizing and quality hold up.
dailychoicecorner – The variety of items surprised me, it’s like a cozy online bazaar.
opennewdoors – A metaphorical name that feels encouraging; ideal for exploration or finding fresh options.
inspiredthinkinghub – I like how they frame the brand as a place for ideas, not just shopping.
startyourjourneytoday – The motivational tone of the brand instantly boosts your mood while browsing.
learnexploreachieve – Inspiring brand name; if they deliver educational tools or courses this could be a good fit.
dreamcreateachieve – Full of ambition in one line—makes me think this brand supports creative and lifestyle change.
Wow that was odd. I just wrote an very long comment but after I clicked submit my comment didn’t appear. Grrrr… well I’m not writing all that over again. Anyway, just wanted to say superb blog!
https://dumka.org.ua/iak-ne-kupyty-pidrobku-haid-po-brendakh-bi-led-lin.html
learnsomethingeveryday – Found this site recently, great place to pick up quick facts.
creativegiftplace – So many thoughtful gift ideas; perfect for finding something personal and unique.
The Real Person!
The Real Person!
Ich bin begeistert von der Welt bei Cat Spins Casino, es schafft eine aufregende Atmosphare. Das Angebot ist ein Paradies fur Spieler, mit Spielen fur Kryptowahrungen. 100 % bis zu 500 € inklusive Freispiele. Der Support ist zuverlassig und hilfsbereit. Gewinne werden ohne Wartezeit uberwiesen, ab und zu regelma?igere Promos wurden das Spiel aufwerten. Am Ende, Cat Spins Casino bietet ein unvergessliches Erlebnis. Zudem die Navigation ist klar und flussig, zum Verweilen einladt. Ein gro?er Pluspunkt die regelma?igen Turniere fur Wettbewerbsspa?, kontinuierliche Belohnungen bieten.
Details lernen|
The Real Person!
The Real Person!
Galera, nao podia deixar de comentar no 4PlayBet Casino porque me impressionou bastante. A variedade de jogos e simplesmente incrivel: roletas animadas, todos com graficos de primeira. O suporte foi bem prestativo, responderam em minutos pelo chat, algo que passa seguranca. Fiz saque em Ethereum e o dinheiro entrou mais ligeiro do que imaginei, ponto fortissimo. Se tivesse que criticar, diria que mais brindes fariam falta, mas isso nao estraga a experiencia. Pra concluir, o 4PlayBet Casino tem diferencial real. Com certeza vou continuar jogando.
18×9 4play wheels|
urbanstylemarket – Great site for seasonal fashion, love how the collections are updated.
trendylifestylehub – Trend-forward lifestyle brand angle worked for me, very modern design.
findsomethingunique – The promise of uniqueness is appealing—if the products deliver, this could be a go-to for special finds.
brightnewbeginnings – The name gives a fresh start vibe; nice for seasonal or motivational purchases.
classytrendcollection – The products feel trendy yet elegant, really fits the “classy” branding well.
The Real Person!
The Real Person!
Ich bin total hingerissen von Cat Spins Casino, es ladt zu spannenden Spielen ein. Die Spielauswahl ist beeindruckend, mit interaktiven Live-Spielen. 100 % bis zu 500 € inklusive Freispiele. Die Mitarbeiter sind schnell und kompetent. Auszahlungen sind schnell und reibungslos, allerdings mehr Bonusvarianten waren ein Hit. Kurz gesagt, Cat Spins Casino garantiert langanhaltenden Spa?. Daruber hinaus die Seite ist schnell und attraktiv, zum Weiterspielen animiert. Ein weiteres Highlight ist das VIP-Programm mit einzigartigen Belohnungen, individuelle Vorteile liefern.
Online besuchen|
The Real Person!
The Real Person!
J’adore l’energie de Sugar Casino, ca transporte dans un monde d’excitation. La selection de jeux est impressionnante, avec des machines a sous aux themes varies. Il offre un coup de pouce allechant. Les agents repondent avec efficacite. Le processus est simple et transparent, cependant quelques free spins en plus seraient bienvenus. Dans l’ensemble, Sugar Casino vaut une visite excitante. En bonus le site est rapide et immersif, ce qui rend chaque session plus excitante. Un bonus les transactions crypto ultra-securisees, qui dynamise l’engagement.
VГ©rifier ceci|
The Real Person!
The Real Person!
Je suis fascine par Sugar Casino, on y trouve une energie contagieuse. Le catalogue de titres est vaste, offrant des tables live interactives. Le bonus de depart est top. Le support est efficace et amical. Les gains sont transferes rapidement, quelquefois des recompenses supplementaires dynamiseraient le tout. Au final, Sugar Casino offre une aventure memorable. En bonus la plateforme est visuellement electrisante, ce qui rend chaque session plus palpitante. Un atout les options variees pour les paris sportifs, garantit des paiements rapides.
Visiter aujourd’hui|
learnsomethingeveryday – The posts are concise yet meaningful—very good for a quick knowledge boost.
The Real Person!
The Real Person!
J’adore le dynamisme de Sugar Casino, on ressent une ambiance de fete. Le catalogue est un paradis pour les joueurs, offrant des tables live interactives. Le bonus initial est super. Le support est rapide et professionnel. Les paiements sont securises et rapides, par contre plus de promotions variees ajouteraient du fun. Pour conclure, Sugar Casino est un incontournable pour les joueurs. A mentionner la plateforme est visuellement dynamique, incite a prolonger le plaisir. A mettre en avant les tournois reguliers pour la competition, propose des avantages uniques.
Aller sur le web|
dailytrendspot – Feels like a stylish marketplace where you can discover cool new finds.
mystylezone – Simple and stylish brand name, great for a fashion-oriented site where personal style matters.
urbanstylemarket – Easy checkout and nice promos today, definitely a site I’ll revisit.
simplystylishstore – Clean aesthetic and clear shopping experience; nice for simple, polished purchases.
The Real Person!
The Real Person!
Ich freue mich sehr uber Cat Spins Casino, es schafft eine mitrei?ende Stimmung. Die Auswahl ist so gro? wie ein Casino-Floor, mit traditionellen Tischspielen. Der Willkommensbonus ist ein Highlight. Verfugbar 24/7 fur alle Fragen. Der Prozess ist unkompliziert, von Zeit zu Zeit mehr Bonusoptionen waren top. Alles in allem, Cat Spins Casino ist ideal fur Spielbegeisterte. Au?erdem ist das Design modern und einladend, zum Verweilen einladt. Ein super Vorteil die dynamischen Community-Veranstaltungen, das die Motivation steigert.
Jetzt ansehen|
learnsomethingeveryday – A nice mix of fun facts and practical tips, great combo.
The Real Person!
The Real Person!
Ich bin vollig uberzeugt von Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Es gibt eine enorme Vielfalt an Spielen, mit klassischen Tischspielen. 100 % bis zu 500 € inklusive Freispiele. Der Support ist effizient und professionell. Gewinne werden schnell uberwiesen, allerdings mehr regelma?ige Aktionen waren toll. Zum Schluss, Cat Spins Casino sorgt fur ununterbrochenen Spa?. Nebenbei die Navigation ist unkompliziert, das Spielvergnugen steigert. Besonders erwahnenswert sind die sicheren Krypto-Transaktionen, reibungslose Transaktionen sichern.
Hier klicken|
The Real Person!
The Real Person!
Ich bin beeindruckt von SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Das Angebot an Spielen ist phanomenal, mit Spielen, die fur Kryptos optimiert sind. Die Agenten sind blitzschnell, verfugbar rund um die Uhr. Die Zahlungen sind sicher und smooth, dennoch zusatzliche Freispiele waren ein Highlight. Alles in allem, SpinBetter Casino garantiert hochsten Spa? fur Online-Wetten-Fans ! Hinzu kommt die Plattform ist visuell ein Hit, gibt den Anreiz, langer zu bleiben. Besonders toll die Community-Events, die den Einstieg erleichtern.
https://spinbettercasino.de/|
Just came from google to your website have to say thanks.
yourstylematters – I like the empowering message behind the name, style should always be personal.
hnzkfj.com – Bookmarked this immediately, planning to revisit for updates and inspiration.
The Real Person!
The Real Person!
J’adore l’energie de Sugar Casino, c’est une plateforme qui pulse avec energie. Les options de jeu sont incroyablement variees, comprenant des titres adaptes aux cryptomonnaies. Le bonus initial est super. Le service d’assistance est au point. Les paiements sont securises et instantanes, parfois des bonus plus varies seraient un plus. Pour finir, Sugar Casino est une plateforme qui fait vibrer. Ajoutons aussi le site est rapide et style, permet une immersion complete. Egalement super les competitions regulieres pour plus de fun, qui booste la participation.
https://sugarcasinobonus777fr.com/|
The Real Person!
The Real Person!
Je ne me lasse pas de Sugar Casino, il cree une experience captivante. Les options de jeu sont infinies, offrant des sessions live immersives. Avec des depots rapides et faciles. Les agents repondent avec efficacite. Les transactions sont d’une fiabilite absolue, toutefois quelques tours gratuits supplementaires seraient cool. Pour finir, Sugar Casino offre une aventure inoubliable. En extra l’interface est intuitive et fluide, ce qui rend chaque session plus palpitante. A signaler les evenements communautaires dynamiques, propose des privileges sur mesure.
Commencer Г lire|
trendshopworld – Global-sounding brand, good for catching wide variety of trends.
Hi Dear, are you in fact visiting this web site regularly, if so after that you will without doubt obtain nice know-how.
https://suitsndresses.co.uk/2025/10/19/melbet-kazino-zerkalo-2025/
findyourinspiration – Very motivating messaging; makes me think of product categories that spark ideas or renew your look.
The Real Person!
The Real Person!
Ich bin suchtig nach Cat Spins Casino, es ist ein Ort voller Energie. Die Spielauswahl ist beeindruckend, mit stilvollen Tischspielen. Er macht den Start aufregend. Die Mitarbeiter antworten prazise. Auszahlungen sind einfach und schnell, allerdings mehr Bonusoptionen waren top. Letztlich, Cat Spins Casino ist ein Top-Ziel fur Spieler. Au?erdem die Oberflache ist benutzerfreundlich, zum Verweilen einladt. Ein hervorragendes Plus die regelma?igen Wettbewerbe fur Spannung, reibungslose Transaktionen sichern.
Das ГјberprГјfen|
findbetteropportunities – Ambitious name—makes me curious about what “better opportunities” means for customers.
The Real Person!
The Real Person!
Je suis enthousiaste a propos de Sugar Casino, c’est une plateforme qui deborde de dynamisme. Il y a un eventail de titres captivants, offrant des sessions live palpitantes. 100% jusqu’a 500 € plus des tours gratuits. Les agents repondent avec rapidite. Le processus est transparent et rapide, mais encore quelques spins gratuits en plus seraient top. En resume, Sugar Casino est un choix parfait pour les joueurs. En complement la plateforme est visuellement dynamique, ajoute une touche de dynamisme. Egalement top les transactions crypto ultra-securisees, qui booste la participation.
Aller sur le web|
fashiondailydeals – Prices look great for trendy pieces, could easily become a go-to fashion stop.
perfectbuyzone – The layout is well done and the offers stand out, nice work overall.
I’m gone to inform my little brother, that he should also go to see this blog on regular basis to get updated from most recent news.
https://claudiadevilafames.net/archivos/3232
findsomethingamazing – Such a fun site to explore, always discovering cool new stuff.
happyhomefinds – Great selection and the site loads smoothly—nice home-inspiration hub.
bestpickscollection – Will definitely revisit this site to check for new arrivals and offers.
yourtrendystop – The layout is clean and modern, made browsing effortless and fun.
createimpacttoday – The posts feel genuine and encouraging—exactly what I needed.
unlocknewpotential – Love the tone — positive, encouraging, and not overly complicated.
theperfectgift – Easily found something for a friend’s birthday — really glad I visited.
yourstylematters – The content feels well-curated and the branding looks professional throughout.
unlocknewpotential – Smooth layout and meaningful content—good mix for daily inspiration.
yourvisionmatters – Will revisit this one—there’s a calm confidence in the content that I appreciate.
thinkbigmovefast – Clean design and punchy tone—makes browsing motivating instead of passive.
freshvalueoutlet – Good value impression so far, I’ll bookmark this for my next shopping session.
Howdy! Would you mind if I share your blog with my zynga group? There’s a lot of folks that I think would really appreciate your content. Please let me know. Thanks
https://dozamieszkaniajedenkrok.pl/melbet-2025-obzor-bukmekerskoj-kontory/
findyourfocus – Great site for mental clarity tips, everything’s short, simple, and useful.
discovergreatoffers – I like how every deal is clearly shown and easy to compare.
I used to be able to find good info from your articles.
https://www.iamfilipino.com/melbet-kazino-skachat-obzor-2025/
brightstylecorner – The layout’s bright energy instantly catches your eye, really uplifting vibe overall.
perfectbuyzone – Solid choices and clear presentation, made me feel confident about buying.
yourvisionmatters – Empowering message and good styling; strong brand identity right out of the gate.