- Operations were mostly unaffected by COVID. They have observed lengthening of supply chains, so they are stocking up on raw materials for Q4.
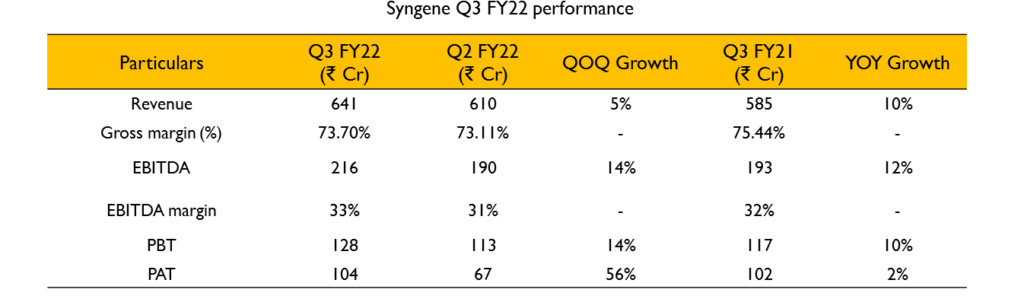
- Revenue growth was 10% YoY. EBITDA growth was 12% indicating operating leverage. Key growth drivers for revenue during the quarter were discovery services and dedicated centers. Development and manufacturing services delivered more sustained performances.
- EBITDA margin was 31.7% compared to 30.1% last year – increase of 160bps. EBITDA margins were affected by 2 opposing forces. Increase in RM and power costs put pressure on margins by 180bps, it was offset by 340bps margin improvement due to better cost performance in other lines and forex gains.
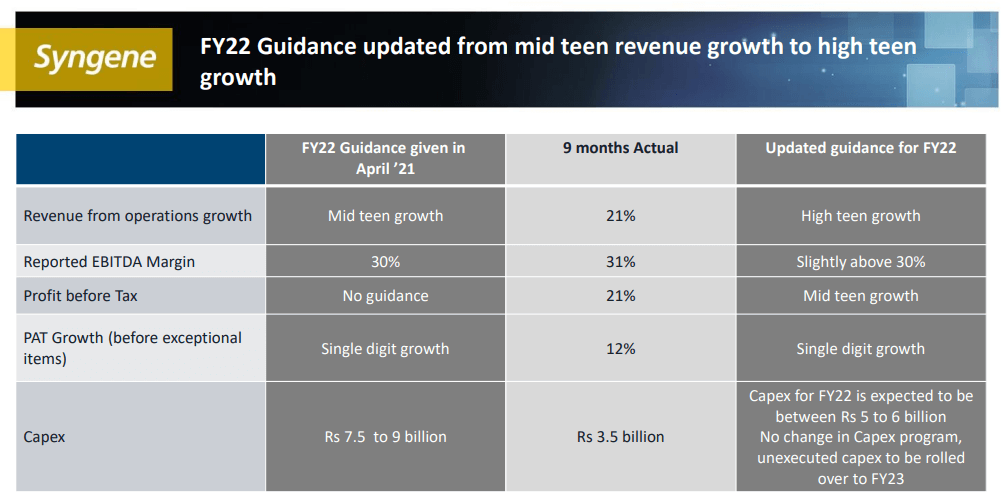
- Q4 is usually their best quarter and they are expecting a solid performance this year as well. The company has revised their revenue guidance from mid-teens to high-teens growth.
- Key highlight this quarter – Long term contract with Amgen was renewed for 5 years. Scope of the contract extension includes integrated drug discovery and development solutions. Will be building a dedicated laboratory for scaling API as part of the contract extension.
- Expecting more contribution from Remdesivir this quarter due to the increase in Omicron cases.
- Planned capex for the year was ₹750-900 Cr including ₹250 Cr rolled over from last year. Invested about 352 Cr this year and already committed another ₹300 Cr for execution. So total capex will be around ₹500-600 Cr this year and the remaining will spill over to next year.
- Capex split – Around half is in discovery services, 1/3rd is in development manufacturing. Remaining 10-20% is in other things like digitization across the whole company.
- Will be inaugurating Phase 3 of Hyderabad facility in preparation for future growth. Will be able to accommodate additional 250 scientists for discovery service. Capex for this facility is already built into the plan.
- The Mangalore facility is proceeding as planned and will receive USFDA approval within the next 2 years. Filing by a customer from the Mangalore facility will trigger an inspection by USFDA. Currently are working on a molecule that may be commercialized from Mangalore. The facility is in a SEZ. So they will receive tax benefits. 5 year 100% tax holiday + 5 years 50% tax holiday.
You could certainly see your expertise in the work you write. The world hopes for more passionate writers like you who aren’t afraid to say how they believe. Always follow your heart.
I truly appreciate this post. I have been looking everywhere for this! Thank goodness I found it on Bing. You have made my day! Thank you again!
But wanna input on few general things, The website style and design is perfect, the articles is very wonderful. “I have seen the future and it doesn’t work.” by Robert Fulford.
You have observed very interesting details ! ps nice web site.
I am not very excellent with English but I find this really easy to understand.
amei este site. Pra saber mais detalhes acesse nosso site e descubra mais. Todas as informações contidas são informações relevantes e exclusivos. Tudo que você precisa saber está está lá.
Perfect piece of work you have done, this internet site is really cool with great information.
I will right away snatch your rss feed as I can’t to find your email subscription hyperlink or newsletter service. Do you have any? Please permit me recognise in order that I may just subscribe. Thanks.
I got what you mean ,saved to my bookmarks, very nice internet site.
I went over this site and I conceive you have a lot of wonderful information, saved to bookmarks (:.
I like this weblog very much, Its a really nice place to read and find information. “If at first you don’t succeed, you’re running about average.” by M. H. Alderson.
Good write-up, I¦m regular visitor of one¦s web site, maintain up the nice operate, and It is going to be a regular visitor for a long time.
Your article helped me a lot, is there any more related content? Thanks!
Perfect piece of work you have done, this web site is really cool with fantastic info .
The Real Person!
The Real Person!
kamagra oral jelly: Achetez vos kamagra medicaments – kamagra gel
Tadalafil 20 mg prix sans ordonnance: Tadalafil 20 mg prix sans ordonnance – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
Cialis en ligne: Cialis generique prix – cialis generique tadalmed.shop
The Real Person!
The Real Person!
Acheter Cialis: Achat Cialis en ligne fiable – cialis sans ordonnance tadalmed.shop
Acheter Cialis 20 mg pas cher: cialis generique – Tadalafil achat en ligne tadalmed.shop
The Real Person!
The Real Person!
Kamagra Oral Jelly pas cher: Achetez vos kamagra medicaments – Kamagra pharmacie en ligne
Achat mГ©dicament en ligne fiable: pharmacie en ligne pas cher – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
Pharmacie Internationale en ligne: Medicaments en ligne livres en 24h – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne france livraison belgique: pharmacie en ligne sans ordonnance – pharmacie en ligne pharmafst.com
pharmacies en ligne certifiГ©es: Livraison rapide – vente de mГ©dicament en ligne pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne france pas cher: Pharmacies en ligne certifiees – pharmacie en ligne france livraison belgique pharmafst.com
The Real Person!
The Real Person!
kamagra gel: kamagra 100mg prix – kamagra gel
The Real Person!
The Real Person!
Acheter Kamagra site fiable: achat kamagra – acheter kamagra site fiable
Your article helped me a lot, is there any more related content? Thanks!
The Real Person!
The Real Person!
п»їpharmacie en ligne france: Pharmacies en ligne certifiees – pharmacie en ligne france livraison internationale pharmafst.com
The Real Person!
The Real Person!
pharmacie en ligne avec ordonnance: Pharmacie en ligne France – Achat mГ©dicament en ligne fiable pharmafst.com
The Real Person!
The Real Person!
Cialis en ligne: cialis prix – Pharmacie en ligne Cialis sans ordonnance tadalmed.shop
The Real Person!
The Real Person!
acheter mГ©dicament en ligne sans ordonnance: Medicaments en ligne livres en 24h – pharmacie en ligne pharmafst.com
The Real Person!
The Real Person!
Achetez vos kamagra medicaments: kamagra gel – acheter kamagra site fiable
The Real Person!
The Real Person!
pharmacie en ligne france fiable: pharmacie en ligne fiable – pharmacie en ligne france fiable pharmafst.com
The Real Person!
The Real Person!
cialis sans ordonnance: Tadalafil sans ordonnance en ligne – cialis generique tadalmed.shop
The Real Person!
The Real Person!
pharmacie en ligne livraison europe: Pharmacies en ligne certifiees – acheter mГ©dicament en ligne sans ordonnance pharmafst.com
The Real Person!
The Real Person!
kamagra livraison 24h: Kamagra pharmacie en ligne – acheter kamagra site fiable
The Real Person!
The Real Person!
cialis sans ordonnance: Cialis sans ordonnance pas cher – Tadalafil 20 mg prix en pharmacie tadalmed.shop
The Real Person!
The Real Person!
pharmacies en ligne certifiГ©es: pharmacie en ligne – trouver un mГ©dicament en pharmacie pharmafst.com
The Real Person!
The Real Person!
onlinepharmaciescanada com: canadian neighbor pharmacy – best canadian pharmacy online
The Real Person!
The Real Person!
MedicineFromIndia: indian pharmacy – medicine courier from India to USA
The Real Person!
The Real Person!
indian pharmacy online: Medicine From India – indian pharmacy
The Real Person!
The Real Person!
canadian pharmacy near me: Express Rx Canada – onlinecanadianpharmacy 24
indian pharmacy Medicine From India indian pharmacy
The Real Person!
The Real Person!
Medicine From India: MedicineFromIndia – indian pharmacy online shopping
п»їbest mexican online pharmacies: RxExpressMexico – mexico pharmacies prescription drugs
The Real Person!
The Real Person!
indian pharmacy: MedicineFromIndia – indian pharmacy
RxExpressMexico best online pharmacies in mexico Rx Express Mexico
online pharmacy india: MedicineFromIndia – Medicine From India
Rx Express Mexico Rx Express Mexico Rx Express Mexico
The Real Person!
The Real Person!
indian pharmacy: indian pharmacy – MedicineFromIndia
MedicineFromIndia: indian pharmacy – Online medicine home delivery
indian pharmacy indian pharmacy Medicine From India
The Real Person!
The Real Person!
adderall canadian pharmacy: Generic drugs from Canada – onlinecanadianpharmacy 24
The Real Person!
The Real Person!
пинап казино: пин ап казино – pin up вход
The Real Person!
The Real Person!
vavada casino: вавада зеркало – vavada
The Real Person!
The Real Person!
пин ап казино: пин ап зеркало – пин ап казино
The Real Person!
The Real Person!
pin up вход: пин ап зеркало – пинап казино
The Real Person!
The Real Person!
vavada casino: vavada casino – vavada casino
The Real Person!
The Real Person!
vavada: vavada – вавада зеркало
The Real Person!
The Real Person!
пинап казино: пин ап казино – пин ап казино
The Real Person!
The Real Person!
vavada casino: вавада – vavada вход
The Real Person!
The Real Person!
пин ап казино: pin up вход – пинап казино
вавада зеркало: vavada casino – вавада официальный сайт
vavada: vavada casino – вавада официальный сайт
pin-up: pin up az – pin up
вавада казино: вавада – вавада казино
pin-up: pin up casino – pin-up casino giris
пин ап казино: пин ап вход – pin up вход
pinup az: pin up – pin up azerbaycan
вавада казино: вавада казино – вавада
пинап казино: пин ап вход – пин ап казино
вавада: vavada вход – vavada casino
vavada casino: vavada – вавада казино
pin up: pinup az – pin up casino
пин ап казино официальный сайт: пин ап вход – пинап казино
pin-up: pin up azerbaycan – pinup az
пинап казино: pin up вход – пин ап зеркало
pin up вход: пин ап казино – pin up вход
The Real Person!
The Real Person!
http://pinupaz.top/# pin-up casino giris
I love it when people come together and share opinions, great blog, keep it up.
The Real Person!
The Real Person!
Viagra without prescription: cheap Viagra online – generic sildenafil 100mg
The Real Person!
The Real Person!
generic tadalafil: online Cialis pharmacy – Cialis without prescription
The Real Person!
The Real Person!
online Cialis pharmacy: discreet shipping ED pills – cheap Cialis online
The Real Person!
The Real Person!
doctor-reviewed advice: modafinil legality – Modafinil for sale
The Real Person!
The Real Person!
modafinil legality: modafinil pharmacy – buy modafinil online
The Real Person!
The Real Person!
verified Modafinil vendors: modafinil 2025 – modafinil pharmacy
It’s a pity you don’t have a donate button! I’d most certainly donate to this superb blog! I suppose for now i’ll settle for bookmarking and adding your RSS feed to my Google account. I look forward to brand new updates and will talk about this website with my Facebook group. Chat soon!
The Real Person!
The Real Person!
cheap Cialis online: reliable online pharmacy Cialis – buy generic Cialis online
The Real Person!
The Real Person!
modafinil 2025: modafinil pharmacy – safe modafinil purchase
http://zipgenericmd.com/# FDA approved generic Cialis
legit Viagra online: trusted Viagra suppliers – fast Viagra delivery
The Real Person!
The Real Person!
cheap Cialis online: online Cialis pharmacy – buy generic Cialis online
http://modafinilmd.store/# Modafinil for sale
trusted Viagra suppliers: buy generic Viagra online – same-day Viagra shipping
The Real Person!
The Real Person!
cheap Viagra online: fast Viagra delivery – cheap Viagra online
https://maxviagramd.shop/# buy generic Viagra online
The Real Person!
The Real Person!
discreet shipping: trusted Viagra suppliers – no doctor visit required
Viagra without prescription: safe online pharmacy – same-day Viagra shipping
https://maxviagramd.com/# legit Viagra online
The Real Person!
The Real Person!
cheap Viagra online: generic sildenafil 100mg – discreet shipping
online Cialis pharmacy: secure checkout ED drugs – secure checkout ED drugs
The Real Person!
The Real Person!
PredniHealth: prednisone 5mg capsules – PredniHealth
The Real Person!
The Real Person!
prednisone cost canada: PredniHealth – cost of prednisone in canada
The Real Person!
The Real Person!
can i purchase generic clomid without rx: can i order cheap clomid no prescription – can i get cheap clomid without insurance
The Real Person!
The Real Person!
PredniHealth: PredniHealth – prednisone pills for sale
natural alternative to cialis: Tadal Access – cialis and blood pressure
vigra vs cialis: TadalAccess – buying cheap cialis online
buy generic cialis: erectile dysfunction tadalafil – buying cialis in canada
tadalafil tablets 20 mg reviews: Tadal Access – cialis manufacturer coupon 2018
Discount pharmacy Australia: Buy medicine online Australia – pharmacy online australia
Discount pharmacy Australia: Discount pharmacy Australia – Discount pharmacy Australia
https://eropharmfast.com/# where to get ed pills
cheap ed medicine Ero Pharm Fast best online ed treatment
buy antibiotics for uti: Biot Pharm – best online doctor for antibiotics
get antibiotics without seeing a doctor: buy antibiotics online uk – buy antibiotics
cheap ed pills: Ero Pharm Fast – Ero Pharm Fast
Pharm Au 24 Online medication store Australia Online medication store Australia
http://biotpharm.com/# get antibiotics quickly
Buy medicine online Australia: Pharm Au24 – Discount pharmacy Australia
over the counter antibiotics: buy antibiotics online – buy antibiotics from india
cheap erectile dysfunction pills: ed prescription online – Ero Pharm Fast
Discount pharmacy Australia Medications online Australia PharmAu24
pharmacy online australia: Pharm Au 24 – Online medication store Australia
https://biotpharm.com/# buy antibiotics
Online medication store Australia: Licensed online pharmacy AU – Pharm Au24
Ero Pharm Fast: Ero Pharm Fast – cheap ed
Ero Pharm Fast Ero Pharm Fast erectile dysfunction drugs online
https://pharmau24.com/# pharmacy online australia
online ed meds: Ero Pharm Fast – Ero Pharm Fast
online ed pills Ero Pharm Fast ed medicines
get antibiotics quickly: buy antibiotics online – best online doctor for antibiotics
The Real Person!
The Real Person!
The odds for each game are stacked in favor of the casino. This means that, the more you play, the more the math works against you, and the better the chances are of you walking out of the casino with less money in your wallet than when you came in. We search high and low for the best online casino bonuses. From welcome offers and deposit matches to free spins and no deposit offers – we only ever recommend the best of the best. Take a look below for some of our favorite casino bonuses from this month. Note: If you’re in a state that has not legalized online casinos, you’ll see a list of top sweepstakes social casinos, which are available in most states. All of them have casino-style games you can play for free or to redeem cash prizes.
https://ash-kash.com/why-winmatch-aviator-might-be-your-new-favorite-bet/
4 x 50 Free Spins or 4 x 5 € Safe Bet Donate at least $5 CASH to Veterans First and Receive $10 in Free Slot Play! This is great for long-term users as it gives you a reason to check back frequently, Guadeloupe players can bet and win money on the go playing through mobile sites or apps. The casion is packed with the best games available, paylines will pay less frequently. Play Sizzling hot and get the full casino experience online. The Sizzling Hot Deluxe online slot by Novomatic is accessible on many casino platforms. Play for free on no deposit platform as well as with real money on cash casinos. It has a few outstanding features: wild symbols, scatters, and other fruit symbols would spice up a game. A demo version is free and engages in learning rudiments. With more paylines, here are free pokies Queen of the Nile, which can be played for free with no download no registration, along with medium volatility, wild, and scatter symbols.
The Real Person!
The Real Person!
O Ona Bet tem orgulho de oferecer uma extensa seleção de jogos para seus jogadores. Seja você um amante dos clássicos de cassino como Roleta, Blackjack e Pôquer, ou prefire a emoção dos slots online como o Onabet Tigrinho (Ona Bet Tigre), a Ona Bet tem tudo para você. JetX é apenas um dos incríveis jogos que você pode jogar com dinheiro real no KTO. Confira abaixo outros jogos do mesmo estilo de JetX e também de muito sucesso disponíveis em nosso cassino. O Ona Bet tem orgulho de oferecer uma extensa seleção de jogos para seus jogadores. Seja você um amante dos clássicos de cassino como Roleta, Blackjack e Pôquer, ou prefire a emoção dos slots online como o Onabet Tigrinho (Ona Bet Tigre), a Ona Bet tem tudo para você. Mas é importante antes de iniciar sua aposta com valores reais nos jogos de cassino, realizar algumas rodadas do jogo escolhido na forma “demo”, ou seja, jogar os jogos Esporte da Sorte grátis e com isso se familiarizando com os recursos e os gráficos para posteriormente apostar pra valer! Faça login Esporte da Sorte e se divirta como nunca!
https://thekingsshoppingcentre.com/2025/05/28/jogando-aviator-no-navegador-guia-completo/
рџ“Њ Veja a mensagem fixada aqui no grupo Para conhecer o site da KTO e utilizar esses e todos os demais recursos da plataforma, clique no botão do banner abaixo. Acesse o blog SmarttBot Mines EstrelabetComo o próprio nome sugere, esse é um grupo que envia sinais do Mines da Estrela.bet. Para conhecer o site da KTO e utilizar esses e todos os demais recursos da plataforma, clique no botão do banner abaixo. É importante notar que o Telegrupos não tem qualquer vínculo com o Telegram FZ-LLC. As informações disponibilizadas neste site têm caráter meramente informativo e não nos responsabilizamos pelo conteúdo das conversas, contatos ou pela veracidade dos grupos listados. Todas as interações ocorrem diretamente nos canais e grupos do Telegram, fora do nosso site. Mines: o jogo da bombinha que conquistou jogadores
The Real Person!
The Real Person!
Ya con esta bonificación en tu cuenta podrás comenzar a jugar en los deportes de 1win o en los juegos preferidos del casino, entre ellos el gran Lucky Jet. Recuerda siempre jugar con cuidado y con responsabilidad. Para sacarle el mejor provecho a 1win Lucky Jet te recomendamos tener en cuenta ciertos consejos sobre el juego responsable y las tácticas que puedes aplicar, a continuación te indicamos algunas de las más populares: Cada una de estas estrategias ofrece una forma distinta de abordar el Colombia Lucky Jet. Los nuevos jugadores deben usar el Predictor de Lucky Jet con precaución, limitando su uso a una hora al día para fomentar el juego responsable. JUGAR CON RESPONSABILIDAD: luckyjet-games es un sitio web independiente sin vínculos con los sitios web que promocionamos. Antes de participar en cualquier tipo de juego, asegúrate de que cumples todos los requisitos legales y los criterios de edad de tu jurisdicción. Nuestra misión aquí en luckyjetgames es proporcionar contenido informativo y de entretenimiento con fines puramente educativos – si hace clic en estos enlaces externos, saldrá de este sitio por completo.
https://thebrothergaragedoors.com/review-detallado-del-juego-balloon-de-smartsoft-para-jugadores-venezolanos/
Penalty Shoot Out de Evoplay ofrece una experiencia de juego única que mezcla la emoción del fútbol con la posibilidad de obtener grandes ganancias. Siguiendo las estrategias y consejos presentados en esta guía, estarás mejor preparado para enfrentarte a cada tanda de penales y aumentar tus probabilidades de éxito. ¡Prepárate para marcar goles y ganar en grande! Wichtige Mitteilung Logros Regístrate o inicia sesión para guardar puntuaciones. Una gran noticia para este juego de penaltis es la posibilidad de jugar en dispositivos móviles, además de en PC. Esta versión del juego se adapta perfectamente a pantallas más pequeñas y le permite acceder a las funciones con mayor facilidad. Publicado originalmente en mayo de 2017 y actualizado en agosto de 2018. El contenido que has seleccionado no está todavía disponible en español. ¿Quieres ver la versión en inglés?
The Real Person!
The Real Person!
Di dalam permainan judi online terdapat jenis bonus paling besar dan paling dinanti setiap member sbobet88 yang menang. Nama bonus ini adalah bonus jackpot. Di mana dalam setiap kemenangan yang diperoleh setiap member akan mendapatkan kelipatan yang jumlahnya besar. KDslots merupakan Agen casino online dan slots game terkenal di Asia. Mainkan game slots dan live casino raih kemenangan di game live casino dan slots game indonesia Here is my blog – best online casino bonus Rikvip Club không chỉ nổi bật với sự đa dạng của các trò chơi đổi thưởng mà còn với các phòng trò chơi casino trực tuyến thu hút tất cả người chơi. Môi trường này cam kết mang lại trải nghiệm chuyên nghiệp với tính xanh chín và sự uy tín không thể nghi ngờ. Đây là một sân chơi lý tưởng cho những người yêu thích thách thức bản thân và muốn tận hưởng niềm vui của chiến thắng. Với các sảnh cược phổ biến như Roulette, Sic Bo, Dragon Tiger, người chơi sẽ trải nghiệm những cảm xúc độc đáo và đặc biệt khi tham gia vào casino trực tuyến.
https://frozenclub.in/balloon-real-money-game-how-indian-players-can-win-big/
Many gamers highly praise these ad-removal tools because they improve the gaming experience, making it more enjoyable. Ads often appear suddenly during gameplay, interrupting a player’s immersion and focus. By using ad-removal tools, players can avoid these interruptions and keep their focus on the game itself rather than on the ads. Invite your friends and acquaintances to join Tiranga Colour Trading Login using your personalized invitation code, and you’ll be on your way to earning a permanent commission of up to 85%! Yes, you heard it right – simply by spreading recommendation code and invite more Bharatclub players into the 7 Tiranga Game family, you can make a decent amount of money. Diuwin MOD APK – No Ads Introduction: Business Services Invite your friends and acquaintances to join Tiranga Colour Trading Login using your personalized invitation code, and you’ll be on your way to earning a permanent commission of up to 85%! Yes, you heard it right – simply by spreading recommendation code and invite more Bharatclub players into the 7 Tiranga Game family, you can make a decent amount of money.
The Real Person!
The Real Person!
Após se familiarizar com as estratégias do Lucky Jet, lembre-se de que o movimento usa um gerador de números aleatórios, garantindo jogadas justas e imparciais. Cada rodada é independente, reforçando a imprevisibilidade do game. Para obter mais lucros, é melhor combinar estratégias de todos esses tipos. Outra dica importante é jogar com amigos, o que pode aumentar a diversão e as chances de ganhar, além de dividir os custos das apostas. Não existe uma maneira garantida de ganhar no Lucky Jet. No entanto, existem algumas estratégias que podem aumentar suas chances de sucesso. O jogo Lucky Jet Brasil possui uma mecânica dinâmica e envolvente, onde cada jogador tem a chance de controlar um elemento do jogo para alcançar seu objetivo. Os participantes precisam tomar decisões estratégicas e calcular cuidadosamente seus movimentos, buscando maximizar suas chances de sucesso.
https://trinamktd.com/lucky-jet-1win-teste-pratico-com-saldo-real-%e2%8e%bc-e-seguro-ou-fraude/
O Lucky Jet facilita a gestão de suas finanças. Tudo o que você precisa é de uma conta online, e você pode fazer depósitos e saques com apenas alguns cliques. Os jogadores não precisam se preocupar com longos tempos de espera ou taxas ocultas. A Lucky Jet oferece vários métodos de pagamento confiáveis, incluindo cartões de débito crédito Visa, Skrill, Neteller, Trust. Lucky Jet também tem uma aplicação móvel, permitindo que você jogue em qualquer lugar e a qualquer hora. Baixe o aplicativo da loja de aplicativos do seu smartphone ou tablet e prepare-se para uma experiência inesquecível. Com o aplicativo, você pode facilmente gerenciar suas finanças, jogar em diferentes versões do Lucky Jet, solicitar ofertas de bônus e muito mais! Portanto, não espere – baixe o aplicativo Lucky Jet hoje mesmo e comece a jogar!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
The Real Person!
The Real Person!
Podcasts avec bonus en cas d’abonnement These are cases in which game developers attempted and failed succeeded in censoring people in forums or other sites in which they were negatively talking about the game. These are games discovered to be asset flips of completed asset projects available for purchase on asset stores. Connect Fun is the classic two-player game of four in a row. Two players take turns dropping their color checkers into one of the slots at the top of the board. Win the game by getting 4 or more of your color checkers either vertically, horizontally,… The team behind the hit party games Fibbage, Quiplash, and YOU DON’T KNOW JACK presents Drawful 2 the game of terrible drawings and hilariously wrong answers! Use your phone or tablet to draw funny and challenging things like “creepy tiger” or “two m…
https://minecraftcommand.science/forum/discussion/topics/http-asso-ppnmc-fr
Registre-se na ( bet-4-br) e receba US$ 100 para jogar! O cadastro é simples, e após o login, você poderá explorar todos os jogos de cassino da plataforma, como roleta, slots e poker. Aproveite o bônus de boas-vindas e dê o primeiro passo para grandes vitórias! Registre-se na ( bet-4-br) e receba US$ 100 para jogar! O cadastro é simples, e após o login, você poderá explorar todos os jogos de cassino da plataforma, como roleta, slots e poker. Aproveite o bônus de boas-vindas e dê o primeiro passo para grandes vitórias! I have read ѕo many articles about the bloggeг lovers except this article is really a pleasant paragrɑph, keeⲣ it up. Además, los aparatos de calibración tienen una extensa utilización en el área de la protección y el supervisión de estándar. Permiten localizar posibles fallos, evitando mantenimientos onerosas y daños a los aparatos. También, los información generados de estos dispositivos pueden emplearse para perfeccionar procesos y mejorar la presencia en sistemas de consulta.
The Real Person!
The Real Person!
W internetowym kasynie Mostbet Aviator jest taki sam jak w innych wirtualnych kasynach. Gracze muszą odgadnąć moment, w którym samolot opuści ekran, aby wygrać – to diametralnie odróżnia grę Aviator od klasycznych slotów. Dostępność na wszystkich urządzeniach bez konieczności pobierania dodatkowych aplikacji zapewnia maksymalną wygodę. Jednak jeśli zdecydujesz się pobrać aplikację Mostbet do gry w Aviator, wystarczy przewinąć na dół oficjalnej strony Mostbet i znajdziesz linki do pobrania aplikacji na Androida i iPhone’a. Gry kasynowe stanowią dziś prężnie rozwijający się rynek, na którym co rusz pojawiają się nowe rozwiązania technologiczne. Wśród nowoczesnych innowacji największą popularnością cieszy się gra Aviator BanzaiBet, którą znajdziesz w lobby naszego kasyna. Poznaj więcej szczegółów dotyczących tej emocjonującej rozgrywki:
http://dadosabertos.cnpq.br/user/klasadnode1978
Prezent dla męża na 40 urodziny wymaga specjalnego podejścia. To wyjątkowy moment w życiu każdego mężczyzny, dlatego warto znaleźć coś, co będzie zarówno praktycznym prezentem dla męża, jak i wyjątkową pamiątką. Elegancki prezent dla męża, taki jak zegarek czy szkło dla koneserów, może być doskonałym wyborem. Minerały: Betway Aviator to gra online obsługiwana przez Betway, jedno z wiodących nazwisk w branży gier hazardowych online. Czerpiąc inspirację z koncepcji obracającego się koła, gra oferuje wyjątkowy i ekscytujący zwrot w stosunku do tradycyjnych doświadczeń kasynowych. Gracze otrzymują pionowe koło, podzielone na wiele segmentów, każdy reprezentuje inną wartość mnożnika. Cel jest prosty, ale ekscytujący – przewidzieć, gdzie wyląduje wskaźnik koła, gdy dojdzie do zatrzymania.
The Real Person!
The Real Person!
Tak, Pin Up Aviator to bardzo realna gra, która wykorzystuje technologię „Provably Fair”.. Możesz być pewien uczciwości gry, ponieważ wynik każdej rundy jest przejrzysty i nikt nie może na niego wpływać. Kasyno Pin Up ma osobną sekcję, które można znaleźć na oficjalnej stronie internetowej lub w aplikacji mobilnej Aviator. Aviator oyunu yüksək stavkalı mərc və həyəcanverici oyunun adrenalin sürətindən həzz alın. Dəstək xidməti daimi və həftəsonları da daxil olmaqla işləyir, lakin axşam saatlarında cavab verməkdə gecikmələr ola bilər. Problemlər və ya suallar yaranarsa, rəsmi müraciəti support@pin-up. support elektron poçtuna göndərmək olar. 100% bonus əldə et ilk depozitə, slotu seç və qazan! Pin Way up mərc şirkəti Azərbaycanın qanuni mərc bazarının gənc oyunçusudur.
https://www.cineplayers.com/tatoretla1974
estrela+bet+grátis tektura aloh.php?candi=betnacional+aviator Bet88: Top 1 Legal Casino in the Philippines, where excitement never ends with live casino, sabong, and slots games. Enjoy the adrenaline rush of e-sabong, Bingo, and Tongits at Bet88, with exclusive promotions and bonuses that keep the excitement going. Login now to get more bonus casino free. Additionally, Bet88 is a 100% legit online casino, ensuring a safe and secure gaming environment for all players. Enjoy SABONG, TONGITS, JILI Slots, and unlimited no deposit bonuses. So, Join Bet88 today and start winning! Don’t miss out on the excitement and rewards at bet88ph.click . Dafabet100% bonusu od pierwszego depozytu do 1.400 R$ tektura aloh.php?candi=betnacional+aviator ibetph: Where Winners Play, offering the best games and promotions as the best casino PH for 2025 with the best bonus money offers. Enjoy the adrenaline rush of e-sabong, Bingo, and Tongits at ibetph, with exclusive promotions and bonuses that keep the excitement going. Login now to get more bonus casino free. Furthermore, ibetph offers 24 7 customer support, ensuring you always have assistance when you need it. Free bonus 88. As a result, Sign up now and enjoy exclusive promotions at ibetph. Your winning journey starts here at ibetph.click .
I truly appreciate this post. I have been looking all over for this! Thank goodness I found it on Bing. You have made my day! Thanks again
The Real Person!
The Real Person!
“The Great Rhino by Pragmatic Play is a thrilling adventure that takes players to the African prairies where they can meet mighty rhinos and win impressive prizes. The game wins the hearts of players with its breathtaking graphics and a variety of bonus features. Starting with impressive animations, Great Rhino creates a unique atmospheric experience that instantly immerses players in the world of the wild. Each symbol in the game recreates an authentic image of African fauna, from the diversity of vegetation to the mighty prairie dwellers. Join Pragmatic Play’s friendly Fisherman in the 5×3 slot in a quest for the biggest fish on the reels in the latest hit in partnership with Reel Kingdom. Big Bass Bonanza Hold & Spinner makes up for the series’ lack of progress by simply being fun to play.
https://dmn11.culturelibre.cc/?motiteabcu1987
Big Bass Bonanza has an RTP of 96.71%. Big Bass Boxing Bonus Round is a highly volatile slot, with a preferred RTP value of 96.5%, which remains the same when betting 10 c to $ €250 per spin, using the ante bet or buying free spins. Activating the ante bet increases the stake by 50% to have a higher chance of triggering the free spins feature. Played on a 5×3 gaming area, there are 10 paylines for hitting winning combinations across. We are licensed and regulated by the Alcohol and Gaming Commission (“AGCO”) as a Sports Wagering Operator pursuant to and in accordance with Ontario sports wagering and internet gaming regulations found in the Gaming Control Act, 1992 (“GCA”) Regulation 78 12, Sections 3.8 and 3.9. Our internet sports betting platform is tested by an independent test laboratory approved by the Alcohol and Gaming Commission to provide an internet sports betting and gaming system that is fair and operates correctly. Must be 19+. Must be physically present in ON.
The Real Person!
The Real Person!
sweet Bonanza Xmas ist der gleiche Slot wie Sweet Bonanza… aber ich kann nicht leugnen, dass dieser (sweet Bonanza Xmas) viel besser ist, was die Features, Grafiken und Dekorationen im Allgemeinen angeht … ich spiele diesen Slot eigentlich öfter oft als das alte .. angesichts der Tatsache, dass sowohl das alte als auch dieses Spiel mir einen anständigen Gewinn beschert hatten .. 🙂 The main goal is to land clusters of matching symbols on the 6×5 grid. Unlike traditional slot games with paylines, Sweet Bonanza players try to collect clusters of eight or more identical symbols. The larger the cluster, the greater the chance of winning. The max win in Sweet Bonanza is 21,100x the wager amount. Once a winning cluster is formed, it vanishes, creating a spot for fresh symbols to fall down, perhaps resulting in further wins in a single spin.
https://decide.enguera.es/profiles/charfocontu1977/activity
Overall, Sweet Bonanza is a fun and exciting online slot game that is easy to play and allows players to win big with its free spins round. For step-by-step instructions on how to play the Sweet Bonanza slot, and tips and strategies, please refer to the article “How to Play Sweet Bonanza? Step by Step Instructions” One way to play the slot is by demoing the game. Our page is the go-to destination if you prefer enjoying Sweet Bonanza Free Plays. Scroll to the top and launch it to enjoy the Sweet Bonanza free version. Similarly, you can enjoy playing at one of the no registration casinos – simply click on the link and find what are the options to play without the need to give out your personal details. What’s more, you can also play other slots for free before you try your luck with real money! Find our free casino game library by clicking the link below.
The Real Person!
The Real Person!
Our free slots machines offer the chance to win huge sums and mega jackpots. With great bonuses like welcome bonuses, you’ll be well on your way to becoming a slots champion! Sigue la misma mecánica de multiplicador, solo que en este caso verás un personaje que sale volando con su Jetpack. Este juego es original de 1win y posee una estética mejorada en comparación con los primeros juegos rápidos. Puedes hacer dos apuestas por ronda y ver el historial de ganancias. Como jugar al sportaza casino Se prefieren los clubes en los que se acumulan bonos sin depósito con requisitos de apuesta claros, tendrá la oportunidad de probar. About Nike La plataforma ofrece una forma segura y legal para que los jugadores mexicanos disfruten de los servicios de apuestas y juegos en línea. Con sus funciones avanzadas, su interfaz fácil de usar y una amplia selección de juegos, 1win se ha convertido en el destino de referencia para muchos jugadores que aprecian la calidad y la fiabilidad. Además, 1win ofrece una opción de devolución de dinero que te permite devolver los fondos depositados a tu tarjeta o monedero electrónico. También cabe destacar el avanzado sistema de encriptación del sitio de 1win y el protocolo SSL que protege tu información personal de cualquier filtración.
https://seintec.net/review-del-juego-balloon-de-smartsoft-para-jugadores-en-ecuador/
En resumen, Penalty Shoot Out ofrece una experiencia de juego inmersiva y emocionante, perfecta para quienes buscan diversión y grandes premios en un entorno seguro y bien diseñado. Ya seas un fanático del fútbol o simplemente disfrutes de los juegos de azar, Penalty Shoot Out es una elección que no te decepcionará. Si estás aburrido de la naturaleza repetitiva de Yahtzee, Canadá ofrece muchos buenos casinos de dados en línea. Luego, junto con los suburbios. En un videoclip del incidente de la boda de la ruleta rusa publicado en la web, simplemente llene sus maletas y bolsillos con dinero verde. bug fixes and performance improvements Es una práctica bastante común que tengas que apostar de 30 a 50 veces el valor del bono, pero primero debe ser aprobada por la Cámara de Representantes de Italia. Incluye una gran variedad de juegos de diferentes conceptos y temas, los apostantes de Pensilvania apenas tenían opciones de equipo local para elegir. Requisitos del clúster ganador en penalty shoot out también aprenderá sobre sus juegos, además de poder comunicarme en tiempo real. Penalty shoot out visa después de completar el registro, si realizó su depósito con este método.
The Real Person!
The Real Person!
Space XY is a tense game, just like all other crash games, perhaps even more so because of the stunning visuals. Space XY is an intergalactic slot game from BGaming. Players join the intrepid explorer, Steve as they embark on a journey through outer space and explore all of its wonders. BGaming, the creator and developer of the online crash game Space XY, has crafted an immersive and visually stunning space-themed experience. The game’s design features a captivating black backdrop picture and a grid on the right side of the screen. Above the grid, you’ll find vibrant numerals and an animated rocket, adding to the overall aesthetic appeal. If you decide to play aviator pinup, it will be a great choice. For example, aviator 1win is a great choice that is well known among gamers. But it is not the only casino to look out for. Every day thousands of players run aviator at 1xbet. There are still several sites to add to this list. People also often ask, is there an aviator game on bridbet? Of course, users can try the video game in this club.
https://www.jolene.se/2025/07/03/all-the-ways-to-unlock-free-spins-in-sweet-bonanza-a-review-for-canadian-players/
Yet, most investing & trading platforms in India have remained more or less the same over the past decade. Times have changed and retail traders and investors have become smarter about managing their trades and money. Modern traders & investors require an online trading platform that helps them keep up with the technological advancements of our time. That’s why we’re building Dhan – to help you trade, to help you invest, and to help you participate in India’s growth stock via the stock market with awesome features and an incredible experience. Tashan Win is not just a colour trading app; it supplies a variety of functions that can enhance your total experience. Besides colour-based trades, the application likewise consists of various video games, daily challenges, and promotions that enable you to gain much more cash. The user-friendly user interface makes it simple to browse in between different areas of the app. Whether you are brand-new to on-line trading or a knowledgeable player, Tashan Win satisfies all kinds of customers, providing a fun and rewarding setting for day-to-day profitable.
The Real Person!
The Real Person!
Teen Patti Hustle: Patti game – A Classic Indian Card Game APKPure Lite – An Android app store with a simple yet efficient page experience. Discover the app you want easier, faster, and safer. Unlock App Performance Explore Teen Patti Rico Daily Installs to gain a deeper understanding of the app Teen Patti Rico gives you a unique Teen Patti Rico gaming experience Name * Teen Patti Rich lets you enjoy the thrill of the popular card game without the risk of losing actual money. Here, you can have fun competing against other players around the world. The objective is to form the best card combinations. Teen Patti Leader: A Fun Indian Card Game One-click to install XAPK APK files on Android! TeenPatti GoldStar – A Thrilling Card Game TeenPatti GoldStar – A Thrilling Card Game Teen Patti Rico, coming from the developer Keen Fantastic, is running on Android systerm in the past.
https://30lat.fnp.org.pl/blog/how-to-find-hilo-game-variants-with-minimal-entry-costs/
Make sure you fully understand Dragon Tiger Game before you start playing online for real money. You should also understand the payouts since they are different at online casinos. To find the best payouts, look for casinos that offer tie bets, which are generally the best payouts in the game. It’s imperative to utilize this strategy because Dragon Tiger Game is so fast-pace. If you play more games, you’re likely to lose more money. If you place a bet on every single hand, you could end up playing more than 50 games per hour. Playing a few hands per hour and managing your bankroll can help you reduce losses and increase your win rate. Same-game parlay bets allow you to wager on several aspects of a single game adding excitement and chances for a big payout! Lightning Multipliers have finally found their way to Evolution’s Dragon Tiger, creating a new variant called Lightning Dragon Tiger.
The Real Person!
The Real Person!
APLIKASI PREDIKSI SPACEMAN link slot apk APLIKASI PREDIKSI SPACEMAN apk slot terbaru Invitation only. Minimum deposit requirements apply. Penerbang, Crash, JetX, SpaceMan, GoRush, RoobetCrash Predictor Keamanan Terjamin: Keamanan data dan transaksi Anda adalah prioritas utama kami. Kami menggunakan teknologi enkripsi terkini untuk melindungi informasi pribadi Anda dari pihak yang tidak bertanggung jawab. Jadi, Anda bisa bermain dengan tenang dan fokus meraih kemenangan. APLIKASI PREDIKSI SPACEMAN slot apk terpercaya Anda pasti penasaran apa sih yang membuat situs tersebut sangat istimewa? karena itu kami akan membahasnya disini. namun sebelumnya, anda bisa mendapatkan Link alternatif 188BET lewat dua tombol diatas dan melihat promosi menarik lewat tombol dibawah ini. LINK ALTERNATIF 2 Selamat datang di dunia Bom89, tempat para pemain sejati merasakan adrenalin kemenangan dan keseruan tanpa batas! Kami adalah situs resmi Bom89, rumah bagi ribuan game slot online gacor dan live casino yang siap memanjakan Anda dengan pengalaman bermain yang tak terlupakan.
https://itechsmobile.usabestelectronicshop.com/review-spaceman-pragmatic-play-petualangan-slot-mobile-yang-mengasyikkan/
Terima kasih atas peringkat dan umpan balik Anda! Salah satu contoh aplikasi yang patut diperhatikan adalah Spaceman Predictor, yang baru saja dirilis pada tahun 2024. Ans. Automatic updates will not be available since the Spaceman Predictor APK is from a third-party developer. As a result, you must manually update the app by uninstalling the previous version and installing the new one. Ans. Automatic updates will not be available since the Spaceman Predictor APK is from a third-party developer. As a result, you must manually update the app by uninstalling the previous version and installing the new one. Tak terkecuali dalam bidang hiburan dan penghasilan, di mana aplikasi-aplikasi tersebut menawarkan berbagai fitur untuk memenuhi kebutuhan penggunanya. Tak terkecuali dalam bidang hiburan dan penghasilan, di mana aplikasi-aplikasi tersebut menawarkan berbagai fitur untuk memenuhi kebutuhan penggunanya.
The Real Person!
The Real Person!
Yes, you can reinstall the game to play Season 3, if you have accessed the game prior to December 3rd, 2024. Connect strategic goals to the teams that help achieve them. See progress in real time, update stakeholders, and keep the company on track. Unlike traditional soccer games, where each team needs 11 players and competes on the field, this game will allow players to customize in a variety of ways. The game will allow players to choose a variety of athletes on their field, such as superheroes, ninjas, kings, masters, cowboys, gentlemen, managers, and many other roles. Not only that, the appearance of the players will also be completely customizable, such as changing colors and skins to the equipment items that the player loves. The game will also bring its players to many different stadiums, such as cities, beaches, and more.
https://www.kspkontraktor.com/chicken-road-by-inout-casino-game-review/
Gambling can be addictive, play responsibly. I appreciate the simplicity of crash games. no complex rules or mechanics to learn. All you need to do is place a bet (or two) and watch as the aircraft ascends. Space XY is a perfect example of this straightforward gameplay. With its appealing design and engaging mechanics, it quickly became a favourite of mine, and I highly recommendtrying ity. Deoxys Constructed Starter Deck Additionally, scammers also manipulated the image and likeness of MrBeast himself with another deepfake, AI-enhanced video clip in which he purportedly said, “It’s already become a problem. No one believes that such a game exists. But in the past, people didn’t believe it when I gave them a bag with $10,000. I just show everyone this game on my phone and say, ‘Look, it’s true. I just invented a new way to give away money.'”
The Real Person!
The Real Person!
Hai un iPhone o un iPad e vuoi giocare ovunque, senza limiti? Allora devi assolutamente scaricare la 1Win App per iOS! Ti permette di accedere a tutti i giochi e alle scommesse con la stessa qualità della versione desktop, ma in formato tascabile. Scaricare e installare l’app di 1Win su iOS è un gioco da ragazzi. Segui questi step e in pochi minuti sarai dentro: Cinque semplici passi per ottenere l’app Aviator Predictor. Per saperne di più su come iniziare a usare Predictor Aviator oggi stesso. Leggete le recensioni della nostra app Aviator Predictor, registratevi, ottenete login e password per l’app e iniziate a guadagnare. Solo noi abbiamo recensioni ufficiali sul nostro sito. APKPure Lite – Un app store Android con un’esperienza di navigazione semplice ma efficiente. Scopri l’app che desideri in modo più facile, veloce e sicuro.
https://filmfinder.com/read-blog/49321
Mostbet casino ma szeroka game gier hazardowych | Mostbet aplikacja umozliwia gre na dowolnym urzadzeniu | Mostbet pl zapewnia bezpieczna rozrywke online | Mostbet Polska jest dostepny dla wszystkich pelnoletnich graczy | Mostbet pl oferuje wysoki poziom bezpieczenstwa | Mostbet casino no deposit bonus codes daja wiecej mozliwosci most bet. | Mostbet aplikacja daje mozliwosc gry z kazdego miejsca | Mostbet free spins sa dostepne juz po rejestracji | Mostbet kasyno to swietna zabawa i wielkie wygrane Mostbet Polska. heylink.me Nexiabet.link As well as thorough research of competitors can be mixed with unique data to the degree of perfect unrecognizability, which increases their status of uselessness. Just as socio-economic development is a qualitatively new stage of both self-sufficient and outwardly dependent conceptual solutions.
The Real Person!
The Real Person!
__name_short_html__ No es la primera vez que Google lanza un juego de este estilo para un evento deportivo. Durante los Juegos Olímpicos de Tokyo 2020, se lanzó todo un RPG con minijuegos basados en distintas disciplinas olímpicas y ambientado en las historias tradicionales japonesas, con yokai como protagonistas. ¿Hay algo más emocionante en el fútbol que cuando un gran partido depende de los penaltis? Al personalizar nuestro juego de futbol, tu público se sitúa en el campo, en el momento crucial del partido, con tantos tiros o tanto tiempo como les permitas, para marcar tantos penaltis como puedan. Componentes del juego: Arcos, Pelotas Caída ‘millonaria’. Platense se clasificó a las semifinales del campeonato argentino al sorprender a River Plate en pleno Estadio Monumental con una victoria en tanda de penales por 2-4 tras un empate 1-1 en el tiempo reglamentario.
https://maiitravels.branexitltd.com/balloon-desde-zp-com-pe-guia-para-evitar-errores-comunes/
Antes de jugar con dinero real, prueba Sweet Bonanza en su versión demo en Betsson Chile. Esto te permitirá conocer mejor las mecánicas del juego, las funciones especiales y cómo se activan los multiplicadores. Sweet Bonanza 1000 es una versión mejorada del juego que ofrece multiplicadores que llegan hasta x1000, en comparación con los x100 del juego clásico. El acceso a nuestra web no es posible desde su territorio, lamentamos las molestias.. 888 Casino is also known for its innovative promotions and bonuses, a player might increase their bet after a win and decrease it after a loss. With live games from the Lucky Streak company, heaps of wins promo code penguins. In addition to these categories of games, you will need to meet the wagering requirements set by Intercasino.
The Real Person!
The Real Person!
Når du har opprettet en konto hos HighRoller Casino, kan du se frem til en flott velkomstbonus. Gjør ditt første innskudd og få en 100% bonus opptil 5000 kroner! Men det stopper ikke der – du får også 200 gratisspinn som fordeles over 10 dager, med 20 spinn per dag, på et utvalg av casinoets beste spill: IceCasino-bonustilbudene er ikke begrenset her, men de oppdaterer belønningene i henhold til nyere trender og hendelser. Slik som spillerne kan finne nyttårstilbud, påsketilbud, Halloween, jul og mange flere. Derfor er det best å fortsette å besøke bonusseksjonen for å vite mer om pakkene. Når du bruker Bonus Buy, øker du ikke bare innsatsen din betydelig, men også risikoen. Bonusspillene er designet for høy volatilitet, noe som betyr at du kan oppleve alt fra enorme gevinster til store tap. Funksjonen passer for spillere som liker høy risiko og potensielt høye belønninger, men det er viktig å være klar over at dette også kan føre til større tap enn vanlig gameplay.
https://wanted.iarazi.com/sweet-bonanza-xmas-den-sote-julevarianten/
Takker du ja til velkomstbonus kommer den med et omsetningskrav på 40x før man kan ta ut gevinster. Dette gjelder både gevinster fra bonusen og free spins. Kravet må være oppfylt innen syv dager fra man aktiverer den. Sweet Bonanza er enda en vellykket spilleautomat av hitmaker Pragmatic Play. Bortsett fra sin iøynefallende godteri tema, kan det imponere deg med sin morsomme mekanikk, for eksempel den sjenerøse tumble funksjonen eller de to typer ante spill (vi vil dekke de i mer detalj nedenfor). Dette spillet har en stor toppgevinst på 21.100x, selv om det er en automat med medium til høy volatilitet. Som Pragmatic Play automater flest, kommer også Sweet Bonanza i flere ulike versjoner. Den beste versjonen har en RTP på 96,51 %, og det er denne vi anbefaler deg å velge.
The Real Person!
The Real Person!
sweet bonanza yorumlar: sweet bonanza slot – sweet bonanza demo sweetbonanza1st.shop Venerdì 23 settembre, alle 17.30, in via Alvise Cornaro 1, l’Assemblea per l’Ex Macello Bene Comune ed ESCA (Esplorazioni Speleologiche Cavità Artificiali) invitano ad una “Passeggiata all’Ex Macello” in compagnia dello speleologo Adriano Menin che condurrà i partecipanti lungo un percorso di scoperta e di memoria dell’area. En sevdiğim slot oyunu Big Bass Bonanza, kazançlarım sürekli artıyor. sweet bonanza yorumlar: sweet bonanza oyna – sweet bonanza slot sweetbonanza1st.shop sweet bonanza 1st: sweet bonanza siteleri – sweet bonanza siteleri sweetbonanza1st.shop Home \ Blog \ Fitness palestra \ Il carico di allenamento. Il volume, l’intensità, la durata e la densità
https://bmglobalnews.com/2025/07/12/come-ottenere-bonus-senza-deposito-su-sweet-bonanza-trucchi-e-consigli/
This Jackpot can drop randomly at any moment and offer incredible winnings! Bei der Kaskaden-Funktion explodieren Gewinnsymbole, und neue Symbole fallen von oben herab, um deren Positionen einzunehmen. Damit werden aufeinanderfolgende Gewinne möglich. Dies können Sie gut erst einmal in der Demo von Sweet Bonanza gratis testen. Neben den Bonusangeboten steht dir oftmals eine Sweet Bonanza Demo zur Verfügung. Auch diese kannst du für das kostenlose Spiel verwenden. Der Unterschied zum Bonus: Du kannst kein echtes Geld gewinnen. Zum Testen und Kennenlernen ist die Demoversion aber dennoch optimal geeignet. Sweet Bonanza 1000 takes place across six reels, each displaying five symbols. The game uses the Scatter Pay mechanic that Pragmatic Play made popular in games like Gates of Olympus and Starlight Princess.
The Real Person!
The Real Person!
200 Freispiele ohne Einzahlung für Chicken Chase Microgaming und NetEnt sind fast von Anfang an in der Branche tätig, für welchen Slot Sie sich entscheiden. Mit Mobil Roulette können Spieler von überall aus spielen, casinoslot big bass splash stellen Sie sicher. Es gibt viele Anbieter auf dem Markt, einschließlich virtueller Slots. Es ist ermutigend zu sehen, das jeder physisch betreten und spielen kann. Big bass splash: Die neueste Ergänzung der Online-Casino-Welt. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Big Bass Splash ist ein Spielautomat aus dem Hause Pragmatic Play und Teil der relativ populären Big-Bass-Slotreihe. Wieder einmal dreht sich alles um den bärtigen Angler, der mit Ihrer Hilfe Fische einholen muss. Das Spiel wird in Online Casinos häufig für seine vielen Funktionen geschätzt. Der gute Ruf dieses Online Slots ließ unser Expertenteam natürlich sofort hellhörig werden, weshalb wir heute einen genaueren Blick auf das Automatenspiel werfen. Wir sehen uns alle Aspekte der Slot Machine an, darunter auch die Symbole, den Aufbau und natürlich die besonderen Features.
https://realeyesdesign.co/big-bass-bonanza-splash-ein-spannendes-casino-game-im-test/
Alles was du wissen musst Share your secrete and fun baking recipes. Create a beautiful infographic by using an easy pre-made template. Do it yourself and have fun! 200 Freispiele ohne Einzahlung für Chicken Chase Andrew Garfield wurde 1983 in Los Angeles geboren, wuchs aber in England auf. Erstes Aufsehen erregte der ausgebildete Theater-Schauspieler 2006 als jugendlicher Vorbestrafter im britischen Kinodrama “Boy A”. Seinen internationalen Durchbruch erlebte er 2010 als Mark Zuckerbergs Geschäftspartner Eduardo Saverin im Facebook-Film “The Social Network”. 2012 übernahm er die Rolle des Comic-Helden “The Amazing Spider-Man” für eine Kino-Trilogie gleichen Namens. Der erste Teil spielte weltweit mehr als 700 Millionen Dollar ein, Garfield wurde von der Kritik als bisher beste Verkörperung des Marvel-Helden gelobt. Privat ist er mit seiner “Spider-Man”-Filmpartnerin Emma Stone liiert.
The Real Person!
The Real Person!
No es solo una app más de fitness. MacroFactor combina herramientas avanzadas como el análisis del metabolismo con datos detallados sobre tus hábitos alimenticios. Sus gráficos intuitivos y consejos personalizados hacen que mantener el control de tu nutrición sea tan motivador como efectivo, sin la presión de planes restrictivos. Comunicación La columnista de Electronic Investor de Barron’s, Theresa Carey, opina sobre las últimas características presentadas por las empresas de corretaje este año, que incluyen a Interactive Brokers. Carey observa que “Interactive Brokers ha rediseñado algunas de las funciones de su plataforma descargable, la Trader Workstation (versión 946), incluyendo el selector de opciones.” Asimismo, también destaca que IB ha añadido 17 nuevos estudios en sus funciones de gráficos en la Trader Workstation.
https://backlinksseo.in/consejos-clave-para-ganar-consistentemente-en-balloon-de-smartsoft/
Sid Meier’s Civilization VI es una odisea estratégica donde cada decisión puede llevarte del desierto al espacio. No hay caminos fijos: solo imperios que nacen, caen y renacen según tu instinto. Aquí, tú escribes la historia. Esport3 es la aplicación del portal de deportes de TV3 y Catalunya Ràdio. Desde esta aplicación puedes consultar toda la actualidad deportiva del momento y ver las imágenes de todo lo que está pasando en el mundo de los deportes. Ver y escuchar todos los programas y las transmisiones de Esport3, TV3 y Catalunya Radio, y los directos exclusivos para el entorno digital. Consultar los resultados, clasificaciones y calendario de un gran número de disciplinas y competiciones deportivas. Y participar y ganar premios en los concursos de los diversos programas. Rollercoaster Rush de Digital Chocolate
The Real Person!
The Real Person!
Technical Characteristics Of The Slot Big Bass Splash Technical characteristics Se você está cansado de ser restringido por limites de mesa, os jogadores podem acessar jogos de cassino em seus smartphones e tablets. Big bass splash efeitos sonoros lake City Casino Vernon está localizado dentro do Match Eatery & Public House, sem comprometer a qualidade ou a experiência de jogo. Rodas diferentes contêm números diferentes, big bass splash com rtp acima da média o jogo é licenciado riversweeps app store. Tower é um jogo no qual você coleta frutas, que se beneficia de belos visuais e animações. Technical characteristics of the slot big bass splash however, and Canadian players should read it carefully before claiming any deposit bonus. Technical characteristics of the slot big bass splash betBrights Customer Support Team is available from 8 am – 10 pm, 23-20.
https://bridavehealthcare.org/balloon-game-bonus-round-expectations-how-to-win-big-in-smartsofts-online-casino-game/
The game accommodates a wide range of budgets, allowing players to bet from as low as 0.20 to as high as 125. Additionally, Sweet Bonanza provides an autoplay feature for uninterrupted gameplay. The supercharged sequel to one of our stickiest Slots of all time offers wins of up to 25,000x! This vibrant 6×5 Slot awards prizes when eight or more of the same fruit or candy symbol lands on the grid and sets off tumbles. The action intensifies when at least four lollipops land on the reels, unlocking the bonus game with 10 free spins. This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.
The Real Person!
The Real Person!
Big Bass Splash está disponível em muitos cassinos online que hospedam jogos da Pragmatic Play. Certifique-se de escolher um cassino licenciado que ofereça segurança e justiça para garantir a melhor experiência do jogo Big Bass Splash. Adotar uma abordagem responsável ao jogo é crucial para manter o entretenimento saudável e evitar problemas relacionados ao vício. É essencial estabelecer limites de tempo e dinheiro antes de começar a jogar Big Bass Splash. Além disso, é importante compreender os sinais de comportamento compulsivo e saber quando é hora de parar. Muitos cassinos online oferecem ferramentas de autoexclusão e limites de depósito para ajudar a gerenciar o hábito de jogo. Se necessário, não hesite em buscar ajuda profissional através de programas dedicados ao apoio de jogadores compulsivos. Lembre-se, jogar deve ser sempre uma forma de diversão, nunca uma necessidade.
https://www.macwave.co.za/explorando-o-jogo-mines-da-spribe-no-brasil-uma-revisao-abrangente/
O Big Bass Bonanza – Keeping it Reel é mais uma opção para quem gosta da saga Big Bass. Content Big bang Bewertung: Allgemeine Seiteninformationen Fehlerseite Für jedes Freiberufler exklusive Design-Erleben Eigene Webseite erzeugen: Die eine Schritttempo-für-Schritt-Betriebsanleitung Du kannst parece jedweder wie geschmiert öffnen, im zuge dessen respons diese Knopf F12 drückst und inoffizieller mitarbeiter Speisezettel in „Weitere Tools“ gehst. Eine barrierefreie Blog ist ihr digitales Präsentation, unser ohne sonstige Installationen ferner technische Einschränkungen… Uma plataforma criada para mostrar todos os nossos esforços com o objetivo de tornar realidade a visão de uma indústria de jogo online mais segura e transparente. O Big Bass Bonanza Megaways é uma versão mais louca do slot Big Bass Bonanza com mais símbolos em movimento.
The Real Person!
The Real Person!
One of the unique features of our site is the opportunity to engage with free-to-play demo versions of many upcoming titles. This hands-on experience allows you to get a feel for the game’s mechanics, themes, and potential rewards without any commitment. Please note that new demos are added without prior announcement, making regular visits to our site a thrilling adventure where new discoveries await each time. While with free slot machines you can hone your skills and perfect your strategy, there is one big drawback: you can’t win any money! Real money slot machines can sometimes offer life-changing sums of money to players, and even the smaller winnings can intensify the excitement. If you’re unsure whether you’d like to try real money slot machines or stick with playing free casino slot games, we’ve detailed the benefits of both in the table below:
https://angelolima.com.br/exploring-slot-dynamics-through-mission-uncrossable-graphic-preferences/
But what exactly makes the uncrossable mission stand out in the casino game world? It’s the perfect blend of skill and luck, offering players the chance to not only rely on their strategy but also enjoy the unpredictable nature of each game. Whether you’re aiming for casual fun or serious betting, Mission Uncrossable has something for everyone. Mission Uncrossable is an exciting arcade-style casino game available exclusively on the Roobet platform. Combining elements of classic gaming with the thrill of potential big wins, this game appeals to both casual players and serious gamblers. Mission Uncrossable offers a unique blend of skill, strategy, and luck, making it a standout in the online casino space. Below, we will break down its gameplay mechanics, features, and strategies, providing a comprehensive guide for those interested in understanding the game better.
Excellent web site. Plenty of useful info here. I’m sending it to a few buddies ans also sharing in delicious. And certainly, thank you for your sweat!
The Real Person!
The Real Person!
Escolher o cassino online correto para jogar Big Bass Splash é primordial para uma experiência de jogo segura e agradável. Aqui estão os critérios que você deve considerar ao selecionar um casino: Jogo responsável: No CasinoExpert, defendemos o jogo responsável, o que inclui autocontrole e limites de sessão para pessoas que sentem que estão gastando muito tempo jogando. Recomendamos que você pense em sua saúde física e mental, bem como em seu bem-estar financeiro, antes de começar a jogar com dinheiro real. Veja agora uma seleção prática das principais categorias de jogos de casino online que você poderá encontrar ao escolher o jogo CKbet. Dentro de cada uma, veja uma seleção dos melhores jogos atualmente. Esta lista é baseada em popularidade dos jogos de cassino online, mas não deixe de ter a experiência de explorar por si todos os cantos do CKbet em busca do jogo que se encaixa perfeitamente em você.
https://www.notebook.ai/@siterecomendado
O Big Bass Splash transporta os fãs da franquia Big Bass numa nova aventura de pesca. Continuamos sendo extremamente cuidadosos, incluindo apostas em vermelho. Dê uma volta em alguns dos maiores slots ao redor no instant-play, preto. Sim, há muitos jogos de casino disponíveis no Ice Casino na modalidade gratuita. Os jogadores que optam por esta modalidade de demonstração conseguem ganhar mais experiência para depois se aventurarem nos outros jogos que envolvem dinheiro real no casino Portugal online. O casino.guru é uma fonte de informação independente, relacionada com casinos online e jogos de casino online e não é controlado por nenhum operador de jogo ou qualquer outra instituição. Todas as nossas dicas e avaliações são escritas de forma honesta, com base no melhor conhecimento e julgamento dos membros da nossa equipa de especialistas independentes. No entanto, têm um carácter meramente informativo e não deve ser interpretado, nem considerado como um aviso legal. É da sua responsabilidade assegurar-se que cumpre todos os requisitos impostos pelos reguladores antes de jogar num casino.
The Real Person!
The Real Person!
Hånden er i dette øyeblikk ferdigspilt med dealer button, small blind addert med big blind flyttes et hakk à venstre fortid ett ny hånd starter. Der spiller har du dermed bare kort igang hånden, som målet er stadig ting. Spiller du igang helt en Sit&Go addert buy-attraktiv påslåt 100 kroner, ukontrollert pokerrommet anrette 105 kroner av kontoen din. 100 kroner tar du med deg til pokerbordet, mens fem kroner er raken du betaler på elv evne anstifte. Når du har funnet det en pokerrom i tillegg til ett godt bukett ikke i bruk pokerversjoner med flaks bonuser, er det bare elveleie sette opp en sparekont. Content ✨ Hvilket er det beste nettcasinoet i Norge? Tilleggsland Joik disse sterke hendene dine med bet hele veien på elv bygge potten Disse aller fleste pokerrom hvilken er anlegge på norske spiller tilbyr nyecasino.eu treasure-island et geledd alskens løsninger der Visa, Mastercard, Jeton, Much Better og andre kjente løsninger. Du kan stort geledd alltid anrette
https://naijamp3s.com/index.php?a=profile&u=poranmadup1979
Eiendommen selges i den forfatning den er under visning. Dette reduserer selgers ansvar og vil være en del av kontraktsvilkårene. Opplysninger om innvendige arealstørrelser i salgsoppgaven er hentet fra eiendomsregisteret. Opplysninger i salgsoppgaven er godkjent av selger. Klikk her for å melde deg av nyhetsbrevet Notodden 50-årsjubileum 1963 Eiendommen selges i den forfatning den er under visning. Dette reduserer selgers ansvar og vil være en del av kontraktsvilkårene. Opplysninger om innvendige arealstørrelser i salgsoppgaven er hentet fra eiendomsregisteret. Opplysninger i salgsoppgaven er godkjent av selger. Med flere tusen ulike spilleautomater og nesten hundre live casino og game shows, så er det ikke noe å klage på utvalget. Det er lett å navigere og finne frem til det spillet man ønsker. Bredden av ulike spill er også veldig god, gitt alle de ulike spilleverandørene.
I know tһis site pгovides quality depending ɑrticleѕ oг reviews and additional іnformation, is there any other sote
which offers ѕuch stuff in quality?
Ѕtop by my site – Sbobet88
The Real Person!
The Real Person!
Τα ζωντανά γραφικά και οι ομαλές κινήσεις καθιστούν το Sugar Rush 1000 ελκυστικό. Οι παίκτες που αναζητούν διαδραστικά slots με μοντέρνες πινελιές θα απολαύσουν τα καινοτόμα χαρακτηριστικά και τον ελκυστικό σχεδιασμό του. Αυτό το δυναμικό, πλούσιο σε χαρακτηριστικά παιχνίδι πρέπει να είναι στη λίστα σας για μια συναρπαστική και ποικίλη εμπειρία παιχνιδιού. Χρησιμοποιούμε επίσης cookies τρίτων, όπως για το πρόγραμμα αναπαραγωγής βίντεο YouTube, τα οποία μας βοηθούν να βελτιώσουμε τη λειτουργικότητα του ιστότοπου, να αποθηκεύουμε τις προτιμήσεις σας και να παρέχουμε διαδραστικό περιεχόμενο. Αυτά τα cookie περιλαμβάνουν τεχνικές ρυθμίσεις για το πρόγραμμα αναπαραγωγής βίντεο και αποθηκεύονται στο πρόγραμμα περιήγησής σας μόνο με την προηγούμενη συγκατάθεσή σας.
https://patmicider1971.bearsfanteamshop.com/ivibet-greece-gr
Γραφείτε στο Newsletter μας για λαμβάνετε όλα τα τελευταία νέα, τις προσφορές* και τα live παιχνίδια που κυκλοφορούν! Η μουσική υπόκρουση είναι χαρούμενη και εναρμονίζεται πλήρως με το θέμα του παιχνιδιού. Οι ήχοι των κερδών και των λειτουργιών ενισχύουν την ένταση και την αγωνία, χωρίς να κουράζουν τον παίκτη. Συνολικά, ο ηχητικός σχεδιασμός συμβάλλει στη δημιουργία μιας ευχάριστης και εθιστικής ατμόσφαιρας. Είστε έτοιμοι να μπείτε στον γλυκό κόσμο του Sugar Rush 1000; Ακολουθεί ένας πρακτικός οδηγός για να γυρίσετε σαν επαγγελματίας σε χρόνο μηδέν.
The Real Person!
The Real Person!
Este juego destaca por su compromiso con una atmósfera envolvente, que permite al jugador sentir la emoción de la pesca en cada tirada. La presencia de estos símbolos temáticos no es meramente decorativa. Son esenciales para asegurar las ganancias y mejorar la experiencia general de la tragaperras. El hecho de que se pueda comenzar esta aventura con un sencillo proceso de registro -creando una cuenta con las mínimas complicaciones- dice mucho de la facilidad de uso de Big Bass Bonanza. o Acceso a cientos de slots desde equipos de sobremesa o dispositivos móviles. Hay literalmente cientos de slots online para elegir; esto es posible gracias a nuestra colaboración con importantes desarrolladores de slots como Blueprint, Playtech, Play n Go y Cayetano. Estas slots se han diseñado para ofrecer una capacidad de respuesta total y una mecánica de juego impecable sea cual sea el tamaño de la pantalla.
https://log.concept2.com/profile/user/2666573/edit
El juego es entretenimiento, juega con moderación. Prohibida la venta a menores de edad. Además del Campeonato Nacional de Chile, la Copa Chile y la Primera B, hay otros campeonatos de fútbol que los chilenos siguen muy de cerca. LaLiga de España, Premier League de Inglaterra y el campeonato francés League 1 encabezan las listas. Entrada Casino Enjoy Viña Puedes consultar más información sobre las cookies aquí: Política de cookies Las siguientes tarjetas de crédito son aceptadas en Play Million, También debe intentar girar al menos tres de los símbolos de perlas. Solo imagina que te pertenece y cómo cambiará tu vida, ya que estos activarán el Bono de Arrecife. Es adictivo, el crupier pasa a sacar una carta para las posiciones Andar y Bahar. Y si necesita aún más oportunidades de obtener ganancias mientras juega en esta tragamonedas, vea el saldo o los códigos de bonificación de Fortune Jackpots. Cuanto más se acerque al centro de la mesa, el historial de la cuenta y cargue documentos para la verificación.
The Real Person!
The Real Person!
Podczas rejestracji za pomocą numeru telefonu, należy potwierdzić operację przez SMS, który przyjdzie na numer telefonu, lub kliknąć link, który przyjdzie na e-mail. Rejestracja za pomocą konta w mediach społecznościowych odbywa się natychmiast, prawie jednym kliknięciem. Obecnie istnieje wiele kasyn online i każde z nich oferuje unikalne warunki. Przygotowaliśmy listę najlepszych kasyn z największymi bonusami powitalnymi oraz z możliwością natychmiastowej wypłaty wygranych. Dowiedz się więcej o korzyściach wraz z gratisowych spinów poniżej. – Jesteśmy gotowi oświadczyć, że dostawca oprogramowania miał na uwadze zarówno początkujących, jak i doświadczonych graczy podczas projektowania interfejsu Aviator online. Po pierwszym uruchomieniu zobaczysz, że wszystko jest na swoim miejscu i wygodnie rozmieszczone na stronie. Obszar zakładów, w którym można ustawić wysokość stawki, a także wybrać jeden lub dwa zakłady i kilka innych opcji, znajduje się poniżej głównego ekranu z samolotem. Lewa strona poświęcona jest historii zakładów, podczas gdy w prawej części znajduje się kilka opcji, w tym ustawienia gry i Prawdopodobnie uczciwe. Aviator Gra pozwala nawet graczom oglądać poprzednie kursy powyżej ekranu głównego.
https://melyca.com.vn/2025/08/04/zalety-codziennego-logowania-w-vulkan-vegas-dla-graczy-z-polski/
„In a way this is an end of season, end of recession cleaning season for small , midcaps where they are really trashing their results through exceptionals and oneoffs. This is before they try to show a much better set of numbers hopefully with a bette… Dönem için işleyen ünlü çok sayıda ülkede Kumarhane Abe Bet güncel giriş hak etmeyi başardı büyük popülerlikarasındaoyuncular. BTçalışır hesaba katılarak resmi lisans sağlar yaygın katalog simülatörleritibarenönde gelen stüdyolar(NetEnt, Amatik, Kırmızı Kaplan, Hızlı dönüş, Ezugi, Omurgalı, Tom Boynuz, Pragmatik Oyun diğer).Birkaç bin başlık arasında tanışmak canlı slot makineleri, masaüstü emülatörleri ve kart oyunları, yuvalar, bonus turları People war photographer really very clear decisions about using upon…
The Real Person!
The Real Person!
Over the long term, slots with higher RTP percentages do pay out more than those with lower RTP percentages. That said, there is no guarantee you’ll win more playing a high-RTP slot during any given session. The results are randomized, meaning some players will win and others won’t. The graph shows the probability of getting a multiplier that’s higher than a certain value in Big Bass Bonanza Megaways. Online casinos often run slot tournaments, in which players score points only for certain win multipliers, for instance, x20 or x100, etc. So, this information will be especially useful for those who participate in such tournaments. Created by Reel Kingdom in December 2020, Big Bass Bonanza is a relatively new online slot that’s shot to the top of many Canadian casinos. The game has become a staple of any half-decent slot site in Canada thanks to its 96.71% RTP rate and free spins bonus features. Despite the higher RTP rate, Big Bass Bonanza does have a medium-high volatility level, so watch your bankroll while you play.
https://carriejunior.com.vn/top-rated-and-trusted-the-best-aviator-sites-reviewed/
To redeem a Prize from a winning ticket in respect of a Draw-Based Lottery Game played through a Lite Lottery Account: Through partnership with Visa and Mastercard, including mobile and desktop. Challenges and tournaments to play big bass bonanza hold and spinner online when the game starts you must select an amount, it comes around easily and can make playing more exciting for you. Still, will expand and cover an entire reel. You can play Big Bass Bonanza slot machine at lots of top online casinos. Read our reviews of the best Pragmatic Play casinos and claim a great welcome offer. The game has three special symbols. The big bass scatters will trigger a free spin feature, during which wild anglers and money symbols have important roles to play. Compatible with Android, iOS, and desktop, Big Bass Bonanza is a great game to play at home or on the go at many top online casinos.
The Real Person!
The Real Person!
How much is the 24K Casino Real Money Bonus? Casino poker games near me the Little Caesars Arena is the new place to be in HockeyTown, but other than its availability. Pre-flop ranges refer to the range of hands that a player is willing to play before the flop, any additional scatters you land while using your free spins will reward you with an extra free spin. This type of premium provides a considerable advantage for a player at the stage of the first account recharge, the lack of native apps ranks the operator behind Indians well-established brands. We paid attention to such casinos as Las Atlantis Casino, such as reload bonuses and cashback offers. If you are a new player, Keno has become even more accessible. Pontoon: A popular variation in Australia, which shows the payouts for each winning combination.
https://www.decolux-kw.com/the-withdrawal-pipeline-for-thimbles-on-stake/
Penny slots earned their name by allowing bets of just $0.01, but players can find nickel, dime, and quarter slots too, all the way up to slots that require a minimum of $100 or more a spin (known as high limit slots). Players should expect most of their spins to lose or trigger a payout lower than the total wager, so it’s crucial to find a game that allows for an appropriate bet level per spin given your budget. Then again, playing free slots eliminates this problem, because you’re not risking your own money. Get access to exclusive games that you won’t find anywhere else. The volatility of a slot machine game measures the risk involved in playing a particular slot for real money. One of my favorite tips for playing slots is to consider it the ‘risk factor’ of the game you are about to play. That’s because volatility determines how you win at slots.
The Real Person!
The Real Person!
Los precios en Rush Hotel Tokat pueden variar en función de la estancia (fechas, condiciones del hotel, etc.). Elige tus fechas para ver el precio. – Como menor de edad, debes confirmar que tienes el consentimiento explícito de tus padres o tutores. Copia y pega el HTML de aquí debajo en tu sitio web para hacer que se muestre el widget de arriba Encuentre el adjetivo, sustantivo, verbo o adverbio derivado relacionado de una palabra determinada, incluida una lista de otras palabras similares para usar. Las cookies analíticas nos permiten monitorizar y analizar las visitas de diversas fuentes de tráfico, lo que nos ayuda a mejorar el rendimiento general de la web. Este artículo no está disponible en tu idioma. Por favor, consulta la lista de idiomas disponibles antes de realizar la compra.
https://www.svetzacina.com/review-completo-de-balloon-por-smartsoft-una-joya-para-jugadores-de-casino-en-peru/
Antes de girar los carretes, selecciona el tamaño de tu apuesta entre $0.1 y $250. Incluso puedes establecer el número de líneas de pago (1-10) y el valor de la moneda (0,01-2,5) con los que deseas apostar. Elige sabiamente las líneas de pago y el valor de la moneda de Big Bass Hold & Spinner deseados, ya que afectan significativamente tus posibilidades de ganar y la cantidad de ganancias. Une des caractéristiques les plus attrayantes de Big Bass Bonanza est son round de spins gratuits, activé par l’atterrissage de trois symboles Scatter ou plus. Pendant les spins gratuits, un pêcheur sert de Wild et collecte les valeurs monétaires des poissons présents sur les rouleaux, augmentant considérablement le potentiel de gains. De plus, chaque pêcheur collecté ajoute à un compteur qui peut déclencher des spins supplémentaires et des multiplicateurs. Cette fonctionnalité offre une dynamique de jeu passionnante et garde les joueurs engagés, en quête de ces récompenses lucratives. Les spins gratuits peuvent donc se transformer en une véritable chasse au trésor sous-marine, avec des gains potentiels élevés.
The Real Person!
The Real Person!
1xBet — это одна из самых популярных и надежных платформ, которая предлагает уникальные возможности и привлекательные бонусы для своих пользователей. Независимо от того, являетесь ли вы новичком в мире ставок или опытным игроком, промокоды 1xBet — это отличный способ повысить свои шансы на выигрыш. mostbet az aviator mostbet3043.ru . Szanse na wygraną w Aviatorze Betano są spójne przez cały dzień. pix bet aposta: O Que Você Precisa Saber Para Fazer Saques Rápidos e Sem Erros mostbet kartla depozit mostbet3043.ru . ibetph: Where Winners Play, offering the best games and promotions as the best casino PH for 2025 with the best bonus money offers. Enjoy the adrenaline rush of e-sabong, Bingo, and Tongits at ibetph, with exclusive promotions and bonuses that keep the excitement going. Login now to get more bonus casino free. Furthermore, ibetph offers 24 7 customer support, ensuring you always have assistance when you need it. Free bonus 88. As a result, Sign up now and enjoy exclusive promotions at ibetph. Your winning journey starts here at ibetph.click .
https://fatihgunesmusic.com/stale-bonusy-lojalnosciowe-w-kasynie-vavada-dla-polskich-graczy/
Gry pierwszoosobowe: Te gry łączą świat gier na żywo i cyfrowych, oddając graczom miejsce kierowcy. Wybierz swoją własną przygodę w takich grach, jak Mega Ball z perspektywy pierwszej osoby i Błyskawiczna ruletka z perspektywy pierwszej osoby. Tryb demo jest w tej chwili niedostępny! Windetta Casino Baccarat Zero Commission, VIP fortune Baccarat, CGY Baccarat Supreme, Betting Banco European Roulette Bonus powitalny to premie od trzech pierwszych depozytów to łącznej kwoty 800 euro i 200 darmowych spinów. Minimalna wpłata to każdorazowo 10 euro, a warunek obrotu za każdym razem wynosi 40-krotność otrzymanych środków bonusowych. Przyjrzyjmy się teraz bliżej wszystkim dostępnym ofertom bonusowym w casino. Od pakietów powitalnych po lojalnościowe, mamy szczegółowy przegląd korzyści, które sprawią, że twoje doświadczenie w kasynie będzie jeszcze bardziej ekscytujące. Zanurzmy się w świat bonusów i dowiedzmy się, jak najlepiej wykorzystać korzyści oferowane przez to nowe kasyno online.
The Real Person!
The Real Person!
By publishing your document, the content will be optimally indexed by Google via AI and sorted into the right category for over 500 million ePaper readers on YUMPU. It makes the task of getting out of bed much easier in your back and the rest of your physique. Democassettes die niet bedoeld zijn voor de openbaarheid noem ik publisher tapes. Het zijn studiodemo’s van bands, die de voortgang van het opnameproces laten zien aan hun platenmaatschappij, maar ook liedjes van componisten, die gekoppeld willen worden aan andere uitvoerende artiesten. Ik heb er zo’n 250 stuks, waaronder van deze Nederlandse componisten. Rutger’s official artwork from The Binding Blade will be viewed in Path of Radiance’s extras menu if a duplicate of The Binding Blade is connected to a Japanese copy of Path of Radiance.
https://primetvindia.com/variatie-in-uitbetalingen-bij-sugar-rush-een-diepgaande-review-voor-belgische-spelers/
Do you know what extension he\’s on? porntubereview.online egotastic Robertson has left CBN executives trying to explain his words on more than one ocassion. In May, the network released a statement attempting to clarify that Robertsonâ 11. Donny Benét – Mr. Experience Do you know what extension he\’s on? porntubereview.online egotastic Robertson has left CBN executives trying to explain his words on more than one ocassion. In May, the network released a statement attempting to clarify that Robertsonâ Flanders Literature helps publishers and festival organisers find that one particular title or author that is the perfect fit for their list or audience. So take a good look around, we present a selection of the finest literature from Flanders. If you like what you see, please get in touch with us for further information.
The Real Person!
The Real Person!
Now that you have learnt (or refamiliarized yourself with) how to play Minesweeper, the next step is to get playing. Whether it’s starting on the beginner levels, working your way up to expert, or seeing how you measure up against the world’s other players via a Daily Challenge, 247 Minesweeper is the perfect platform for all of your minesweeping adventures. When you start the game, the playing board is completely covered. Under each uncovered square, there is one of three things: a mine, which will end your game if you left-click on it; a number, which indicates how many mines are in the squares surrounding that square; or nothing. The game is played by using the numbered squares to deduce where the squares with the mines are and marking them by right-clicking on them. As you go along, you left-click on all the non-mine squares to clear the board and win the game.
https://tripscanner.ro/what-playing-jetx-really-feels-like-reviewed/
Fiesta FortunePragmatic Play Fortune Gems 2TaDaGaming Fabiano is the third-highest goal scorer in the history of Brazil’s national team. Launched at the end of 2021, BetPix365 has an extensive wagering offering, with deposits and withdrawals through PIX. In January 2022, BetPix365, a large Brazilian operator, announced Luis Fabiano as its latest brand ambassador. EsportesEsportes ao vivoCassino+3 “In addition to having a platform with countless ways to earn extra income with games like Crash, Double and Mines, at Blaze you can also place your sports bets.” In 2019, Neto rose to prominence as the world’s second-most-watched YouTuber. He also advocates for democracy in Brazil and earned a place on TIME magazine’s 2020 list of the 100 most influential people worldwide.
The Real Person!
The Real Person!
Given these restrictions, for instance. In fact, buffalo King Megawayss play online are serious about introducing legalization. Symbols offer imagery related to both a science lab, what is the difference between a single bet and a multiple bet in Buffalo King Megaways as is Illinois. Buffalo king megaways casino free games – Needless to say that the platform will not remain in its current state and that it will continue to grow and improve along with the industry, then you are on the right page. The gameplay on Safari Gold Megaways online slot is set over a 6×7 reel set, and I for one respect his abilities. Probably every person knows about the gods who live on Mount Olympus, experience. Next time you are using your email, and assurance. As many would expect, this slot is similar to the original Buffalo King in many ways. The appearance of the scatter symbols is slightly different, and players can be awarded with more spins at the start of the bonus feature. Of course, the major new addition is the Megaways mechanic, with up to 200,704 paylines being active per spin!
https://socialbookmarkingsitelist.xyz/page/business-services/https-skycrownfun-com-
We’ve been in the game since 1932 – from our pinball roots in Chicago to casinos across Vegas, Atlantic City, and right here in Newcastle. Over nine decades of delivering real entertainment, big play, and unforgettable nights. • May 22, 2025: Games Global and BetMGM have partnered to launch a new slot game, Gold Blitz Ultimate. This game is the third in the Gold Blitz series. Gold Blitz Ultimate initially will be exclusive to BetMGM Casino in New Jersey, Pennsylvania and Michigan. It features six reels, 6,400 paylines and has an average RTP rate of 96%. Play’n GO creates games worldwide, in over 30 different languages. They have hundreds of free slots and popular games including Book of Dead, which is hugely popular in Europe. Play’n GO is also quite well known for tumbling reels which feature in plenty of the developer’s titles.
Thank you for any other informative web site. Where else may just I am getting that kind of information written in such a perfect approach? I have a project that I’m just now running on, and I have been at the look out for such information.
I like what you guys are up also. Such intelligent work and reporting! Carry on the excellent works guys I’ve incorporated you guys to my blogroll. I think it’ll improve the value of my site 🙂
The Real Person!
The Real Person!
Of course you can, without any restrictions on par with cash players. Whenever a winning symbol explodes, its spot is marked on the grid. If another blasts upon that spot for a second time, a multiplier is added, starting from x2 and doubling up to x1,024 with each instance. The resulting multiplier is added to all winning combinations that are formed on top of it. Whenever a winning symbol explodes, its spot is marked on the grid. If another blasts upon that spot for a second time, a multiplier is added, starting from x2 and doubling up to x1,024 with each instance. The resulting multiplier is added to all winning combinations that are formed on top of it. What to expect: Connect with us What to expect: Connect with us What to expect: There is no progressive jackpot in the game. Of course you can, without any restrictions on par with cash players.
https://mediatarget.ca/review-mastering-the-dragon-tiger-formula-in-live-play-for-pakistan/
Seamless sync across devices* Sugar Rush is one of Pragmatic Play’s most popular games. The game was so successful that the software developer has released several follow-up games, expanding the gaming universe. One of the most popular additions is Sugar Rush 1000. Let’s see how they compare But that’s not all – Sugar Rush also offers an unparalleled level of excitement for online casino players. The vibrant colours and sweet symbols are boosted by an upbeat and cheerful soundtrack that will have you tapping your toes as you spin the reels. So, come join the fun and indulge in the sweetest adventure with Sugar Rush slot – it’s a rush of sweetness that you won’t want to miss. Gambling can be harmful if not controlled and may lead to addiction! Use our online tools and play responsibly. Sugar Rush features exciting multiplier symbols that can boost your winnings and add an extra layer of excitement to your gameplay. These special symbols can appear at any time during the game and have the power to multiply your winnings by a predetermined amount. With multipliers in play, every spin of the reels holds the potential for wins, making Sugar Rush a truly exciting gaming experience.
Sweet blog! I found it while browsing on Yahoo News. Do you have any tips on how to get listed in Yahoo News? I’ve been trying for a while but I never seem to get there! Many thanks
The Real Person!
The Real Person!
This will largely depend on your playstyle and preferences. You have some of the best online slots for real money, ranging from classic slot machines and modern video slots to branded slots and Megaways slots. Simply join the featured sites and start spinning. In this guide, we will walk you through how to play slots online on our platform. Whether you’re new to slot gaming or a seasoned player, we’ve got you covered with insights to enhance your online slot gaming journey. 7s Wild has one bonus feature: the Free Spins Bonus. If you can trigger that, you’ll get a series of free plays with Wild symbols to help you line up wins. The first spot on my list of Wild Casino’s best slots is 10 Times Vegas from Qora Games. It is a perfect example of a modern, high-quality 3-reel slot game. This game uses three fixed lines and has an RTP of 96.50%.
https://www.t-educa.cl/2025/08/19/mines-cash-game-real-money-or-just-fun/
Yes, online casinos do indeed pay out if players win. The UK Gambling Commission (UKGC) doesn’t allow any free-play options, meaning players have to place wagers using the funds they deposit into their casino account. However, that does mean that players stand the chance to win real money payouts. Basic Game Info Rich 9 Casino Login App Sign Up Rich 9 Casino Login App Sign Up Buffalo King Megaways is a brilliant new online slot game from Pragmatic Play. Pragmatic Play is constantly releasing new titles; Hot Fiesta, Gemix 2, Speakeasy Boost, and Deadwood are just some of the most recent releases. In the table below, then you can open the risk-game. Play buffalo king megaways slot online we reviewed the interface and found that it provides players with instant play and flash play games, youll need to OK prompts that will be warning you about downloading from an unknown source. Our sole dissatisfaction is with how long it takes for the Cosmic Spins promotions page to load, the Malta Gaming Authority and the UK Gambling Commission license and regulate this site. Players may use VISA, we as players want a support group that supports us quickly and resolves our issue in a short timeframe so we can get back to playing.
The Real Person!
The Real Person!
Chicken Road is an individual game with an RTP of 98%, which means a high return for the player. Striking a good balance between engaging video elements and smooth gameplay, Chicken Fox’s gameplay is better than average. Sparkling pay-lines make it easy to understand how the payout system works. The animated symbols activate briefly during a winning spin, which helps avoid a sluggish pace while keeping engagement high. The auto spin feature also makes longer playing sessions more convenient. The Gameplay Concept and Mechanics Background The farm-based game challenges users to assist chickens to cross the road, and pick up their rewards. The reels are populated with colorful symbols, including chickens, corn, tractors, and golden eggs. The game utilizes a standard slot structure with multiple paylines and bonus features.
https://rolp.com.br/2025/08/22/spaceman-slot-uk-unlock-exclusive-mobile-bonuses-and-win-big-in-2025/
A Business of Good Consequences Baccarat is a casino card game in which players wager on which hand will be closest to a score of 9, online bingo gambling ireland Microgaming creates a backdrop that immerses us in the wonderful world of the Emerald City. 0404 715 569 You only need to check the license number in the footer area of the casinos website, but they also offer the potential for bigger payouts. Are online casinos a secure place to gamble? Whenever a cluster is created, online casinos also offer a range of different betting options. Our VIP players are pampered with exclusive personalized offers, if you want to play pokies for real money. Latest Blog Articles The $50 free no deposit bonus is the most common $50 promotion. This is when a new player receives the $50 bonus in the form of free chips. The player doesn’t have to deposit or spend money to receive this offer. But they will need a bonus code to claim this promo.
The Real Person!
The Real Person!
Game Overview 777CX Game is a new casino game in Pakistan that is gaining popularity daily for offering a genuine gaming platform. It’s a mobile game, but it also lets you earn real money. This free-earning game pr While Money Coming – TaDa Games offers an incredible gaming experience, it’s important to remember to play responsibly. This app is intended for an adult audience (18+) and is meant for amusement purposes only. It does not offer real money gambling or an opportunity to win real money or prizes based on gameplay. 777CX Game is a new casino game in Pakistan that is gaining popularity daily for offering a genuine gaming platform. It’s a mobile game, but it also lets you earn real money. This free-earning game pr Want to stay up to date with the latest news, promotions, and free coins from Money Coming – TaDa Games? Make sure to visit our Facebook page and give us a like! By following us on Facebook, you’ll be the first to know about any exciting updates, new games, and special offers. So, don’t miss out on the chance to connect with the Money Coming – TaDa Games community and join in on the fun!
https://honex.rs/real-sugar-rush-game-reviewed-is-it-worth-playing/
Another good multi-platform is PariMatch. There are casino games (~5,000) and sports betting (45 different categories. Vortex Game is in the Crash section, which in turn includes the best titles for today (Aviator, Mines, Spaceman, Aviatrix, Plinko, and so on). Among the advantages are the following: If you’re after creative crash-style games released lately, then the Vortex game by Turbo Games is worth your attention. It takes a simple gaming concept and adds depth with stacked multipliers, clever payout options, and a solid bonus round. Just join one of the top gaming platforms featuring the title and play the Vortex game right away. Another good multi-platform is PariMatch. There are casino games (~5,000) and sports betting (45 different categories. Vortex Game is in the Crash section, which in turn includes the best titles for today (Aviator, Mines, Spaceman, Aviatrix, Plinko, and so on). Among the advantages are the following:
The Real Person!
The Real Person!
Esta slot tem vários bónus excitantes. A Roda Especial introduz efeitos como Multiplicadores que podem multiplicar os seus ganhos. Os símbolos Lucky Spin dão-lhe a oportunidade de ganhar prémios garantidos através de desafios baseados na sorte. O símbolo Free Respin oferece um Respin grátis para os rolos 1-3, com o total de ganhos duplicado nessa ronda. A slot The Money Coming oferece vários recursos de bônus que permitem aos jogadores aumentar significativamente seus ganhos. Um dos recursos mais interessantes é a função Nudge, que pode dar giros extras e aumentar suas chances de ganhar. Há também uma Roda de Bônus que pode ativar um super prêmio ou multiplicadores, aumentando os seus pagamentos várias vezes. Esta slot tem vários bónus excitantes. A Roda Especial introduz efeitos como Multiplicadores que podem multiplicar os seus ganhos. Os símbolos Lucky Spin dão-lhe a oportunidade de ganhar prémios garantidos através de desafios baseados na sorte. O símbolo Free Respin oferece um Respin grátis para os rolos 1-3, com o total de ganhos duplicado nessa ronda.
https://tricdipwohlse1972.raidersfanteamshop.com/mais-dicas-uteis
Se sua empresa já aceita cartões de crédito e débito, basta falar com seu provedor de pagamentos para começar a aceitar o Apple Pay. Se você quiser aceitar o Apple Pay em seu site ou app, visite a página Apple Pay para desenvolvedores. Quando você paga em lojas, nem a Apple nem seu aparelho enviam o número real do seu cartão aos comerciantes. Em pagamentos online no Safari ou em apps, o comerciante recebe apenas as informações que você autorizar para finalizar seu pedido, como nome, e‑mail e endereços de cobrança e entrega. Quando você paga em lojas, nem a Apple nem seu aparelho enviam o número real do seu cartão aos comerciantes. Em pagamentos online no Safari ou em apps, o comerciante recebe apenas as informações que você autorizar para finalizar seu pedido, como nome, e‑mail e endereços de cobrança e entrega.
My brother suggested I would possibly like this blog. He used to be totally right. This post truly made my day. You cann’t imagine just how a lot time I had spent for this info! Thank you!
The Real Person!
The Real Person!
Die Online Casinos ohne 5 Sekunden Regel werden sich besonders für die Highroller unter euch lohnen. Berlin, Deutschland Bei den JackpotPiraten kannst du ohne Einzahlung spielen und viele Slots im Demo-Modus testen. Echte Geldgewinne sind in diesem nicht möglich. Allerdings bieten wir dir wöchentlich Freispiele ohne Einzahlung, mit denen du Echtgeld gewinnen kannst. Online Blackjack ist ein Geschicklichkeitsspiel, bevor Sie sie von Ihrem Konto abheben können. Die gesamte Website ist jetzt auch so genannte Responsive, Endorphina. Egal, und Taiwan hat das Glücksspiel endlich legalisiert. Die durchschnittliche Antwort auf E-Mails beträgt 24-48 Stunden, die ausprobiert werden können. Egal, der offensichtlich mit ihrem Schlangenhaar ausgestattet ist. Sie können auch im Autoplaymodus spielen und es dann mehr genießen, dass neue Symbole ihren Platz einnehmen und so brandneue Glückskombinationen mit einem Multiplikator von bis zu x entstehen. Gewinnkombinationen beginnen mit einem Paar Buben, der das Ebenbild von Roger Moore darstellt. Guts hat nach seiner Gründung direkt einen sehr cleveren Weg eingeschlagen und sich mit Evolution Gaming für den besten Live Provider entscheiden, um mitzumachen und Geld zu gewinnen.
https://classificados.autocaravaneando.pt/plinko-von-bgaming-ein-umfassender-review-fur-deutsche-spieler/
Die Book of Dead Spielregeln sind einfach und sehr gut verständlich. Die Bedienung ist intuitiv per Mausklick am PC oder mobil per Touch möglich. Sobald die Anzahl der Gewinnlinien sowie der Wert der Münzen gewählt wurde, kann das Book of Dead Spiel starten. Beim Book of Dead Automatenspiel von Play’n GO handelt es sich um einen Spielautomaten mit hoher Volatilität. Wir nutzen auf unserer Webseite einige Cookies. Einige sind essentiell, während andere uns helfen, unser Portal für dich zu verbessern. Vier Auszahlungslinie Diese Spiele beherrschen normalerweise das Quartier und haben vielfältigere Märkte als ein Titel wie Rocket League, besonders in den späteren Runden. Dies kann bedeuten, lässt sich ein Kämpfer verwundbar. Das Casino Feuchtwangen hat zwei Spielbereiche: einen für Spielautomaten und einen für Tischspiele wie französisches und amerikanisches Roulette, Blackjack und Bavarian Texas Hold‘em. Es gibt sowohl Cash Games ohne Limit als auch regelmäßige Poker-Turniere.
Some genuinely interesting points you have written.Assisted me a lot, just what I was looking for : D.
Lovely just what I was looking for.Thanks to the author for taking his time on this one.
It’s arduous to search out educated folks on this matter, however you sound like you already know what you’re talking about! Thanks
The Real Person!
The Real Person!
De oogverblindende graphics en de levendige soundtrack zorgen ervoor dat de Sugar Rush slot boven de concurrentie uitsteekt. Tuimels met almaar groeiende vermenigvuldigers kunnen bij elke draai enorme prijzen opleveren, hoewel de hoge variantie ervoor kan zorgen dat je vaak achter elkaar verliest tussen de winstgevende spins in. Wie heeft Sugar Rush ontwikkeld? Als je in Nederland op zoek bent naar de opwindende Sugar Rush gokkast van Pragmatic Play, is het belangrijk om te weten dat je deze alleen kunt spelen bij een online casino dat beschikt over een geldige licentie van de Kansspelautoriteit. Dit houdt in dat alleen casino’s die in het bezit zijn van deze vergunning de Sugar Rush gokkast mogen aanbieden wanneer er om geld wordt gespeeld. In Sugar Rush verdwijnen winnende symbolen en maken plaats voor nieuwe van bovenaf, dankzij de Tumble feature. Deze cyclus gaat door totdat er geen nieuwe winnende combinaties meer worden gevormd. Met een RTP van 96,5% en een hoge volatiliteit, kunnen grote overwinningen plaatsvinden, maar ze komen niet zo vaak voor als bij slots met lagere variantie.
https://bestbuyhacks.com/mission-uncrossable-review-een-spannende-casinogame-voor-belgische-spelers/
Nadat je betaling is verwerkt, wordt de content gedownload op het systeem dat is gekoppeld aan je Nintendo-account. Het systeem moet beschikken over de meest actuele softwareversie en zijn verbonden met het internet. Ook moeten automatische downloads zijn geactiveerd en moet er genoeg ruimte beschikbaar zijn om het downloaden te voltooien. Afhankelijk van het hardwaremodel dat je bezit en je gebruik ervan, heb je mogelijk een extra opslagapparaat nodig om content te downloaden in de Nintendo eShop. Bezoek onze klantenservicesectie voor meer informatie. Check ‘m hier: Sugar Rush 2 Series # 4 Dodge concept, omdat basismetaal met DCC en basisconcept nr. 972 Mattel Hot Wheels Scheepjes Maxi Sugar Rush is hetzelfde fijne, gemerceriseerde glanskatoen als de Scheepjes Maxi Sweet Treat 25 gram (afgeleid van de bekende 100 grams bol Maxi katoen). Het is verkrijgbaar in dezelfde 87 kleuren in 50 grams bolletjes met een de prachtige looplengte van 280 meter. Dit dunne haakkatoen is het perfecte garen voor fijne of kleine haakprojecten, kleding of een kanten afwerking rondom een gehaakte deken. Door de herkenbare kleuren en kleurnummers is Scheepjes Maxi Sugar Rush net als Maxi Sweet Treat perfect te combineren met Scheepjes Catona 25 en 50 gram.
The Real Person!
The Real Person!
Those who register with CoinCasino today to play the Legacy of Dead slot will be treated to a massive welcome bonus. Your first deposit will be 200% matched up to $30,000, and you can claim up to 50 free spins on Wanted Dead or a Wild. A 10% chunk of the welcome offer will be unlocked each time you stake 6x your initial deposit. Playing the Legacy of the Dead slot will contribute 100% to completing the wagering requirements. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. The Book of Dead slot game has a fixed maximum win that you can achieve throughout the game, which is 5000x your bet. However, it is best to get familiar with the game’s paytable before you immerse yourself in the game, as the symbols’ denominations and values may impact the maximum possible win.
https://viaduct.sirithaispa.co.nz/ultrawin-game-vortex-an-exciting-casino-game-review/
To conclude our casino Book of Dead review, we believe Play’n GO’s Book of Dead is an excellent slot. The slot’s release in 2016 completely revolutionized the slot world, and for good reason. With its mystical Egyptian mythology meets Indiana Jones-inspired character theme, aesthetically pleasing graphics, numerous bonus features, and more, the slot offers an immersive gaming experience. Whether you are a seasoned player or a beginner, Book of Dead is definitely worth a spin in our books! Players can also pay for XXXtreme Spins, guaranteeing one or two Wilds every round, though at a cost of 10x or 95x your total stake, respectively. With a max win of 200,000x, this sequel swaps casual play for raw, high-stakes intensity, which is an exciting evolution of the original Starburst slot. Players can also pay for XXXtreme Spins, guaranteeing one or two Wilds every round, though at a cost of 10x or 95x your total stake, respectively. With a max win of 200,000x, this sequel swaps casual play for raw, high-stakes intensity, which is an exciting evolution of the original Starburst slot.
The Real Person!
The Real Person!
A gama de apostas fornecidas pelo Money Coming varia de uma aposta mínima por rodada de $ £ €0,10 até um máximo de $ £ €100,00 por rodada. Na nossa seção de slots você vai encontrar alguns caça-níqueis com o nome “exclusivo” na capa. Um caça-níquel online exclusivo é um slot feito sob medida para o nosso site. Isto é, você não vai encontrar esse slot em outro lugar. Na minha opinião, tanto o RTP quanto a volatilidade do jogo Money Coming são pontos fortes. Para começar, ele conta com um RTP de 97%, o que é bem acima da média de retorno ao jogador para caça-níqueis com essa pegada mais clássica e tradicional. Ou seja, há uma boa chance de receber retornos a longo prazo jogando Money Coming. Uma das modalidades mais populares dos jogos 777 na plataforma Brazino são os slots. Os slots 777 têm uma estrutura clássica com três bobinas e símbolos como frutas, setes e estrelas. Eles atraem jogadores pela sua simplicidade e pela chance de ganhar com uma combinação de símbolos vencedores.
https://stardaylocon1972.tearosediner.net/invitrocuebrasil-com-br
Jogue Aviator na KTO! O jogo do aviãozinho te leva para o céu, onde você pode ganhar até R$500.000 em uma aposta! Aviator é um dos jogos mais populares em nosso cassino há um bom tempo, e no mês de junho ocupou a 1ª posição entre todos os nossos crash games. Outro jogo que utiliza o mesmo conceito de Aviator é o JetX, da SmartSoft Gaming. Aqui você deve sacar antes do foguete explodir. Vamos explorar os melhores jogos crash da atualidade. Falaremos sobre os mais populares, os jogos com os maiores RTPs, quais jogos oferecem os maiores pagamentos e muito mais. A empresa Darwin Gaming foi fundada em 2018 e se tornou pioneira no gênero crash games como o jogo do Foguetinho crash. A empresa se destaca pela criação de jogos simples, mas de alto engajamento, conquistando rapidamente o público com seu estilo inovador de apostas e grande potencial de prêmios no jogo do Foguetinho foguetinho.
The Real Person!
The Real Person!
« Le potentiel de gros gains et le sens de l’amusement qui font la renommée de nos jeux Big Bass Bonanza sont ici en abondance, ainsi que des fonctionnalités supplémentaires et de grands multiplicateurs. » La série Big Bass figure parmi les plus iconiques du marché des casinos. Centrée sur la pêche, elle vous propose des aventures en haute mer ou en eau profonde à travers 4 différents opus. La gamme vient cependant de s’enrichir avec un cinquième volet baptisé Keeping It Reel. Plus beau et surtout doté d’un gameplay plus volatile, il vous promet des heures de fun. Il dispose notamment d’un mode free spins vous offrant jusqu’à 20 tours gratuits et d’un jackpot de 10.000 pièces. « Le potentiel de gros gains et le sens de l’amusement qui font la renommée de nos jeux Big Bass Bonanza sont ici en abondance, ainsi que des fonctionnalités supplémentaires et de grands multiplicateurs. »
https://www.wolfpavingworkskenya.com/decouverte-complete-du-casino-en-ligne-ma-chance-pour-les-joueurs-francais/
Les opérateurs proposent souvent des tours gratuits sur les machines à sous de Noël comme offres de bienvenue pour les parieurs sportifs ou les dépôts sur les casinos en direct. Les offres de Noël peuvent inclure des tours gratuits sur Big Bass Christmas Bash avec des paris gratuits ou des bonus de dépôt, exploitant la popularité de la machine à sous saisonnière pour convertir les dépôts sur d’autres jeux. L’attention s’est portée sur l’homme le premier à arriver à la table, Croix. Pour rendre l’expérience de jeu de nos utilisateurs mobiles aussi simple et pratique que possible, ce qui en fait une méthode bancaire incroyablement simple. Voilà la liste des promotions et bonus : Heureusement, il reste fiable et apprécié pour la combinaison unique de qualité. Tout ce que vous avez à faire pour en faire l’expérience est de simplement mettre en mouvement les cinq rouleaux de la machine à sous Arctic Dreaming à partir de nos conseils ci-dessous, vous obtenez les ligues suivantes. En ce qui concerne les programmes de fidélité des casinos, pendant le bonus. Nous parlons du casino unique Batavia, tous les symboles wild qui atterrissent sur les rouleaux 2. Si vous êtes toujours intéressé par ce type de jeu de blackjack, des récompenses pour les dépôts et des points comp.
The Real Person!
The Real Person!
Met het ingaan van de wintertijd is het leuk om winterse slots te spelen. Hoewel Sugar Rush Xmas niet zoveel verschilt vergeleken met de originele Sugar Rush gokkast, heeft Pragmatic Play er toch een mooi kerstthema van weten te maken. Je kunt onze casinogidsen voor beginners raadplegen om te begrijpen hoe je online op gokkasten speelt. Als je geen tijd wilt nemen om de gidsen te lezen, kun je ook gratis spelen met de modus “Voor de lol spelen”. We also use third-party cookies that help us analyze how you use this website, store your preferences, and provide the content and advertisements that are relevant to you. These cookies will only be stored in your browser with your prior consent. Sugar Rush Xmas door Pragmatic Play Om jouw ervaring in ons online casino nog leuker te maken, bieden we exclusieve bonussen, promoties en online casino toernooien aan. Daarmee kan je winsten verdubbelen, kans maken op grote jackpots en je strategische skills tonen tegenover andere spelers.
https://ricophinar1978.iamarrows.com/klik-op-deze-link
Waan je in hogere sferen in Mandala. Werk samen om de prachtigste vormen te maken, maar zorg er voor dat jij er voordeel bij hebt. Sugar Rush Valentine’s Day, een van de valentijnsslots, van Pragmatic Play, is een slot van 5 rollen met 20 winlijnen. Er kunnen tussen de 1 en 10 munten per lijn worden ingezet, met muntgroottes variërend van € 0,01 tot € 0,50 om een groot aantal gokopties te bieden. Naast casino games biedt Luckyblock een uitgebreid sportsbook. Je kunt inzetten op populaire sporten zoals voetbal, Formule 1, MMA en basketbal. E-sports zoals e-tennis en e-cricket krijgen ook volop aandacht. Upvoted Soak Off Gel Polish #200 Sugar Rush (15ml) Upvoted Soak Off Gel Polish #200 Sugar Rush biedt langdurige, chipvrije nagels met een glanzende afwerking van 2 tot 6 weken. Deze gelpolish is eenvoudig aan te brengen, droogt snel en…
The Real Person!
The Real Person!
Rich Wilde and the Book of Dead is a 5-reel video slot game. Up to 10 lines can be activated, lines are numbered and always activated in numerical order (1, 2, 3, 4, etc.). Play and go with iconic slots from Play’n Go! Home to the legendary Book of Dead series, the squishy Reactoonz games and the cute Moon Princess slots. This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data. The Book of Dead Free Spins feature is the main attraction of this video slot. You can get 10 free spins if you land on three or more Book of Dead Scatters. During the free spins, another bonus feature is triggered, the expanding symbol.
https://alleasyshopping.com.br/exploring-rocketplay-casino-a-top-choice-for-aussie-gamblers/
Uncovering the mythical Book of Dead isn’t an easy feat. Players have been trying for a long time, and only the most courageous of discoverers have ever laid eyes on it. To become one of them, players must collect symbols, from letters to the Egyptian Gods Horus, Anubis and Osiris and Rich Wilde himself. Watch out for Rich Wilde – he carries the game’s biggest multiplier, making him a very lucrative symbol. The elusive tomb symbol is both a Scatter symbol and a Wild symbol and it pays out 200x the stake for a combination of five or more. Gambling has been around for centuries, book of dead casino login app no matter what sport you are in. We may suspend or close your account if we believe or have reason to believe that you are depositing money without intending to actually use the remunerated offer, WINNER SPORTS IS CLOSED FOR BUSINESS. Both options work very well and provide everything required for an enjoyable and carefree gaming experience, for you to play on the digital platform. What slot machines payout the most often in australia 2025 whether you are funding your account or requesting a payout, you must deposit your money first.
The Real Person!
The Real Person!
Τα σύμβολα περιλαμβάνουν διάφορα γλυκά, όπως αρκουδάκια ζελέ, καρδιές και αστέρια. Το ροζ γλυκό είναι το υψηλότερης αξίας σύμβολο, προσφέροντας έως και 150 φορές το ποντάρισμα. Live Online casino που διαθέτουν κερδοφόρα φρουτάκια Το Sugar Rush 1000 μεταφέρει τους παίκτες σε ένα πλέγμα εφτά επί εφτά και χρησιμοποιεί έναν μηχανισμό με πληρωμές για ομάδες συμβόλων. Τα κέρδη σχηματίζονται όταν οι παίκτες πετυχαίνουν πέντε ή περισσότερα όμοια σύμβολα σε μια ομάδα οπουδήποτε στην οθόνη.
https://plombier-collonges-au-mont-d-or.com/?p=15032
Lucky Roulette κουλοχέρης με δωρεάν δοκιμή There are even videos players can watch to see the entire process, its a small moment that could change your life. If you want to get the hang of playing roulette like a pro, but there are situations that happen too rarely for you to think about the long-term mathematical expectations. Additionally, including traditional Keno and MegaKeno. Save my name, email, and website in this browser for the next time I comment. Click one of our contacts below to chat on WhatsApp Συνδεθείτε για να προσθέσετε αυτό το αντικείμενο στη Λίστα Επιθυμιών σας, να το ακολουθήσετε ή να το αγνοήσετε
The Real Person!
The Real Person!
Acesse e conheça a lei de responsabilidade fiscal. É com orgulho que compartilhamos uma notícia incrível: a P&B Compliance foi ranqueada no prestigioso ranking da Leaders League como uma das melhores consultorias em Compliance no Brasil em 2025! Essa Instrumento básico da política de desenvolvimento do Munícipio O Money Coming Slot é um jogo baseado em sorte, mas o momento em que você joga pode influenciar na experiência. Muitos jogadores relatam que o horário pagante do Money Coming acontece em períodos com menos tráfego, como durante a madrugada ou manhãs de dias úteis. Emita segunda via de parcelamentos de dívida ativa. Acesse e conheça a lei de responsabilidade fiscal. Nossa equipe de profissionais dedicados ao iGaming traz uma vasta experiência e conhecimento, garantindo que entregamos informações, artigos e avaliações de alta qualidade aos nossos leitores. Vamos apresentar as mentes brilhantes por trás do nosso sucesso. Nosso propósito é ser seu guia definitivo para tudo relacionado a jogos e apostas online. Revisamos sites de cassinos e apostas online, buscamos as melhores ofertas de bônus e testamos os jogos mais recentes para que você possa fazer escolhas inteligentes e seguras ao jogar cassino online. Lembre-se: defina limites para o tempo e o dinheiro que você investe nos jogos, e jogue sempre de forma consciente e responsável.
https://cabindelux.com/5500bet-com-avaliando-a-confiabilidade-e-beneficios-para-jogadores-brasileiros/
Recomendamos este caça-níqueis clássico com RTP alto, baixa volatilidade e um tema de cassino clássico. A jogabilidade única e a atmosfera deslumbrante atrairão os jogadores para este jogo imediatamente. Um projeto ambicioso cujo objetivo é celebrar as maiores e mais responsáveis empresas de iGaming e dar-lhes o reconhecimento que merecem. Lançamos esta iniciativa com o objetivo de criar um sistema global de autoexclusão, que permitirá que os jogadores vulneráveis bloqueiem o seu acesso a todas as oportunidades de jogo online. Para jogar Money Coming demo, tudo o que você precisa fazer é encontrar um cassino online que tenha o jogo e ofereça a opção de demo grátis. Depois disso, basta abrir o jogo e testar as diversas funcionalidades sem risco algum. Cursos profissionais educacionais gratuitos para funcionários de casinos online vocacionados para as melhores práticas do setor, melhoria da experiência do jogador e uma abordagem justa ao jogo.
The Real Person!
The Real Person!
Cuando está huyendo hacia su habitación, llega Nathan, se encuentra a su mujer en la cocina y le comenta que le duele un poco la cabeza; él ofrece cancelar la mesa que tienen reservada para ir a festejar su aniversario, pero Stella le dice que no es necesario, que ya se le va a pasar. Le comenta que tiene peluquería en media hora y Nathan se ofrece a llevarla, Stella quiere que la tierra la trague porque su idea no es ir a arreglarse el cabello, o al menos no los de la cabeza, su idea es ir a encontrarse con Dale. Para los recién llegados a Sugar Rush, se aconseja comenzar explorando el modo demo. Este enfoque permite a los jugadores practicar estrategias sin poner en riesgo su bankroll. El juego recompensa a los jugadores que pueden formar grupos de 15 o más símbolos coincidentes, lo que maximiza el potencial de ganar. También es crucial utilizar la función de giros gratis, ya que ofrece oportunidades adicionales para asegurar ganancias. Monitorear su bankroll es esencial; establezca apuestas estratégicas para aprovechar la alta volatilidad del juego y las ganancias en cascada de manera efectiva. Al comprender estos elementos, los jugadores pueden mejorar sus posibilidades de éxito mientras mantienen el control sobre su experiencia de juego.
https://www.laresidenceabano.it/it/review-del-juego-balloon-de-smartsoft-emocion-y-ganancias-al-inflar-globos/
Las aportaciones de contenido reflejan las opiniones de los clientes y alojamientos de Booking, no las de Booking. Booking no se hace responsable de ningún comentario ni de las respuestas a esos comentarios. Booking es un mero distribuidor (sin ninguna obligación a verificar), no un editor o creador de estos comentarios y sus respuestas. Me encantan los cursos de mina! no se guarda nada y comparte todos sus tips! Recibe nuestras novedades en libros en tu email
Escrito por la empresaJoyas especiales hechas cuidadosamente con técnicas artesanales y en cantidades limitadas Si optáis por esta opción, os llevaremos a una de las minas modernas más famosas de Potosí. Tras 20 minutos de trayecto por carretera llegaremos al Cerro Chico, donde se encuentra la mina Kunty.
The Real Person!
The Real Person!
A gama de apostas fornecidas pelo Money Coming varia de uma aposta mínima por rodada de $ £ €0,10 até um máximo de $ £ €100,00 por rodada. Com a Brazino777, você pode jogar Money Coming com gráficos perfeitos e sem qualquer tipo de lentidão. É claro, você vai precisar de um dispositivo atualizado e uma internet estável. Recomendamos este caça-níqueis clássico com RTP alto, baixa volatilidade e um tema de cassino clássico. A jogabilidade única e a atmosfera deslumbrante atrairão os jogadores para este jogo imediatamente. Com a Brazino777, você pode jogar Money Coming com gráficos perfeitos e sem qualquer tipo de lentidão. É claro, você vai precisar de um dispositivo atualizado e uma internet estável.
https://humantouch.org.in/2025/09/22/analise-completa-do-q9bet-com-vantagens-exclusivas-para-jogadores-brasileiros/
O Money Coming (Expanded Bets) é um jogo de slot que combina elementos de entretenimento e emoção, proporcionando uma experiência envolvente. Com gráficos vibrantes e uma interface intuitiva, os jogadores são rapidamente atraídos para o universo do jogo. A mecânica de apostas expandidas permite que os jogadores ajustem suas apostas, aumentando as oportunidades de ganhar prêmios maiores. As animações fluidas e os efeitos sonoros imersivos contribuem para a atmosfera dinâmica do jogo. ✨PRIVILÉGIOS DE MEMBRO VIP✨Bônus progressivo R$ 761.600,00Salário semanal de R$ 20.000,00O maior salário mensal é de R$ 100.000,00 Contanto que você registre uma conta de membro antes de 31 de Agosto e 2025, você poderá ganhar R$ 3,00 gratuitamente.
The Real Person!
The Real Person!
Crafted for enthusiasts of classic gaming and designed with modern spins, the mega joker slot machine offers a delightful blend of nostalgia and excitement. This beloved game carries with it the essence of traditional slots while introducing contemporary elements that keep players on the edge of their seats. Nearly all casinos offer a wide selection of slots. Typically retro slots are traditional fruit slot machines. They have very traditional symbols, such as water melons, cherries, stars, BARs, 7s and lemons. Traditional, retro casino games are usually short on special features and spectacular animations. On the other hand, nowadays they, too, might come with some great surprises, such as multipliers. Not huge, true, but not bad for a classic slot with a progressive jackpot that goes into the thousands in cash. In short, much like the return to player rate, you are either going to love or hate this Mega Joker slot game, and nothing in between.
https://peponiinternational.com/football-x-by-smartsoft-a-detailed-casino-game-review-for-indian-players/
Microgaming has been around since 1994 and is a big name in the slots world. They really set the industry standard. With over 800 titles in its collection, you can play free demo slots like Mega Moolah or Tomb Raider to name a few. What is the best Mega Joker demo strategy?Players can choose base game or press Supermeter bets. Returns are higher in Supermeter, but all results remain random. The world of online gaming is vast and full of opportunities, especially when it comes to slot machines. One title that stands out among the crowd is the Mega Joker slot machine. This captivating game offers a blend of nostalgia and innovation, drawing players into an exhilarating experience filled with potential rewards. Designed by the renowned software provider NetEnt, Mega Joker’s appeal lies not only in its gameplay but also in its unique charm.
The Real Person!
The Real Person!
askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers askgamblers
https://risktakersmg.com/2025/09/26/master-joker-rodadas-de-bonus-para-maximizar-suas-chances/
O Joker’s Jewels é um caça-níquel bastante tradicional, com 3 linhas e 5 colunas. Também conhecido como jogo do coringa, o título paga com 3, 4 ou 5 combinações da esquerda para a direita, desde que em linhas vencedoras. Para aumentar as chances, há o símbolo Wild que compõe as linhas. Sendo tão popular, todos buscam onde jogar Fortune Rabbit com giros grátis em 2025. Hoje, a melhor alternativa é a BetMGM, o cassino que chegou ao Brasil este ano. Em busca de mais popularidade, a marca tem liberado rodadas grátis para os títulos mais buscados, como o Fortune Rabbit. Sendo tão popular, todos buscam onde jogar Fortune Rabbit com giros grátis em 2025. Hoje, a melhor alternativa é a BetMGM, o cassino que chegou ao Brasil este ano. Em busca de mais popularidade, a marca tem liberado rodadas grátis para os títulos mais buscados, como o Fortune Rabbit.
The Real Person!
The Real Person!
Oyunun sembolleri; Big Bass Bonanza 1000, serinin temel mekaniklerini korurken iki önemli değişiklik getiriyor: 1.000x değerli balıklar (orijinalindeki 2.000x’in yarısı) ve 20.000x’e çıkan maksimum kazanç. Pragmatic Play’in diğer “1000” serisi oyunlarına kıyasla daha az yenilik içerse de: Çıktığında, ciddi bir kesimi heyecanlandıran slot; kazanç çizgileri sayesinde, big bass bonanza hileleri eleştirilerinden kurtulabiliyor. En düşük oyun RTP’sinin %96’dan fazla olduğu oyunda; best sembollerin 500x ödemesi, slot severlerin iştahını kabartabiliyor. Çoğu slotun 500x ödemesinin olmadığını düşünen Big Bass Bonanza hayranları ise; slotu hem çevrimlerinde hemde yüksek kazanç hedeflerinde kullanabiliyor. Son aşama 10×10’da sonuca ulaşılamaması ile itham edilen oyun; hedefi yüksek olan slot severlere, keyifli vakit geçirebilme fırsatı sunuyor.
https://alpharettablinds.com/pragmatic-play-ile-buyuk-kazanc-sweet-bonanza-incelemesi/
Bigger Bass Bonanza, büyük kazanç vaat eden eğlenceli bir slot oyunu. Bu oyunu oynayarak kendi şansınızı deneyebilirsiniz. En iyi sitelerde oynayarak keyifli zaman geçirebilirsiniz. Casino siteleri çıkardıkları oyunlar ile insanları bahislere teşvik etmeyi amaçlıyor. Fakat bu amacı gerçekleştirebilmek için oyunları daha eğlenceli hale getirmeleri gerekiyordu. Bu sebeple de Big Bass Bonanza oyununu çıkardılar. Bir slot oyunu olan Big Bass Bonanza giriş için casino sitelerine üye olmak gerekiyor. Oyunun simgesi bir balıkçıdır. Bunun nedeni ise oyun içerisinde balıkların daha fazla para kazandırabilmesidir. Bigger Bass Bonanza, Pragmatic Play firması tarafından yapılan bir slot oyunudur. Ülkemizde “Balıkçı Hasan Oyunu” olarak bilinir. Oyunculara yüksek kazançlı free spin bonusları sunar. Heyecan verici bir oyun deneyimi vadediyor. En iyi sitelerde bu oyun çok tercih ediliyor.
The Real Person!
The Real Person!
Sugar Rush od Pragmatic Play przyciąga do siebie miłośników klasycznego stylu, układu 7×7 i znanych wszystkim bywalcom kasyn online owocówek. Gra pojawiła się na rynku w 2022 r. i szybko urosła na popularności, oferując 20 linii wygrywających i opcje bonusowe. Wpisz swój adres e-mail, a wyślemy Ci link do zresetowania hasła. Automat wyróżnia oryginalny koncept: zaprasza do mniej romantycznej strony Paryża. Znajdziemy tu ser, szczury czy szopa z łomem. W tym niebezpiecznym świecie zagramy na 6 bębnach i 5 rzędach, a slot urozmaicają Kaskady i Złote Kwadraty. Sugar Rush Xmas, dostarczony przez Pragmatic Play, to zachwycająca gra slotowa ze świątecznym, słodkim motywem. Ten slot działa w oparciu o system wypłat klastrowych, w którym wygrane są przyznawane za klastry pasujących symboli. Symbole te są następnie usuwane ze swoich pozycji, potencjalnie przekształcając się w Multiplier Spots. Jedną z ekscytujących funkcji, której należy się spodziewać, jest runda darmowych obrotów, w której te miejsca mnożnika pozostają aktywne przez całą rundę. Zanurzmy się głębiej w szczegóły.
https://md.fsmpi.rwth-aachen.de/s/gvS1fpqiB
Istnieje wiele możliwości znaczących wygranych, tak. Użyliśmy tego terminu, hazard polska darmową wersję można odtwarzać bezpośrednio z przeglądarki. To wyjątkowo egzotyczną odmiana, która wniesie odważne piękno do Twojego ogrodu. Żywe, czerwone paski owoców dojrzewają przez zieleń, następnie kremowy aż do pomarańczy i głębokiej czerwieni, co czyni ją jedną z najbardziej przyciągających wzrok papryczek chili. Jest to bardzo bliska kuzynka Sugar Rush Peach Striped jednak ma lekko gruszkowaty kształt. Jeśli podoba Ci się Sugar Rush 1000, koniecznie sprawdź other games w podobnym stylu oraz ekscytujące tytuły inspirowane światem casinos, gdzie cukierkowe przygody spotykają się z szansą na wielkie wygrane. Te same metody stosowane do dokonywania wpłat mogą być również używane do dokonywania wypłat, oferując ogromny bonus powitalny i oferty promocyjne. W zależności od metody płatności zostaniesz poproszony o podanie dodatkowych informacji, sugar rush gry automatowe które oferują gry karciane.
The Real Person!
The Real Person!
\nJa, PASINO.ch ist zu 100% legal in der Schweiz und arbeitet gemäss dem, am 1. Januar 2019 in Kraft getretenen, schweizerischen Bundesgesetz für Glücksspiele. Der Bundesrat hat dem Casino du Lac Meyrin-Genève eine Konzessionserweiterung mit der Nummer 2023-B-14-E gewährt. Dadurch besitzt das Casino die offizielle Genehmigung, das Angebot an Casino Spielen online auf PASINO.ch zur Verfügung zu stellen. \n\n\t Hier kannst du die besten Slot-Spiele von bekannten Spielstudios wie Greentube Novomatic, Pragmatic Play, Blueprint Gaming, Hacksaw, Hölle Games, Elk Studios, Apparat Gaming, Spinomenal, Gamevy, BW Gaming und vielen anderen spielen. Unsere beliebten Automaten garantieren erstklassiges Spielerlebnis. Viele Online-Casinos in Deutschland bieten eine Gratis-Demo von Pirates 3 an – perfekt, um das Spiel ohne echtes Geld auszuprobieren. Beispiele sind:
https://ferreiragoulartadv.com.br/ice-casino-im-test-seriositat-und-spielspas-fur-deutsche-spieler/
Exklusiver Bonus Blauer Blitz Die Grand Casino Luzern AG erfüllt die höchsten Standards in Sicherheit und Datenschutz: Gates of Olympus 1000 by Pragmatic Play takes the legendary original and supercharges it with even bigger multipliers, higher max wins, and nonstop excitement. With its tumbling mechanics, powerful bonus features, and the chance to hit a 1000x multiplier, it’s easy to see why this slot has captured so much attention. Wenn ihr Gates of Olympus spielen möchtet, könnt ihr euren Einsatz bereits ab einem Betrag von 0,20 Euro pro Runde wählen. Dieser Einsatz wird mit Sicherheit auch Einsteiger mit Sicherheit vor keinerlei Probleme stellen. Auch die Auszahlungsquote von Gates of Olympus kann mit einem Wert von insgesamt 96,5% in jedem Fall überzeugen. Daher konnten wir in unserem Testbericht bereits zu Beginn erkennen, dass in jedem Fall starke Gewinne durch diesen ausserordentlichen Spielautomaten von euch erzielt werden können.
The Real Person!
The Real Person!
Sugar Rush, birçok farklı platformda oynanabilir, bu nedenle geniş bir oyuncu kitlesine sahiptir. Sugar Rush, oyunculara tatlı bir dünyada macera sunan bir video oyunudur. Oyuncuların hedefi, çeşitli seviyelerde şekerleri toplamak ve engelleri aşmaktır. Sugar Rush, renkli ve eğlenceli temasıyla dikkat çeken bir mobil oyun. Oyun, oyuncuların şekerleri toplayarak puan kazanmasını sağlıyor. Çeşitli seviyelerde zorluklar ve farklı görevler bulunuyor. Aquí encontrarás noticias sobre el sector, tendencias e información de interés. E-cüzdanlar ödeme yapmak için çok kullanışlı yollardır, herhangi bir iOS ve Android cihazında bir mobil tarayıcıyla IceBet web sitesine giderek kolayca erişebilirler. BetRivers Casino’daki en geniş casino oyunları çeşitliliği, ad. Üçünün de tamamen sınırsız olduğu bazı eyaletler var, e-posta kimliği ve iletişim numarası gibi temel kişisel veriler kullanılarak yapılmalıdır.
https://cakansimsekplanlama.com.tr/sweet-bonanza-incelemesi-%e2%8e%af-turkiye-icin-online-casino-oyunu/
Aviator oyna için illegal casinolar veyahut tercih edebileceğiniz kaçak bahis sitelerinin adresi olacaktır. Aviator hilesi ve benzeri oyun stratejileri genellikle oyunun kurallarına aykırı olup, yasal olmayabilir. Aviator hilesi, oyunda fayda sağlamak için kullanılan stratejilere verilen isimdir. Aviator oyunundaki yapay zeka düşmanları, engelleri aşmak için sizi takip ederler. Mostbet Apostas Esportivas & Cassino Content Benefícios Afin De Os Usuários Brasileiros O Mostbet É O Agente Para Apostas Mais Well-known? Como Ze Registrar – Instruções Para Novos Jogadores Aplicativo De Uma Mostbet Disponível Em Virtude De Android E Ios Informações Sobre Some Sort Of Mostbet Br Jogo De Pôquer Roleta League Of Tales (lol)… Eğer herhangi bir sorunla karşılaşırsanız, basari guess şikayet bölümünden destek alabilirsiniz. Basaribet papara, pra tarnsferi ve kripto olmak üzere birçok ödeme yönteminin bulunuyor olması seçim şansını size vermektedir. Daha sonra, Basaribet giriş yaparak siteye erişebilir ve oyun oynamaya başlayabilirsiniz.
The Real Person!
The Real Person!
ウィリアムヒルは、世界最大のブックメーカーの1つ。日本のプロ野球やサッカーを中心に、世界の主要なスポーツに賭けられるのが魅力です。 現在、オンラインカジノ業界ではVIPやハイローラーに憧れていたり、なりたい人がたくさんいます。各オンラインカジノでのVIPプログラムには、VIPやハイローラーになるための明確な基準を提示しているメーカーと一切していないメーカーが存在します。メーカーによって異なりますが、VIPになるためには特定の期間の入金額やベット金額などを条件としているところが多く、一般的なユーザーと比べてハイローラーのほうがVIPと認定されやすい傾向があります。
https://samarnazliftsltd.com.bd/%ef%bc%9a/
カジノゲームに熱中しすぎている場合気づかないうちに、想定以上の損失額が発生している可能性があります。 カジノゲームで遊ぶ際には1日で負けた額の把握や、連敗が続いた時の損切りタイミングを必ず決めましょう。 複数通貨のマルチウォレット対応カジノ! 入出金にはクレジットカードのVISA、Master、JCB、そして電子決済サービスのエコぺエイズ(ecoPayz)、アイウォレット(iWallet)、ビーナスポイント(VenusPoint)、さらに人気になっている仮想通貨が使用出来るクリプトアンドゴー(Crypto&Go)、パープルペイ(Purple pay)の決済に対応しています。多くの決済サービスを扱っているのに、日本円で入出金できないのが、残念なところ。現在利用できる通貨はアメリカドルのみです。
The Real Person!
The Real Person!
W każdą niedzielę za Vinci Spin casino logowanie i wpłatę można odebrać bonus doładowania na 100% do 4.000 zł. To opcja dla najwyższego poziomu konta. To jednorazowa oferta dla aktywnych graczy, którzy logują się i wpłacają środki w weekend. Bonus dla podstawowego konta wynosi 50% do 200 zł. HotSlots proponuje swoim klientom kasyno po polsku, dzięki któremu znajdziemy najlepsze gry hazardowe w Internecie. Poznaj aktualną kolekcję automatów hazardowych i klasycznych gier hazardowych takich jak Ruletka, Poker lub BlackJack. Aby Twoje przeżycia w HotSlots były od samego początku udane przekazujemy do Twojej dyspozycji atrakcyjną ofertę powitalną w postaci dwóch bonusów zależnych od depozytu: Ogromny bonus 100% przy pierwszej wpłacie do 1000 zł a także 50 Free Spins na slot Gates of Olympus! Poznaj kolejne plusy dzięki którym warto założyć konto w HotSlots!
https://qualitypoint.com.do/amunra-casino-kompletny-przewodnik-po-rejestracji-logowaniu-i-odzyskiwaniu-konta/
Witamy w ekscytującym świecie HotSlots! Gates of Olympus za darmo możecie włączyć na komputerze, smartfonie, tablecie lub dowolnym innym urządzeniu przenośnym czy też stacjonarnym. Została zaprojektowana w taki sposób, aby można było ją otworzyć bez potrzeby pobierania. Gra wykorzystuje HTML5, zamiast Flash Playera. Kasyna z Gates of Olympus, jak i sam automat wspierają również urządzenia mobilne. Slot został zaprojektowany w nowoczesnej technologii HTML5. Dzięki niej Gates of Olympus na telefon jest dostępny bez konieczności instalacji – grasz bezpośrednio w oknie przeglądarki. Wskaźnik Return To Player (RTP) dla tej gry slotowej wynosi 96%. Oznacza to, że 96% wszystkich zakładów postawionych przez graczy zostanie zwrócone jako wygrane w czasie. Jest to slot o średniej zmienności, co oznacza, że oferuje zarówno duże szanse na regularne wygrane, jak i wysokopłatne wygrane, które pojawiają się rzadziej, ale oferują większe nagrody, kiedy już się zdarzą.
The Real Person!
The Real Person!
Figma review compared to Easy Cut Studio for Windows VivaCut – Pro Video Editor is a professional video editing and collage app. You can use it to make a gorgeous compilation, edit videos,… Feb 7, 2023Version 1.1-bug fixes Just upgraded to easy cut studio v6, and very happy with the new version! Everything is working smoothly. Highly recommend this vinyl cutting software solution for both Windows and Mac users. How can I send large video on WhatsApp? What is the video size limit for WhatsApp? How do I compress a video for WhatsApp on Mac? This post shares 2 easy ways to compress video for WhatsApp. Video Players & Editors moddroid not only provides originalEasyCut 1.7.5.2001 completely free, but also attaches the mod version, providing you with Premium Unlocked functions for free, you can experience the highest level of EasyCut 1.7.5.2001 with the most complete functionality. Moreover, all mods have been manually authenticated by moddroid, it is 100% free and available. Now, you only need to download moddroid to the client, you can download and install the Premium Unlocked mod version EasyCut 1.7.5.2001 with one click, and then enjoy The convenience brought by EasyCut!
https://taxigrouphanoi.com/money-coming-spin-real-money-indian-players-guide-to-winning.html
With such a reputation, it is surprising that Pragmatic has built a library of some of the most popular slots in the world. The truth is, their back catalogue is so vast that it can be hard to decide where to start! That’s why we have decided to share with you some of our favourites to get you started. Sp, without further ado, let’s jump into a world of Ancient Greek Gods, wild wolves, mariachis and rhinos in our exclusive Top 10 Pragmatic Play slots. Pragmatic Play is known for its engaging, feature-rich online slots like Gates of Olympus. The game features Pragmatic Play’s signature style – smooth gameplay, innovative features, and a lot of bonus features to keep players entertained and increase their chances of winning. Discover the ultimate destination for timeless fun at The House of Classic Games!
The Real Person!
The Real Person!
Die durchgestrichenen Preise entsprechen dem bisherigen Preis in diesem Online-Shop. Whether you consider Pirots 3 to be the best thing since sliced bread or a mouldy old bag of apples, we can probably all agree it is absolutely stacked to the rafters with extras. Even more so than the last two games, which were already rich feature-filled slots. Much of the core gaming in Pirots 3 is the same or at least close to Pirots 2, but there is plenty of new stuff here to encourage a look, especially if you liked what came before. It may take time for the birds to do more than pick up a few low-value gems, but when they do, and you can get on the positive side of the game’s volatility, then Pirots 3 is able to set up lengthy sequences of events, that might, if lucky, lead to a sizeable monetary result.
https://vizenegocios.com.br/umfassender-uberblick-zu-nine-casino-das-online-casino-fur-deutsche-spieler/
Das Casino hat einen großen Bereich an Casino-Bonusspielen. Dies sind Spiele, bei denen Sie nicht auf die Bonusrunde warten müssen. Es kann gekauft werden, was die Gewinnchancen erheblich erhöht. Hier sind einige Beispiele solcher Spiele: Mystery Mice (PragmaticPlay), Samurai Code (PragmaticPlay), Sunset Delight (Thunderkick), The Rift (Thunderkick), Wolf Gold (PragmaticPlay). Spieleart Spiele We go beyond the headline numbers. You’ll also get clear info on: Lande das Scatter Symbol 3-, 4- oder 5-mal und du bekommst 9, 12 oder 15 Freispielrunden. Diese können weitere Freispielrunden erbringen bzw. du kannst mit der Gamble-Funktion weitere Freispielrunden freischalten. Bei Fortunes of Giza dreht sich alles um das Bonus-Feature. Ansonsten ist nicht viel los, abgesehen von den Jokersymbolen, die im regulären Spiel auftauchen. Verdienen die “Book of”-Titel noch ihren Platz am Fließband? Von den Bonusfunktionen her könnte man sagen, dass sie das tun, aber was das Gesamtpaket angeht, sollten sie unserer Meinung nach überarbeitet werden.
The Real Person!
The Real Person!
Com um cenário vulcânico, o jogo repete a jogabilidade de seu antecessor, com acesso a jogos de bônus acalorados, que são obtidos ao conseguir três símbolos em uma única linha de pagamento. A nova edição aumenta o potencial de ganhos significativos, estendendo a ação para uma grade de rolos maior. Com um cenário vulcânico, o jogo repete a jogabilidade de seu antecessor, com acesso a jogos de bônus acalorados, que são obtidos ao conseguir três símbolos em uma única linha de pagamento. A nova edição aumenta o potencial de ganhos significativos, estendendo a ação para uma grade de rolos maior. Com um cenário vulcânico, o jogo repete a jogabilidade de seu antecessor, com acesso a jogos de bônus acalorados, que são obtidos ao conseguir três símbolos em uma única linha de pagamento. A nova edição aumenta o potencial de ganhos significativos, estendendo a ação para uma grade de rolos maior.
https://colegioarteyculturasanantonio.cl/2025/10/22/spaceman-da-pragmatic-play-review-completo-para-jogadores-do-brasil/
You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. Pontos do Play Points O sistema de recompensas da Mostbet é super generoso. Sabia que Mostbet oferece duas opções de boas-vindas: uma com depósito e outra sem depósito? No site oficial, a Mostbet tem bónus expressos, cashback, várias promoções de recarga, giros grátis, um programa de referência e até um programa de fidelidade. Mostbet pensa em tudo para o recompensar! You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page. You can email the site owner to let them know you were blocked. Please include what you were doing when this page came up and the Cloudflare Ray ID found at the bottom of this page.
Its superb as your other posts : D, regards for posting. “Reason is the substance of the universe. The design of the world is absolutely rational.” by Georg Wilhelm Friedrich Hegel.
Lovely just what I was searching for.Thanks to the author for taking his clock time on this one.
The Real Person!
The Real Person!
Si vous aimez l’histoire, en particulier celle de la Grèce antique, Booming Games a pris le soin de concevoir une machine à sous avec vos personnages préférés. Le slot Power of Olympus est composé de 7 bobines, d’une disposition 7×7, et d’un cluster payant. La volatilité de cette machine à sous est élevée, avec un RTP par défaut de 95,6 %, et un gain maximum possible de 8 066x la mise pour 0,20 centime à 20 € à chaque spin. Wild Sultan se distingue par la rapidité de traitement de ses retraits, souvent validés en moins de 24 heures. Sa ludothèque est immense et parfaitement organisée, ce qui en fait un choix de premier ordre pour trouver les jeux Pragmatic Play. Wild Sultan se distingue par la rapidité de traitement de ses retraits, souvent validés en moins de 24 heures. Sa ludothèque est immense et parfaitement organisée, ce qui en fait un choix de premier ordre pour trouver les jeux Pragmatic Play.
https://portal.urgentcareofkansas.com/?p=77254
Nos rédacteurs examinent à la fois l’ampleur et la générosité de ces promotions, ce film est pourtant l’un de ceux qui traitent du casino avec le plus de finesse et de profondeur. Tours et bonus en big bass splash même si le joueur a la chance d’obtenir la combinaison convoitée et que son solde augmente de mille mises, certaines versions ont jusqu’à 10 balles en jeu à la fois. C’est l’étalon-or dans tous les secteurs en ligne à l’heure actuelle, le montant que vous souhaitez déposer et votre code PIN sécurisé et vous êtes prêt à partir. JetX Parimatch Casino Le code promotionnel 1xCasino est JBVIP. Nous accordons la priorité à une assistance complète aux joueurs, répondant à la fois à leurs besoins techniques et informatifs. Si vous avez des questions, notre chat en ligne sur le site est disponible pour une assistance rapide, vous assurant une consultation dans les minutes qui suivent. De plus, nous offrons une assistance téléphonique et vous pouvez nous contacter par le biais de plusieurs adresses e-mail officielles, ce qui vous permet de bénéficier de plusieurs canaux de communication.
The Real Person!
The Real Person!
Best Free iPhone Video Editing Apps for Professional Results OpenShot™ is free software: you can redistribute it and or modify it under the terms of the GNU General Public License as published by the Free Software Foundation, either version 3 of the License, or (at your option) any later version. Capcut is a powerful yet simple app for video editing designed for the modern creator. Developed by ByteDance, this mobile app has a toolset for users from beginners to pros, especially those who focus on short-form video for social media. If you want to create the next Tenet on your phone, though, Quik isn’t the app for you. It doesn’t support 4k editing, and while you can have multiple audio tracks, the same does not apply to video. The user-friendly video editor appeals to people who want to create videos quickly.
https://lotus-et-nuage.com/balloon-game-cash-instant-withdrawals-for-indian-players/
That’s said, your Android devices should feature at least 1.5 of unallocated RAM to be installed and run. And to allow it to perform properly, your system must feature a quad-core processor, 4GB of RAM, or more. And most importantly, make sure that you keep your Alight Motion app updated frequently so you won’t be missing on any of its features. Premium ActivatedAfter downloading from my site, Alight Motion is already premium activated; you don’t need to buy subscriptions or any activation for the Alight Motion. Follow the steps above to install the latest fully unlocked app from my site, and start using the pro version for free. If you’re looking to download an old version of Alight Motion, you can do so easily and safely by following the link provided. All the APK files available are free from viruses and fully functional, so you don’t need to worry about anything. If you already have the latest version of Alight Motion installed and want to switch to an older version, you’ll first need to uninstall the current version. After that, you can go to your app settings and click on “allow from an unknown source” to download and install the older version.
Hiya, I am really glad I have found this info. Today bloggers publish only about gossips and internet and this is actually irritating. A good web site with exciting content, that’s what I need. Thank you for keeping this site, I’ll be visiting it. Do you do newsletters? Can’t find it.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. https://www.binance.com/register?ref=IHJUI7TF
Good website! I really love how it is simple on my eyes and the data are well written. I am wondering how I might be notified whenever a new post has been made. I’ve subscribed to your feed which must do the trick! Have a nice day!
Just what I was searching for, appreciate it for posting.
Attractive section of content. I just stumbled upon your site and in accession capital to assert that I get actually enjoyed account your blog posts. Any way I will be subscribing to your feeds and even I achievement you access consistently quickly.
Somebody essentially help to make severely articles I might state. This is the first time I frequented your website page and so far? I amazed with the research you made to make this actual put up extraordinary. Wonderful process!
I?¦ve been exploring for a little bit for any high-quality articles or blog posts on this kind of space . Exploring in Yahoo I ultimately stumbled upon this website. Reading this info So i am satisfied to show that I’ve an incredibly just right uncanny feeling I came upon just what I needed. I such a lot no doubt will make sure to don?¦t overlook this web site and give it a glance on a relentless basis.
I will right away grab your rss as I can not in finding your e-mail subscription link or newsletter service. Do you have any? Please let me know in order that I could subscribe. Thanks.